Abstract
A Hydrogenophaga pseudoflava strain was able to synthesize poly(3-hydroxybutyric acid-co-4-hydroxybutyric acid) [P(3HB-co-4HB)] having a high level of 4-hydroxybutyric acid monomer unit (4HB) from γ-butyrolactone. In a two-step process in which the first step involved production of cells containing a minimum amount of poly(3-hydroxybutyric acid) [P(3HB)] and the second step involved polyester accumulation from the lactone, approximately 5 to 10 mol% of the 3-hydroxybutyric acid (3HB) derived from the first-step culture was unavoidably reincorporated into the polymer in the second cultivation step. Reincorporation of the 3HB units produced from degradation of the first-step residual P(3HB) was confirmed by high-resolution 13C nuclear magnetic resonance spectroscopy. In order to synthesize 3HB-free poly(4-hydroxybutyric acid) [P(4HB)] homopolymer, a three-stage cultivation technique was developed by adding a nitrogen addition step, which completely removed the residual P(3HB). The resulting polymer was free of 3HB. However, when the strain was grown on γ-butyrolactone as the sole carbon source in a synthesis medium, a copolyester of P(3HB-co-4HB) containing 45 mol% 3HB was produced. One-step cultivation on γ-butyrolactone required a rather long induction time (3 to 4 days). On the basis of the results of an enzymatic study performed with crude extracts, we suggest that the inability of cells to produce 3HB in the multistep culture was due to a low level of 4-hydroxybutyric acid (4HBA) dehydrogenase activity, which resulted in a low level of acetyl coenzyme A. Thus, 3HB formation from γ-butyrolactone is driven by a high level of 4HBA dehydrogenase activity induced by long exposure to γ-butyrolactone, as is the case for a one-step culture. In addition, intracellular degradation kinetics studies showed that P(3HB) in cells was completely degraded within 30 h of cultivation after being transferred to a carbon-free mineral medium containing additional ammonium sulfate, while P(3HB-co-4HB) containing 5 mol% 3HB and 95 mol% 4HB was totally inert in interactions with the intracellular depolymerases. Intracellular inertness could be a useful factor for efficient synthesis of the P(4HB) homopolymer and of 4HB-rich P(3HB-co-4HB) by the strain used in this study.
Many microorganisms synthesize poly(3-hydroxybutyrate) [P(3HB)] intracellularly and accumulate it in granular inclusion bodies as a carbon and energy reserve (2). They also synthesize different types of polyesters composed of various kinds of monomers depending on the fermentation conditions and the carbon source. More than 100 different monomer units are known to be incorporated into the polymer chain (25), but only a few bacterial homopolyesters are known. These bacterial homopolyesters include P(3HB), poly(3-hydroxyvalerate), poly(4-hydroxybutyrate) [P(4HB)], and poly(3-hydroxy-5-phenylvalerate) (10, 15, 20, 23, 25). The first three of these homopolymers are crystalline when they are isolated from cells, but poly(3-hydroxy-5-phenylvalerate) is amorphous, and the first three homopolymers have melt transition temperatures of 175, 112, and 53°C, respectively, and glass transition temperatures of 15, 0, and −40°C, respectively (10, 15, 16). P(4HB) is much more ductile (200 times higher elongation-to-break) than P(3HB) (20). Thus, the thermal, crystalline, and mechanical properties depend on the type of the monomer unit.
Introduction of the 4-hydroxybutyrate monomer unit (4HB) was first described by Doi et al. (10, 16). These workers synthesized copolyesters having different ratios of 3-hydroxybutyrate (3HB) and 4HB by using Ralstonia eutropha H16 (formerly Alcaligenes eutrophus H16) (28). Recently, Saito and Doi isolated Comamonas acidovorans DS-17, which can accumulate the P(4HB) homopolymer at levels up to approximately 21 to 28% (wt/wt) of the dry weight when it is grown on 4-hydroxybutyric acid (4HBA) or 1,4-butanediol (20). P(4HB) homopolyester was also synthesized in a recombinant Escherichia coli strain containing hybrid plasmids harboring the R. eutropha PHA synthase gene (phaC) and the Clostridium kluyveri orfZ gene encoding a 4HB-coenzyme A (CoA) transferase (12).
In most bacterial strains, intracellular degradation usually follows the exponential accumulation period during batch cultivation (2, 13). Because of this, it is generally believed that intracellular polyesters are energy reserve compounds. The polyesters are usually composed of 3-hydroxy acid units. However, several short-chain polyhydroxyalkanoate (PHA)-producing bacteria can also incorporate unusual monomer units that are oxidized at different positions, such as 4HB, 4-hydroxyvalerate, 5-hydroxyvalerate units, etc., into the polymer (2, 6, 10, 20, 25). In light of the important physiological role of PHA inclusions, it is surprising that there have been no studies of the intracellular degradation of the unusual polyesters. Thus, we concluded that studying the intracellular degradation of the unusual polyesters would be important not only from the physiological point of view but also from the process optimization point of view.
In a previous study, we reported that Hydrogenophaga pseudoflava can produce 3HB-4HB copolyesters having 4HB contents of up to 66 mol% (35% of the dry cell weight) when it is grown on 10 g of glucose per liter and 3 ml of γ-butyrolactone per liter by using a one-step cultivation method (6). Higher lactone concentrations in the medium significantly inhibited cell growth. Thus, 3HB-4HB copolyesters containing more than 66 mol% 4HB could not be prepared by the one-step cultivation procedure. However, the data suggested that the level of 4HB in the 3HB-4HB copolyester could be increased in this bacterium by using a two-step cultivation technique. In addition, we were interested in preparing a P(4HB) homopolymer. Except for P(3HB), a two-step cultivation procedure was usually used previously for synthesis of the homopolymers desired (10, 20). However, small amounts of P(3HB) homopolymer resulting from the first culture step were problematic in obtaining 3HB-free homopolymers by the two-step cultivation procedure (22). Therefore, it was necessary to develop a new culture technique in order to synthesize 3HB-free P(4HB) homopolymer.
In this report, we show that H. pseudoflava can synthesize the P(4HB) homopolymer efficiently in a three-stage cultivation process because the polymer does not degrade during prolonged incubation. For a similar reason, poly(3-hydroxybutyric acid-co-4-hydroxybutyric acid) [P(3HB-co-4HB)] copolymers containing high levels of 4HB were efficiently synthesized in a two-step cultivation process. The intracellular inert nature of the polymers was verified by performing degradation studies. The final purpose of this study was to investigate how, depending on the cultivation conditions, the 3HB/4HB ratio in H. pseudoflava is modulated in order to synthesize 3HB-4HB copolyesters and the P(4HB) homopolymer from γ-butyrolactone.
MATERIALS AND METHODS
Organism and culture media.
Strain ATCC 33668 of H. pseudoflava was purchased from the American Type Culture Collection. Inocula were grown in 5-ml test tubes containing nutrient-rich media (1% yeast extract, 1.5% nutrient broth, 0.2% ammonium sulfate) for 18 h. The following two media were used for first-step cultivation: (i) Luria-Bertani medium (10 g of tryptone, 5 g of yeast extract, and 10 g of NaCl in 1 liter of distilled water) and (ii) modified PHA synthesis mineral medium (6, 7). All growth experiments were performed under aerobic conditions in a temperature-controlled shaker (Korea Instrument Co., Seoul, Korea) at 35°C and 190 rpm.
Growth of the strain on γ-butyrolactone in a single-step cultivation process.
Precultured cells were transferred to modified PHA synthesis mineral medium containing an appropriate amount of γ-butyrolactone plus ammonium sulfate (0.6 g/liter). Cell growth was monitored by measuring the optical density of the medium at 660 nm.
Polyester accumulation in a two-step cultivation process.
Precultured cells were transferred to Luria-Bertani medium and cultured for 22 h. The cells were harvested by centrifugation with a Beckman model J2-HS centrifuge (6,000 rpm, 4°C, 10 min) and then were transferred to PHA synthesis mineral medium containing 2 ml of γ-butyrolactone per liter plus 0.6 g of ammonium sulfate per liter and cultivated for 48 h.
P(4HB) homopolymer synthesis in a three-step cultivation process.
Nutrient broth-grown cultures were transferred to Luria-Bertani medium and cultivated for 22 h. The cells were isolated by centrifugation and were incubated in carbon-free mineral medium (medium containing the same minerals as PHA synthesis mineral medium) containing 0.3 or 0.6 g of ammonium sulfate per liter for 10 h. The cells recovered from the second cultivation step were transferred to nitrogen-free PHA synthesis mineral medium containing 2 ml of γ-butyrolactone per liter and cultivated for 36 h.
Monitoring cell growth.
Five milliliters of a growth culture was removed every 4 to 5 h in order to analyze the medium and the cells. The amount of NH4+ remaining in the medium was measured by the Nessler reagent method (7). The amount of residual γ-butyrolactone and the monomer unit composition of polyesters in cells were determined by gas chromatography with a Hewlett-Packard model HP5890A gas chromatograph equipped with a Carbowax 20M column and a flame ionization detector (7). The lactone remaining in the medium was extracted with an appropriate amount of chloroform, dried over Na2SO4, and analyzed by gas chromatography. For analysis of the polyesters in cells, 10 mg of dried cells was added to a mixed solution containing 1 ml of chloroform, 0.85 ml of methanol, and 0.15 ml of concentrated sulfuric acid. The reaction mixture (in a closed screw-cap tube) was incubated at 100°C for 3 h. The organic layer containing the reaction products was separated, dried over Na2SO4, and analyzed by gas chromatography. Each peak was standardized by using standard methyl esters which were obtained by methanolysis of purified P(3HB) and P(4HB) homopolymers or of a copolyester with a known composition, as determined by 1H nuclear magnetic resonance (NMR) analysis. The 4HB unit produced two peaks, each of which was associated with 4HBA methyl ester and γ-butyrolactone (6). At least two or more measurements were obtained for each sample. The averaged values were within ±6%.
Polyester isolation and characterization.
Polyesters were extracted from an appropriate amount of cells which had been dried overnight at 50°C under a vacuum; extraction was performed with hot chloroform in a Pyrex Soxhlet apparatus for 6 h. The concentrated solvent extract was precipitated in rapidly stirred cold methanol. The polymers isolated were dried overnight under a vacuum at the ambient temperature and then weighted. Quantitative determinations of the monomer units in the polyesters were performed by gas chromatography as described above and by 1H NMR with a Bruker-DRX 500-MHz spectrometer (6, 7). Thermal transitions of the polyesters were measured with nitrogen purging by using a TA differential scanning calorimeter (DuPont model 2100, DSC V4.0B) equipped with a data station. The heating rate was 10°C/min. The scanning range was between −100 and 200°C.
Intracellular degradation of P(3HB) and 4HB-rich P(3HB-co-4HB) in carbon-free media.
Two types of cells, P(3HB)- and P(3HB-co-4HB)-containing cells, were recovered by centrifugation and were transferred to carbon-free mineral medium (medium containing the same minerals as PHA synthesis mineral medium) containing ammonium sulfate (1.0 g/liter) (or not containing ammonium sulfate for the control experiment). The cells were incubated under aerobic shaking conditions at 35°C and 190 rpm. The cells and the remaining NH4+ were analyzed as described above.
Determination of enzyme activity.
To prepare crude enzyme extracts, cells were washed in 100 mM potassium phosphate (pH 7.4). The washed cells (approximately 0.1 g [wet weight]) were suspended in 1 ml of 50 mM Tris-HCl buffer (pH 7.0) and disrupted with an ultrasonic homogenizer operated at 50 W (Cole-Parmer Co., Chicago, Ill.). The preparation was sonicated for 5 min by using 5-s bursts, each of which was followed by a 5-s break. During disruption, the samples were cooled on an NaCl-ice mixture. The disrupted cells were centrifuged for 10 min at 14,000 × g with a high-speed centrifuge (model Micro 17R; Hanil Science Industrial Co., Seoul, Korea). The supernatant fluid was used to determine enzyme activity.
The activities of 3-hydroxybutyric acid (3HBA) dehydrogenase and 4HBA dehydrogenase were determined spectrophotometrically by using a modification of previously described methods (11, 21, 27). The dehydrogenation reaction was started by adding 5 μl of crude extract to a solution containing 1 ml of 400 mM Tris-HCl buffer (pH 8.0) supplemented with 20 mM NAD+ and 25 mM 3HBA or 25 mM 4HBA. Protein concentrations were determined by the Bradford method (4).
RESULTS
Characteristics of H. pseudoflava growth on γ-butyrolactone during single-step cultivation.
H. pseudoflava was able to grow on γ-butyrolactone at a relatively low concentration, 0.8 ml/liter, as the sole carbon source in PHA synthesis mineral medium containing ammonium sulfate (0.66 g/liter), but this lactone concentration was the highest lactone concentration at which the bacterium was able to grow (Table 1). A long induction period (3 to 4 days) was required for growth of the strain. An additional 24 h of cultivation led to a plateau steady-state growth period during which the polyester weight was only 1.3% of the cell weight. An additional 1 day of cultivation resulted in a plateau polyester content of 6 to 8% (wt/wt). The polyester was composed of two monomer units, 3HB and 4HB. The molar ratio of 3HB to 4HB was relatively constant (45:55) throughout cultivation. The molar C/N ratio in the medium changed from the initial value, 2.1 at zero time, to 4.0 after 168 h of cultivation. Such a slight change in the C/N ratio may mean that polyester accumulation is not a critical function of the C/N ratio in the medium. However, growth of H. pseudoflava appears to be composed of two-phase cell growth, followed by polyester accumulation.
TABLE 1.
Growth of and polyester accumulation by H. pseudoflava grown on γ-butyrolactone (0.8 ml/liter; 10.4 mM) as the sole carbon source by using the one-step cultivation processa
| Culture time (h) | Dry cell wt (g/liter) | Polyester content (%, wt/wt) | Polyester composition (mol%)b
|
Amt of remaining nitrogen (g/liter)c | Amt of remaining γ-butyrolactone (ml/liter) | C/N ratio | |
|---|---|---|---|---|---|---|---|
| 3HB | 4HB | ||||||
| 120d | 0.69 | 1.3 | 37 | 63 | 0.18 (1.4)e | 0.41 (5.3)e | 3.8f |
| 144 | 0.69 | 6.1 | 45 | 55 | 0.16 (1.2) | 0.35 (4.6) | 3.8 |
| 168 | 0.66 | 8.0 | 44 | 56 | 0.15 (1.1) | 0.34 (4.4) | 4.0 |
| 192 | 0.64 | 7.8 | 45 | 55 | 0.16 (1.2) | 0.33 (4.3) | 3.6 |
Two additional sets of batch experiments produced similar results.
Calculated from gas chromatographic data.
The initial concentration of ammonium sulfate was 0.66 g/liter (5.0 mM).
The culture time includes the 96-h induction period.
The values in parentheses are molar concentrations.
The C/N ratio was defined as the ratio of the molar concentration of γ-butyrolactone to the molar concentration of ammonium sulfate remaining in the medium.
Synthesis of 3HB-4HB copolymer containing a high level of 4HB units by using a two-step cultivation process.
In the usual two-step cultivation procedure for PHA synthesis, the first step results in maximal cell accumulation, and the second step results in PHA accumulation. Cells grown in the first-step Luria-Bertani medium were transferred to PHA synthesis mineral medium containing nitrogen plus γ-butyrolactone (2.2 g/liter) and were grown for 48 h. It is important to note that H. pseudoflava accumulated substantial amounts of P(3HB) even under these nutrient-rich conditions (Table 2). Thus, as Table 2 shows, the residual P(3HB) from the first-step culture could affect the final product in the second step. We noted that the 3HB unit content of the second-step cells was comparable to or far less than the 3HB unit content of the first-step cells, depending on the absence or presence of additional salts (NaCl and KCl).
TABLE 2.
Effect of the residual P(3HB) formed during first-step cultivation of H. pseudoflava in Luria-Bertani medium for 22 h on the composition of polyesters that accumulated during second-step cultivation in which the strain was grown on γ-butyrolactone (2.2 g/liter) as the sole carbon source for 48 ha
| First-step cultivation
|
Second-step cultivation with γ-butyrolactone
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Addition to medium | Dry cell wt (g/liter) | P(3HB) content (%, wt/wt) | Concn of residual P(3HB) (g/liter)b | Dry cell wt (g/liter) | Polyester content (%, wt/wt) | Polyester composition (mol%)b
|
Concn of residual 3HB in the polymer (g/liter) | % γ-Butyrolactone
|
||
| 3HB | 4HB | Used for 4HBc | Unused | |||||||
| Noned | 1.61 ± 0.02 | 3.23 ± 0.12 | 0.052 ± 0.003 | 2.40 ± 0.02 | 30.4 ± 0.1 | 11 ± 1 | 89 ± 1 | 0.083 ± 0.004 | 29 ± 2 | 13 ± 1 |
| NaCl (170 mM)e | 1.87 ± 0.02 | 11.1 ± 0.20 | 0.206 ± 0.003 | 2.25 ± 0.04 | 33.3 ± 0.8 | 9 ± 1 | 91 ± 1 | 0.067 ± 0.007 | 31 ± 2 | 11 ± 1 |
| KCl (170 mM)e | 1.73 ± 0.02 | 3.51 ± 0.08 | 0.061 ± 0.001 | 2.20 ± 0.08 | 30.6 ± 0.6 | 5 ± 1 | 95 ± 1 | 0.045 ± 0.006 | 29 ± 2 | 11 ± 1 |
Cells grown in Luria-Bertani medium were transferred to PHA synthesis mineral medium containing γ-butyrolactone (2.2 g/liter) and 0.6 g of ammonium sulfate per liter for polyester accumulation.
Calculated from gas chromatographic data.
Percent conversion of γ-butyrolactone (2.2 g/liter) to 4HB in the polymer.
All values are averages for three different experiments.
All values are averages for six different experiments.
Luria-Bertani medium contains 10 g of NaCl per liter, so we assumed that salinity might play a role in PHA synthesis in H. pseudoflava. Table 2 shows the influence of salinity on PHA synthesis. P(3HB) accumulation in the first step increased in the presence of additional NaCl, but a further increase in the salinity of the medium to 3% (wt/vol) eventually inhibited cell growth (data not shown). However, salinity had little effect during the second-step cultivation (Table 2). When NaCl was replaced with KCl at the same molar concentration, the P(3HB) level decreased to the level in the control experiment. We believe that the salt effect observed was not nutritional but was probably associated with osmotic adaptation that led to changes in PHA-related enzymatic properties or with an active role of Na+ ions in transport mechanisms in the cells.
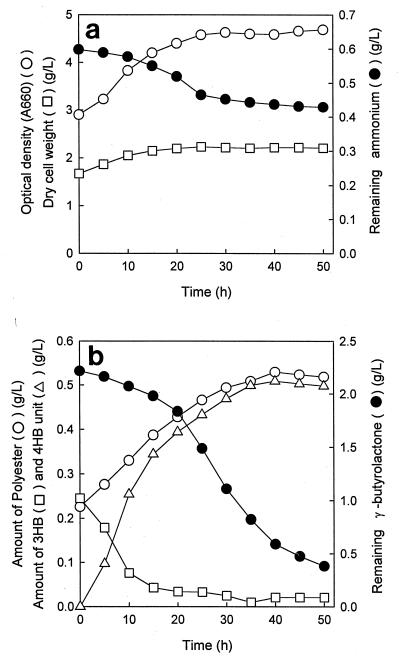
Considering the significant decrease in the proportion of 3HB during the second-step cultivation, we decided to determine the time course of PHA composition. The time course data in Fig. 1 are data for second-step cultivation in PHA synthesis mineral medium containing γ-butyrolactone (2.0 ml/liter) of first-step cells which were grown in Luria-Bertani medium containing 170 mM NaCl. In our initial procedure to remove the first-step residual P(3HB), a series of ammonium sulfate concentrations were added to the second-step γ-butyrolactone-containing medium. In the absence of ammonium sulfate, the resulting second-step PHA contained a significant amount of 3HB, up to 15 mol% (data not shown). The optimum concentration of ammonium sulfate for removal of the residual P(3HB) was approximately 0.6 to 1.0 g/liter, so 0.6 g of ammonium sulfate per liter was added to PHA synthesis mineral medium to remove the residual P(3HB) (Table 2). As Fig. 1a shows, NH4+ was steadily consumed during the initial 24 h of the second-step cultivation, and an increase in biomass was observed during the same culture period. The optical density also increased. It is not known whether the increase in optical density was due to an increase in cell number or to an increase in the PHA content (22).
FIG. 1.
(a) Growth and increase in optical density during second-step cultivation of H. pseudoflava on γ-butyrolactone (2 ml/liter) as a sole carbon source in a two-step procedure. Zero time was the beginning of the second-step cultivation. (b) Time course of 4HB monomer incorporation during the second-step cultivation of H. pseudoflava on γ-butyrolactone (2 ml/liter) as a sole carbon source in a two-step procedure. Zero time was the beginning of the second-step cultivation. A660, absorbance at 660 nm.
Figure 1b shows that the decrease in the 3HB monomer content occurred during the same period that NH4+ consumption occurred. This result suggested that addition of NH4+ accelerated degradation of the residual P(3HB) during the second-step cultivation procedure (5, 19). Figure 1b also shows that polymerization of 4HB occurred simultaneously with degradation of P(3HB) in cells. Thus, some of the 3-hydroxybutyryl-CoA monomers derived from the hydrolyzed product may have been reincorporated into the polyester chain along with 4-hydroxybutyryl-CoA to form a 4HB-3HB copolymer in which 3HB was the minor component.
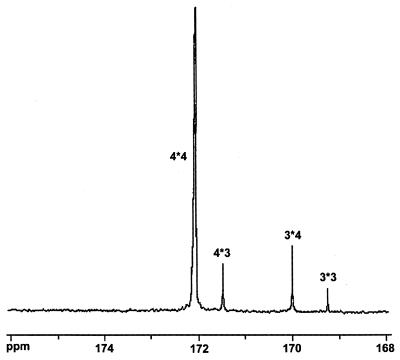
If no 3HB-CoA formed from γ-butyrolactone during the second-step cultivation, the hydrolyzed 3HB monomer units might have been present in the form of comonomers in the 4HB-3HB polymer isolated. Otherwise, the undegraded remaining P(3HB) would have been present in the form of a blend of the two homopolymers, P(3HB) and P(4HB). This problem was solved by analyzing the microstructure sequence of the polymer, which was determined by 13C NMR spectroscopy (10, 15). The NMR splitting of the absorption bands resulting from each monomer depended on the type of neighboring monomers. Thus, the splitting pattern determined the microstructure sequences, such as dyad, triad, tetrad, etc. An analysis of the carbonyl absorption region (around 170 ppm) for the 3HB-4HB polymer revealed its dyad sequence distribution (Fig. 2). The presence of the two dyad sequences, 4*3 and 3*4, demonstrated that some of the 3HB-CoA derived from the first-step residual P(3HB) was copolymerized with 4HB-CoA during the second-step cultivation.
FIG. 2.
Dyad sequence distribution in the C13 NMR carbonyl absorption region of the 3HB-4HB (5 mol% 3HB–95 mol% 4HB) polymer synthesized from γ-butyrolactone during two-step cultivation of H. pseudoflava. The 3HB unit was designated 3, and the 4HB unit was designated 4.
The value of the NMR parameter D, which was equal to the ratio of the fractions of the four dyads, (F33F44)/(F34F43), was calculated to be 5.7 for the 3HB-4HB polymer (5 mol% 3HB, 95 mol% 4HB) synthesized by the two-step culture procedure from γ-butyrolactone. A statistically random copolymer has a D value of 1. The high value obtained indicated that the copolymer had a blocky nature, which suggested that some partially undegraded 3HB oligomers might have been inserted in 4HB chains during polymerization in the second step and/or that some undegraded P(3HB) chains might have contributed to the NMR signal associated with the 3*3 dyad. One of our goals in this study was to prepare a P(4HB) homopolymer by using the second-step cultivation method. However, from the NMR results, we concluded that partial incorporation of 3HB units (∼5 to 10 mol%) into the 4HB chains was unavoidable under the two-step cultivation conditions used.
Synthesis of the P(4HB) homopolymer by using a three-step cultivation process.
The first-step residual P(3HB) was completely removed by culturing the cells from the first step in carbon-free ammonium sulfate (0.3 g/liter)-containing mineral medium (Table 3). Growth of the P(3HB)-free cells on γ-butyrolactone resulted in production of the P(4HB) homopolymer (third-step culture). Increasing the ammonium sulfate concentration to 0.6 g/liter in the second-step cultivation yielded essentially the same results.
TABLE 3.
P(4HB) homopolymer synthesis during three-step cultivation of H. pseudoflavaa
| Culture step | Dry cell wt (g/liter) | Polyester content (%, wt/wt) | Polyester composition (mol%)b
|
|
|---|---|---|---|---|
| 3HB | 4HB | |||
| First | 1.94 ± 0.08 | 10.1 ± 0.6 | 100 | |
| Second | 1.68 ± 0.07 | NDc | ||
| Third | 2.42 ± 0.06 | 19.6 ± 0.5 | 100 | |
The second-step carbon-free medium contained 0.3 g of ammonium sulfate per liter. The P(3HB)-free cells recovered were transferred to γ-butyrolactone-containing PHA synthesis mineral medium (the third culture). Duplicate experiments were carried out.
Calculated from gas chromatographic data.
ND, not detected by gas chromatographic analysis.
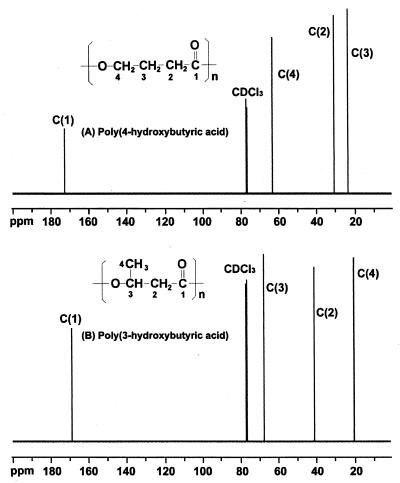
The 13C NMR spectrum of the polymer isolated is shown along with the spectrum of the P(3HB) homopolymer in Fig. 3. The chemical shifts (172.65, 63.52, 30.66, and 24.00 ppm) associated with the four carbons in 4HB (Fig. 3A) agreed well with previously published values (10, 20). The four signals at 169.19, 67.66, 40.84, and 19.80 ppm associated with 3HB (Fig. 3B) were barely detectable in the spectrum of the polymer from the third-step γ-butyrolactone-grown cells. This suggested that the 3HB units in the two-step product, P(3HB-co-4HB) containing 5 mol% 3HB and 95 mol% 4HB, originated from the first-step residual P(3HB), not from γ-butyrolactone.
FIG. 3.
125-MHz 13C NMR spectra of P(4HB) homopolymer (A) and P(3HB) homopolymer (B).
Methanol-reprecipitated, dried P(4HB) homopolymer was analyzed by differential scanning calorimetry. This homopolymer had a melting temperature of 69°C, a glass transition temperature of −43°C, and a melting enthalpy of 61 J/g. In comparison with the precipitated and dried sample, the sample that was melted and then recrystallized melted at 56°C, had an enthalpy value of 49 J/g, and had a glass transition temperature of −44°C. The thermal transition data for the latter sample agreed with previously published values (20).
Inertness of 4HB-rich P(3HB-co-4HB) polyesters in interactions with the intracellular depolymerase.
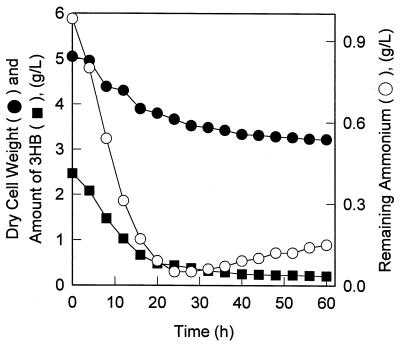
As Fig. 1b shows, in spite of the rapid degradation of the residual P(3HB), the amount of 4HB and the total polyester content never decreased during cultivation even though there were extra NH4+ ions. This result indicated that the depolymerase(s) in H. pseudoflava may be highly specific for P(3HB) and totally inactive against 4HB-rich chains. In order to prove this hypothesis about the specificity of the intracellular depolymerases, we investigated the degradation kinetics (5, 19) of two polyesters, P(3HB) and P(3HB-co-4HB) containing 6 mol% 3HB and 94 mol% 4HB, in cells prepared separately by using glucose and γ-butyrolactone. The cells were cultivated by using the same two-step procedure. As shown in Fig. 4, intracellular P(3HB) was almost completely degraded within 30 h of inoculation and exhibited degradation kinetics similar to those shown in Fig. 1b. The decreases in the profiles for P(3HB) content, cell dry weight, and NH4+ content were consistent with one another. Only 10 to 15% P(3HB) degradation was observed after 60 h of cultivation in the control experiment in which no nitrogen was added (data not shown). Thus, intracellular degradation of P(3HB) was stimulated by adding NH4+ ions (5). In contrast, however, P(3HB-co-4HB) containing 94 mol% 4HB in cells grown on γ-butyrolactone was not degraded under the same incubation conditions, as estimated from the constant 4HB concentration (0.4 g/liter) and the constant amount of biomass (1.9 g/liter) over 60 h of incubation. None of the NH4+ ions (1.0 g/liter as ammonium sulfate) were consumed. The 3HB contents of the copolymer also remained constant throughout the incubation (data not shown). Such complete inactivity of the depolymerases toward the 4HB-rich polymer demonstrated that the enzymes were very specific for P(3HB).
FIG. 4.
Stimulation of intracellular P(3HB) degradation in H. pseudoflava by ammonium sulfate (1.0 g/liter). The cells initially contained 49% (wt/wt) P(3HB) homopolymer.
Activities of 3HBA and 4HBA dehydrogenases.
In 4HBA-utilizing bacteria, the initial step involves oxidative conversion of the hydroxyl group to the aldehydic group, which is catalyzed by a 4HBA dehydrogenase (27). We assumed that γ-butyrolactone could be metabolized in a similar manner except for the ring-opening reaction. Therefore, in order to understand the characteristics of PHA synthesis from γ-butyrolactone depending on the culture conditions, the activities of the 3HBA and 4HBA dehydrogenases were determined under various culture conditions (Table 4). The two activities in R. eutropha H16 were also measured for comparison. For both strains, cells grown in Luria-Bertani medium did not exhibit any 4HBA dehydrogenase activity. The activity appeared after the cells were grown on γ-butyrolactone in the second step. With of the one-step γ-butyrolactone-grown cells, the activity increased with culture time. An abrupt increase in the activity was observed after 120 h of cultivation. This was the time when the cells began to accumulate 3HB-4HB copolymers. A similar trend in enzyme activity and enzyme induction with culture time was also observed for 4HBA-grown cells. Compared to the one-step-grown cells, only 10% of the activity was detected in the cells grown on γ-butyrolactone for 48 h in the second step of the two-step process. Therefore, we concluded that during the relatively short period of exposure of H. pseudoflava to γ-butyrolactone in the multistep cultivation (36 to 48 h), few 3HB monomers might be formed at such a low level of 4HBA dehydrogenase activity, probably because of the resulting insufficient supply of acetyl-CoA, which is discussed in detail below.
TABLE 4.
Activities of 3HBA and 4HBA dehydrogenases in H. pseudoflava and R. eutropha H16 grown on γ-butyrolactone under various culture conditions
| Organism | Culture medium | Culture time (h) | Total protein concn (mg/ml) | Sp Act (U/mg) of:
|
|
|---|---|---|---|---|---|
| 3HBA dehydrogenase | 4HBA dehydrogenase | ||||
| H. pseudoflava | Luria-Bertani medium | 22 | 6.83 | 0.254 | NDc |
| Medium containing γ-butyrolactone (second step)a | 48 | 3.21 | 2.75 | 0.064 | |
| Medium containing 0.8 ml of γ-butyrolactone per liter (one step)b | 96 | 1.06 | ND | 0.089 | |
| 108 | 2.31 | 0.031 | 0.170 | ||
| 120 | 2.85 | 0.095 | 0.492 | ||
| 132 | 3.13 | 0.183 | 0.531 | ||
| 144 | 3.61 | 0.338 | 0.557 | ||
| Medium containing 0.8 g of 4HBA per liter (one step)b | 96 | 1.54 | 0.075 | 0.030 | |
| 108 | 2.01 | 0.188 | 0.092 | ||
| 120 | 2.47 | 0.231 | 0.162 | ||
| 132 | 2.93 | 0.243 | 0.285 | ||
| 144 | 3.01 | 0.277 | 0.369 | ||
| R. eutropha H16 | Luria-Bertani medium | 22 | 3.81 | 1.69 | ND |
| Medium containing γ-butyrolactone (second step)a | 48 | 4.33 | 3.15 | 0.071 | |
Cells grown in Luria-Bertani medium were transferred to PHA synthesis mineral medium containing γ-butyrolactone (2 ml/liter) plus ammonium sulfate (0.66 g/liter).
PHA synthesis mineral medium also contained 0.66 g of ammonium sulfate per liter.
ND, not detected.
In addition, for H. pseudoflava, a 10-fold increase in 3HBA dehydrogenase activity was observed with cells transferred to γ-butyrolactone medium compared to first-step cells grown in Luria-Bertani medium. The rapid degradation of the first-step residual P(3HB) may have been related to this significant increase in 3HBA dehydrogenase activity.
DISCUSSION
H. pseudoflava cells pregrown in Luria-Bertani medium during the first step were not able to produce 3HB-CoA when they were grown on γ-butyrolactone for 36 to 48 h in the second or third step and produced only 4HB-CoA for PHA synthesis. When cells were grown on γ-butyrolactone-containing PHA synthesis mineral medium (one-step culture), they were able to produce both 3HB-CoA and 4HB-CoA for use in PHA synthesis (Table 1). Thus, the culture history of cells strongly affects the composition of the polyester produced from a precursor.
H. pseudoflava can also metabolize 4HBA in order to synthesize 4HB-rich P(3HB-co-4HB) and P(4HB) homopolyester (data not shown). We observed a PHA synthesis trend similar to the trend observed when γ-butyrolactone was the substrate. For example, when cells were grown on 4HBA (2.0 g/liter) in PHA synthesis mineral medium, P(3HB-co-4HB) containing 90 mol% 4HB was produced and accounted for 14% (wt/wt) of the dry weight. During metabolism of γ-butyrolactone, the ring-opening reaction should occur first. γ-Butyrolactone was probably transported into the cells without rupturing of the lactone ring, a conclusion which was supported by the absence of 4HBA released into the medium during cultivation (data not shown). Ring opening probably occurred within the cell, followed by further metabolism.
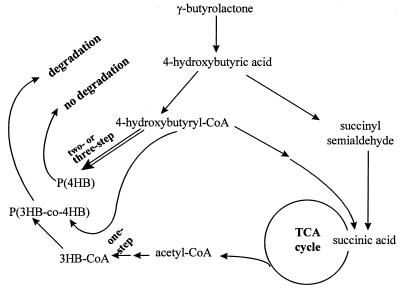
With the two- and three-step cultivation procedures, efficient synthesis of 4HB-rich P(3HB-co-4HB) or P(4HB) homopolymer in H. pseudoflava from γ-butyrolactone is characterized by blockage of two metabolic pathways, formation of 3HB-CoA, and depolymerization of the polymers (Fig. 5). During multistep cultivation, hydrolyzed 4HBA is converted into 4HB-CoA, which is principally utilized as a substrate for PHA synthase instead of being converted into 3HB-CoA via acetyl-CoA. In contrast, R. eutropha H16 was able to metabolize γ-butyrolactone or 4HBA in order to synthesize copolymers containing 3HB and 4HB units at a 3:1 mole ratio in the two-step cultivation procedure (18, 27) because this bacterium possesses a pathway for converting acetyl-CoA to 3HB-CoA (27). Similarly, H. pseudoflava was also able to synthesize 3HB-CoA without any lag in induction when it was grown on saccharides and other lactone, such as γ-caprolactone (6) and γ-valerolactone, in a one-step cultivation process. In the case of R. eutropha H16, the NMR analysis of 13C-labeled 4HBA degradation showed that 4HBA is degraded via succinate and intermediates of the tricarboxylic acid cycle (27). We suggest that during one-step cultivation of H. pseudoflava, the 4HBA hydrolyzed from γ-butyrolactone was probably degraded via a catabolic pathway similar to that in R. eutropha H16 (Fig. 5). Growth of H. pseudoflava on γ-butyrolactone in mineral salts medium requires a long induction period, during which the related enzymes associated with the pathway for conversion of γ-butyrolactone to succinic acid (an intermediate of the tricarboxylic acid cycle) must be produced. However, R. eutropha H16 utilizes γ-butyrolactone as a sole carbon source for growth and PHA production without a long lag period before induction, as in H. pseudoflava. In the one-step culture procedure, R. eutropha produced P(3HB-co-4HB) containing 95 mol% 3HB and 5 mol% 4HB when it was grown on γ-butyrolactone (3.0 ml/liter) in mineral salts medium for 72 h. In both bacteria, 4HBA dehydrogenase activity was induced only after growth on γ-butyrolactone (Table 4). In the two-step cultivation process, H. pseudoflava produced 4HB-rich P(3HB-co-4HB) containing 90 mol% 4HB, while R. eutropha H16 produced 3HB-rich P(3HB-co-4HB) containing 85 mol% 3HB. The higher level of 3HB in the PHA of the one-step-grown R. eutropha H16 cells than in the two-step-grown cells could have been due to a requirement for a high level of acetyl-CoA for growth during the one-step cultivation procedure. The two bacteria exhibited similar levels of 4HBA dehydrogenase activity after 48 h in the two-step cultivation procedure. If PHA synthesis occurs via similar degradation pathways, as suggested above, there probably are regulatory enzymes that control the levels of acetyl-CoA and 4HB-CoA available for PHA synthesis. In H. pseudoflava, one of the key enzymes involved in modulation of 3HB formation from γ-butyrolactone may be the 4HBA dehydrogenase which converts ring-opened 4HBA to succinate semialdehyde. As shown in Tables 1 and 4, however, 3HB was not formed until 4HBA dehydrogenase activity reached a certain level. Thus, the pathway leading to 3HB was inactive, probably due to an insufficient supply of acetyl-CoA at low 4HBA dehydrogenase activity. Therefore, it is thought that the level of acetyl-CoA determining the driving force for formation of 3HB monomers may be related to the level of 4HBA dehydrogenase activity. The long lag period for induction in H. pseudoflava thus makes it possible to synthesize P(4HB) homopolymer and 4HB-rich P(3HB-co-4HB) copolymers. In R. eutropha H16, however, in spite of the low level of 4HB dehydrogenase activity, most γ-butyrolactone was converted to 3HB, while a small amount of the lactone was converted to 4HB-CoA that was used for PHA synthesis in the two-step cultivation. A more detailed study should be performed to understand how γ-butyrolactone or 4HBA is split into the two paths leading to 4HB-CoA and 3HB-CoA for PHA synthesis.
FIG. 5.
Putative metabolic pathway for PHA synthesis from γ-butyrolactone in H. pseudoflava. The intracellular degradability of P(3HB-co-4HB) copolymers depends on the 3HB/4HB ratio of the copolyesters. TCA, tricarboxylic acid.
Depolymerization of P(4HB) and 4HB-rich P(3HB-co-4HB) did not occur in H. pseudoflava, presumably because of the inactivity of the intracellular depolymerases against the 4HB polymers. There is another possibility, namely, that the intracellular depolymerases were not expressed during cultivation on γ-butyrolactone. However, this possibility was eliminated by our finding that in cells containing inclusions composed of two types of polymers, P(3HB) homopolymer and 4HB-rich P(3HB-co-4HB) copolymers, only P(3HB) was degraded (8). Most bacterially produced PHAs are chiral compounds (2, 25). 4HB, however, has no chiral center. The lack of intracellular degradability is thought to be due to the absence of a chiral center, as well as to the unusual oxidation site in the 4HB monomer unit.
Native granules of PHA in cells are completely amorphous (1, 3, 13, 24), and the intracellular degradation of storage PHA by intracellular depolymerases is not understood (13). The intracellular degradation data obtained in this study, however, reflected intermolecular interactions between the depolymerase and the native noncrystalline PHA substrate. It can be speculated that as in the degradation of denatured, crystalline PHA granules or PHA films by extracellular or intracellular depolymerases, the contribution of the crystallinity of the polymers to degradation kinetics (9, 13, 14, 18) could influence the specific nature of the enzymes originating principally from true molecular interactions free from any constraints imposed by neighboring crystalline domains. Therefore, the much higher specificity of the H. pseudoflava intracellular depolymerase for P(3HB) in cells than for P(4HB) in cells may represent the true specific nature of the P(3HB) depolymerase.
It is generally thought that bacteria synthesize PHA from excess carbon under unbalanced nutritional conditions and accumulate the polymers in the form of inclusion bodies for use as a carbon and energy source (2). However, H. pseudoflava synthesizes a polyester even though the polyester cannot be degraded by the intracellular depolymerases and utilized. In this respect, therefore, P(4HB) and 4HB-rich P(3HB-co-4HB) are not energy reserve compounds for the bacterium, and it is not known why bacteria accumulate this type of apparently useless polymer inclusion.
If polyesters can be synthesized without degradation, as is apparently the case for the 4HB-rich polyesters of H. pseudoflava, then the organism must possess some advantage over other organisms which have active depolymerases. A lack of activity of PHA depolymerases may be an advantage during efficient production of high-molecular-weight PHA, as in the case of recombinant Escherichia coli strains harboring P(3HB) synthesis genes but lacking the P(3HB) depolymerase gene (17, 26). Even though such bacteria are intracellularly not capable of degrading a polyester chain to obtain energy, they can synthesize and accumulate the polymer chains in the form of inclusion bodies in their cells. This type of organism, which has polymerase-depolymerase PHA synthesis machinery similar to that of H. pseudoflava, could also be used to synthesize high-molecular-weight PHA.
ACKNOWLEDGMENTS
This work was supported by Korea Science and Engineering Foundation project 97-0401-03-01-3.
We express our sincere thanks to the reviewers for their kind suggestions.
REFERENCES
- 1.Amor S R, Rayment T, Sanders J K M. Polyhydroxyalkanoate in vivo: NMR and X-ray characterization of the elastomeric state. Macromolecules. 1991;24:4583–4588. [Google Scholar]
- 2.Anderson A J, Dawes E A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990;54:450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonthrone K M, Clauss J, Horowitz D M, Hunter B K, Sanders J K M. The biological and physical chemistry of polyhydroxyalkanoates as seen by NMR spectroscopy. FEMS Microbiol Rev. 1992;103:269–278. [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Cho C H, Jeong S J, Kim J W. Stimulation of poly(3-hydroxybutyrate) degradation by nitrogen nutrient in Alcaligenes eutrophus H16. Korean Biochem J. 1994;27:316–322. [Google Scholar]
- 6.Choi M H, Song J J, Yoon S C. Biosynthesis of copolyesters by Hydrogenophaga pseudoflava from various lactones. Can J Microbiol. 1995;41(Suppl. 1):60–67. [Google Scholar]
- 7.Choi M H, Yoon S C. Polyester biosynthesis characteristics of Pseudomonas citronellolis grown on various carbon sources, including 3-methyl-branched substrates. Appl Environ Microbiol. 1994;60:3245–3254. doi: 10.1128/aem.60.9.3245-3254.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi, M. H., and S. C. Yoon. Microstructure dependence of polyhydroxyalkanoic acid degradation in Hydrogenophaga pseudoflava. Submitted for publication. [DOI] [PubMed]
- 9.Doi Y, Kitamura S, Abe H. Microbial synthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Macromolecules. 1995;28:4822–4828. [Google Scholar]
- 10.Doi Y, Kunioka M, Nakamura Y, Soga K. Nuclear magnetic resonance studies on unusual bacterial copolyesters of 3-hydroxybutyrate and 4-hydroxybutyrate. Macromolecules. 1988;21:2722–2727. [Google Scholar]
- 11.Hardman J K. γ-Hydroxybutyrate dehydrogenase from Clostridium aminobutyricum. Methods Enzymol. 1962;5:778–783. [Google Scholar]
- 12.Hein S, Söhling B, Gottschalk G, Steinbüchel A. Biosynthesis of poly(4-hydroxybutyric acid) by recombinant strains of Escherichia coli. FEMS Microbiol Lett. 1997;153:411–418. doi: 10.1111/j.1574-6968.1997.tb12604.x. [DOI] [PubMed] [Google Scholar]
- 13.Jendrossek D, Schirmer A, Schlegel H G. Biodegradation of polyhydroxyalkanoic acids. Appl Microbiol Biotechnol. 1996;46:451–463. doi: 10.1007/s002530050844. [DOI] [PubMed] [Google Scholar]
- 14.Kasuya K, Inoue Y, Doi Y. Adsorption kinetics of bacterial PHB depolymerase on the surface of polyhydroxyalkanoate films. Int J Biol Macromol. 1996;19:35–40. doi: 10.1016/0141-8130(96)01097-5. [DOI] [PubMed] [Google Scholar]
- 15.Kim B K, Yoon S C, Nam J D, Lenz R W. Effect of C/N ratio on the production of poly(3-hydroxyalkanoates) by the methylotroph Paracoccus denitrificans. J Microbiol Biotechnol. 1997;7:391–396. [Google Scholar]
- 16.Kunioka M, Kawaguchi Y, Doi Y. Production of biodegradable copolyesters of 3-hydroxybutyrate and 4-hydroxybutyrate by Alcaligenes eutrophus. Appl Microbiol Biotechnol. 1989;30:569–573. [Google Scholar]
- 17.Kusaka S, Abe H, Lee S Y, Doi Y. Molecular mass of poly[(R)-3-hydroxybutyric acid] produced in a recombinant Escherichia coli. Appl Microbiol Biotechnol. 1997;47:140–143. doi: 10.1007/s002530050902. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura S, Doi Y, Scandola M. Microbial synthesis and characterization of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) Macromolecules. 1992;25:4237–4241. [Google Scholar]
- 19.Saito T, Takizawa K, Sagegusa H. Intracellular poly(3-hydroxybutyrate) depolymerase in Alcaligenes eutrophus. Can J Microbiol. 1995;41(Suppl 1):187–191. [Google Scholar]
- 20.Saito Y, Doi Y. Microbial synthesis and properties of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in Comamonas acidovorans. Int J Biol Macromol. 1994;16:99–104. doi: 10.1016/0141-8130(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 21.Shuster C W, Doudoroff M. A cold-sensitive d(−)β-hydroxybutyric acid dehydrogenase from Rhodospirillum rubrum. J Biol Chem. 1962;237:603–607. [PubMed] [Google Scholar]
- 22.Song J J, Shin Y C, Yoon S C. P(3HB) accumulation in Alcaligenes eutrophus H16 under nutrient-rich condition and its induced production from saccharides and their derivatives. J Microbiol Biotechnol. 1993;3:115–122. [Google Scholar]
- 23.Song J J, Yoon S C. Biosynthesis of novel aromatic copolyesters from insoluble 11-phenoxyundecanoic acid by Pseudomonas putida BM01. Appl Environ Microbiol. 1996;62:536–544. doi: 10.1128/aem.62.2.536-544.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song J J, Yoon S C, Yu S M, Lenz R W. Differential scanning calorimetric study of poly(3-hydroxyoctanoate) inclusions in bacterial cells. Int J Biol Macromol. 1998;23:165–173. doi: 10.1016/s0141-8130(98)00046-4. [DOI] [PubMed] [Google Scholar]
- 25.Steinbüchel A, Valentin H E. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol Lett. 1995;128:219–228. [Google Scholar]
- 26.Valentin H E, Dennis D. Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in recombinant Escherichia coli grown on glucose. J Biotechnol. 1997;58:33–38. doi: 10.1016/s0168-1656(97)00127-2. [DOI] [PubMed] [Google Scholar]
- 27.Valentin H E, Zwingmann G, Schönebaum A, Steinbüchel A. Metabolic pathway for biosynthesis of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from 4-hydroxybutyrate by Alcaligenes eutrophus. Eur J Biochem. 1995;227:43–60. doi: 10.1111/j.1432-1033.1995.tb20358.x. [DOI] [PubMed] [Google Scholar]
- 28.Yabuuchi E, Kosako Y, Yano I, Hotta H, Nishiuchi Y. Transfer of two Burkholderia and an Alcaligenes species to Ralstonia gen. nov. Microbiol Immunol. 1995;39:897–904. doi: 10.1111/j.1348-0421.1995.tb03275.x. [DOI] [PubMed] [Google Scholar]