Abstract
Background
Known in the past for its toxic aspect as the main urban pollutant, in the last few decades, ozone has been gaining greater visibility for its possible antimicrobial, antiviral, and antioxidant effects when used in human dermatological pathologies. Despite the reports of clinical benefits, the standard dosage for clinical efficacy and safety are yet not clear, nor are its means of application and its true acting mechanism.
Objective
We conducted a review to determine the efficacy and safety of ozone therapy for a variety of dermatological conditions.
Methods
We considered clinical trials (both randomized and non-randomized) published between December 2020 and March 2021 as long as they provided some PICO information, i.e., population (P), intervention (I), and study design. The skin dermatological conditions researched were: acne, dermatitis, psoriasis, systemic sclerosis, herpes, aging, ulcers, and skin scarring.
Results
A total of 326 articles were identified and 150 remained after duplicates were removed. After titles, abstracts and full articles were read, 17 articles were included in the systematic review (with 643 patients).
Conclusion
Ozone therapy seems promising for some dermatological conditions; however, the articles included in this review had methodological limitations and did not sufficiently demonstrate sound evidence for safe therapy. Therefore, more studies with better methodological standards and longer-term assessments of side effects should be conducted to achieve better standards and safety in ozone therapy for dermatological conditions.
Keywords: dermatological affections, dermatological pathologies, ozone, ozone therapy
Ozone has been used in medicine for over 100 years in an alternative and empirical way; however, in the last decade, there have been great advances in the therapy due to new technological resources, with greater precision in gas concentration and a better understanding of its possible action mechanisms. Thus, ozone therapy has turned out to be an open research field, in which studies aim to clarify its effectiveness and biological performance, as to ascertain the control of its therapeutic actions.1,2
Ozone is defined as an oxygen (O2) tri-atomic molecule. Its first report occurred in 1840, when Christian Friedrich Schonbien found that, via electron sharing, the properties of atoms allowed them to originate ≥1 simple and different substances, like ozone (O3).2,3
In the mid-19th century, the first ozone generator was developed following the concept of transformation of pure oxygen via a high-voltage gradient, generating as final product a mixture of gases that was 95% oxygen and 5% ozone. However, these first devices did not always possess a precise photometer to calculate the percentage of ozone in the mixture.3,4
The gas is colorless, with a sour odor and explosive in its liquid or solid form. Its half-life is 40 min at 20°C and about 140 min at 0°C. O3 is hazardous to humans, especially when inhaled, but for therapeutic purposes the gas must be produced from medical grade oxygen and administered in precise therapeutic doses, never by inhalation.1–3,5
It is believed that ozone therapy, together with many other therapies, was methodically eliminated from the educational process in the United States; however, this suppression was milder in Europe. Hence, in Germany and other countries, ozone therapy is common. More than 7,000 doctors in Germany use ozone therapy daily. Nowadays in the United States, only 13 states practice ozone therapy. In countries like Cuba, Spain, Italy, Russia, Ukraine, and Greece, the practice is a little more widespread.4,5
As the gas is extremely unstable, there are some forms used in ozone therapy in dermatology. They are mainly categorizable as topical application with ozonated water or oils, ozone bags, subcutaneous applications, rectal insufflation, and major or minor autohemotherapy. 1,6 (Table 1).
TABLE 1.
Administration routes of ozone therapy in dermatology
| ADMINISTRATION | PREPARATION | METHOD | ADVANTAGES | DISADVANTAGES |
|---|---|---|---|---|
| Topical | Ozone in water or oil | Immersion or compress | Therapy dosage maintenance/No age restrictions/Low adverse risk | Rapid ozone degradation when in water |
| Ozone bag | Ozone and oxygen | Topical with containment of gases with appropriate plastic. | Action on bigger lesions | Rapid ozone degradation |
| Subcutaneous | Ozone and oxygen | Subcutaneous | Local action | No systemic action |
| Rectal insufflation | Ozone and oxygen | Rectal | Immediate stimulus to cell metabolism | Rapid ozone degradation |
| Major and minor autohemotherapy | Ozone with individual’s own blood | Intravenous | Immediate stimulus to immune system | Contamination risk |
As ozone is a potent oxidative physiological response, therapy has been used to disinfect and treat diseases for over 150 years, being recommended to treat infections, wounds, and multiple diseases. During World War I, doctors conversant with the antibacterial properties of O3 applied it topically to infected wounds and discovered that not only did O3 heal the infection, it also had hemodynamic and anti-inflammatory properties.5
Starting from its physiological effects, researchers began to assess the use of ozone therapy in skin conditions. The first evidence of beneficial effects of ozone on skin diseases was given by Shpektorova in 1964 and soon made apparent by Białoszewski et al. in 2003, when patients with chronic infected wounds that were resistant to antimicrobial therapy underwent topical ozone therapy. Results showed inhibition of the infections and a faster healing process.7–9
Other types of dermatological conditions, such as acne, dermatitis, psoriasis, herpes, systemic sclerosis, aging, ulcers, and skin scarring, have been being included in ozone therapy treatment protocols; nonetheless, there is no clear evidence of the real efficacy or safety nor guidelines to such interventions.1,6,7,8
Hence, this systematic review is justified as an evaluation of whether ozone therapy has efficacy and safety and if it may be recommended as therapy for those dermatological conditions.
METHODS
Protocol and registration. A systematic review was carried out, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 checklist. The research was registered in the International Prospective Register of Systematic Reviews (PROSPERO no. CRD42021238665).
Eligibility criteria. Eligibility criteria followed some PICO elements, i.e., population (P), intervention (I), and study design. The studies included children, men, and women with skin dermatological conditions such as acne, dermatitis, psoriasis, systemic sclerosis, herpes, aging, ulcers, and skin scarring. Eligible trials of intervention were those that assessed the efficacy, safety, and application benefits of ozone therapy in the aforementioned types of dermatological conditions.
Randomized, non-randomized, and quasi-randomized clinical trials were considered. This research was conducted without date, sample size, or language restrictions for the evaluation of publications. Trials that associated ozone with other therapies in the same area of treatment were excluded.
Information sources and search strategy. Scientific databases assessed for the identification of relevant trials were the Cochrane Central Register of Controlled Trials (CENTRAL), National Center for Biotechnology Information (PubMed), ScienceDirect, Biblioteca Científica Eletrônica Online (SciELO), and Embase. The following medical scientific terms (Medical Subject Headings) were considered to develop the research strategy: [("ozone therapy") AND ("acne") AND ("dermatitis") AND ("psoriasis") AND ("herpes") ("systemic sclerosis") AND ("aging") AND ("ulcers and scarring skin”)].
Trial selection. The search strategy in scientific databases was conducted by 2 independent researchers (D. A. O. M. and R. C. F.), and any disagreements on whether to include a trial in the systematic review were resolved by a third senior researcher (K.C .R.), who was invited to aid in the analysis and inclusion of the article. Randomized, non-randomized, and quasi-randomized clinical trials were included in this systematic review. Studies were primarily selected by their title, then secondly by their abstract, underwent verification of the type of trial, and, in case they contained the proposed subject matter, were accessed in full form for final evaluation and inclusion.
Moreover, the references present in the articles were also checked, with the purpose of identifying any other study missed by the search strategy. After reading the full versions of selected studies, in cases deemed necessary, authors were contacted and requested to provide the data presented in the articles. Data extraction from the articles was done via a data-collection form and tabulated in an electronic spreadsheet, with the following data for each study: database, type of research, article, author/year, recommendation, aim of study, studied population, intervention, results, and conclusions.
Data collection process. Results were expected to demonstrate if ozone therapy is safe, effective, and present benefits in healing of skin dermatological conditions, such as acne, dermatitis, psoriasis, herpes, systemic sclerosis, aging, ulcers, and skin scarring.
Risk of bias in individual studies. The risk of bias was assessed according to the checklist by Downs and Black, which was already used in another review study previously published.10
Synthesis of results. Trials were divided according to their recommendations: acne, dermatitis, psoriasis, systemic sclerosis, herpes, aging, ulcers, and scarring. For trials that compared ozone therapy to other techniques, only the arm treated with ozone was considered.
RESULTS
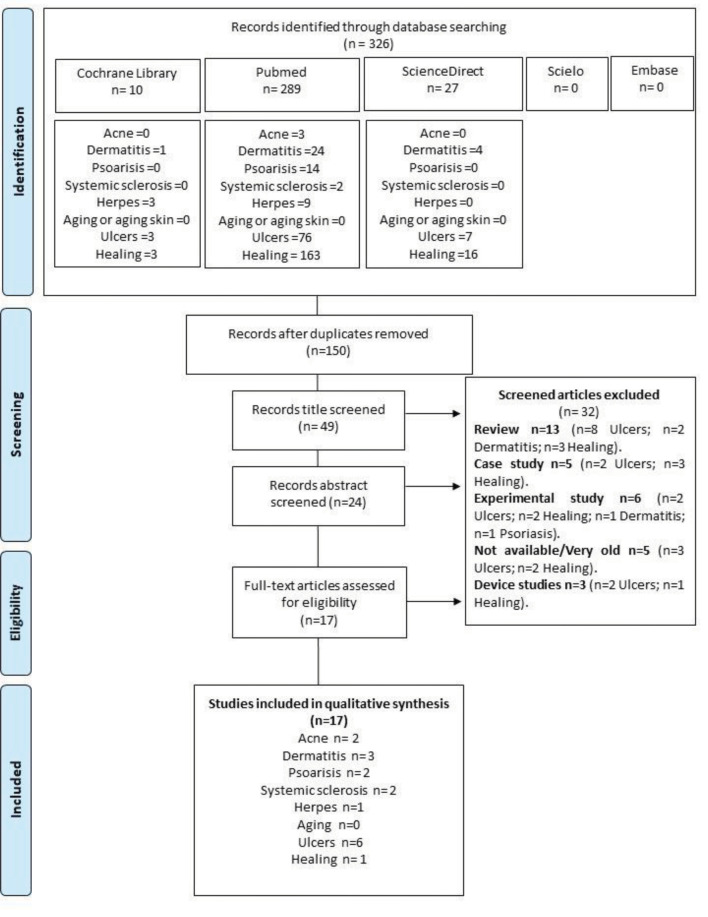
Search history. Initially, 326 articles from January to March 2021 were identified by searching the databases for the key words. After the removal of duplicate articles, 150 were selected. After examination of the titles, 49 were chosen and, after reading of abstracts, 24 articles remained. After reading of full articles, 17 were included in this systematic review (643 patients, Figure 1).
FIGURE 1.
Flowchart of the studies included in the systematic review.
Study characteristics. Table 2 describes the main characteristics and results of the 17 studies with the objective of enhancing the clinical state of dermatological conditions, subdivided by conditions included in the review. As for study design, 3 trials were non-randomized10–12 and 14 were randomized.13–26 Moreover, considering the ozone therapy recommendation, 2 articles were on acne,10,13 3 were on atopic dermatitis,14–16 2 on psoriasis,11,17 2 were on systemic sclerosis,12,18 1 was on herpes-zoster,19 6 were on ulcers,20–25 and 1 was on scarring.25 No study on aging was found during the data search.
TABLE 2.
Characteristics of the studies included in the systematic review
| AUTHOR/YEAR | DERMATOLOGICAL CONDITIONS | TYPE OF STUDY | NUMBER OF PATIENTS | GROUP CONTROL | INTERVENTION GROUP |
|---|---|---|---|---|---|
| Davatdarova and Kazimov, 2008 | Acne | RTC | 72 | Standard drug treatment | Autohemotherapy and topical ozone |
| Gloor and Lipphardt, 1976 | Acne | Not RTC | 16 | NA | Topical ozone |
| Zeng et al., 2020 | Dermatitis | RCT split sides | 12 | Routine care | Ozonated water immersion and topical ozonated oil |
| Guizhi et al., 2018 | Dermatitis | RTC | 60 | Common water immersion | Ozonated water immersion and topical ozonated oil application |
| Jianyun et al., 2018 | Dermatitis | RCT split sides | 12 | Common water wash and common oil application | Ozonated water immersion and ozonated camellia oil |
| Gao et al.,2019 | Psoriasis | Not RCT | 20 | - | Ozonated oil |
| Lina et al., 2018 | Psoriasis | RCT | 40 | Methone ointment with 0.2 mg/g of flumetasone | Ozonated oil |
| Hassaniem et al., 2018 | Systemic sclerosis | RCT | 50 | Drugs (Epilat Retard 40 mg/day) | Drugs (Epilat Retard 40 mg/day) associated with ozone bags |
| Nowicka, 2017 | Systemic sclerosis | Not RCT | 42 | - | Ozonated water immersion |
| Jian et al., 2018 | Herpes zoster | RTC | 60 | Routine care: drugs, low-intensity laser, and topical ointment | Routine care associated to ozonated water compress and topical ozonated oil |
| Kasmawati et al., 2020 | Diabetic foot ulcer | RTC | 27 | Antimicrobial dressings | Antimicrobial dressings associated to ozone bags |
| Izadi et al., 2019 | Diabetic foot ulcer | RTC | 100 | Routine care (debridement and dressings) | Association of ozone bags and ozonated gel; subcutaneous application around the wound and systemic use by rectal insufflation |
| Solovâstru et al., 2015 | Chronic Venous Ulcers | RTC | 29 | Routine care (debridement and dressings) | Routine care associated to topical ozonated sunflower oil |
| Zhang et al., 2014 | Diabetic foot ulcer | RTC | 50 | Routine care (debridement and dressings) | Ozone bag |
| Wainstein et al., 2011 | Diabetic foot ulcer | RTC | 32 | Routine care (debridement and dressings) and placebo ozone therapy (inactive form) | Routine care associated with ozone bag |
| Martínez-Sánchez et al., 2005 | Diabetic foot ulcer | RTC | 51 | Only drug therapy | Ozone bag, ozonated oil and rectal insufflation |
| Campanati et al., 2013 | Healing | RTC split sides | 30 | Topical hyaluronic gel | Ozonated oil |
| AUTHOR/YEAR | OZONE CONCENTRATION | NUMBER OF SESSIONS/TIME | CONCLUSION | RISK OF BIAS |
|---|---|---|---|---|
| Davatdarova and Kazimov, 2008 | 100 mL of blood with 150 mL of ozonated saline solution/topical not reported | Not reported/topical 3–4 times/week | Forms of ozone therapy were effective when compared to drug therapy | Not reported |
| Gloor and Lipphardt, 1976 | Not reported | Once daily for 1 week | Ozone therapy was ineffective against P. acne and other microorganisms | Not reported |
| Zeng et al., 2020 | Ozonated water (3.0 ± 1.5 mg/L); oil not reported | Once daily for 15 minutes and topical oil twice daily for 3 days | Compared to the control group, ozone therapy may significantly mitigate lesions | Not reported |
| Guizhi et al., 2018 | Ozonated water (3.0 ± 1.5 mg/L). Oil not reported | 3–5 times/week for 15 min for 2 weeks | Ozone therapy is safe and efficient | Not reported |
| Jianyun et al., 2018 | Ozonated water (3.0 ± 1.5 mg/L). Oil not reported | Twice daily for 7 days | Reduced effects of S. aureus colonization | Not reported |
| Gao et al.,2019 | 13.5% ozone | Twice daily for 4 weeks | Ozonated oil may be a safe and efficient treatment | Not reported |
| Lina et al., 2018 | Not reported | Twice daily for 4 weeks | Ozonated oil is safe and effective , equivalent to glucocorticoids topical ointments | Not reported |
| Hassaniem et al., 2018 | Bag: 52 mg/mL of ozone (total volume, 20–50 mL) | Once daily for 30 min for 20 days | Stimulated early wound healing | Not reported |
| Nowicka, 2017 | Ozone at 2.42 mg/min | Twice daily for 10 min for 10 days | Increased joint mobility and skin temperature and decreased its thickness | Not reported |
| Jian et al., 2018 | Ozonated water (3.0 ± 1.5 mg/L); oil not reported | Once daily for 5 days | Reduced pain in herpes-zoster acute phase and shortened virus duration | Not reported |
| Kasmawati et al., 2020 | Bag: 70 μg/mL of ozone | Once every 3 days for 21 days; bag time not reported | Standard treatment combined with ozone therapy reduced the number of bacterial colonies | Not reported |
| Izadi et al., 2019 | Not reported | 30 min of ozone bag; topical ozonated oil every 12h; subcutaneous and rectal routes not reported; sessions conducted until complete healing of ulcer: average, 69.44 ± 36.055 days | Efficient as complement to conservative management | Not reported |
| Solovâstru et al., 2015 | 15% ozonated sunflower oil (9.6% O3) | Once daily for 30 days | Promising therapeutic option with calming and anti-inflammatory effects | Not reported |
| Zhang et al., 2014 | with 52 μg/ mL ozone (total volume: 20–50 mL) | Bag 30 min once daily for 20 days | Ozone therapy promoted early healing of ulcer | Not reported |
| Wainstein et al., 2011 | Step 1: 96% oxygen and 4% (80 1g/mL) ozone Step 2: 98% oxygen and 2% (40 lg/ mL) ozone. | Step 1: 4 times/week for 4 weeks; step 2: 2 times/week for 12 weeks | Clinical benefit when combined with conservative management | Selection bias; study data collection logistics |
| Martínez-Sánchez et al., 2005 | Bag: 60 mg/L of ozone; oil not reported; systemic use by rectal insufflation: 10 mg ozone and 50 mg/L concentration | Bag: 1 h every day; rectal insufflation for 20 sessions | Therapy considered efficient and may be used as alternative treatment | Not reported |
| Campanati et al., 2013 | Not reported | Once daily for 12 weeks | As effective as hyaluronic acid | Not reported |
Ozone therapy application routes. Choosing the route of application for ozone therapy is an important factor for the results. In this review, 12 trials used ozone therapy as a topical agent (ozonated oil) or immersion in hydrotherapy with ozone10–17,19–22,25; 5 trials used only ozone bags,18,20,23,24,25; 1 trial combined topical use with ozone bag, subcutaneous, and rectal insufflation21,25; and 1 trial used autohemotherapy.13 Ozone concentrations varied according to the route of application; however, in 4 studies, information on the concentration used was not found.
Efficacy, benefits, safety, and adverse events in ozone therapy.
Only Gloor and Lipphardt11 reported ozone therapy as non-effective for acne treatment, because the therapy did not present the expected effect on Propionibacterium acnes and other microorganisms. The other included studies reported a possible safety of the therapy, through the use of adequate ozone concentrations for the treatment of each type of dermatological condition, with the dose used not being sufficient to cause damage and adverse effects to the research participants.
Benefits reported for acne were reduction in the inflammatory process and, for dermatitis, were reduction of size of lesions and prurigo.12–14 In psoriasis, the therapy reduced painful states, enhanced circulation, and the immune system15,16; in systemic sclerosis, it aided in early healing and enhanced joint mobility,17,18; for herpes-zoster, it significantly reduced duration of the pathological condition19; and, for ulcers and scarring, it helped with the repair and regeneration processes, increasing blood circulation and combating bacteria and infectious agents.20–26
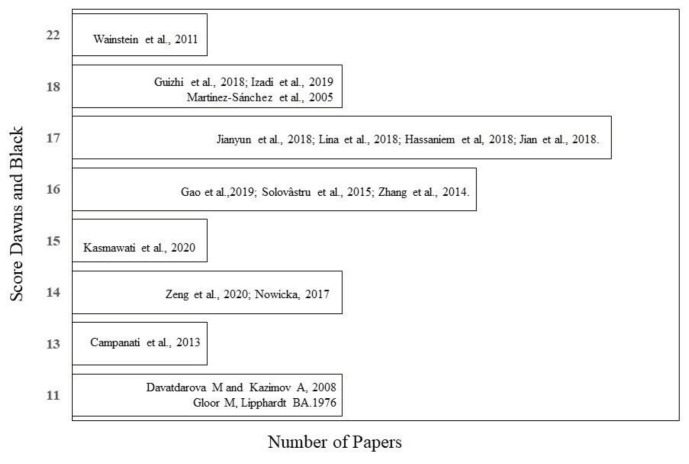
Methodology quality assessment. The 17 trials included in this review were assessed to discern the methodological quality using the Downs and Black checklist, which is used for the assessment of methodological quality of randomized and non-randomized trials. The checklist categorizes the quality of trials according to the following points: 26–28 points, excellent; 20–25 points, good; 15–19 points, regular; and 14 points, poor.27
In evaluating the included studies, 512,15,17,25,28 scored 11–14 points and were considered methodologically poor, 1112,13,4,16,19–23,25 scored 15–19 points and were considered to have regular methodological quality, and only 1 study24 scored 22 points and was thus considered to have good methodological quality (Figure 2).
FIGURE 2.
Downs and Black scores of methodological quality for the systematically reviewed articles.
The greatest bias risks were observed concerning the absence of information on confusion factors, dispersion estimation, reports of adverse events, loss to follow-up, probability values, alternatives to blind patients and researchers, reports of post-hoc exploratory analysis, types of randomization, and reports of possible protocols of treatment intent.
DISCUSSION
In spite of being an old therapy, little or no evidence on its use in dermatological conditions can be found nowadays. Through our review research, we observed a somewhat greater foundation for the therapy in other health areas, such as rehabilitation and odontology.29–32
In 2006, Bocci already forewarned of the scarcity of studies on ozone therapy and their concern on the therapy dissemination by unqualified professionals, for whom the most important information is the ozone dosage, instead of understanding how it acts on tissues and what we can do to avoid its toxicity. Moreover, the lack of a precise ozone generator and of dosage standards lead up to the absence of sound scientific and clinical data published under adequate methodological conditions. All this favors the skepticism towards the possible recommendations for ozone therapy.33
According to Wang, ozone therapy is recent in dermathology and can be a complementary therapy for skin conditions caused by bacteria, viruses, fungi, yeasts, and/or parasites because it acts on the inactivation of these micro-organisms. In such cases, ozone oxidizes the compound phospholipids and lipoproteins of the cell walls of bacteria; hence, they lose their integrity, and their growth and multiplication are inhibited.34
On skin conditions caused by viruses, such as in herpes, ozone damages the virus structure known as the “viral capsid” via its lipid peroxidation mechanism, which prevents the viral proliferation in the host cells, breaking its reproductive means, while causes cell cytotoxicity in the already infected cells, which may lead to cell death by apoptosis or necrosis.32–36
In the present review, we found studies that reported the use of ozone in treating acne, atopic dermatitis, and ulcers, in which therapy aided in reducing bacterial colonization, such as of Staphylococcus aureus, showing the shortening on the duration of bacterial presence, or virus in the case of herpes-zoster, contributing to the repair process and the regeneration of dermic lesions, especially in cases of patients with diabetic ulcers.11,12–14,19,28
For treatment of venous or diabetic ulcers, stimulus of lesion healing can be triggered in varied ways, and, for wounds that do not heal with conventional treatments, ozone therapy may become an alternative.20–25
According to Kushmakov et al., besides its antibacterial and antifungal effects, ozone has the capacity to enhance platelet aggregation. In non-hemostatic functions, platelets are important in inflammation and wound healing; they interact with leukocytes and release vasoactive amines; cytokines; mitogens; and growth factors such as vascular endothelial growth factor, transforming growth factor β, and platelet-derived growth factor. In such a manner, they promote chemotaxis, cell proliferation and differentiation, neovascularization, and extracellular matrix deposition. This process stimulated by ozone aids in the faster healing of wounds/lesions.38
In cases like diabetic ulcers, ozone has an even more specific actuation. When the glycemic state of these patients is not under control, it causes mitochondrial damages in repair cells and even their apoptosis. There is an increase in free radicals and a lower level of antioxidants, leading to vascular damage that hinders the wound healing. In these cases, the ozone’s power of induction for an antioxidant response is beneficial, for it acts on the balance between glycemic level and free radicals and stimulates a rise in antioxidant levels, enhancing the blood microcirculation and thus increasing oxygen delivery to tissues.20–23,37
Since ozone is a potent inducer for the antioxidant system, this effect helps the physiological process of damaged cells and tissues, for it contributes to the modulation of oxidant and antioxidant systems of the organism, since it also activates the nuclear factor erythroid-derived 2, enhancing the activity of antioxidant enzymes and reducing the levels of pro-inflammatory cytokines. The oxidant molecules, among which is oxygen, play an important role in physiological processes. The reactive oxygen species, also known as free radicals, induce oxidative stress in cells and activate lymphocytes and monocytes and the release of cytokines in circulation and tissues, even stimulating the production of Interferon, which has powerful antiviral activity.33–37,38
Atopic dermatitis and psoriasis both usually present with an imbalance in the skin microbiota that affects immune and anti-inflammatory responses, thus contributing to the severity of the disease. In many cases, there is an association of bacterial colonization that adds to the exacerbation and relapse of these diseases. We have included in this review 3 articles that have used ozone therapy as an alternative treatment for atopic dermatitis and 2 articles that reported its use in psoriasis. Authors have reported that, compared to conventional therapy, ozone may be a beneficial treatment alternative for both diseases; nonetheless, it must be used as complimentary to drug therapy. The reason for its success suggested is that its antibacterial action is associated with the ability to stimulate the balance of the immune system and its potent antioxidant action alters the epigenetics of the disease and generates skin restoration via microbiological regulation of the damaged skin.12–14,16
Another skin condition for which ozone therapy has been being used is systemic sclerosis. This disease has a high morbidity rate, since, besides damaging the skin, it also affects internal organs. Its main characteristic is skin fibrosis with vascular diseases, which causes ulcers, mainly digital. Researches report that ozone therapy enhances endogenous growth factors, stimulating fibroblasts, increasing then in the microcirculation via the release of nitric oxide and rebalancing the immunological function, favoring the skin restoration and revitalizing the repair and regeneration processes of ulcers and wounds.17,18,33,34
Generally speaking, the articles report that ozone therapy may aid in certain types of dermatological conditions of the skin, for it favors the increase in blood flow with consequent increase in oxygen flow, through stimulus of the oxygen absorbing capacity of the erythrocytes, enhancing their metabolism. Moreover, ozone may balance the immune system and prompt growth factors, cytokines, and chemokines involved in the inflammatory response, modulating pain and altering the cell biology in benefit of its initial characteristic, all due to its antioxidant effect.29–35
However, authors report that evidence on ozone use for the dermatological conditions included in this review is yet extremely doubtful because most trials do not present follow-up studies and do not report in a trustworthy fashion the doses used during therapy. Besides, adverse effects are seldom taken into account.24,25,33
Furthermore, the biological effects of ozone may be dubious, depending on its concentration and site of application. Very low concentrations are practically useless—at best, they act as placebo. Very high concentrations may provoke a negative effect, such as ill-being and fatigue, and even severe adverse effects, like lowering the pulmonary function and dyspnea, among others. In the articles included in this review, some did not report the concentration of ozone when used with water or in the form of ozonized oil. Therapy forms of administration also differed among studies. For the most part, ozone therapy was used as a topical agent, as ozonated oil, immersion in hydrotherapy, or simply with oxygen via the ozone bag. Rectal insufflation, autohemotherapy, or subcutaneous applications were used in few studies. A lack of information on forms of administration and concentration and doses of ozone hinder the efficacy assessment of the resource and efforts to standardize its use.33–36,41
Our review has shown that evidence on the use of ozone therapy for skin dermatological conditions is scarce. Regarding methodological quality, the findings demonstrate there are few randomized studies and, among the 17 included, all presented diverse methodological aspects. The Downs and Black checklist was used to assess randomized and non-randomized trials. It offers a score for quality of the study and a profile for the research external validity in order to enable understanding on the adequacy of the selected studies.27
Only Wainstein et al.24 scored 22 points on the Downs and Black checklist—that is, only this 1 study was considered to have good methodological quality. The objective of the study was to assess the effect of ozone therapy on the healing of ulcers on diabetic feet. The methodological quality of 11 studies was considered regular and the rest had poor methodological quality.
Studies included in this review presented an apparent lack of information regarding confusion factors, dispersion estimate, adverse effects reports, loss of follow-up, probability values, and patient and researcher blinding alternatives. The data not reported by the studies are considered of utmost importance, and their absence hinders an assessment of the studies regarding therapy recommendation and the qualification of their indication associated with the evidence level.
Considering the information and methodological evaluation of the studies included in this review, it is still not possible to support an evidence level of ozone therapy for treatment of dermatological conditions such as acne, dermatitis, psoriasis, herpes, systemic sclerosis, and aging. This indicates the need for new, methodologically adequate trials with follow-up assessments. For ulcers and scarring dermatological conditions, we have observed that ozone therapy presents greater evidence for its efficacy and safety. However, more studies in these areas are still important.
It is necessary to stress the pertinence of this literature systematic review, in which a yet low number of studies on the subject matter were found. Among the studies included here, some presented adequate and similar methodology in the application of ozone therapy. It was evident that short-term therapy may even present beneficial results for the dermatological conditions cited in the present study, especially ulcers and scarring processes. However, we point out the limitations of this systematic review related to the lack of information in the follow-up period of the studies after the end of treatment, to assess possible long-term adverse effects, and the methodological quality of the studies, which for the most part did not present standards for ozone therapy application.
CONCLUSION
Based on the above, this review concludes that evidence on ozone therapy for treatment of dermatological conditions of the skin seems promising for short term use, especially for ulcers and scarring. Nonetheless, long-term efficacy and safety still present limitations since the studies do not assess adverse effects or long-term results after the end of treatment. Moreover, all studies included show a scarcity of data regarding therapy, which makes setting standards for ozone therapy for the treatment of the studied conditions more difficult. More research is needed, under better methodological standards, with dosage and ozone concentration reports, equal forms of application for each treatment, and a follow-up period that covers a longer time frame so as to assess the possibility of permanent adverse effects.
Contributor Information
Débora Aparecida Oliveira Modena, Dr. Oliveira Modena is with the Department of Surgery, Medical Sciences Institute, University of Campinas (Unicamp), Campinas, São Paulo, Brazil and the Departamento de Medicina Celular e Molecular, Faculdade de Medicina do ABC, Santo André, São Paulo, Brasil..
Rafael de Castro Ferreira, Mr. de Castro Ferreira is with the Departamento de Medicina Celular e Molecular, Faculdade de Medicina do ABC, Santo André, São Paulo, Brasil..
Patricia Meyer Froes, Dr. Froes is with the Federal University of Rio Grande do Norte (UFRN), Natal, Brazil..
Katya Cristina Rocha, Dr. Rocha is with the Departamento de Patologia, Faculdade de Medicina do ABC, Santo André, São Paulo, Brasil..
References
- 1.Zeng J, Lu J. Mechanisms of action involved in ozone-therapy in skin diseases. Int Immunopharmacol. 2018;56(138):235–241. doi: 10.1016/j.intimp.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Valacchi G, Van der Vliet A, Schock BC et al. Ozone exposure activates oxidative stress responses in murine skin. Toxicology. 2002;179(1–2):163–170. doi: 10.1016/s0300-483x(02)00240-8. [DOI] [PubMed] [Google Scholar]
- 3.Suh Y, Patel S, Kaitlyn R et al. Clinical utility of ozone therapy in dental and oral medicine. Med Gas Res. 2019;9(3):163–167. doi: 10.4103/2045-9912.266997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoemaker J. Ozone therapy: history, physiology, indications, results. J Nat Sci Biol Med. 2011;2(1):66–70. doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elvis AM, Ekta JS. Ozone therapy: a clinical review. J Nat Sci Biol Med. 2011;2(1):66–70. doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz-Tapia A, Martínez-Sánchez G, Sabah F Madrid declaration on ozone therapy. 2015. p. 50. ISCO3.
- 7.Dariusz Białoszewski MK. Superficially, longer, intermittent ozone theraphy in the treatment of the chronic, infected wounds. Ortop Traumatol Rehabil. 2003;30(5):652–658. [PubMed] [Google Scholar]
- 8.Mauro R DI, Cantarella G, Bernardini R et al. The biochemical and pharmacological properties of ozone: the smell of protection in acute and chronic diseases. Int J Mol Sci. 2019;20(3):634. doi: 10.3390/ijms20030634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valacchi G, Fortino V, Bocci V. The dual action of ozone on the skin. Br J Dermatol. 2005;153(6):1096–1100. doi: 10.1111/j.1365-2133.2005.06939.x. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira Modena DA, Godoy Miranda AC, Grecco C et al. Efficacy and safety of ND:YAG 1064;nm lasers for photoepilation: a systematic review. Lasers Med Sci. 2020;35(4):797–806. doi: 10.1007/s10103-019-02939-6. [DOI] [PubMed] [Google Scholar]
- 11.Gloor M, Lipphardt BA. Studies on ozone therapy of acne vulgaris. Z Hautkr. 1976;51(3):1976. [PubMed] [Google Scholar]
- 12.Zeng J, Dou J, Gao L et al. International Immunopharmacology Topical ozone therapy restores microbiome diversity in atopic dermatitis. Int Immunopharmacol. 2020;80:106191. doi: 10.1016/j.intimp.2020.106191. [DOI] [PubMed] [Google Scholar]
- 13.Guizhi QIN, Jinhua H, Yizhi PAN et al. Topical ozone application: an innovative therapy for infantile atopic dermatitis [in Chinese]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43(2):163–167. doi: 10.11817/j.issn.1672-7347.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Jianyun LU, Miaomiao LI, Jian H et al. Effect of ozone on Staphylococcus aureus colonization in patients with atopic dermatitis [in Chinese]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43(2):157–162. doi: 10.11817/j.issn.1672-7347.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Gao L, Dou J, Zhang B et al. Ozone therapy promotes the differentiation of basal keratinocytes via increasing Tp63-mediated transcription of KRT10 to improve psoriasis. J Cell Mol Med. 2020;24(8):4819–4829. doi: 10.1111/jcmm.15160. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lina TAN, Jian H, Jing LU, Jianyun LU. Clinical efficacy of ozonated oil in the treatment of psoriasis vulgaris [in Chinese]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43(2):173–178. doi: 10.11817/j.issn.1672-7347.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Nowicka D. Thermography improves clinical assessment in patients. Biomed Res Int. 2017;2017:5842723. doi: 10.1155/2017/5842723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassanien M, Rashad S, Ghaly M. Non-invasive oxygen-ozone therapy in treating digital ulcers of patients with systemic sclerosis. Acta Reumatol Port. 2018;43(3):210–216. [PubMed] [Google Scholar]
- 19.Huang J, Huang J, Xiang Y et al. Topical ozone therapy: an innovative solution to patients with herpes zoster [in Chinese]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43(2):168–172. doi: 10.11817/j.issn.1672-7347.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Kadir K, Syam Y, Yusuf S, Zainuddin M. Ozone therapy on reduction of bacterial colonies and acceleration of diabetic foot ulcer healing. Home Healthc Now. 2020;38(4):215–220. doi: 10.1097/NHH.0000000000000889. [DOI] [PubMed] [Google Scholar]
- 21.Izadi M, Kheirjou R, Mohammadpour R. Efficacy of comprehensive ozone therapy in diabetic foot ulcer healing. Diabetes Metab Syndr. 2019;13(1):822–825. doi: 10.1016/j.dsx.2018.11.060. [DOI] [PubMed] [Google Scholar]
- 22.Solovăstru LG, Stîncanu A, De Ascentii A et al. Randomized, controlled study of innovative spray formulation containing ozonated oil and α-bisabolol in the topical treatment of chronic venous leg ulcers. Adv Skin Wound Care. 2015;28(9):406–409. doi: 10.1097/01.ASW.0000470155.29821.ed. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Guan M, Xie C et al. Increased growth factors play a role in wound healing promoted by noninvasive oxygen-ozone therapy in diabetic patients with foot ulcers. Oxid Med Cell Longev. 2014;2014:273475. doi: 10.1155/2014/273475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wainstein J, Feldbrin Z, Boaz M, Harman-boehm I. Efficacy of ozone-oxygen therapy for the treatment of diabetic foot ulcers. Diabetes Technol Ther. 2011;13(12):1255–1260. doi: 10.1089/dia.2011.0018. [DOI] [PubMed] [Google Scholar]
- 25.Martínez-Sánchez G, Al-Dalain SM, Menéndez S et al. Therapeutic efficacy of ozone in patients with diabetic foot. Eur J Pharmacol. 2005;523(1-3):151–161. doi: 10.1016/j.ejphar.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Campanati A, De Blasio S, Giuliano A et al. Topical ozonated oil versus hyaluronic gel for the treatment of partial- to full-thickness second-degree burns: a prospective, comparative, single-blind, non-randomised, controlled clinical trial. Burns. 2013;39(6):1178–1183. doi: 10.1016/j.burns.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Modena OAD, Miranda GCA, Grecco C et al. Efficacy and safety of ND:YAG 1064;nm lasers for photoepilation: a systematic review. Lasers Med Sci. 2020;35(4):797–806. doi: 10.1007/s10103-019-02939-6. [DOI] [PubMed] [Google Scholar]
- 28.Davatdarova M, Kazimov A. Comparative estimation of medicinal and complex therapy influence on immunological indexes in the patients with acne disease [in Russian]. Georg Med News. 2018;163:80–83. [PubMed] [Google Scholar]
- 29. Liu J, Zhang P, Tian J. et al. Ozone therapy for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2015. 2015 10CD008474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baranova I. The use of the functional state of the joints for the estimation of the effectiveness of the application of oxygen/ozone therapy for the rehabilitative treatment of the patients suffering from knee arthritis [in Russian]. Vopr Kurortol Fizioter Lech Fiz Kult. 2018;95(3):42–48. doi: 10.17116/kurort201895342. [DOI] [PubMed] [Google Scholar]
- 31.Giombini A, Menotti F, Di Cesare A et al. Comparison between intrarticular injection of hyaluronic acid, oxygen ozone, and the combination of both in the treatment of knee osteoarthrosis. J Biol Regul Homeost Agents. 2016;30(2):621–625. [PubMed] [Google Scholar]
- 32.Domb WC. Ozone therapy in dentistry. A brief review for physicians. Interv Neuroradiol. 2014;20(5):632–636. doi: 10.15274/INR-2014-10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bocci VA. Scientific and medical aspects of ozone therapy. State of the art. Arch Med Res. 2006;37(4):425–435. doi: 10.1016/j.arcmed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Bocci VA. Tropospheric ozone toxicity vs. usefulness of ozone therapy. Arch Med Res. 2007;38(2):265–267. doi: 10.1016/j.arcmed.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Wang X. Emerging roles of ozone in skin diseases. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43(2):114–123. doi: 10.11817/j.issn.1672-7347.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Valacchi G, Fortino V, Bocci V. The dual action of ozone on the skin. Br J Dermatol. 2005;153(6):1096–1100. doi: 10.1111/j.1365-2133.2005.06939.x. [DOI] [PubMed] [Google Scholar]
- 37.Hems RS, Gulabivala K, Ng YL et al. An in vitro evaluation of the ability of ozone to kill a strain of Enterococcus faecalis. Int Endod J. 2005;38(1):22–29. doi: 10.1111/j.1365-2591.2004.00891.x. [DOI] [PubMed] [Google Scholar]
- 38.Wen Q, Chen Q. An overview of ozone therapy for treating foot ulcers in patients with diabetes. Am J Med Sci. 2020;360(2):112–119. doi: 10.1016/j.amjms.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Martínez-Sánchez G. La ozonoterapia gana evidencias científicas en el campo clínico. Revista Cubana de Farmacia. 2013;47(1):1–4. [Google Scholar]
- 40.Kushmakov R, Gandhi J, Seyam O et al. Ozone therapy for diabetic foot. Med Gas Res. 2018;8(3):111–115. doi: 10.4103/2045-9912.241076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Re L, Mawsouf MN, Menéndez S et al. Ozone therapy: clinical and basic evidence of its therapeutic potential. Arch Med Res. 2008;39(1):17–26. doi: 10.1016/j.arcmed.2007.07.005. [DOI] [PubMed] [Google Scholar]