Abstract
Background
Immune checkpoint inhibitors (ICI) are becoming increasingly common in treating several cancer types. Durvalumab is a human IgG1 monoclonal antibody that blocks PD-L1 binding to PD-1 and CD80 and has recently been approved for the treatment of extensive-stage small-cell lung cancer (ES-SCLC) and locally advanced unresectable (NSCLC). The present review aimed to analyse immune-mediated uveitis, secondary to durvalumab treatment, through a review of the literature and a presentation of two clinical cases.
Patients and methods
A literature review using PubMed search was conducted to identify cases of uveitis secondary to durvalumab and cases of uveitis with optic disc oedema secondary to ICI use that were reported prior to November 14, 2021. Additionally, we report two cases of uveitis consequent on durvalumab treatment.
Results
Five cases of uveitis secondary to durvalumab use were identified in the literature. Anterior, posterior uveitis and vasculitis were reported. Additionally, we present a case of bilateral intermediate uveitis with bilateral optic disc oedema and a case of bilateral posterior uveitis. Our further search revealed 12 cases of uveitis with optic disc oedema secondary to ICI use, with the majority of cases reported secondary to PD-1 inhibitors.
Conclusions
Rarely reported, uveitis secondary to durvalumab can present various clinical pictures and requires a thorough diagnostic workup. Once the diagnosis is established, treatment, commonly with a local or systemic corticosteroid, should be adapted to the severity of the inflammation.
Key words: durvalumab, uveitis, optic disc oedema, immune checkpoint inhibitor, adverse effects
Introduction
Durvalumab is a human monoclonal antibody belonging to immune checkpoint inhibitors (ICI) group. It is registered for the treatment of extensive-stage small-cell lung cancer (ES-SCLC) and locally advanced unresectable non-small cell lung cancer (NSCLC).1,2 Interaction of programmed death-ligand 1 (PD-L1) expressed on the surface of cancer cells with programmed cell death protein 1 (PD-1) and the cluster of differentiation 80 (CD80) molecules expressed on the surface of T-cells results in inhibition of T-cell activation. By selectively blocking PD-L1/PD-1 and PD-L1/CD80 interactions, durvalumab increases the likelihood of immunologic attack and destruction of cancer cells.3 Under normal conditions, PD-L1/PD-1 interaction is involved in maintaining immune tolerance to self-antigens.4 Blocking PD-L1 interactions durvalumab thus not only facilitates the destruction of cancerous cells but also induces various immune-mediated adverse effects.2,5 The present review aimed to analyse immune-mediated uveitis, secondary to durvalumab treatment, through a review of the literature and a presentation of two additional clinical cases.
Methods
Published cases of inflammatory eye conditions secondary to treatment with durvalumab were collected through a literature search on PubMed, using the following search terms: ’’durvalumab’’, ’’eye’’, ’’uveitis’’, ’’inflammation’’. Moreover, published cases of optic disc oedema with uveitis secondary to immune checkpoint inhibitors were collected with PubMed search, using the following search terms: ’’uveitis’’, ‘’immune checkpoint inhibitor/s’’, ’’optic disc oedema’’, ’’papillitis’’. Only papers accessible in the English language were included.
Additionally, we report two cases of uveitis secondary to durvalumab treatment that presented to our clinic. Clinical report adhered to the general principles outlined in the Declaration of Helsinki. Informed consent was obtained from the patients.
Results
Uveitis secondary to durvalumab
Numerous cases of uveitis have been described as consequent on the immune checkpoint inhibitors5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, with only a few occurring secondary to durvalumab treatment.6, 7, 8 This is likely due to both: fewer patients treated with durvalumab in proportion to the other immune checkpoint inhibitors6,16 and more frequent and severe side effects of cytotoxic T lymphocyte-associated protein 4 (CTLA-4) inhibitors compared to the PD-1 and PD-L1 inhibitors.6
Our search, described in the Methods above, yielded five reports of uveitis secondary to durvalumab treatment. Additionally, two patients presented to our clinic between November 2020 and March 2021 and are added in Table 1. The clinical presentation varies – anterior, intermediate, posterior uveitis, and retinal vasculitis have been described and were managed with a local or a systemic steroid. Immune checkpoint inhibitor was discontinued in four out of seven (57%) cases.
Table 1.
Published cases reporting uveitis secondary to durvalumab (PD-L1 inhibitor) treatment
| Author, year | Number of cases reported | Indication | Ocular inflammation specification (% eyes) | Treatment | Discontinuing immune checkpoint inhibitor |
|---|---|---|---|---|---|
| Dow et al. 20206 | 3 | n/a | Anterior uveitis 80% Posterior uveitis 20% |
Local corticosteroid Systemic corticosteroid |
No |
| Parikh et al., 20207 | 1 | Non-small cell lung cancer | Anterior uveitis | Local corticosteroid | Yes |
| Andrade et al., 20208 | 1 | Non-small cell lung cancer | Retinal vasculitis | Systemic corticosteroid | Yes |
| Vrabic et al., 2021 | 2 | Small cell lung cancer, non-small cell lung cancer | Intermediate uveitis, Posterior uveitis | Systemic corticosteroid | Yes |
Other inflammatory eye conditions secondary to durvalumab
Although rare, various other immune-mediated ocular adverse effects have been linked with durvalumab. Carrera reported a drug-induced myopathy of extraocular muscles in a patient treated with durvalumab and tremelimumab.17 Optic neuropathy with disc oedema has been described as secondary to durvalumab use by Noble et al.18 Drug-induced Graves’ orbitopathy has been observed in a patient treated with tremelimumab and durvalumab.19 Moreover, in one of the largest reviews of databases of ocular adverse events in patients treated with PD-1/PD-L1 inhibitors, Young et al. recently reported two cases of serious ocular adverse events, with no data on the ocular diagnosis available.16
Patients
We report two cases of uveitis secondary to durvalumab treatment. The first patient is a 57-year-old female who presented to our clinic in November 2020 with a one-month history of blurred vision and floaters. Due to ES-SCLC the patient was treated with four cycles of combined chemo-immunotherapy with carboplatin, etoposide, and durvalumab, starting in April 2020 and then continued maintenance durvalumab treatment, receiving a total of eight cycles by November 2020. Both clinical and radiological remissions were achieved. She presented to our clinic after having received the eighth cycle of durvalumab. Her other past medical and ocular history was unremarkable.
Ophthalmic examination revealed aqueous cells 1+ and vitreous cells 1+ as well as bilateral oedema of the optic discs (Figure 1). The best-corrected decimal visual acuity (BCVA) was 0.7 and 0.8 p. for the right and left eye, respectively.
Figure 1.
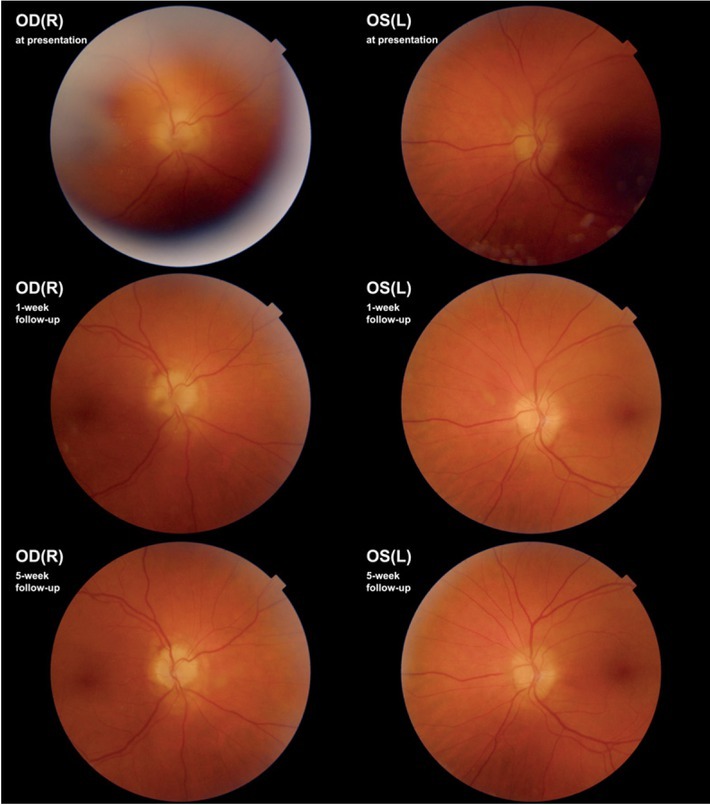
Case I. Fundoscopy, at presentation, on one-week and five-week follow-up. Follow-up images show diminishment of vitreous haze and regression of optic disc swelling.
OD(R) = oculus dexter (right); OS(L) = oculus sinister (left)
Bilateral optic nerve drusen were revealed on fundus autofluorescence (FAF) and confirmed on ocular ultrasound and optical coherence tomography (OCT) (Figure 2). Fluorescein angiography (FA) was performed (Figure 3), and a diagnosis of intermediate uveitis was made with optic disc drusen as an incidental finding.
Figure 2.
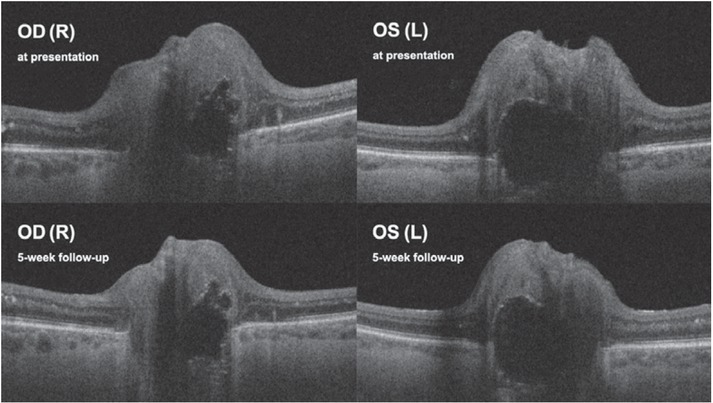
Case I. Optical coherence tomography (OCT) of optic discs. Optic disc drusen at presentation and on a five-week follow-up. No alteration in optic disc drusen size is visible. Up images show diminishment of vitreous haze and regression of optic disc swelling.
OD(R) = oculus dexter (right); OS(L) = oculus sinister (left)
Figure 3.
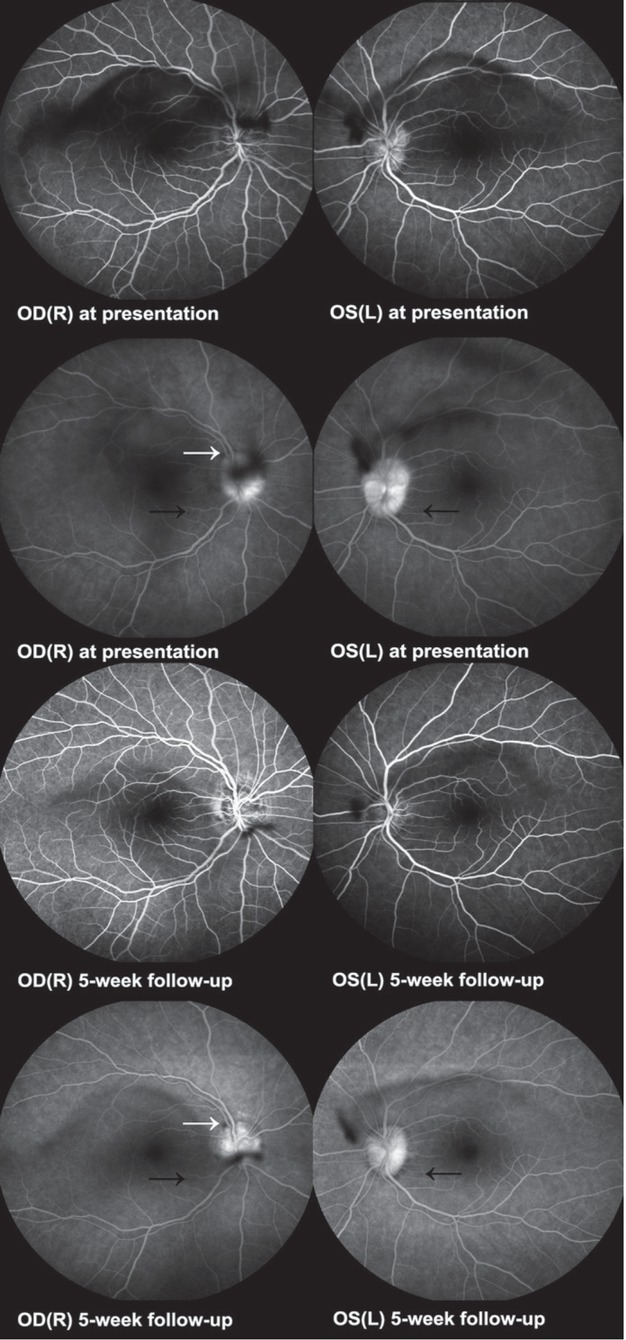
Case I. Fluorescein angiography at presentation. Early and late frames. Contrast blockage in the upper temporal part of the right optic disc (white arrow), corresponding to a peripapillary haemorrhage. Hypofluorescent dots (black arrow) corresponded to hyperreflective spots on optical coherence tomography (OCT). Fluorescein angiography five weeks on methylprednisolone treatment. Early and late frames. Diminishment of the contrast blockage in the upper temporal part of the right optic disc (white arrow) corresponding to a peripapillary haemorrhage. Hypofluorescent dots (black arrow) are less prominent.
OD(R) = oculus dexter (right); OS(L) = oculus sinister (left)
No signs of intracranial hypertension were revealed on MRI, nor did it show clear signs of intracranial metastasis or other abnormalities in the course of the optic nerves. After excluding other common uveitic aetiologies (Supplementary File 1. Supplementary Table 1), the ocular inflammation was thought to be consequent to durvalumab treatment. The patient was treated with three applications of intravenous methylprednisolone, receiving 500 mg per day and continued with 48 mg of oral methylprednisolone per day. The dose was then tapered over six weeks. Monthly maintenance treatment with durvalumab was discontinued not only due to uveitic reaction but also due to the progression of the disease.
We observed stable best-corrected visual acuity (BCVA) of the left eye on a five-week follow-up, and a slight improvement of BCVA of the right eye. On ophthalmoscopy, diminishment of inflammation was observed with clear anterior chambers and vitreous cells 0.5 +. In addition, decreased swelling of the optic discs was noted (Figure 4).
Figure 4.
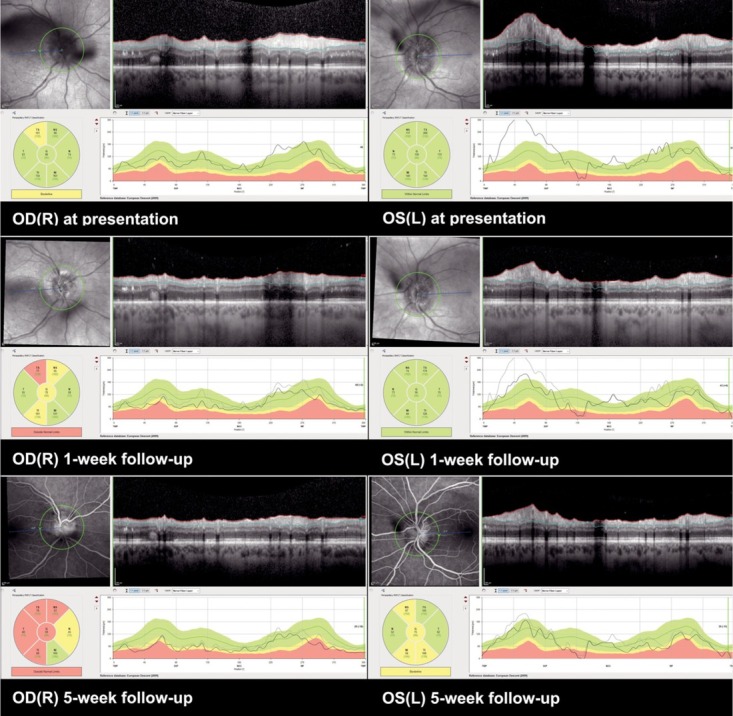
Case I. Optical coherence tomography (OCT) of optic discs, at presentation, one week on methylprednisolone treatment, five weeks on methylprednisolone treatment. Follow-up shows regression of optic disc swelling.
OD(R) = oculus dexter (right); OS(L) = oculus sinister (left)
The second case was a 55-year old female, sent to our clinic in March 2021 for a complaint of blurry vision that she first noted one week prior to consulting. Her past medical history was significant for K-RAS positive adenocarcinoma in the right middle pulmonary lobe, diagnosed in 2019. She was treated with concurrent radical chemo-radiotherapy. Clinical and radiological remissions were achieved. She then continued with immunotherapy and received 26 applications of durvalumab in the period between January 2020 and January 2021.
The patient had been previously seen at our clinic eight years prior due to peripheral pigmented retinal changes, which were at that time thought to be extensive retinal pigment epithelium (RPE) hypertrophy and small choroidal nevi (Figure 5A). Follow-up was advised, but the patient failed to present for further check-ups.
Figure 5.
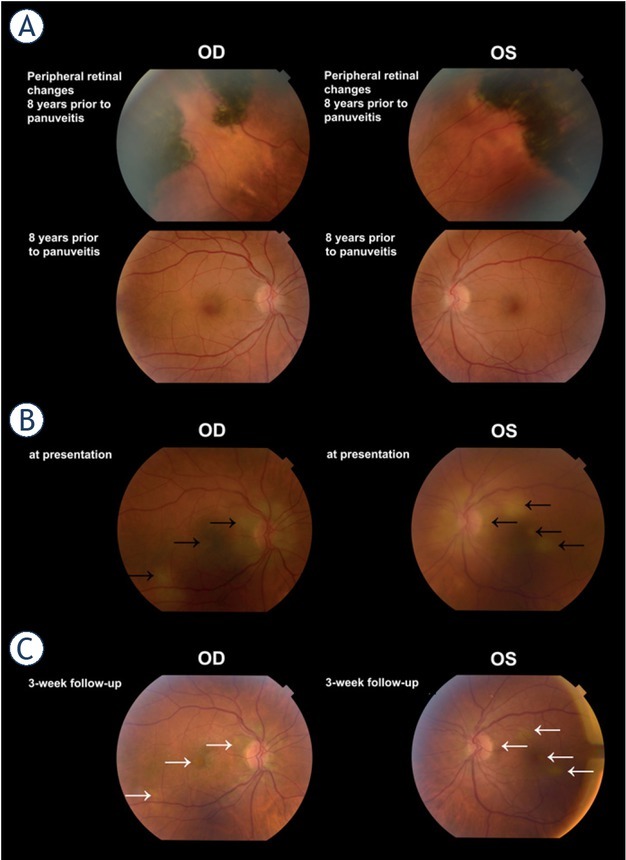
Case II. (A) Fundoscopy, prior to non-small cell lung cancer diagnosis in 2013: posterior pole and peripheral retinal changes. (B) Colour fundus photography at presentation in March 2021: multiple flat, grey-white lesions (black arrows) are visible at presentation. (C) Three weeks on methylprednisolone treatment in April 2021: a diminishment of lesions (white arrows) is observed.
OD = oculus dexter; OS(L) = oculus sinister
In March 2021, the patient was diagnosed with bilateral iridocyclitis and prescribed corticosteroid and nonsteroid anti-inflammatory eye drops with the addition of a systemic corticosteroid, 8 mg of oral methylprednisolone daily. By her first consultation at our clinic six days later, her initial BCVA of 0.6 in the right and 0.4 in the left eye improved to 0.7 p. and 1.0, respectively. Slit-lamp examination revealed bilateral aqueous cells 1+ and vitreous cells 2+, blurred papillary margins, and multiple flat, grey-white lesions on the posterior pole with macular and peripapillary predilection (Figure 5B). Hypofluorescent areas in the peripapillary and macular region on indocyanine-green angiography (ICGA) corresponded to the areas of ellipsoid zone loss on OCT (Figure 6A, Figure 7). The patient was diagnosed with bilateral posterior uveitis.
Figure 6.
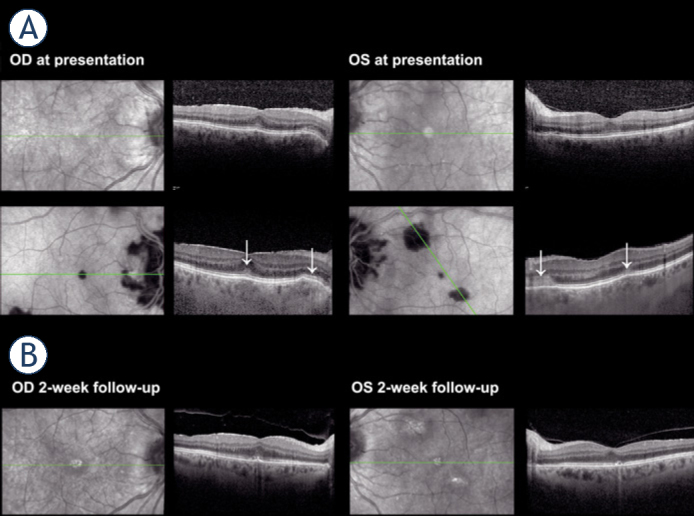
Case II. (A) Macular optical coherence tomography (OCT) presentation and (B) two weeks on methylprednisolone treatment. Loss of ellipsoid zone and hyperreflective haze in outer plexiform and outer nuclear layers is evident at presentation. Indocyanine angiography at presentation shows hypofluorescent lesions corresponding to ellipsoid zone disruptions (white arrows). Two weeks on methylprednisolone treatment ellipsoid zone appears granular.
OD = oculus dexter; OS(L) = oculus sinister
Figure 7.
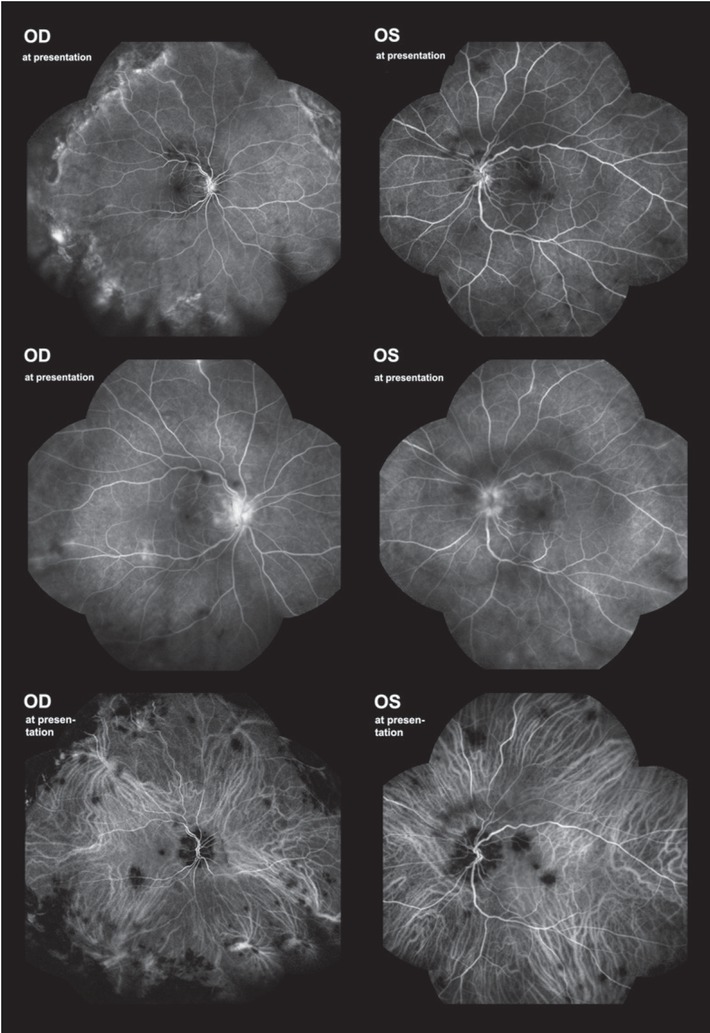
Case II. Fluorescein angiography at presentation, early and late frames. Note surface capillary net dilatation and late staining of the papilla and hypofluorescent areas on the posterior pole and periphery. Indocyanine angiography at presentation showing hypofluorescent lesions.
OD = oculus dexter; OS(L) = oculus sinister
Laboratory and radiological workups were performed to exclude common causes of uveitis (Supplementary File 1. Supplementary Table 2). CT scan of the thorax revealed no progress of the lung cancer, nor did CT scan of the head and ultrasound of the orbits show signs of cranial or orbital metastasis.
Table 2.
Published cases reporting uveitis and optic disc oedema/papillitis secondary to other immune checkpoint inhibitors
| Author, year | Immune checkpoint inhibitor | Target receptor | Cancer diagnosis | Clinical findings at presentation | Complications | Initial treatment | Discontinuing immune checkpoint inhibitor |
|---|---|---|---|---|---|---|---|
| Hahn et al., 20169 | Ipilimumab | CTLA-4 | Malignant melanoma | AC inflammation, keratic precipitates | Panuveitis, papillitis, intraretinal, subfoveal fluid | Topical prednisolone acetate, brimonidine tartrate timolol maleate, oral prednisolone 20 mg/day | Discontinued |
| Aaberg et al., 201710 | Pembrolizumab | PD-1 | Uveal melanoma | AC inflammation, vitreous cells and haze | Optic disc oedema, posterior uveitis, retinal vasculitis | Intraocular dexamethasone implant | No. |
| Wang et al., 201911 | Nivolumab | PD-1 | Renal carcinoma | AC inflammation, keratoprecipitates, posterior synechiae, vitreous floaters | Panuveitis, papillitis, serous retinal detachment | Intravenous methylprednisolone 500 mg/day, followed by oral prednisolone 30 mg/day tapered over 2 months; recurrence of uveitis managed with periocular methylprednisolone, and intraocular dexamethasone implant | Discontinued, resumed 6 weeks after discontinuation, recurrence of uveitis 2 weeks after resumption |
| Reid et al., 201812 | Pembrolizumab | PD-1 | Malignant melanoma | AC vitreous inflammation, cells, choroidal thickening, posterior synechiae | Panuveitis, optic disc oedema, hypotony | Oral prednisolone 75 mg/day 7 days, tapered over 3 weeks | Discontinued after 12 months commenced on nivolumab therapy |
| Navarro- Perea et al., 201913 | Pembrolizumab | PD-1 | Malignant melanoma | AC inflammation, irido-crystalline synechiae | Optic disc oedema | Topical dexamethasone, cyclopentolate, tropicamide, phenylephrine, oral prednisolone 40 mg/day tapered over 2 months | Discontinued, replaced by vemurafenib and cobimetinib |
| Sun et al., 20205 | Nivolumab | PD-1 | Malignant melanoma | AC inflammation, vitreous cells, and haze | Optic disc oedema, ocular hypertension | Topical prednisolone acetate, oral prednisolone 60 mg/day | n/a |
| Sun 2020et 5 al., | Pembrolizumab | PD-1 | Malignant melanoma | AC inflammation | Hypotony, Papillitis | Topical difluprednate, subtenon triamcinolone acetonide | n/a |
| Sun et al., 20205 | Ipilimumab | PD-1 | Malignant melanoma | AC inflammation, vitreous cells | Macular oedema, Papillitis | Tiamcinolonetransseptal followed by retrobulbar) | n/a |
| Kim et al., 202014 | Pembrolizumab | PD-1 | Renal carcinoma | n/a | Panuveitis, papillitis | Topical steroid, posterior subtenon triamcinolone injection | Discontinued |
| Kim 2020et 14 al., | Pembrolizumab | PD-1 | Uveal melanoma | n/a | Panuveitis, papillitis | Systemic steroid | Discontinued |
| Kim 2020et 14 al. | Pembrolizumab | PD-1 | Lung cancer | n/a | Panuveitis, uveal effusion papillitis, | Systemic steroid | Discontinued |
| Kikuchi et al. 202015 | Nivolumab | PD-1 | Hypopharyngeal cancer | Granulomatous mutton-fat keratic precipitates, AC inflammation, posterior synechiae | Panuveitis, papillitis, serous retinal detachment; Vogt- Koyanagi-Harada disease-like uveitis | Sub-tenon triamcinolone acetonide, followed by methylprednisolone 1000 mg/day 3 days, followed by oral methylprednisolone 50 mg/day tapered by 5 mg every week | Discontinued due to the patient’s deteriorating health |
| Vrabic et al. | durvalumab | PDL-1 | NSCLC | AC inflammation, vitreous cells | Intermediate uveitis, optic dics oedema | Systemic steroid 500 mg/day 3 days, followed by oral methylprednisolone 48 mg/day tapered over 6 weeks | Discontinued |
AC = anterior chamber; CTLA-4 = cytotoxic T lymphocyte-associated protein 4; N/A not available; NSCVLC = non-small cell lung cancer; PD-1 = programmed cell death protein 1
Bilateral posterior uveitis was then considered most likely secondary to durvalumab. The patient was treated with 500 mg of intravenous methylprednisolone daily for three consecutive days. She continued with 48 mg of oral methylprednisolone daily, tapered over four weeks until the maintenance dose of 12 mg of oral methylprednisolone per day was reached.
On a three-week follow-up, amelioration of right eye BCVA (0.9) and stable left eye BCVA (1.0 p.) was observed. No aqueous cells were detected while vitreous exudation with cells 1+ in the right and 2+ in the left eye remained without clear dynamics. Diminishment of grey-white lesions on the posterior pole was observed on fundoscopy (Figure 5C) and FAF revealed hyperautofluorescent spots (Figure 8). Due to the current remission of the pulmonary disease, treatment with durvalumab is not foreseen in the near future regardless of the uveitic reaction.
Figure 8.
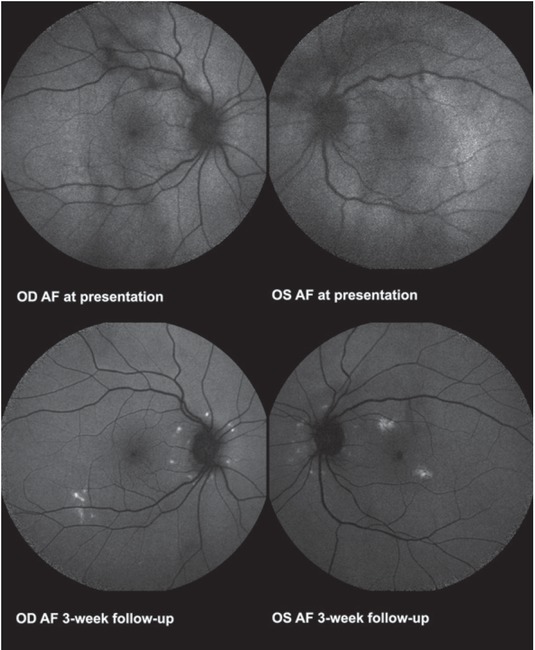
Case II. Fundus autofluorescence images at presentation show no pathologic changes. Hyperautofluorescent spots are visible three weeks on methylprednisolone treatment.
AF = fundus autofluorescence; OD = oculus dexter; OS(L) = oculus sinister
Uveitis with optic disc oedema secondary to immune checkpoint inhibitor treatment
While posterior uveitis secondary to durvalumab has been previously reported6, the constellation of uveitis and optic disc oedema consequently to durvalumab treatment has been, to our knowledge, described here for the first time. Uveitis with either optic disc oedema or papillitis was previously linked with the use of other ICIs (Table 2). Even though CTLA-4 inhibitors are deemed to cause more frequent and severe inflammatory ocular side effects than PD-1, and PD-L1 inhibitors6, most published cases of uveitis with optic disc oedema or papillitis were secondary to a PD-1 inhibitor (85%), with only one case following the use of CTLA-4 inhibitor. In 7 patients (53%), optic disc oedema occurred with panuveitis. In 5 patients (39%), signs of anterior uveitis with or without vitreous exudation were present, and in 1 patient (8%) optic disc oedema occurred with posterior uveitis and vasculitis. Systemic steroid (with or without a local steroid) was the treatment of choice in 69%, while 31% patients were managed with a local steroid only. Only one case was reported not discontinuing the ICI treatment.
Discussion
A plausible explanation for durvalumab-induced uveitis, one of the most commonly reported ophthalmic complications linked with the durvalumab use2, lies in the proposed constitutive expression of PD-L1 receptor by the eye tissue.20 PD-L1 receptor was found near the blood-ocular barriers, including corneal epithelium, corneal endothelium, iris/ ciliary body, and RPE.20 The explanation is consistent with various clinical presentations of uveitis secondary to durvalumab treatment. Inflammation can be located in different eye regions - anterior, intermediate, posterior uveitis, as well as vasculitis, have now all been reported.6, 7, 8
Uveitis commonly appears within the first six months of starting ICI immunotherapy.6 This interval may be longer in durvalumab-associated uveitis and vasculitis, as reported by Parikh et al. and Andrade et al. (13 and 20 months, respectively) as well as our cases (6 and 15 months).7,8When the first episode of uveitis is observed in a patient treated with an ICI, the basic workup should exclude common uveitic aetiologies.7 Overlooking infectious causes of uveitis can have harmful consequences for the patient, especially when treated with corticosteroids. Moreover, intravitreous metastasis should also be considered in the differential diagnosis, especially in patients with malignant melanoma.21
When bilateral optic disc oedema is present, the patient should undergo head imaging to exclude space-occupying lesions and intracranial progression of the malignancy. In addition, MRI should be performed when other neuro-ophthalmic complications related to ICI treatment, such as optic neuritis, are suspected.22
After excluding common causes of uveitis and the distant spread of underlying malignancy, paraneoplastic aetiology remains an important consideration. In the first presented case, the constellation of underlying ES-SCLC, subacute painless bilateral visual loss, visual field loss, and bilateral optic disc oedema might invoke differential diagnosis of paraneoplastic optic neuropathy.23, 24, 25 However, visual loss in paraneoplastic optic neuropathy tends to be severe and is usually insensitive to methylprednisolone therapy.24 Though vitreous cells are often present, anterior uveitis is atypical.25 Moreover, MRI commonly shows abnormalities in the course of the optic nerves24, and other neurological deficits usually accompany the disease25, which were not present in this case. When paraneoplastic optic neuropathy is likely, lumbar puncture and paraneoplastic autoanti-body screening may be helpful.23,25
In the second case, cancer-associated retinopathy (CAR) might be considered in the differential diagnosis. Although uncommon, CAR has been reported to occur with NSCLC.26,27 Some authors base their diagnosis of CAR on distinctive ophthalmological manifestations28, although the presence of circulating autoantibodies against retinal antigens is required to confirm this diagnosis.29 Several findings in our case were compatible with CAR: acute visual acuity deterioration, anterior and posterior segment inflammation, and pathological changes in the outer retinal layers. Other findings characteristic of CAR such as photopsia, night blindness, outer retinal atrophy with foveolar sparing, vessel attenuation, and arteriolar narrowing on fundos-copy were absent in our case.28, 29, 30 Although retinal autoantibody testing, visual field, and electroretinogram testing were not performed, the diagnosis of CAR was thought to be unlikely in our case.
There is no standardised approach to treating the ocular inflammatory adverse effects occurring with the ICI treatment. Uveitic complications secondary to ICIs are commonly treated with a local and/or systemic corticosteroid.5,6, 7, 8, 9, 10, 11, 12, 13, 14, 15 There is little experience in treating uveitic complications of ICI with other immunomodulatory therapy.
Some uveitic cases resolve with discontinuation of ICI therapy7,13; in others, the inflammation may persist after ICI discontinuation.18 In our first case, the patient was discontinued from durvalumab treatment not only due to grade 3 ocular toxicity according to Common terminology criteria for adverse events (CTCAE) and disease progression but also due to the progression of the underlying ESSCLC. In the second case, the patient completed durvalumab therapy, and remission of NSCLC was achieved prior to presenting with bilateral posterior uveitis. Both patients were treated with a systemic corticosteroid. Slight amelioration of BCVA and diminution of inflammation were achieved in both cases. Moreover, we observed decreased swelling of the optic discs in the first case. Visual field defects largely persisted and were likely the consequence of the underlying bilateral optic disc drusen.31 Bilateral atrophy of the retinal nerve fiber layer (RNFL) and ganglion cell layer (GCL) noted at presentation in the first case were believed consequent to optic disc drusen (Supplementary File 1. Supplementary Figure 1).32 Since no alteration of the optic disc drusen was observed on corticosteroid treatment (Figure 2), progression of RNFL and GCL atrophy (Supplementary File 1. Supplementary Figure 1) was considered consequent to the optic disc oedema. In this patient, hyperreflective spots in the inner nuclear and outer plexiform layers on the macular OCT scan possibly corresponded to activated microglia.33Inflammatory eye conditions secondary to immune checkpoint inhibitors require a thorough diagnostic workup. Once the diagnosis is established, treatment, commonly with a local or systemic corticosteroid, should be adapted to the severity of the inflammation. CTCAE is a helpful tool in deciding upon discontinuing the immunomodulatory treatment.
Supporting Information
Supplementary Tables
Disclosure
No potential conflicts of interest were disclosed.
References
- 1.Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–39. doi: 10.1016/S0140-6736(19)32222-6. et al. [DOI] [PubMed] [Google Scholar]
- 2.Imfinzi, INN-durvalumab. 2020. https://www.ema.europa.eu/en/documents/product-information/imfinzi-epar-product-information_en.pdf European Medicines Agency. [cited 30 Nov 2020]. Available at.
- 3.Lee HT, Lee JY, Lim H, Lee SH, Moon YJ, Pyo HJ. Molecular mechanism of PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and durvalumab. Sci Rep. 2017;7:5532. doi: 10.1038/s41598-017-06002-8. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun MM, Levinson RD, Filipowicz A, Anesi S, Kaplan HJ, Wang W. Uveitis in patients treated with CTLA-4 and PD-1 checkpoint blockade inhibition. Ocul Immunol Inflamm. 2020;28:217–27. doi: 10.1080/09273948.2019.1577978. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dow ER, Yung M, Tsui E. Immune checkpoint inhibitor-associated uveitis: review of treatments and outcomes. Ocul Immunol Inflamm. 2021;29:20311. doi: 10.1080/09273948.2020.1781902. [DOI] [PubMed] [Google Scholar]
- 7.Parikh RA, Chaon BC, Berkenstock MK. Ocular complications of checkpoint inhibitors and immunotherapeutic agents: a case series. Ocul Immunol Inflamm. 2021;29:1585–90. doi: 10.1080/09273948.2020.17660828. [DOI] [PubMed] [Google Scholar]
- 8.Andrade AR, Moll-Udina A, Martin R, Cilveti E, Subirà O, Disfetano L. Retinal vasculitis secondary to durvalumab. Case Rep Ophthalmol. 2020;11:161–6. doi: 10.1159/000507609. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahn L, Pepple KL. Bilateral neuroretinitis and anterior uveitis following ipilimumab treatment for metastatic melanoma. J Ophthal Inflamm Infect. 2016;6:14. doi: 10.1186/s12348-016-0082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aaberg MT, Aaberg TM. Pembrolizumab administration associated with posterior uveitis. Retin Cases Brief Rep. 2017;11:348–51. doi: 10.1097/ICB.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Lam WC, Chen L. Recurrent grade 4 panuveitis with serous retinal detachment related to nivolumab treatment in a patient with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2019;68:85–95. doi: 10.1007/s00262-018-2260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid G, Lorigan P, Heimann H, Hovan M.. Management of chronic hypotony following bilateral uveitis in a patient treated with pembrolizumab for cutaneous metastatic melanoma. Ocul Immunol Inflamm. 2019;27:1012–15. doi: 10.1080/09273948.2018.1459733. et al. [DOI] [PubMed] [Google Scholar]
- 13.Navarro-Perea C, Garcia-Gonzalez J, Perez-Blazquez E. Case report: bilateral uveitis and papillitis secondary to treatment with pembrolizumab. Indian J Ophthalmol. 2019;67:2075–7. doi: 10.4103/ijo.IJO_1161_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YJ, Lee JS, Lee J, Lee SC, Kim TI, Byeon SH. Factors associated with ocular adverse event after immune checkpoint inhibitor treatment. Cancer Immunol Immunother. 2020;69:2241–52. doi: 10.1007/s00262-020-02635-3. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kikuchi R, Kawagoe T, Hotta K. Vogt-Koyanagi-Harada disease-like uveitis following nivolumab administration treated with steroid pulse therapy: a case report. BMC Ophthalmol. 2020;20:252. doi: 10.1186/s12886-020-01519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young L, Finnigan S, Streicher H, Chen HX, Murray J, Sen HN. Ocular adverse events in PD-1 and PD-L1 inhibitors. J Immunother Cancer. 2021;9:e002119. doi: 10.1136/jitc-2020-002119. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrera W, Baartman BJ, Kosmorsky G. A case report of drug-induced myopathy involving extraocular muscles after combination therapy with tremelimumab and durvalumab for non-small cell lung cancer. Neuro-Ophthalmol. 2017;41:140–3. doi: 10.1080/01658107.2017.1291686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noble CW, Gangaputra SS, Thompson IA, Yuan A, Apolo AB, Lee JM. Ocular adverse events following use of immune checkpoint inhibitors for metastatic malignancies. Ocul Immunol Inflamm. 2020;28:854–9. doi: 10.1080/09273948.2019.1583347. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabini E, Sframeli A, Marinò M. A case of drug-induced Graves’ orbitopathy after combination therapy with tremelimumab and durvalumab. J Endocrinol Invest. 2018;41:877–8. doi: 10.1007/s40618-018-0906-0. [DOI] [PubMed] [Google Scholar]
- 20.Yang W, Li H, Chen PW, Chen HX, Murray J, Sen HN. PD-L1 expression on human ocular cells and its possible role in regulating immune-mediated ocular inflammation. Invest Ophthalmol Vis Sci. 2009;50:273–80. doi: 10.1167/iovs.08-2397. et al. [DOI] [PubMed] [Google Scholar]
- 21.Francis JH, Berry D, Abramson DH, Barker CA, Bergstrom C, Demirci H. Intravitreous cutaneous metastatic melanoma in the era of checkpoint inhibition. Ophthalmology. 2020;127:240–8. doi: 10.1016/j.ophtha.2019.09.018. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun MM, Seleme N, Chen JJ. Neuro-ophthalmic complications in patients treated with CTLA-4 and PD-1/PD-L1 checkpoint blockade. J Neuroophthalmol. 2021;41:519–30. doi: 10.1097/WNO.0000000000001148. et al. 10.1097/WNO.0000000000001148. [DOI] [PubMed] [Google Scholar]
- 23.Gordon L, Dinkin M. Paraneoplastic syndromes in neuro-ophthalmology. Continuum (Minneap Minn) 2019;25:1401–21. doi: 10.1212/CON.0000000000000788. [DOI] [PubMed] [Google Scholar]
- 24.Xu Q, Du W, Zhou H, Zhang X, Liu H, Song H. Distinct clinical characteristics of paraneoplastic optic neuropathy. Br J Ophthalmol. 2019;103:797–801. doi: 10.1136/bjophthalmol-2018-312046. et al. [DOI] [PubMed] [Google Scholar]
- 25.Cross SA, Salomao DR, Parisi JE, Kryzer TJ, Bradley EA, Mines JA. Paraneoplastic autoimmune optic neuritis with retinitis defined by CRMP-5-IgG. Ann Neurol. 2003;54:38–50. doi: 10.1002/ana.10587. et al. [DOI] [PubMed] [Google Scholar]
- 26.Adamus G, Champaigne R, Yang S. Occurrence of major anti-retinal autoantibodies associated with paraneoplastic autoimmune retinopathy. Clin Immunol. 2020;210:108317. doi: 10.1016/j.clim.2019.108317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yagyu K, Ueda T, Miyamoto A, Uenishi R, Matsushita H, Tanaka T. Cancerassociated retinopathy with neuroendocrine combined large-cell lung carcinoma and adenocarcinoma. Intern Med. 2019;58:3289–94. doi: 10.2169/internalmedicine.2313-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoogewoud F, Butori P, Blanche P, Brézin AP. Cancer-associated retinopathy preceding the diagnosis of cancer. BMC Ophthalmol. 2018;18:285. doi: 10.1186/s12886-018-0948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makiyama Y, Kikuchi T, Otani A, Oishi A, Guo C, Nakagawa S. Clinical and immunological characterization of paraneoplastic retinopathy. Invest Ophthalmol Vis Sci. 2013;54:5424–31. doi: 10.1167/iovs.13-11868. et al. [DOI] [PubMed] [Google Scholar]
- 30.Pepple KL, Cusick M, Jaffe GJ, Mruthyunjaya P. SD-OCT and autofluorescence characteristics of autoimmune retinopathy. Br J ophthalmol. 2013;97:13944. doi: 10.1136/bjophthalmol-2012-302524. [DOI] [PubMed] [Google Scholar]
- 31.Lee KM, Woo SJ, Hwang JM. Factors associated with visual field defects of optic disc. PLoS One. 2018;13:e0196001. doi: 10.1371/journal.pone.0196001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casado A, Rebolleda G, Guerrero L, Leal M, Contreras I, Oblanca N. Measurement of retinal nerve fiber layer and macular ganglion cell–inner plexiform layer with spectral-domain optical coherence tomography in patients with optic nerve head drusen. Graefes Arch Clin Exp Ophthalmol. 2014;252:1653–60. doi: 10.1007/s00417-014-2773-5. et al. [DOI] [PubMed] [Google Scholar]
- 33.Coscas G, De Benedetto U, Coscas F, Li Calzi CI, Vismara S, Roudot-Thoraval F. Hyperreflective dots: a new spectral-domain optical coherence tomography entity for follow-up and prognosis in exudative age-related macular degeneration. Ophthalmologica. 2013;229:32–7. doi: 10.1159/000342159. et al. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables


