Abstract
Psychiatric disorders co-occur very frequently with epilepsy. This guideline covers the etiopathogenesis, presentation, evaluation and management of various psychiatric disorders in epilepsy such as mood, anxiety, psychotic and substance use disorders. It also provides an approach to important special issues in this population.
Keywords: Epilepsy, management, psychiatric disorders
The International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy defined an epileptic seizure in 2005 as “a transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain.” It is a disorder of the brain that leads not only to induction of seizures but also to neurological, cognitive, and psychosocial consequences.[1] In 2014, ILAE gave an operational definition of epilepsy which requires “ two unprovoked seizures separated by more than 24 h, or one unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after two unprovoked seizures, occurring over the next 10 years or diagnosis of an epilepsy syndrome.”[2]
Epilepsy is a common neurological disorder. It is seen in nearly 70 million people worldwide. 90% of cases of epilepsy belong to developing countries. The prevalence rate in India varies from 1.2 to 11.9/1000 among adult population.[3]
Psychiatric disorders occur very often in patients with epilepsy. Comorbidity with epilepsy is a condition that occurs along with epilepsy. It may pre-exist even before the onset of epilepsy, may be the cause or the consequence of epilepsy, and may occur any time during the course of the disorder.[3] Psychiatric disorders commonly seen in epilepsy include depression, anxiety disorders, psychosis, personality change, cognitive abnormalities, and attention deficits.
Psychiatric disorders go unnoticed in these patients as control of seizures becomes the focus of management, and many a times, clinicians are not aware that psychiatric disorders may occur in patients with epilepsy.[4] Psychiatric disorders cause poor response to treatment, affect the quality of life of the patient, and increase the risk of early death due to suicide or accidents. Hence, it is important to treat them.[5]
Psychiatric disorders are more prevalent in patients with epilepsy than in general population. Various population studies have reported a prevalence of psychiatric disorders ranging from 5.9% to 54.9%, maximum being 80% in some patients of temporal lobe epilepsy (TLE). In two Indian studies, psychiatric disorders were found to be more prevalent than in those with other chronic illnesses, such as asthma, and healthy controls.[3,6]
Hospitalized psychiatric patients showed more prevalence of epilepsy than general population. A British psychiatric hospital had a prevalence of 4.7% and it was found to be 9.7% in a US Veterans Affairs psychiatric hospital. Approximately 30% of patients attending epilepsy clinics had a history of psychiatric hospitalization, and 10%–20% were on some psychiatric medication.[3]
Etiology and risk factors for developing psychiatric disorders in epilepsy are summarized in Table 1.[3,6,7]
Table 1.
Etiology and risk factors for psychiatric disorders in epilepsy
| Etiology | Risk factors |
|---|---|
| Biological | Type of epilepsy, severity of epilepsy, ictal and interictal neuronal activity, disturbances in sleep–wake schedule, traumatic brain injury |
| Psychosocial | Stigma, constraints in lifestyle, low self-esteem, limitations in vocations and educational achievement, dependence on others for socioeconomic needs, poor social support |
| Treatment related | Poor adherence to treatment, polytherapy, side effects of antiepileptic medication |
BIDIRECTIONAL HYPOTHESIS
Various epidemiological studies suggest a bidirectional relationship between epilepsy and psychiatric disorders. Depression, anxiety disorders, and psychotic disorders do not occur only secondary to epilepsy but can also precede epilepsy, indicating that there may be some common psychopathology involved. In depression, the proposed common pathophysiological changes are seen in hyperactivity of hypothalamic–pituitary–adrenal axis and changes in glutamatergic and GABAergic systems. Structural changes in the form of decreased volume of hippocampus and frontal lobes are seen both in depression and epilepsy. Psychotic disorders share a common pathophysiology with epilepsy in the form of dopamine overactivity in mesial temporal regions and limbic system along with a decreased dopamine activity in ventrolateral and dorsolateral prefrontal cortices.[3,8]
DEPRESSION
A meta-analysis by Scott et al. in 2017 gave a pooled prevalence of depression in epilepsy as 22.9%.[9] The Canadian Community Health Survey conducted in 36,984 people described a prevalence of major depressive disorders as 17.4%[10] and EPIC study from the US gave a prevalence of 32.5%.[11] Disability due to epilepsy, stigma experienced by persons with epilepsy (PWE), and poor social support act as the risk factors for developing depression. PET studies have demonstrated deficits in 5HT1A receptor binding in medial temporal regions seen both in depression and TLE. Some of the neuronal networks in the frontal and temporal regions may be involved both in causation of frontal and temporal epilepsy and in dysregulation of mood and behavior. Depression adversely affects the course of the illness in epilepsy. It leads to poorer quality of life, poorer response to treatment, and poorer results after surgery.[12]
PSYCHOSIS
Psychosis is seen in 7%–12% patients of epilepsy. The risk of psychosis increases 5–8 times in patients with epilepsy as compared to that in general population. 7% of patients of TLE develop psychotic disorders as against 5.6% in other types of epilepsy.[13]
Risk factors for developing psychotic disorders are:[8,13]
Severe cases of epilepsy
Family history of epilepsy and family history of schizophrenia
Epilepsy of temporal lobe origin (TLE), especially in those having a history of febrile seizures
Hippocampal sclerosis on magnetic resonance imaging
Presence of autoantibodies such as anti-NMDA, anti GABA-B receptor, and anti-voltage gated potassium channel (seen in 10% of patients).
Psychosis can occur as ictal, postictal, and interictal phenomenon. A phenomenon of “forced normalization” has been described in patients in whom psychotic episode emerges when seizures seem to be under control. A chronic schizophrenia-like psychosis is also seen.
ANXIETY DISORDERS
A meta-analysis by Scott et al. in 2017 gave a pooled prevalence of anxiety disorders in epilepsy as 20.2%. EPIC study from the US gave a prevalence of 22.4%.[9,11] Anxiety disorders in patients with epilepsy go unnoticed because of number of reasons. Anxiety disorders coexist with depression very often. Many a times, a panic attack is mistaken for ictal fear.[8] Sometimes, they are considered as a natural emotional reaction to having a diagnosis of epilepsy and a consequence of functional limitations caused by it. Panic disorder, generalized anxiety disorder, agoraphobia, social phobia, and rarely obsessive–compulsive disorder are seen in patients with epilepsy. While diagnosing these anxiety disorders, thyroid and other endocrine abnormalities and medication side effects need to be ruled out.[7]
ICTAL, POSTICTAL, AND INTERICTAL PSYCHIATRIC DISORDERS
Behavioral disturbances and psychiatric syndromes occur during ictal, peri-ictal, postictal, and interictal period. They have characteristic features. They need to be identified and treated along with the seizure disorder. Table 2 gives a summary of such psychiatric syndromes.[14,15]
Table 2.
Ictal, peri-ictal, postictal, and interictal psychiatric disorders
| Depression | Anxiety | Psychosis | |
|---|---|---|---|
| Ictal | Less common than anxiety disorders, present with guilt, hopelessness, worthlessness, and suicidal ideation | Fear as a part of aura, one-third of patients of partial seizures involving right temporal foci | Associated with partial seizures. Present with ill-defined visual, gustatory or auditory hallucinations |
| Postictal | May last for 2 weeks. May range from mild to severe associated with suicidal ideas. More common in the right temporal and frontal foci | Seen less commonly than depression | Follows a cluster of complex partial seizures with or without secondary generalization. Onset of psychosis is after 12-72 h of lucid interval. Symptoms of delusions, hallucinations, manic features Course is transient, but may persist for many weeks. May be recurrent |
| Interictal | Seen in drug-resistant epilepsy, TLE with hippocampal sclerosis, hypoperfusion of bifrontal and temporal regions, Stigma, dissatisfaction with life also seen. Symptoms of sadness of mood, loss of interest, anhedonia, and sleep or appetite disturbances | Associated with left-sided TLE. Stigma, functional difficulties may cause anxiety | Chronic schizophrenia-like disorder. Onset is after more than 10 years of epilepsy. More often in patients with an early age of onset, poor response to treatment and bitemporal foci, more with left-sided foci |
TLE – Temporal lobe epilepsy
EVALUATION AND ASSESSMENT
A detailed evaluation for psychiatric disorders in epilepsy is provided in Table 3.
Table 3.
Evaluation and assessment
| Clinical history of epilepsy | Age at onset, clinical features including impairment in consciousness, nature of seizures, generalized/focal, type of seizure |
| Clinical history of psychiatric disorders | History suggestive of depression, anxiety, psychosis, attention deficit disorder, cognitive impairment, suicidality, aggression. Relationship to seizures, e.g., postictal, ictal, interictal |
| Past and family history | Psychiatric disorders, epilepsy |
| Impact of illness | Patient’s understanding about the illness, their concerns, functional difficulties, perceived social support, and stigma |
| Treatment history | Epilepsy, psychiatric disorders - response, side effects |
| Assessment of caregivers | Caregiver burden, understanding of the nature of the illness, their concerns, social support, and stigma |
| Clinical examination | Neurological examination, mental status examination |
| Investigations | EEG, video EEG, MRI, CT scan |
| Psychometric tests | Rating scales, cognitive tests |
EEG – Electroencephalography; MRI – Magnetic resonance imaging; CT – Computed tomography
A variety of psychometric scales and tests have been used to assess, quantify, and monitor psychiatric issues in epilepsy and have been specifically validated for use in this population.[16,17,18,19] Table 4 lists the same. Since the status of availability of scales can change from time to time, it is recommended to check the latest updates online before using them.
Table 4.
Scales for assessment of psychiatric disorders in epilepsy
| Disorder/domain | Validated tools |
|---|---|
| Depression | NDDI-E |
| PHQ-9 | |
| Beck depression inventory-II | |
| HADS | |
| MDI | |
| Anxiety | GAD-7 |
| HADS-A | |
| Personality disorders | BFI |
| MMPI-2 | |
| Aggression | BDHI |
| AQ | |
| Suicidality | Item 4 of the NDDI-E |
| Item 9 of the PHQ-9 | |
| MINI suicidality module | |
| Cognitive deficits | MoCA |
| MMSE | |
| WAIS and WISC |
NDDI-E – Neurological disorders depression inventory for epilepsy; PHQ-9 – Patient health questionnaire-9; HADS – Hospital Anxiety and Depression Scale; MDI – Major depression inventory; GAD-7 – Generalized anxiety disorder-7; HADS-A – Hospital Anxiety and Depression Scale for anxiety; BFI – Bear-Fedio inventory; MMPI-2 – Minnesota multiphasic personality inventory-2; BDHI – Buss-Durkee hostility inventory; AQ – Aggression questionnaire; MINI – Mini-international neuropsychiatric interview; MoCA – Montreal cognitive assessment; MMSE – Mini–mental state examination; WAIS – Wechsler Adult Intelligence Scale; WISC – Wechsler Intelligence Scale for Children
MANAGEMENT OF PSYCHIATRIC DISORDERS IN EPILEPSY
Pharmacotherapy
There is a paucity of data on the treatment of psychiatric issues in epilepsy patients due to lack of systematic studies [Table 5]. Existing guidelines rely heavily on data from clinical experience and open-label studies and generally recommend following similar treatment considerations as those in nonepilepsy individuals. However, there are certain principles of pharmacological management which the clinicians need to know while prescribing psychotropic medications in patients with epilepsy.
Table 5.
Dosages of commonly used drugs for treatment of psychiatric issues in epilepsy
| Name | Usual adult dose range (mg) |
|---|---|
| Antidepressants | |
| TCAs | |
| Amitriptyline | 100-200 |
| Nortriptyline | 50-200 |
| SSRIs | |
| Citalopram | 20-60 |
| Escitalopram | 5-30 |
| Fluoxetine | 10-80 |
| Sertraline | 25-200 |
| Paroxetine | 10-60 |
| Fluvoxamine | 100-300 |
| SNRIs | |
| Venlafaxine | 75-375 |
| NaSSAs | |
| Mirtazapine | 15-60 |
| NDRIs | |
| Bupropion | 150-450 |
| Antipsychotics | |
| FGAs | |
| Haloperidol | 2-20 |
| Trifluoperazine | 10-30 |
| Chlorpromazine | 200-1000 |
| SGAs | |
| Olanzapine | 5-20 |
| Risperidone | 2-16 |
| Quetiapine | 150-800 |
| Lurasidone | 40-160 |
| Amisulpride | 300-1200 |
| Aripiprazole | 10-30 |
| Ziprasidone | 40-160 |
TCAs – Tricyclic antidepressants; SSRIs – Selective serotonin reuptake inhibitors; SNRIs – Serotonin and norepinephrine reuptake inhibitors; NaSSAs – Noradrenergic and specific serotonergic antidepressants; NDRIs – Norepinephrine and dopamine reuptake inhibitors; FGAs – First generation antipsychotics; SGAs – Second generation antipsychotics
Principles of pharmacotherapy
Pharmacokinetic interactions
The older Anti-epileptic drugs (AED) such as carbamazepine (CBZ), phenytoin, and phenobarbitone cause induction of the cytochrome P450 (CYP450) and UDP-glucuronosyltransferase (UGT) enzyme systems. On the contrary, valproate (VPA) has a broad-spectrum inhibitory action on CYP and UGT enzymes. As a result, pharmacokinetic interactions with a lot of antidepressants, antipsychotics, and other psychotropic agents are expected [Table 6].[20] Fewer pharmacokinetic interactions are seen with newer AEDs. Particularly, oxcarbazepine and topiramate (TPM) have been shown to be weak inducers, particularly at high doses.[21]
Table 6.
Drug interactions
| Drug class | Examples | Drug interactions with AEDs |
|---|---|---|
| Antidepressants | ||
| TCAs | Amitriptyline, imipramine, clomipramine, nortriptyline, maprotiline | Pharmacokinetic interactions with inducers (generally not clinically relevant). Seizures (>200 mg) |
| SSRIs | Citalopram, escitalopram, fluoxetine, sertraline paroxetine, fluvoxamine | Pharmacokinetic interactions with inducers |
| SNRIs | Venlafaxine, duloxetine, desvenlafaxine, milnacipran | Interactions with enzyme inducers |
| NaSSAs | Mirtazapine | Interactions with enzyme inducers |
| NDRIs | Bupropion | Interactions with enzyme inducers. Seizures (>450 mg) for immediate release formulation |
| Antipsychotics | ||
| FGAs | Chlorpromazine, fluphenazine, haloperidol, pimozide, thioridazine, trifluoperazine | Interactions with enzyme inducers |
| SGAs | Olanzapine, risperidone, amisulpride, quetiapine, aripiprazole, ziprasidone, asenapine, paliperidone, clozapine | Pharmacokinetic interactions with inducers. Increased risk of seizures with clozapine |
| Psychostimulants | Methyphenidate, atomoxetine, dexamphetamine | Interactions with enzyme inducers (methyphenidate) |
TCAs – Tricyclic antidepressants; SSRIs – Selective serotonin reuptake inhibitors; SNRIs – Serotonin and norepinephrine reuptake inhibitors; NaSSAs – Noradrenergic and specific serotonergic antidepressants; NDRIs – Norepinephrine and dopamine reuptake inhibitors; FGAs – First-generation antipsychotics; SGAs – Second-generation antipsychotics; AEDs – Anti epileptic drugs
Among antidepressants, a 25% reduction in the plasma levels of selective serotonin reuptake inhibitors (SSRIs), mirtazapine, venlafaxine, and bupropion (up to 90%) is seen.[21] Interactions between older AEDs and tricyclic antidepressants (TCAs) are generally not clinically relevant. VPA has been seen to cause a slight increase in the plasma levels of O-desmethylvenlafaxine (active metabolite of venlafaxine) but does not cause interactions with other antidepressants. Similarly, CBZ causes a reduction in the plasma levels of all typical antipsychotics. Among atypical antipsychotics, reductions in the plasma levels have been noted for aripiprazole, clozapine, olanzapine, paliperidone, quetiapine (can reduce to undetectable levels), risperidone, and ziprasidone.[21] Interactions between VPA and antipsychotics generally have no clinical relevance and may be considered only on an individual basis.
Pharmacodynamic interactions
There are both positive and negative types of pharmacodynamic interactions. For example, interactions between AEDs and second-generation antipsychotics (SGAs) are well known and are commonly utilized in the management of mania. The negative ones are more commonly known due to their propensity to cause side effects [Table 7]. In general, negative pharmacodynamic interactions between antipsychotics and AEDs that can be of clinical relevance include sedation, weight gain, and hematologic side effects.
Table 7.
Side effects of medications
| Drug class | Examples | Side effects |
|---|---|---|
| Antidepressants | ||
| TCAs | Amitriptyline, imipramine, clomipramine, nortriptyline, maprolitine | Sedation, weight gain, sexual dysfunction, urinary retention |
| SSRIs | Citalopram, escitalopram, fluoxetine, sertraline, paroxetine, fluvoxamine | Increased risk of hyponatremia, sexual dysfunction, weight gain (especially citalopram) |
| SNRIs | Venlafaxine, duloxetine, desvenlafaxine, milnacipran | No specific pharmacodynamic interactions |
| NaSSAs | Mirtazapine | Weight gain and sedation |
| NDRIs | Bupropion | Seizures (>450 mg) for immediate release formulation |
| Antipsychotics | ||
| FGAs | Chlorpromazine, fluphenazine, haloperidol, pimozide, thioridazine, trifluoperazine | Sedation and weight gain |
| SGAs | Olanzapine, risperidone, amisulpride, quetiapine, aripiprazole, ziprasidone, asenapine, paliperidone, clozapine | Sedation and weight gain (particularly olanzapine) Seizures (clozapine) Agranulocytosis (clozapine with CBZ) |
TCAs – Tricyclic antidepressants; SSRIs – Selective serotonin reuptake inhibitors; SNRIs – Serotonin and norepinephrine reuptake inhibitors; NaSSAs – Noradrenergic and specific serotonergic antidepressants; NDRIs – Norepinephrine and dopamine reuptake inhibitors; FGAs – First-generation antipsychotics; SGAs – Second-generation antipsychotics; CBZ – Carbamazepine
Psychotropics and seizure threshold
When it comes to drug-related seizures, the risk of seizure induction is generally associated with a higher-dose or overdose, rapid up-titration, abrupt withdrawal (for example, benzodiazepines [BZDs]), pharmacokinetic effects (enzyme inhibition, leading to increased levels) or pharmacodynamic effects on seizure threshold [Table 8]. These particularly hold true for inpatients with epilepsy and those with co-existing neurological disorder (brain trauma, dementia). Among antidepressants, seizures are likely to be induced with clomipramine and amitriptyline in doses >200 mg and maprotiline and bupropion immediate release formulation in doses >450 mg.[21]
Table 8.
Psychotropics associated with reduction in seizure threshold
| Antidepressants |
| High risk: TCAs (high doses>200 mg), bupropion (>450 mg), maprotiline |
| Moderate risk: Trazodone, vilazodone, venlafaxine |
| Low risk: SSRIs, mirtazapine |
| Antipsychotics |
| High risk: Clozapine, chlorpromazine, loxapine, zotepine |
| Moderate risk: Olanzapine |
| Low risk: Aripiprazole, risperidone, amisulpride, ziprasidone, haloperidol, trifluoperazine, flupenthixol, fluphenazine |
TCAs – Tricyclic antidepressants; SSRIs – Selective serotonin reuptake inhibitors
Antipsychotics, in general, have low proconvulsant risk. Clozapine is the only antipsychotic which has shown both titration-dependent and dose-dependent risk of inducing seizures. Studies have shown a prevalence of seizures of around 1% for a dose of <300 mg, 2.7% for 300–600 mg, and 4.4% for >600 mg.[21] Clozapine and olanzapine have also been noted to induce electroencephalography (EEG) changes such as epileptiform activity or generalized slowing. However, the association between olanzapine and clinical seizures is less consistent. Other antipsychotics are considered safe in individuals with epilepsy.
Effect of antiepileptics on psychiatric symptoms
AEDs such as VPA, CBZ, and lamotrigine are also established mood stabilizers and are commonly used in the treatment of psychiatric disorders. However, certain AEDs have also been reported to cause worsening or even de novo development of psychiatric symptoms, termed as psychiatric and behavioral side effects (PBSEs) [Table 9].[22] Those with absent seizures, secondary generalized seizures, and intractable epilepsy (poor seizures control on two or more AEDs) are particularly noted to have a higher risk of PBSEs. Levetiracetam has been noted to be associated with the highest incidence of aggression, mood, and psychotic disorders.[23] Similarly, zonisamide is associated with depression, psychosis, and aggression.[23] One should watch for resurgence of symptoms in patients with a history of psychiatric illness.
Table 9.
Psychiatric and behavioral side effects due to antiepileptic drugs
| High risk: Levetiracetam, zonisamide |
| Low risk: Carbamazepine, oxcarbazepine, phenytoin, valproate, clobazam, gabapentin, lamotrigine |
| Uncertain risk: Tiagabine, topiramate |
PBSEs – Psychiatric and behavioral side effects
In case of development of recent-onset psychiatric symptoms in patients on these AEDs or worsening of pre-existing symptoms, the possibility of it being treatment-emergent should be considered by clinicians. Proper history focusing on temporal relation between change of medications and emergence/worsening of symptoms generally helps in clinching the diagnosis. When PBSEs due to AEDs are suspected, it is best to shift to a drug with a low risk while closely monitoring the symptom profile.
Pharmacological management of mood and anxiety disorders in epilepsy
The primary goal of treatment of depression should always be complete remission of symptoms [Figure 1].
Figure 1.
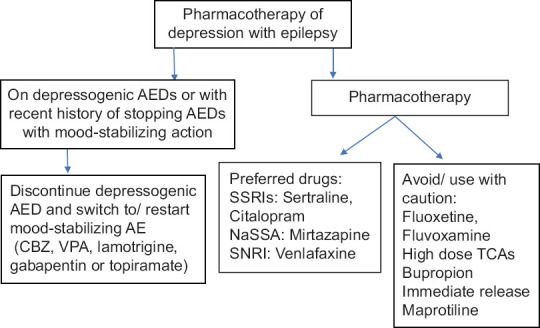
Outline for the pharmacological management of depressive disorders in individuals with epilepsy
Clinicians should keep in mind the other possible side effects due to pharmacodynamic interactions between antidepressants and AEDs such as hyponatremia, sedation, weight gain, and sexual dysfunction.
For the treatment of manic episodes or bipolar depression in PWE, drugs of choice are CBZ, VPA, lamotrigine, gabapentin, and TPM.[24] Lithium is used only as a second choice or as an augmenting agent as it is known to induce EEG abnormalities and in combination with CBZ it may cause encephalopathy.[24]
Electro convulsive therapy (ECT) is an important treatment modality for severe depression with suicidality or treatment resistance, as well as in manic episodes.[24] However, clinicians have to be aware of potential ECT-related issues such as high seizure threshold due to co-administration of AEDs and risk of prolonged or tardive seizures. Subsequently, a higher strength of electrical stimulus as well as close monitoring of seizure duration may be required. Importantly, ECTs are known to increase convulsive threshold over a period of time, which could prove beneficial for managing epilepsy.
For the management of anxiety disorders such as panic disorder, social anxiety disorder, and posttraumatic stress disorder, SSRIs are considered as the first line [Figure 2].[21] Due to its synergistic effects, pregabalin could also be considered as the first line when both epilepsy and generalized anxiety disorder co-exist simultaneously.
Figure 2.
Outline for the management of anxiety disorders and obsessive compulsive disorder in epilepsy
With regard to duration of treatment, it is recommended that clinicians follow general guidelines for the management of anxiety and mood disorders.
The recommendations for the treatment of anxiety disorders in PWE are summarized in Table 10.
Table 10.
Recommendation for management of anxiety disorders
| Disorder | Management |
|---|---|
| Panic disorder | CBT alone or CBT + SSRI (sertraline, citalopram) |
| GAD | Pregabalin |
| Paroxetine, venlafaxine, imipramine | |
| Social anxiety disorder | SSRI (sertraline, paroxetine, escitalopram) |
| Posttraumatic stress disorder | SSRI (sertraline, paroxetine) |
| Obsessive–compulsive disorder | CBT |
| CBT + sertraline | |
| CBT + clomipramine |
CBT – Cognitive behavioral therapy; GAD – Generalized anxiety disorder; SSRIs – Selective serotonin reuptake inhibitors
Pharmacological management of psychosis in epilepsy
Due to lack of direct studies, it is recommended that clinicians follow guidelines of treatment of psychosis outside of epilepsy [Figure 3]. However, using lowest possible therapeutic dose and careful clinical monitoring is recommended. SGAs such as risperidone and aripiprazole are preferred due to their favorable profile with respect to acute extrapyramidal side effects as well as better long-term tolerability. Clozapine should only be used when other antipsychotics have failed to show improvement. Furthermore, it is recommended to start at a low starting dose, titrate slowly, along with careful monitoring of clinical symptoms. Long-acting formulations of antipsychotics are not recommended since it is difficult to discontinue the causative drug immediately in case of seizures.
Figure 3.

Outline for the management of anxiety disorders and obsessive compulsive disorder in epilepsy
Due to lack of sufficient data, psychotic symptoms in interictal psychosis are generally treated in line with the existing protocols for primary psychotic disorders such as schizophrenia. Based on the current literature, antipsychotic treatment should be continued for at least 1 year in patients with first-episode psychosis, and it should be continued for 2–5 years in case of previous history of multiple episodes.[25] Indefinite continuation is recommended in patients with a history of multiple relapses, suicide attempts, or violent and aggressive behavior treatment has to be continued indefinitely.
Treatment of postictal psychosis involves a two-pronged approach of managing acute episode and preventing repeat episodes. Acute episodes, especially in the initial period, can be terminated by oral administration of a BZD.[25] However, nonresponse to a BZD and progressive symptoms warrant oral administration of a BZD and an antipsychotic (for example; risperidone, quetiapine, and olanzapine) in combination.[25] Clinicians should also keep in mind the possibility of a paradoxical response with BZD. In individuals with severe agitation and those with a history of violence during past episodes, intramuscular administration of antipsychotic such as haloperidol with promethazine is recommended.
Postictal psychosis usually improves within a week. Sedatives should be reduced gradually and can be tapered completely on average within 1–3 months of the last episode.[25] Ensuring good seizure control prevents recurrence of further episodes. Hence, continuous administration of antipsychotics is not recommended.
Psychosocial interventions
Psychosocial management forms an important part of management of psychiatric issues in PWE.[26] Table 11 summarizes the various psychological issues pertaining to PWE along with the psychosocial approaches for addressing them. Only those that have shown significant benefits in studies have been enlisted.[23,27,28,29]
Table 11.
Psychosocial interventions
| Domain | Psychosocial intervention |
|---|---|
| Depressive symptoms | Skill-based training and behavioral interventions: Behavioral and social activation, training in problem-solving and goal setting, training in social competencies, and garnering social support |
| Anxiety symptoms | Mindfulness-based interventions |
| CBT | |
| Social problems and stigma | Improving social and communication skills through assertion training, training of epilepsy-related communication, social engagement through community integration, mobilization of social support), and teaching parenting skills |
| Nonadherence | Education and problem-solving strategies |
| Cognitive disturbances | Training in mindfulness to cultivate self-awareness and focused attention |
| Interventions focusing on alternative strategies and cognitive re-training | |
| Acceptance and commitment therapy (nonjudgmental acceptance of cognitive disturbances and learning to focus on achievement of valued life goals in spite of these disturbances) | |
| Psychoeducation | Sessions focusing on knowledge and education about seizures, associated psychiatric conditions, available modes of treatment, and lifestyle challenges |
CBT – Cognitive behavioral therapy
Disability evaluation
The Rights of Persons Disability (RPWD) Act 2016 under the section of “chronic neurological conditions” exemplifies multiple sclerosis and Parkinson’s disease. Nontraumatic epilepsy as such has not been specifically mentioned under this section. However, particularly in those cases which are intractable, it could also be considered as a chronic neurological condition and may be certified accordingly. However, at present, there are no clear guidelines as to how to calculate the severity of disability for epilepsy. Owing to inherent psychosocial issues and stigma related to epilepsy, these individuals have problems in traveling, work, and leading a normal life as a part of the society. Hence, clear guidelines regarding the certification of epilepsy are urgently needed. It should be noted that individuals presenting with seizure disorder along with a psychiatric disorder will have to be certified under the provision of multiple disabilities as per the RPWD Act and will have to be done in conjunction with a neurologist.
SPECIAL ISSUES
A summary of special issues in epilepsy and their management have been provided in Tables 12 and 13.
Table 12.
Special issues in epilepsy
| Special population | Strategies |
|---|---|
| Cognitive impairment | Causes |
| Duration and frequency of seizure | |
| Effect of AEDs, control of seizures | |
| Structural abnormalities on MRI | |
| Subjective cognitive complaints are quite common in epilepsy patients (44%). However, on examination, such cognitive impairment is not present in these patients. This might be due to concurrent depression and anxiety disorders | |
| In elderly patients at the onset itself if cognitive functioning is affected, clinically significant impairment may develop gradually | |
| Generalized cognitive impairment is seen with idiopathic | |
| Generalized epilepsy, whereas TLE is associated with memory impairment.[31] However TLE causes wider network dysfunction leading to other cognitive deficits too. A major concern has been about progressive decline in cognitive functioning. There are mixed findings about this in literature | |
| Another question is whether cognitive impairment persists in spite of seizure control and it has been observed that minor deficits persist especially if there is underlying pre-existent brain damage | |
| Epilepsy surgery is also associated with cognitive impairment. An estimated 44% risk of verbal memory problems and 34% risk of naming difficulties were found in a systematic review. Resection of dominant temporal lobe, normal memory score before surgery, late onset, no hippocampal sclerosis, and poor seizure control are some of the predictors of memory problems postsurgically | |
| Suicidality | There is an increased risk of suicide I patients with epilepsy. 3%–7% patients commit suicide. The risk multiplies 4–5 times than in the nonepileptic population. Temporal lobe involvement in focal dyscognitive seizures increases this risk 25 times |
| Suicidality also shows a bidirectional relationship with epilepsy, thus increasing the risk of epilepsy 5 times in patients with suicidal tendencies[13] | |
| Causes of suicidal thought, suicide attempts, and completed suicides in epilepsy are many. Psychosocial consequences of epilepsy, associated mood disorder in the form of severe depression, command hallucinations during ictal period, agitation, and borderline personality traits are some of the reasons for suicidality. It is also suggested that suicidal risk increases due to some antiepileptic medication, though evidence does not yet support this finding[13] | |
| An assessment for suicidal ideation and suicidal behavior is very essential for prevention and early intervention | |
| Personality changes | Particularly in those with TLE, certain behavioral traits have been classically described, including social viscosity (tendency to prolong social encounters), humorlessness, circumstantiality, hyposexuality, and obsessionalism.[22] These have been seen more commonly with left-sided TLE or GE |
| Other studies have demonstrated hyper-religiosity to be associated with bilateral temporal lobe foci. This specific pattern of inter-ictal personality syndrome has been commonly labelled to as Gastaut Geschwind syndrome[22] | |
| Personality traits such as emotional instability, immaturity and disinhibition have been noted in patients with JME and have been thought to be a consequence of frontal lobe pathology | |
| A thorough evaluation including detailed history of symptomatology and assessment of personality (including psychological tests) is required | |
| The potential role of AEDs in the presentation of certain symptoms (such has irritability, hyposexuality) also needs to be kept in mind since these could be a result of side effects of AEDs | |
| Aggression | Aggression could be a direct consequence of ictal phenomena or can be caused by underlying personality, comorbid psychiatric disorders, and psychosocial stressors |
| Peri-ictal aggression is classically nonspecific, purposeless, disorganized, and generally directed toward things in the immediate vicinity[32] | |
| Instances of aggression have particularly been noted in cases where patients are restrained since it is associated with the worsening of confusion[22] | |
| A detailed evaluation of the type, intensity, frequency of the aggression episodes, its temporal connection with seizures along with video EEG may be required to understand the exact picture | |
| Management of aggression is generally directed towards treatment of the cause. In cases where aggression is suspected to be a result of seizure activity, prompt control of seizures with AEDs will help in preventing fresh episodes of violence | |
| In cases where aggression is related to a comorbid psychiatric disorder such as depression or psychosis, treatment of these conditions may help in reducing instances of aggression | |
| Children | In the younger children, epilepsy is frequently associated with ADHD and autism[33,34] |
| In older children and adolescents, it is associated with behavioral problems, mood and anxiety disorders, personality disorders, and psychotic disorders | |
| ADHD commonly presents as inattentive type and is 2–3 times more common than in general population[34] | |
| As per current literature based on multiple RCTs, methyphenidate 0.3–1 mg/kg can be safely given for ADHD even in children with epilepsy with no added risk of seizure worsening[21] | |
| Data on atomoxetine and amphetamines are lacking, hence should only be prescribed in case of nonresponse to methylphenidate based on an informed decision and with proper clinical monitoring | |
| Epilepsy surgery | Following epilepsy surgery, mood disturbances in the form of depressive features or lability occur in the first 6–12 weeks. This is seen in almost 25% of patients and especially in those with temporal lobe surgery. In 10% of patients, depressive features persist requiring treatment for the same[7] |
| Interictal psychosis may arise for the first time after surgery | |
| A large multicenter study has shown that there is improvement in depressive features following surgery if there is good seizure control postsurgically[14] | |
| Hence, it is necessary to evaluate for mood disturbances after surgery, follow up regularly to see if they persist, also see the seizure control with surgery, and accordingly decide to treat these patients | |
| It is essential to rule out depression in presurgical evaluation, as it is associated with poorer seizure control after surgery | |
| Substance use disorders | Substance use can lead to seizures in intoxication, in over dose, in withdrawal, and in long-term toxicity. This can lead to nonadherence to treatment in seizure disorder and poor seizure control. In an Indian study conducted in 450 prisoners, the prevalence of epilepsy was 1.4 times higher among substance using prisoners. Alcohol, cannabis, and opioids were the most commonly used substances[35] |
MRI – Magnetic resonance imaging; EEG – Electroencephalography; ADHD – Attention deficit hyperactivity disorder; RCTs - Randomized controlled trials; AEDs – Anti epileptic drugs; TLE – Temporal Lobe epilepsy; GE – Generalized epilepsy; JME – Juvenile myoclonic epilepsy
Table 13.
Substance use disorders and seizure[36]
| Substance use disorder | Pathophysiology | Treatment |
|---|---|---|
| Alcohol: Chronic use and withdrawal | Hypokalemia, head injury, clotting problems with cerebrovascular hemorrhage lead to lowering of seizure threshold, also chances of prolonged seizure activity. During withdrawal there is hyperexcitability of neurons, kindling, sleep deprivation all reducing seizure threshold | Single seizure in withdrawal- Benzodiazepines for 7 days and vitamin supplements for treatment of withdrawal |
| Multiple seizures, withdrawal related - Psychoeducation for prevention of further seizures | ||
| Multiple seizures during withdrawal with uncertain etiology – Long-term treatment with AEDs. Phenytoin, carbamazepine, phenobarbital not preferred (risk of hepatic induction), valproate not preferred (may lead to further hepatic damage). Preferred AEDs - levetiracetam, lamotrigine, topiramate | ||
| Opioid | Opioid receptor changes, mu receptor agonism (seen in animal models) may induce seizures. metabolic disturbances, intracranial pathology can also cause seizures | Benzodiazepines |
| Cocaine | Serotonergic mechanisms. seizures are not dose related | Usually self-limiting |
| Amphetamines | NMDA toxicity, hyponatremia | Management of hyponatremia, psychoeducation, management of amphetamine dependence |
| Benzodiazepines | Unmasking of downregulation of GABAergic inhibition and upregulation of the glutamatergic system due to chronic use during withdrawal | Long-acting benzodiazepine such as diazepam or chlordiazepoxide in gradually tapering doses |
NMDA – N-methyl-D-aspartate, GABA – Gamma amino butyric acid, AEDs – Anti epileptic drugs
FUNCTIONAL NEUROLOGICAL SYMPTOM DISORDER (DISSOCIATIVE CONVULSIONS) WITH ATTACKS OR SEIZURES AND EPILEPSY
Functional neurological symptom disorder with attacks or seizures as per the DSM V or dissociative convulsions as per the ICD 10 (popularly known as psychogenic nonepileptic seizures) is characterized by episodes of seizure-like activity but without any seizure activity on video EEG. Like epileptic seizures, dissociative convulsions are paroxysmal and time-limited. They are characterized by motor, sensory, autonomic, and/or cognitive signs and symptoms. However, dissociative convulsions are not caused by ictal epileptiform activity.[30] Sometimes, it is difficult to differentiate between epilepsy and dissociative convulsions [Table 14]. It is essential to diagnose dissociative convulsions correctly; otherwise, patients get exposed to antiepileptic medication unnecessarily and may suffer from toxicity of these medications. It leads to repeated hospitalizations too.[15,30] There is a psychological basis for the symptoms in dissociative convulsions. Unless a correct diagnosis is made, these psychological issues are not handled and the patient keeps visiting the emergency services repeatedly.
Table 14.
Differences between epilepsy and functional neurological symptom disorder
| Epilepsy | Functional neurological symptom disorder |
|---|---|
| Most common in young children and elderly | Most common in young age group between 20–40 years |
| Risk factors are infections, genetic metabolic disorders | Risk factors are stress, trauma, scholastic difficulties, interpersonal problems, physical abuse, sexual abuse |
| Clinical features | |
| Duration: <2 min | >2 min |
| Movements - synchronous, tonic rigidity at onset of GTC seizure associated with symmetrical clonic activity | Asynchronous movements, asymmetrical, out-of-phase movements, pelvic thrusts sometimes, and hyperarching at times |
| Sleep: Occurs in physiological sleep | Usually occur while awake |
| Head rotation movements: Absent | Present |
| Amnesia for activities during episode | Recall intact during the episode |
| Pupillary reaction altered or dilated pupils | Pupillary reaction unchanged |
| Heart rate: Increases rapidly during the seizure | Inconsistent increase in heart rate |
| Urinary incontinence usually present | Rarely present |
| Epileptic cry - monotonous, meaningless phrases or sounds | With a feeling tone usually sad, coherent speech |
| Eyes mostly open and when closed, not throughout the episode | Eyes closed throughout the episode |
| Tongue bites more common and on lateral side | Tongue bite less common and on tip or on lip or cheeks |
| Fractures or ecchymoses more common, burns occur mostly with epilepsy | Ecchymoses or fractures less common. Rug burns or excoriations along long bone surfaces more common |
| Gradual recovery postictally | Immediate recovery after the episode |
| Focal neurological deficits, stertorous breathing and physical complaints seen | Focal neurological deficits, stertorous breathing and physical complaints not seen |
| Postictal headache seen commonly | Interictal headache present commonly |
| EEG - abnormal epileptiform activity | Motor activity interspersed with normal background activity |
| Video EEG - abnormal discharge and slowing of background | Normal background activity before, during and after the episode |
| Serum prolactin levels - high (>60–>900 IU/ml) | Normal prolactin levels |
EEG – Electroencephalography; GTC – Generalised tonic clonic
Dissociative convulsions are seen in 10%–40% of long-term cases of epilepsy, and around 10%–15% cases of dissociative convulsions have a seizure disorder. According to a study by LaFrance et al., the time gap between the onset of symptoms and diagnosis of dissociative convulsions is between 1 and 16 years.[30]
Dissociative convulsions are seen more commonly in women aged between 26 and 32 years; however, it is seen in children and at times in the elderly too. It is not seen in preschool age group and the incidence increases with age of the child.
National Association of Epilepsy Centers has given a guideline to refer a patient of suspected dissociative convulsions and if seizure disorder is not controlled by AEDS for more than a year to specialized epilepsy clinic.[19]
Diagnosis of dissociative convulsions
Detailed history, clinical features, and investigations help in diagnosing functional neurological symptom disorder.[13,16,19,30]
Management
Patients with dissociative convulsions present in emergency services, majority number of times. The goal of treatment in the emergency setting is to make patient symptom-free.
Use of suggestion for the same
Techniques such as social isolation
Psychoeducation of caregivers and garnering support
Confirm the diagnosis of dissociative convulsions when patient follows up in the outpatient department: Diagnosis of dissociative convulsions is made on the basis of history, nature of seizure such as episode, investigations such as video EEG, which is the “gold standard,” psychological tests when necessary
Rule out seizure disorder, other neurological disorders
Ask neurologist to taper AEDs
Establishing rapport with patient
Establishing the connection between psychological stress/trauma and dissociative convulsions
Cognitive behavior therapy for improving coping skills, detecting cognitive distortions, and correcting them
Psychodynamic interpersonal therapy and group and family therapy whenever necessary
Strengthen psychosocial support
Assess for comorbid anxiety disorder or depression
If diagnosed start medication for the same
Patients with mixed dissociative convulsions and seizure disorder need to be maintained on AEDs. Very high dosages of AEDs may be reduced and polytherapy may be avoided
Communication between treating teams in psychiatry and neurology helps in unnecessary investigations and treatments in patients with a mixed disorder.
One should keep in mind that in primary care setting, EEG, video EEG, or even psychological tests would not be available. Then, a good clinical history is the key to the correct diagnosis.
CONCLUSION
This CPG looks at the common psychiatric issues seen in PWE and provides pharmacological and psychosocial approaches toward the management of these issues. It is important to take a detailed history focusing on understanding the temporal association between psychiatric symptoms and seizure episodes. Clinicians also need to be aware of possible pharmacokinetic and pharmacodynamic interactions between AEDs and other psychotropic drugs as well as the risk of de novo psychiatric symptoms with certain AEDs. Due to the chronic nature of epilepsy, the co-morbid psychiatric issues and the associated stigma due to both, psychosocial interventions have an indispensable role in the management plan. Timely diagnosis of psychiatric disorders in epilepsy and effective management of these disorders areadvantageous for the patient in terms of improved drug adherence, better functioning, and quality of life.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy:Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–2. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 2.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report:A practical clinical definition of epilepsy. Epilepsia. 2014;55:475–82. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 3.Srinivas HV, Shah U. Comorbidities of epilepsy. Neurol India. 2017;65:S18–24. doi: 10.4103/neuroindia.NI_922_16. [DOI] [PubMed] [Google Scholar]
- 4.Lopez MR, Schachter SC, Kanner AM. Psychiatric comorbidities go unrecognized in patients with epilepsy:“You see what you know”. Epilepsy Behav. 2019;98:302–5. doi: 10.1016/j.yebeh.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Kanner AM. Psychiatric comorbidities in new onset epilepsy:Should they be always investigated? Seizure. 2017;49:79–82. doi: 10.1016/j.seizure.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Bragatti JA, Torres CM, Isolan GR, Bianchin MM. Psychiatric comorbidities of epilepsy:A review. J Neurol Neurophysiol. 2011;2:10–20. [Google Scholar]
- 7.Foong J. Psychiatric Disorders in Epilepsy, In EPILEPSY 2017 From Bench to Bedside, A Practical Guide to Epilepsy Lecture Notes, Sixteenth Epilepsy Teaching Weekend 23–24 September 2017, by University of Oxford Mathematical Institute. F.J. Rugg-Gunn, H.B. Stapley., editors. 2017:193–5. [Google Scholar]
- 8.Salpekar JA, Mula M. Common psychiatric comorbidities in epilepsy:How big of a problem is it? Epilepsy Behav. 2019;98:293–7. doi: 10.1016/j.yebeh.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Scott AJ, Sharpe L, Hunt C, Gandy M. Anxiety and depressive disorders in people with epilepsy:A meta-analysis. Epilepsia. 2017;58:973–82. doi: 10.1111/epi.13769. [DOI] [PubMed] [Google Scholar]
- 10.Tellez-Zenteno JF, Patten SB, Jetté N, Williams J, Wiebe S. Psychiatric comorbidity in epilepsy:A population-based analysis. Epilepsia. 2007;48:2336–44. doi: 10.1111/j.1528-1167.2007.01222.x. [DOI] [PubMed] [Google Scholar]
- 11.Ottman R, Lipton RB, Ettinger AB, Cramer JA, Reed ML, Morrison A, et al. Comorbidities of epilepsy:Results from the Epilepsy Comorbidities and Health (EPIC) survey. Epilepsia. 2011;52:308–15. doi: 10.1111/j.1528-1167.2010.02927.x. [DOI] [PubMed] [Google Scholar]
- 12.Tolchin B, Hirsch LJ, LaFrance WC., Jr Neuropsychiatric aspects of epilepsy. Psychiatr Clin North Am. 2020;43:275–90. doi: 10.1016/j.psc.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Paholpak P, Juan Gan J, Mendez M. Neuropsychiatric aspects of epilepsy. In: Sadock BJ, Sadock VA, Ruiz P, editors. Kaplan &Sadock's Comprehensive Textbook of Psychiatry. 10th ed. Kingston upon Thames, England: Wolters Kluwer; 2017. pp. 507–22. [Google Scholar]
- 14.Josephson CB, Jetté N. Psychiatric comorbidities in epilepsy. Int Rev Psychiatry. 2017;29:409–24. doi: 10.1080/09540261.2017.1302412. [DOI] [PubMed] [Google Scholar]
- 15.Berg AT, Altalib HH, Devinsky O. Psychiatric and behavioral comorbidities in epilepsy:A critical reappraisal. Epilepsia. 2017;58:1123–30. doi: 10.1111/epi.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devinsky O. Psychiatric comorbidity in patients with epilepsy:Implications for diagnosis and treatment. Epilepsy Behav. 2003;4(Suppl 4):S2–10. doi: 10.1016/j.yebeh.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Kanner AM. Psychiatric issues in epilepsy:The complex relation of mood, anxiety disorders, and epilepsy. Epilepsy Behav. 2009;15:83–7. doi: 10.1016/j.yebeh.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 18.Krishnamoorthy ES. Psychiatric issues in epilepsy. Curr Opin Neurol. 2001;14:217–24. doi: 10.1097/00019052-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Doss RC, LaFrance WC. Psychogenic non-epileptic seizures. Epileptic Disord. 2016;18:337–43. doi: 10.1684/epd.2016.0873. [DOI] [PubMed] [Google Scholar]
- 20.Mula M. Epilepsy and psychiatric comorbidities:Drug selection. Curr Treat Options Neurol. 2017;19:44. doi: 10.1007/s11940-017-0483-0. [DOI] [PubMed] [Google Scholar]
- 21.Mula M. The pharmacological management of psychiatric comorbidities in patients with epilepsy. Pharmacol Res. 2016;107:147–53. doi: 10.1016/j.phrs.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Kanner AM. Management of psychiatric and neurological comorbidities in epilepsy. Nat Rev Neurol. 2016;12:106–16. doi: 10.1038/nrneurol.2015.243. [DOI] [PubMed] [Google Scholar]
- 23.Marcangelo MJ, Ovsiew F. Psychiatric aspects of epilepsy. Psychiatr Clin North Am. 2007;30:781–802. doi: 10.1016/j.psc.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Marsh L, Rao V. Psychiatric complications in patients with epilepsy:A review. Epilepsy Res. 2002;49:11–33. doi: 10.1016/s0920-1211(02)00008-6. [DOI] [PubMed] [Google Scholar]
- 25.Adachi N, Kanemoto K, de Toffol B, Akanuma N, Oshima T, Mohan A, et al. Basic treatment principles for psychotic disorders in patients with epilepsy. Epilepsia. 2013;54(Suppl 1):19–33. doi: 10.1111/epi.12102. [DOI] [PubMed] [Google Scholar]
- 26.Mula M, Sander JW. Psychosocial aspects of epilepsy:A wider approach. BJPsych Open. 2016;2:270–4. doi: 10.1192/bjpo.bp.115.002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corrigan FM, Broome H, Dorris L. A systematic review of psychosocial interventions for children and young people with epilepsy. Epilepsy Behav. 2016;56:99–112. doi: 10.1016/j.yebeh.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Michaelis R, Tang V, Goldstein LH, Reuber M, LaFrance WC, Jr, Lundgren T, et al. Psychological treatments for adults and children with epilepsy:Evidence-based recommendations by the International League Against Epilepsy Psychology Task Force. Epilepsia. 2018;59:1282–302. doi: 10.1111/epi.14444. [DOI] [PubMed] [Google Scholar]
- 29.Mittan RJ. Psychosocial treatment programs in epilepsy:A review. Epilepsy Behav. 2009;16:371–80. doi: 10.1016/j.yebeh.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 30.LaFrance WC, Jr, Baker GA, Duncan R, Goldstein LH, Reuber M. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures:A staged approach:A report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia. 2013;54:2005–18. doi: 10.1111/epi.12356. [DOI] [PubMed] [Google Scholar]
- 31.Krishnamoorthy ES. Psychiatric issues in epilepsy. Curr Opin Neurol. 2001;14:217–24. doi: 10.1097/00019052-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Bowden VM. The journal of neuropsychiatry and clinical neurosciences. JAMA. 1992;268:1473–4. [Google Scholar]
- 33.Dagar A, Falcone T. Psychiatric comorbidities in pediatric epilepsy. Curr Psychiatry Rep. 2020;22:77. doi: 10.1007/s11920-020-01195-8. [DOI] [PubMed] [Google Scholar]
- 34.Dreier JW, Pedersen CB, Cotsapas C, Christensen J. Childhood seizures and risk of psychiatric disorders in adolescence and early adulthood:A Danish nationwide cohort study. Lancet Child Adolesc Health. 2019;3:99–108. doi: 10.1016/S2352-4642(18)30351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sureka P, Girdhar NK, Kumar M, Singhal V. A study of the relationship of epilepsy with psychoactive substance dependence in a prison population. ASEAN J Psychiatry. 2014;15:153–63. [Google Scholar]
- 36.Leach JP, Mohanraj R, Borland W. Alcohol and drugs in epilepsy:Pathophysiology, presentation, possibilities, and prevention. Epilepsia. 2012;53(Suppl 4):48–57. doi: 10.1111/j.1528-1167.2012.03613.x. [DOI] [PubMed] [Google Scholar]



