Abstract
Background
One of the new treatment options for unresectable locally advanced pancreatic cancer is electrochemotherapy (ECT), a local ablative therapy that potentiates the entry of chemotherapeutic drugs into the cells, by the application of an electric field to the tumor. Its feasibility and safety were demonstrated in preclinical and clinical studies; however, there is a lack of preclinical studies assessing the actions of different drugs used in ECT, their mechanisms and interactions with other target drugs that are used in clinical practice.
Materials and methods
The aim of the study was to determine the cytotoxicity of two chemotherapeutic drugs usually used in ECT (bleomycin and cisplatin) in the BxPC-3 human pancreatic carcinoma cell line and evaluate the interactions of ECT with the targeted drug sunitinib. First, the cytotoxicity of ECT using both chemotherapeutics was determined. In the next part, the interactions of ECT and sunitinib were evaluated through determination of combined cytotoxicity, sunitinib targets and kinetics of cell death.
Results
The results demonstrate that ECT is effective in pancreatic cancer cell line, especially when bleomycin is used, with the onset of cell death in the first hours after the treatment, reaching a plateau at 20 hours after the treatment. Furthermore, we provide the rationale for combining ECT with bleomycin and the targeted drug sunitinib to potentiate cytotoxicity. The combined treatment of sunitinib and ECT was synergistic for bleomycin only at the highest used concentration of bleomycin 0.14 μM, whereas with lower doses of bleomycin, this effect was not observed. The interaction of ECT and treatment with sunitinib was confirmed by course of the cell death, also indicating on synergism.
Conclusions
ECT and sunitinib combined treatment has clinical potential, and further studies are warranted.
Key words: electrochemotherapy, ECT, pancreas, sunitinib, bleomycin, tyrosine-kinase inhibitors, pancreatic cancer
Introduction
Worldwide, the incidence and death rates of pancreatic cancer are growing. The number of deaths, incident cases, and disability-adjusted life-years caused by pancreatic cancer has more than doubled in the last 30 years and is likely to continue due to the aging of the world population.1 The primary treatment for pancreatic cancer is surgical resection; however, many patients present with unresectable locally advanced disease, as their tumors are not limited to the pancreas.2 Therefore, new local ablative therapies are being investigated as new treatment approaches. One of them is electrochemotherapy (ECT), where the application of an electric field to the tumor increases the permeability of the cell membrane and enables the entry of chemotherapeutic drugs into the cell.3
The standard operating procedures for ECT have already been published, and the method is now used in nearly 170 comprehensive cancer centers around the world, mainly for the treatment of easily accessible cutaneous tumors.4, 5 This approach has also been proven feasible, safe and effective for the treatment of deep-seated tumors such as primary hepatocellular carcinoma, with 84.4% complete responses (CR), 12.5% partial responses (PR) and 3.1% stable disease (SD) rate.6, 7 The ECT is commonly performed during open surgery; however, our group has already reported the first successful percutaneous application.8 Moreover, ECT is also successfully used for the treatment of unresectable colorectal liver metastases located in the vicinity of large vessels or in cases when resection is not possible due to small residual liver volume.9, 10
One of the possible new implementations of ECT into clinical practice could be for the treatment of locally advanced pancreatic cancer or other pancreatic tumors. The feasibility and safety of this approach was established in preclinical studies in a porcine and rabbit models, where it was demonstrated that ECT with bleomycin does not cause devastating complications such as acute pancreatitis, pancreatic fistula, or vascular injury.11, 12 The first clinical studies were also conducted, demonstrating the feasibility and safety of the treatment in a palliative setting.13, 14, 15 However, there is a lack of preclinical studies assessing the actions of different drugs used in ECT, their mechanisms and interactions with targeted drugs, which are currently used in clinical practice for the treatment of pancreatic cancer.
Patients with unresectable locally advanced pancreatic tumors receive therapies based on their performance status and can be treated with chemotherapeutic schemes or in combination with targeted drugs such as sunitinib.16, 17 Sunitinib is indicated for the treatment of progressive neuroendocrine pancreatic tumors that cannot be removed by surgery or that have spread to other parts of the body (metastatic cancer).18 Sunitinib inhibits multiple receptor tyrosine kinases that are implicated in cell proliferation, migration and cell death; therefore, it impedes tumor growth, angiogenesis, and metastatic progression. Sunitinib was identified as an inhibitor of platelet-derived growth factor receptors (PDGFRs), vascular endothelial growth factor receptors (VEGFR1/FLT1, VEGFR2/KDR, and VEGFR3), stem cell factor receptor (KIT), Fms-related receptor tyrosine kinase 3 (FLT3), and glial cell line-derived neurotrophic factor receptor (RET).19
To further explore the potential of ECT for the treatment of pancreatic cancer, the aim of the study was to determine the cytotoxicity of two chemotherapeutic drugs usually used in ECT (bleomycin and cisplatin) and to evaluate the interactions of ECT with the targeted drug sunitinib in a BxPC-3 pancreatic adenocarcinoma cell line. First, the cytotoxicity of ECT using both chemotherapeutics was determined. In the next part, the interactions of ECT and sunitinib were evaluated through the determination of the combined cytotoxicity, sunitinib targets and kinetics of cell death.
Materials and methods
Cell lines and drugs
The human pancreatic cancer cell line BxPC-3 (ATCC® CRL-1687™) was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) in 2020 and cultured in Advanced RPMI-1640 medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 5% (v/v) fetal bovine serum (FBS, Gibco), GlutaMAX (100x, Gibco), and penicillin–streptomycin (100x, Sigma Aldrich, Merck, Darmstadt, Germany) in a 5% CO2 humidified atmosphere at 37°C. The cells were routinely tested for mycoplasma infection by a MycoAlertTM PLUS Mycoplasma Detection Kit (Lonza, Basel, Switzerland) and were mycoplasma free.
A stock solution of 3 mg/ml bleomycin (Bleomycin medac, Medac, Germany) was diluted in 0.9% NaCl saline (B. Braun Melsungen AG, Melsungen, Germany) to seven working solutions in the range from 7 μM to 7 x 10-6 μM.
Cisplatin Kabi 1 mg/mL (Fresenius Kabi, Bad Homburg, Germany) was diluted in 0.9% NaCl saline to 4 working solutions: 833.3, 83.33, 8.33 and 0.83 μM.
Suntinib (50 mg, Selleckchem, Houston, TX, USA) was first diluted in dimethyl sulfoxide (DMSO, Sigma Aldrich, D2650-100ML) to a concentration of 10 mM and further diluted in 0.9% NaCl saline to 7 working solutions: 100, 75, 50, 25, 10, 5 and 1 μM.
ECT
Cells were trypsinized, centrifuged and resuspended in electroporation buffer (EP buffer: 250 mM sucrose (Sigma Aldrich, S0389); 10 mM K2HPO4 (Sigma Aldrich; 2.5 mM KH2PO4 (Sigma Aldrich); 2 mM MgCl2 x 6H2O (Sigma Aldrich) to a concentration of 2.5 x107 cells/ml. Each experimental group contained 106 cells in 40 μL of EP buffer and 10 μL of bleomycin or cisplatin solution or 10 μL of saline in the case of controls (untreated control cells- Ctrl or electroporated cells -EP). The final concentrations of cytotoxic drugs were therefore 5 times lower than the working concentrations (1.4, 1.4 x 10-1, 1.4 x 10-2, 1.4 x 10-2, 1.4 x 10-3, 1.4 x 10-4, 1.4 x 10-5, 1.4 x 10-6 μM for bleomycin and 166.7, 16.7, 1.7, and 0.17 μM for cisplatin). Fifty microliters of the mixture was pipetted between two electrodes (2 mm gap) and electroporated (8 pulses, 1300 V/cm, 100 μs duration at frequency 1 Hz) with an Electro Cell B10 electric pulse generator (LEROY Biotech, Saint-Orens-de-Gameville, France). The cell mixture was transferred into the wells of a 24-well ultralow attachment plate (Corning, Corning, NY, USA), and five minutes after pulse delivery, cell culture medium was added, and the cells were seeded for further assays. For the unelectroporated groups, 50 μL of the mixture was transferred into a 24-well ultralow attachment plate, and medium was added.
Cell survival assay
After ECT, 4 x 103 or 2 x 103 cells were seeded into the wells of 96-well plates for 3 or 7 days, respectively, in 7 technical replicates. Cells were incubated at 37°C in a 5% CO2 humidified incubator until measurement. Cell survival was measured with a Presto Blue viability assay (Thermo Fisher Scientific) 3 and 7 days after the treatment. Presto Blue (10 μl/ well) was added to the cells, and 30 minutes thereafter, fluorescence intensity was measured with a Cytation 1 microplate reader (BioTek, Winooski, VT, USA). The viability of the cells after ECT was normalized to the control groups: untreated control cells (Ctrl) for the bleomycin groups and electroporated cells (EP) for the ECT bleomycin groups to eliminate the effect of pulses alone. The concentration that killed 50% of the cells, the EC50 dose, was estimated directly from cell survival curves.
Sunitinib treatment
A monolayer of 80% confluent cells was trypsinized, centrifuged and counted. In each well of a 96-well plate, 4 x 103 or 2 x 103 cells were seeded in 90 μL for 3 or 7 days of incubation with sunitinib, respectively. Ten microliters of sunitinib was added to the cells to obtain final concentrations of 10, 7.5, 5, 2.5, 1, 0.5 and 0.1 μM sunitinib and incubated for 3 or 7 days in a 5% CO2 humidified atmosphere at 37°C. Then, the cell survival assay was performed as described above. The viability of the cells was normalized to that of untreated control cells (Ctrl).
Combined therapy
Further experiments with combined therapy were performed with several doses of sunitinib. For combining ECT cisplatin with sunitinib, the 7-day observation period was selected, since the effect of ECT cisplatin was observed only after several days. Four concentrations of cisplatin (166.7, 16.7, 1.7, and 0.17 μM) and three concentrations of sunitinib were used: 5 μM sunitinib, which represents the approximation of the EC50 dose, and one lower (2.5 μM) and one higher (7.5 μM) tested dose. For combining ECT bleomycin with sunitinib, the 3-day observation period was selected, since bleomycin effects are observed after several hours. Five final concentrations of bleomycin were tested (0.14 - 1.4 x 10-5 μM) in combination with three final concentrations of sunitinib 7.5, 5 and 2.5 μM. After ECT treatment, 4 x 103 or 2 x 103 cells in 90 μL were seeded in 96-well plates for 3 or 7 days, respectively. Ten microliters of sunitinib solution was added to the wells, and the cells were incubated at 37°C in a 5% CO2 humidified incubator for 3 or 7 days in a 5% CO2 humidified atmosphere at 37°C. Then, the cell survival assay was performed as described above. The viability of the cells was normalized to control groups: cells with added sunitinib for bleomycin + sunitinib groups and electroporated cells with added sunitinib for ECT bleomycin + sunitinib groups to eliminate the effect of pulses and sunitinib. The combination effects (additivity, synergism, and antagonism) of the treatments were calculated by the Chou-Talalay computational method by using CompuSyn software (ComboSyn, Inc, Paramus, NJ, USA).20 Combination indexes below 1 were considered synergistic.
Determination of cell death kinetics
Due to the minimal or no effect of bleomycin or cisplatin alone on cell viability, cell death kinetics were determined only in cells treated with EP and ECT. Cell death was determined through kinetic evaluation of diamidino-2-phenylindole (DAPI, Thermo Fisher Scientific) uptake, determining increased cell membrane permeability as a marker of cell death. Briefly, ECT with 0.14 μM bleomycin was evaluated alone or combined with 2.5, 5 and 7.5 μM sunitinib. Afterwards, 5 x 103 control or EP-treated cells and 25 x 103 ECT-treated cells were seeded in each well of a black 96-well plate. Different cell plating densities were used because of prominent cell death that follows instantly after ECT. These dead cells do not attach to the plate and represent a limitation for the usage of imaging techniques. Cells were incubated for 2 hours to allow cells to attach. Thereafter, medium with non-attached dead cells was removed for cell imaging purposes, and 200 μL of fresh phenol-free medium containing the appropriate concentration of sunitinib and 0.5 μM DAPI was added. Brightfield and fluorescence images (one image per well) of cells were captured every 4 h for 48 hours with Cytation 1 (BioTek) with a 4-x objective. For each time point, the number of DAPI-positive nuclei (representing dead cells) was determined with Gen 5 Data analysis software (BioTek).
RNA isolation and quantitative real-time PCR (RT–qPCR)
For the determination of receptor tyrosine kinases, 90% confluent cells in T25 flasks were harvested, and RNA was extracted using a peqGOLD Total RNA Kit (VWR, West Chester, PA, USA) according to the manufacturer’s instructions. RNA concentrations were quantified by spectrophotometry at 260 nm (Cytation 1), and purity was determined by measuring the ratio of absorbance at A260 nm/280 nm and A260 nm/230 nm. Afterwards, 2000 ng of total RNA was reverse transcribed into cDNA using the SuperScript VILO cDNA Synthesis Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. After reverse transcription, 10 ng of cDNA template was used in 20 μL of RT-qPCRs using predesigned primers: Hs.PT.58.45689824 (KIT), Hs.PT.58.19454325 (FLT3), Hs.PT.58.4318912 (RET), Hs.PT.58.3285240 (KDR), Hs.PT.58.22892761 (PDGFR), Hs.PT.58.40906831 (FLT1) or custom made primers: F-AGGTGATGGAAGAAGTGGTG, R-AGGATTTGGTGTGAGCGATC (Glucuronidase Beta; GUSB) (Integrated DNA Technologies, Coralville, IA, USA) and PowerUp™ SYBR™ Green Master Mix (Thermo Fisher Scientific,). The RT-qPCRs were run until the 40th cycle with the following cycling conditions: 2 min at 50°C, 2 min at 95°C, 40 cycles of 15 s at 95°C, and 1 min at 60°C; for the melting curve determination, 15 s at 95°C, 1 min at 60°C, and 15 s at 95°C. The results were analyzed on a QuantStudio™ 3 Real-Time PCR System (Thermo Fisher Scientific). Ct values above the 35th cycle were considered undetermined. Relative expression was calculated by normalization to the expression of the housekeeping gene GUSB using ΔCt method.21
Statistical analysis
The values in this study are presented as the mean (AM) ± standard error of the mean (SEM). Comparisons between two groups were performed using unpaired two-tailed Student’s t test. The comparison of means of more than two groups was statistically evaluated by one-way analysis of variance (one-way ANOVA) followed by Dunnett’s or Tukey’s multiple comparisons test. A p value of <0.05 was considered to be statistically significant. A sample size (n) for each experiment represents biological replicates and is presented in each figure legend. For statistical analysis and preparation of graphs, GraphPad Prism 9 (La Jolla, CA, USA) was used. The combination effects (additivity, synergism, and antagonism) of the treatments were calculated by the Chou-Talalay computational method by using CompuSyn software).20 Combination indexes below 1 were considered synergistic.
Results
ECT with bleomycin is more effective than ECT with cisplatin in pancreatic carcinoma cell line
Bleomycin alone did not significantly affect cell survival, either after 3 days or after 7 days. (Figure 1A, B). Electroporation of cells significantly increased the cytotoxic effect of bleomycin. A 50% decrease in viability was observed at bleomycin concentrations higher than 1.4 x10-3 μM after 3 days of incubation. More precisely, the EC50 for ECT with bleomycin was 6.1 x10-3 μM. ECT at the two highest concentrations, 0.14 μM and 1.4 μM, killed 90% of cells (Figure 1A). After 7 days of incubation, the EC50 dose for ECT with bleomycin could not be determined, since the lowest tested dose of 1.4 x10-6 killed more than 50% of the cells. Bleomycin doses higher than 0.014 μM killed 90% of cells after 7 days of incubation (Figure 1B).
Figure 1.
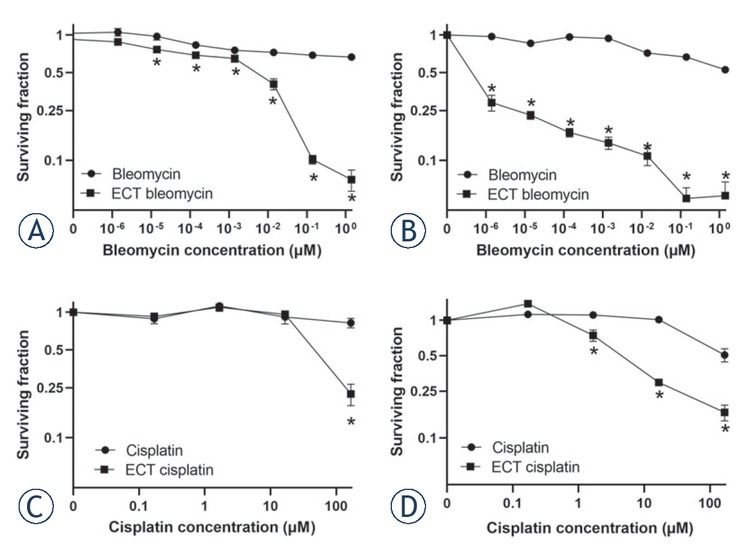
Survival fraction of BxPC-3 cells after electrochemotherapy (ECT). Survival of cells treated with ECT with bleomycin after 3 days (A) and 7 days (B) and survival fraction of BxPC-3 cells treated with ECT with cisplatin after 3 (C) and 7 days (D). The values are presented as the AM ± SEM. *p < 0.05 vs. untreated control cells, n = 3.
When cisplatin was used as a cytotoxic drug in ECT, a significantly lower survival fraction of BxPC-3 cells was observed only when using the highest tested cisplatin concentration, 166.67 μM, 3 days after the treatment (Figure 1C). The EC50 dose for ECT with cisplatin after 3 days of incubation was 60 μM. After 7 days of incubation, cisplatin alone without electroporation at a concentration of 166.67 μM killed approximately 50% of the cells. However, when cells were treated with ECT, a significant decrease in cell viability was observed at a 1.67 μM cisplatin dose. The EC50 for ECT with cisplatin after 7 days of incubation was 6 μM (Figure 1D).
Sensitivity of pancreatic carcinoma cells to sunitinib treatment
First, the expression of potential targets of the tyrosine kinase inhibitor sunitinib was determined in BxPC-3 pancreatic carcinoma cells. All of the tyrosine-kinase receptors that are known targets of sunitinib were expressed in BxPC-3 cells, with FLT3, PDGFR and VEGFR1 being the most highly expressed among them (Figure 2A). After confirmation of sunitinib targets in BxPC-3 pancreatic carcinoma cells, they were treated with different concentrations of sunitinib and incubated for 3 or 7 days to confirm and determine its cytotoxicity. The survival of cells after incubation with lower concentrations of sunitinib, 0.1 μM, 0.5 μM and 1.0 μM, was not significantly reduced compared to control cells (Figure 2 B, C). When the cells were treated with higher concentrations, i.e., > 1.0 μM, survival was significantly reduced, especially after 7 days of incubation (Figure 2C). The EC50 values for sunitinib after 3 days of incubation were 8.1 μM and 5 μM after 7 days of incubation.
Figure 2.
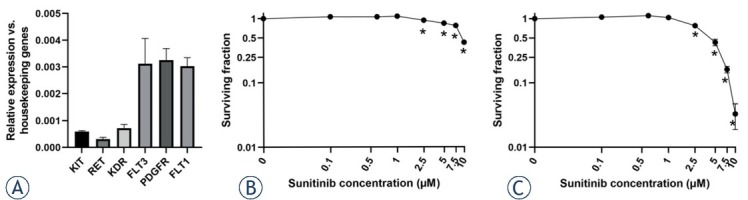
Targeted therapy of BxPC-3 pancreatic carcinoma cells with sunitinib. (A) Expression of different tyrosine kinases as sunitinib targets; n = 2. Surviving fraction of BxPC-3 cells treated with different sunitinib concentrations incubated for (B) 3 and (C) 7 days. The values are presented as the AM ± SEM. *p < 0.05 vs. untreated control cells, n = 3.
FLT1 = fms related receptor tyrosine kinase 1/VEGFR1; FLT3 = fms related receptor tyrosine kinase 3; KDR = kinase insert domain receptor/VEGFR2; KIT = KIT proto-oncogene; RET = ret proto-oncogene; PDGFR = platelet-derived growth factor receptor
Combined treatment of sunitinib and ECT was synergistic for bleomycin but not cisplatin
For combined treatment, five bleomycin concentrations and three sunitinib concentrations, 2.5, 5.0 and 7.5 μM, were tested. The cytotoxic effect was determined 3 days after the treatment due to the fast-cytotoxic action of bleomycin. Compared to ECT with bleomycin in monotherapy, the combination with sunitinib had an increased cytotoxicity, but only at the highest used concentration of bleomycin 0.14 μM (Figure 3A). The synergistic effect of ECT with 0.14 μM bleomycin and sunitinib was confirmed by the Chou-Talalay method with combination indexes of 0.11 and 0.07 for 5 and 7.5 μM sunitinib, respectively.20 When lower doses of bleomycin were used in combination treatment, no significant changes in cell survival were observed compared to ECT in monotherapy (Figure 3A).
Figure 3.
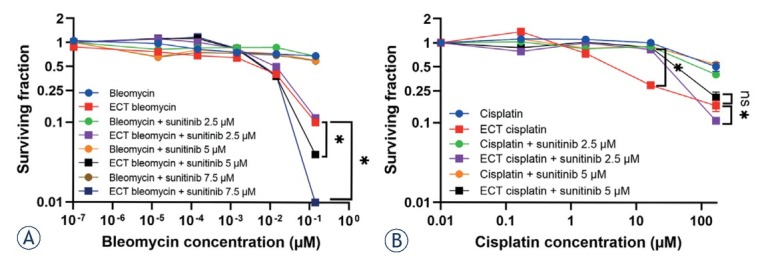
Surviving fraction of BxPC-3 cells treated with ECT monotherapy with bleomycin (A) or cisplatin (B) combined with 2.5, 5 or 7.5 μM sunitinib. The values are presented as the AM ± SEM. *p < 0.05 statistically significant difference vs. annotated groups, n = 3.
For combined treatment of ECT with cisplatin and sunitinib, four cisplatin concentrations and three sunitinib concentrations, 2.5, 5.0 and 7.5 μM, were used. The cytotoxic effect was evaluated 7 days after the treatment due to the slower action of cisplatin, which was observed in previous experiments (Figure 1C, D). Due to this later time point of evaluation compared to ECT with bleomycin, the combination with 7.5 μM sunitinib could not be tested, as this concentration of sunitinib alone was too cytotoxic (Figure 2C). Compared to ECT with cisplatin, the combination with sunitinib did not achieve higher cytotoxicity (Figure 3B). At cisplatin concentrations of 1.67 μM and 16.67 μM, the cytotoxic effect after combined therapy was even lower than that after monotherapy. This may indicate an antagonistic effect of combination therapy with 2.5 or 5.0 μM sunitinib at concentrations of 1.67 μM and 16.67 μM cisplatin; however, this could not be confirmed by the Chou-Talalay method.
Pancreatic carcinoma cells die in the first 48 hours following ECT with bleomycin alone or in combination with sunitinib
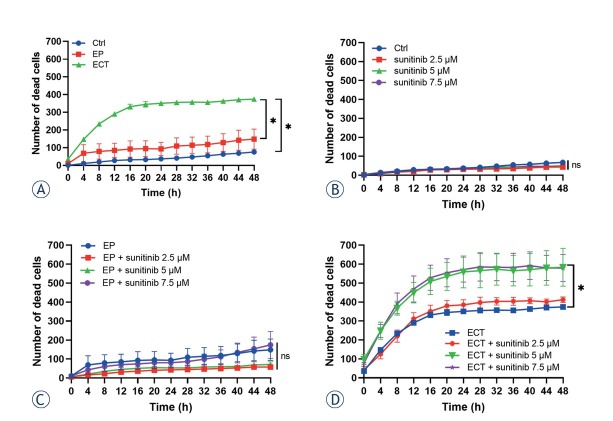
Cell death was determined only after ECT with bleomycin, as it was more effective in the BxPC-3 pancreatic carcinoma cell line than ECT with cisplatin. The kinetic measurements of DAPI uptake by the permeabilized dead cells demonstrated that the majority of cells died soon after ECT, reaching significance at 8 hours after the treatment compared to control (Ctrl) and electroporated cells only (EP) (Figure 4A). The number of dead cells further increased until approximately 20 hours after the treatment, when it reached a plateau (Figure 4A).
Figure 4.
The combination of ECT with bleomycin and sunitinib resulted in a higher number of dead cells. (A) Number of dead cells detected every 4 hours during kinetic measurement (A) after ECT single treatment, (B) after addition of 2.5, 5 or 7.5 μM sunitinib to Ctrl, (C) after addition of 2.5, 5 or 7.5 μM sunitinib to EP and (D) after addition of 2.5, 5 or 7.5 μM sunitinib to ECT. The values are presented as the AM ± SEM. *p < 0.05 vs. annotated groups, n = 3.
Ctrl = untreated control cells; ECT = electrochemotherapy; EP = electroporated cells only
The addition of sunitinib to control untreated cells (Ctrl) or electroporated cells only (EP) did not increase the numbers of dead cells in the first 48 hours, suggesting that cell death induced by sunitinib takes place after the first 48 hours (Figure 4 B, C). However, the combination of ECT with bleomycin and 5 or 7.5 μM sunitinib resulted in significantly higher numbers of dead cells compared to ECT alone (Figure 4D). However, the combination of ECT with bleomycin and 2.5 μM sunitinib did not result in a significant increase in the numbers of dead cells (Figure 4D). The time frame of cell death after combination treatment was similar to that after ECT monotherapy, reaching a plateau in the first 20 hours (Figure 4D).
Discussion
Local ablative therapies are being explored as new treatment options for locally advanced pancreatic cancer. Among them, stereotactic body radiotherapy, radiofrequency ablation (RFA), microwave ablation (MWA), and irreversible electroporation (IRE) are the most frequently used to improve survival in these patients.22, 23, 24 ECT presents a new treatment option for local ablation of pancreatic tumors. Its feasibility and safety were demonstrated in preclinical studies in porcine and rabbit models.11, 12 This was also confirmed in a few clinical studies demonstrating the feasibility and safety of the treatment in a palliative setting.13, 14, 15 However, there is a lack of preclinical studies assessing the actions of different drugs used in ECT, their mechanisms and interactions with targeted drugs that are used in clinical practice. With our study, we demonstrate that in a pancreatic cancer cell line, ECT with bleomycin is more cytotoxic than ECT with cisplatin. The kinetic measurements demonstrated that cells die soon after ECT, reaching a plateau 20 hours after the treatment. Furthermore, we provide the rationale that ECT with bleomycin can be used together with the targeted drug sunitinib with possible potentiation of cytotoxicity.
The effectiveness of ECT has been confirmed in several preclinical and clinical studies, leading to its use in routine clinical practice.3,5 It can be used to treat different tumor types, from superficial tumors to deep-seated tumors, including pancreatic carcinoma.25, 26 The most frequently used chemotherapeutics in ECT are bleomycin and cisplatin, which have different mechanisms of action. Bleomycin causes immediate DNA damage by cleaving double-stranded DNA, causing immediate extensive DNA double-strand breaks.26 In contrast, cisplatin causes DNA intra- and inter-strand adducts, which have a delayed effect on the induced DNA damage at the time point when the cells are trying to replicate.27 Therefore, the time frame of cell death after ECT with both drugs is different, with cisplatin taking longer to exert its cytotoxicity. This was also observed in the current study in the BxPC-3 pancreatic carcinoma cell line. In addition to a different onset of cytotoxic action, ECT with bleomycin was more effective than ECT with cisplatin in the BxPC-3 cell line in our in vitro setting, thus confirming the results published by Jaroszeski et al.28 However, the effectiveness of cisplatin ECT was also previously demonstrated in primary human cells derived from the pulmonary metastasis of pancreatic ductal adenocarcinoma, thus promoting further research in this field, also due to the immunostimulatory effects of cisplatin.29, 30
Pancreatic tumor cells were shown to die from a regulated form of necrotic cell death called necroptosis rather than from apoptosis after ECT with bleomycin, cisplatin, and oxaliplatin.31 In our study, the necrotic type of cell death was also observed after ECT with bleomycin in the BxPC-3 cell line as the cells were permeable. We also demonstrate a quick onset of cell death, confirming the fast action of bleomycin. However, the necrotic type of death in our study could not be distinguished from necroptosis, as the assay was based solely on DAPI uptake through permeable dead cells; therefore, it could also be necroptosis, which is a regulated type of necrotic death with specific molecular characteristics.32
Despite the good cytotoxic effect of ECT, changes in the expression of stemness markers (Nanog and Oct3/4) in pancreatic tumor cells that survived ECT were demonstrated.33 The change in the expression of these markers could potentially contribute to the recurrence of pancreatic cancer after ECT treatment. Therefore, new therapeutic options remain of high importance, and one possible approach is the combination of ECT with targeted drugs that are used to treat pancreatic cancer in a clinical setting. In our study, we evaluated the effect of the combination of ECT with bleomycin or cisplatin and sunitinib, a tyrosine-kinase inhibitor used to treat progressive neuroendocrine pancreatic tumors that cannot be removed by surgery or that have spread to other parts of the body.18 Due to the lack of commercially available neuroendocrine pancreatic tumor cell lines, the adenocarcinoma cell line BxPC-3, which was used for the investigation of sunitinib effects, was used in the current study.34 The cell line was previously confirmed to express the VEGFR1 receptor 35 and the expression of multiple potential sunitinib targets was further confirmed by RT–qPCR in our study. The limitation of the study represents the fact that the investigation of individual targets in relation to response to combined therapy was not performed within the study. Furthermore, the use of single pancreatic cancer cell line represents another limitation of the study, therefore investigation of the combined treatment in additional cell lines together with in vivo studies that resemble a more complex in vivo conditions are warranted.
Our results demonstrate that the highest dose of bleomycin used in combination with sunitinib potentiated the cytotoxic effect of the treatment with a possible synergistic action, as was shown by the Chou-Talalay computational method in our in vitro study. After the combined treatment, the pancreatic carcinoma cells died in a similar time frame as after ECT single treatment.
In conclusion, we demonstrate that ECT is effective in the BxPC-3 pancreatic cancer cell line, especially when bleomycin is used, with the onset of cell death in the first hours after the treatment, reaching a plateau at 20 hours after the treatment. Furthermore, we provide the rationale for combining ECT with bleomycin and the targeted drug sunitinib, which results in the potentiation of cytotoxicity. With these in vitro findings of the intrinsic sensitivity of pancreatic carcinoma cells to the combined treatment, the study provides a basis for further research in a more complex in vivo environment that also comprises blood vessels that are the primary target of sunitinib action.
Acknowledgments
The authors acknowledge the financial support from the state budget by the Slovenian Research Agency, program no. P3-0003 and wish to thank Teja Valant for all the technical help.
Disclosure
No potential conflicts of interest were disclosed.
References
- 1.Pourshams A, Sepanlou SG, Ikuta KS, Bisignano C, Safiri S, Roshandel G. et al. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:934–47. doi: 10.1016/S2468-1253(19)30347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morganti AG, Massaccesi M, La Torre G, Caravatta L, Piscopo A, Tambaro R. et al. A systematic review of resectability and survival after concurrent chemoradiation in primarily unresectable pancreatic cancer. Ann Surg Oncol. 2010;17:194–205. doi: 10.1245/S10434-009-0762-4. [DOI] [PubMed] [Google Scholar]
- 3.Cemazar M, Sersa G. Recent advances in electrochemotherapy. Bioelectricity. 2019;1:204–13. doi: 10.1089/BIOE.2019.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sersa G, Ursic K, Cemazar M, Heller R, Bosnjak M, Campana LG. Biological factors of the tumour response to electrochemotherapy: review of the evidence and a research roadmap. Eur J Surg Oncol. 2021;8:1836–46. doi: 10.1016/j.ejso.2021.03.229. [DOI] [PubMed] [Google Scholar]
- 5.Gehl J, Sersa G, Matthiessen LW, Muir T, Soden D, Occhini A. et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018;57:874–82. doi: 10.1080/0284186X.2018.1454602. [DOI] [PubMed] [Google Scholar]
- 6.Djokic M, Cemazar M, Popovic P, Kos B, Dezman R, Bosnjak M. et al. Electrochemotherapy as treatment option for hepatocellular carcinoma, a prospective pilot study. Eur J Surg Oncol. 2018;44:651–7. doi: 10.1016/J.EJSO.2018.01.090. [DOI] [PubMed] [Google Scholar]
- 7.Djokic M, Cemazar M, Bosnjak M, Dezman R, Badovinac D, Miklavcic D. et al. A prospective phase II study evaluating intraoperative electrochemotherapy of hepatocellular carcinoma. Cancers. 2020;12:1–14. doi: 10.3390/CANCERS12123778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djokic M, Dezman R, Cemazar M, Stabuc M, Petric M, Smid LM. et al. Percutaneous image guided electrochemotherapy of hepatocellular carcinoma: technological advancement. Radiol Oncol. 2020;54:347–52. doi: 10.2478/RAON-2020-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edhemovic I, Brecelj E, Cemazar M, Boc N, Trotovsek B, Djokic M. et al. Intraoperative electrochemotherapy of colorectal liver metastases: a prospective phase II study. Eur J Surg Oncol. 2020;46:1628–33. doi: 10.1016/j.ejso.2020.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Edhemovic I, Brecelj E, Gasljevic G, Marolt Music M, Gorjup V, Mali B. et al. Intraoperative electrochemotherapy of colorectal liver metastases. J Surg Oncol. 2014;110:320–7. doi: 10.1002/JSO.23625. [DOI] [PubMed] [Google Scholar]
- 11.Dežman R, Čemažar M, Serša G, Seliškar A, Erjavec V, Trotovšek B. et al. Safety and feasibility of eElectrochemotherapy of the pancreas in a porcine model. Pancreas. 2020;49:1168–73. doi: 10.1097/MPA.0000000000001642. [DOI] [PubMed] [Google Scholar]
- 12.Girelli R, Prejanò S, Cataldo I, Corbo V, Martini L, Scarpa A. et al. Feasibility and safety of electrochemotherapy (ECT) in the pancreas: a pre-clinical investigation. Radiol Oncol. 2015;49:147–54. doi: 10.1515/RAON-2015-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granata V, Fusco R, Piccirillo M, Palaia R, Petrillo A, Lastoria S. et al. Electrochemotherapy in locally advanced pancreatic cancer: preliminary results. Int J Surg. 2015;18:230–6. doi: 10.1016/J.IJSU.2015.04.055. [DOI] [PubMed] [Google Scholar]
- 14.Granata V, Fusco R, Setola SV, Piccirillo M, Leongito M, Palaia R. et al. Early radiological assessment of locally advanced pancreatic cancer treated with electrochemotherapy. World J Gastroenterol. 2017;15:199. doi: 10.3748/WJG.V23.I26.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casadei R, Ricci C, Ingaldi C, Alberici L, Di Marco M, Guido A. et al. Intraoperative electrochemotherapy in locally advanced pancreatic cancer: indications, techniques and results-a single-center experience. Updates Surg. 2020;72:1089–96. doi: 10.1007/S13304-020-00782-X. [DOI] [PubMed] [Google Scholar]
- 16.Spiliopoulos S, Zurlo MT, Casella A, Laera L, Surico G, Surgo A. et al. Current status of non-surgical treatment of locally advanced pancreatic cancer. World J Gastrointest Oncol. 2021;13:2064–75. doi: 10.4251/WJGO.V13.I12.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tempero MA, Malafa MP, Chair V, Asbun H, Behrman SW, Benson III AB. et al. NCCN Guidelines Version 2.2015, Pancreatic Adenocarcinoma, American Joint Committee on Cancer (AJCC). Available at. https://www2.tri-kobe.org/nccn/guideline/archive/pancreas2015/english/pancreatic.pdf [Google Scholar]
- 18.Raymond E, Dahan L, Raoul J-L, Bang Y-J, Borbath I, Lombard-Bohas C. et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–13. doi: 10.1056/NEJMOA1003825. [DOI] [PubMed] [Google Scholar]
- 19.Carrato Mena A, Grande Pulido E, Guillén-Ponce C.. Understanding the molecular-based mechanism of action of the tyrosine kinase inhibitor: sunitinib. Anticancer Drugs. 2010;21(Suppl 1):S3–11. doi: 10.1097/01.CAD.0000361534.44052.C5. [DOI] [PubMed] [Google Scholar]
- 20.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/METH.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.D’onofrio M, Beleù A, de Robertis R.. Ultrasound-guided percutaneous procedures in pancreatic diseases: new techniques and applications. Eur Radiol Exp. 2019;3:2. doi: 10.1186/S41747-018-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin RCG, McFarland K, Ellis S, Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol. 2013;20((Suppl 3)):S443–9. doi: 10.1245/S10434-012-2736-1. [DOI] [PubMed] [Google Scholar]
- 24.Petrelli F, Comito T, Ghidini A, Torri V, Scorsetti M, Barni S. Stereotactic. body radiation therapy for locally advanced pancreatic cancer: a systematic review and pooled analysis of 19 trials. Int J Radiat Oncol Biol Phys. 2017;97:313–22. doi: 10.1016/j.ijrobp.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Campana LG, Miklavčič D, Bertino G, Marconato R, Valpione S, Imarisio I. et al. Electrochemotherapy of superficial tumors - current status: basic principles, operating procedures, shared indications, and emerging applications. Semin Oncol. 2019;46:173–91. doi: 10.1053/J.SEMINONCOL.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Bonferoni MC, Rassu G, Gavini E, Sorrenti M, Catenacci L, Torre ML. et al. Electrochemotherapy of deep-seated tumors: state of art and perspectives as possible “EPR effect enhancer” to improve cancer nanomedicine efficacy. Cancers. 2021;13:4437. doi: 10.3390/CANCERS13174437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosnjak M, Jesenko T, Markelc B, Janzic L, Cemazar M, Sersa G. PARP inhibitor olaparib has a potential to increase the effectiveness of electrochemotherapy in BRCA1 mutated breast cancer in mice. Bioelectrochemistry. 2021;140:107832. doi: 10.1016/J.BIOELECHEM.2021.107832. [DOI] [PubMed] [Google Scholar]
- 28.Jaroszeski MJ, Dang V, Pottinger C, Hickey J, Gilbert R, Heller R. Toxicity of anticancer agents mediated by electroporation in vitro. Anticancer Drugs. 2000;11:201–8. doi: 10.1097/00001813-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Ursic K, Kos S, Kamensek U, Cemazar M, Scancar J, Bucek S. et al. Comparable effectiveness and immunomodulatory actions of oxaliplatin and cisplatin in electrochemotherapy of murine melanoma. Bioelectrochemistry. 2018;119:161–71. doi: 10.1016/J.BIOELECHEM.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Michel O, Kulbacka J, Saczko J, Mączyńska J, Blasiak P, Rossowska J. et al. Electroporation with cisplatin against metastatic pancreatic cancer: in vitro study on human primary cell culture. BioMed Res Int. 2018;2018:7364539. doi: 10.1155/2018/7364539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandes P, O’Donovan TR, McKenna SL, Forde PF. Electrochemotherapy causes caspase-independent necrotic-like death in pancreatic cancer cells. Cancers. 2019;11:1177. doi: 10.3390/cancers11081177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhuriya YK, Sharma D. Necroptosis: a regulated inflammatory mode of cell death. J Neuroinflammation. 2018;15:199. doi: 10.1186/S12974-018-1235-0.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali MS, Gill KS, Saglio G, Cilloni D, Soden DM, Forde PF. Expressional changes in stemness markers post electrochemotherapy in pancreatic cancer cells. Bioelectrochemistry. 2018;122:84–92. doi: 10.1016/J.BIOELECHEM.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Tran Cao HS, Bouvet M, Kaushal S, Keleman A, Romney E, Kim G. et al. Metronomic gemcitabine in combination with sunitinib inhibits multisite metastasis and increases survival in an orthotopic model of pancreatic cancer. Mol Cancer Ther. 2010;9:2068–78. doi: 10.1158/1535-7163.MCT-10-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wey JS, Fan F, Gray MJ, Bauer TW, McCarty MF, Somcio R. et al. Vascular endothelial growth factor receptor-1 promotes migration and invasion in pancreatic carcinoma cell lines. Cancer. 2005;104:427–38. doi: 10.1002/CNCR.21145. [DOI] [PubMed] [Google Scholar]