Abstract
Studies purporting to show changes in brain structure following the popular, 8-week mindfulness-based stress reduction (MBSR) course are widely referenced despite major methodological limitations. Here, we present findings from a large, combined dataset of two, three-arm randomized controlled trials with active and waitlist (WL) control groups. Meditation-naïve participants (n = 218) completed structural magnetic resonance imaging scans during two visits: baseline and postintervention period. After baseline, participants were randomly assigned to WL (n = 70), an 8-week MBSR program (n = 75), or a validated, matched active control (n = 73). We assessed changes in gray matter volume, gray matter density, and cortical thickness. In the largest and most rigorously controlled study to date, we failed to replicate prior findings and found no evidence that MBSR produced neuroplastic changes compared to either control group, either at the whole-brain level or in regions of interest drawn from prior MBSR studies.
This study found no evidence that 2 months of training in mindfulness meditation among novices produced neurostructural changes.
INTRODUCTION
Research on mindfulness-based interventions has increased in response to a growing interest in alternative treatments for reducing stress and improving well-being. Findings from a few small studies have permeated popular media with the notion that a few weeks of training in mindfulness-based stress reduction (MBSR) can lead to measurable changes in brain structure (1, 2) and have been cited over 3200 times, combined. However, there is a lack of replication (conceptual or direct) or confirmatory analysis of these findings in a fully randomized trial. Moreover, a recent meta-analysis found that the proportion of high-quality publications in this domain have not improved over time, although there are a growing number of high-quality studies being conducted (3).
MBSR is a popular, manualized mindfulness intervention that was originally developed for use in clinical settings to improve patients’ ability to cope with pain (4). MBSR is efficacious for ameliorating symptoms of multiple psychopathologies (5) and for reducing stress (6). Studies have begun elucidating cognitive and neural mechanisms underlying mindfulness training-related changes in affect (7, 8), cognition (9, 10), and pain (11, 12), among other processes. Studies have also examined whether mindfulness meditation practice leads to changes in brain structure, as described in a meta-analysis (13), in light of numerous studies demonstrating changes in brain structure following behavioral training in other domains (14–16). However, only three studies included in the meta-analysis assessed changes specifically following training with MBSR. Most of the included studies focused on cross-sectional research of long-term meditation practitioners from a variety of meditation traditions, who may have preexisting differences relative to nonmeditators and idiosyncratic lifestyle factors associated with engaging in long duration meditation practice and meditation retreats. Conversely, MBSR is a standardized, manualized intervention in which participants receive similar training over an 8-week period, where pre-post design research can control for individual differences at baseline. Thus, we focus the current investigation specifically on the effects of MBSR. Prior research has reported that participants who completed MBSR had increased gray matter density (GMD) in the hippocampus, posterior cingulate cortex (PCC), temporoparietal junction (TPJ), cerebellum, and brainstem (17) and increased gray matter volume (GMV) in the left caudate (18). While prior research on MBSR lacked measures of cortical thickness (CT), research on meditation more broadly has reported regional increases in CT, including in the insula (12). More recent studies of the impact of short-term mindfulness meditation training on brain structure have consisted of pilot trials, with fewer than 15 participants and no control group (19).
Prior studies on MBSR-related changes in brain structure have marked limitations. These include a lack of active control groups and randomization, reliance on circular analysis (20), and small sample sizes—methodological limitations that are prevalent in meditation research more broadly (21). The current study aimed to address these limitations by integrating a waitlist (WL) and a well-matched active control group with larger sample sizes (i.e., a minimum of 70 participants per group), in a set of two rigorous, randomized controlled trials (RCTs) with pre-post designs from which we created a combined dataset. In this way, we were able to test for structural changes that were specific to mindfulness meditation training, rather than nonspecific effects associated with well-being interventions more generally. Recent literature also stresses the need for replication (22, 23) and the risk of false positives with underpowered studies (24). The current study used data that were pooled from two trials, rather than a strict replication of past work, and thus provides a conceptual replication to test the veracity of prior claims of the generalizability of structural brain changes with MBSR training. An exact replication would require the use of older, outmoded analysis pipelines and different recruiting procedures. We elected to conduct a conceptual replication that incorporated the most rigorous current design features and analytic methods.
Prior research used varied measures to gauge structural neuroplasticity, including GMV, GMD, and/or CT. GMV provides a measure of the size of a region of interest (ROI) in cubic millimeters, whereas GMD indicates the concentration of gray matter within an ROI (or within each voxel). CT indicates the thickness of the cortical sheet between the white matter and pial surfaces and thus is not available for subcortical regions. All three measures can be estimated with voxel-based morphometry (VBM). Surface-based analysis can also be used to calculate GMV and CT using information derived from geometric models of the cortical surface. Growth in any of these measures putatively reflects the same underlying processes, including synaptogenesis and gliogenesis (25). However, some prior research has found that surface-based analysis was most effective (e.g., with higher sensitivity and lower variability) for subcortical volume estimation (i.e., in measurement of error-prone regions) (26) and provided the best estimates for change over time in longitudinal models (27). Therefore, we used a surface-based analysis pipeline for the present study, although we subsequently reprocessed the data using VBM methods as well, for sensitivity analysis (see the Supplementary Materials).
Changes in brain structure in association with mindfulness meditation training would provide evidence of structural neuroplasticity and may elucidate potential mechanisms underlying benefits of mindfulness meditation. In prior work, hippocampus and insula were selected as ROIs a priori, because of their role in emotion control and awareness (respectively), their activation during meditative states, and prior associations with long-term meditation training and increased GMV in these regions (13). The insula, amygdala, and anterior cingulate contribute to the salience network, which is associated with emotional reactivity and subjective awareness processes that are hypothesized to change with mindfulness training (28, 29). PCC and TPJ are major nodes within the default mode network, which is implicated in self-referential thought and mind-wandering (30, 31), that have been shown to change with mindfulness training (32). Given the evidence for mindfulness-related changes in function and psychological processes associated with these brain regions (33–35), structural changes in gray matter might also be expected. Prior research provides evidence for mindfulness-related changes in brain structure in the default mode and salience networks, among other regions (13, 17, 18).
We attempted to conceptually replicate prior findings of increased GMD following MBSR in the hippocampus, PCC, TPJ, cerebellum, and brainstem and increased GMV in the caudate, as a subcomponent of a larger study that aimed to examine the impact of MBSR on sleep, cognition, emotion, and well-being. Because the current study included much broader aims than a straightforward replication, we used a more modern and sensitive processing and analysis strategy compared to the prior studies. In addition, we included results for alternative pipelines that were more consistent with prior work as sensitivity analysis (i.e., using VBM in addition to surface-based analysis). In addition to the aforementioned regions, we also assessed structural changes in the amygdala and insula, as these regions are involved in affective processing that may change with mindfulness training (7, 8). While prior research did not find associations between structural changes and amount of MBSR practice time, we tested for such associations, given the broader range of MBSR practice time in the current study relative to prior work (17). We hypothesized that MBSR practice time would be associated with increased GMV, GMD, and CT in all ROIs except amygdala, where we expected an inverse relationship between size and practice time, given the inverse relationship between MBSR-related reductions in amygdala GMD and stress in prior research (36). Thus, the current research sought to conceptually replicate and extend the literature on structural neuroplasticity associated with short-term mindfulness meditation practice in MBSR.
RESULTS
All results (from both whole-brain and ROI analysis) are consistent regardless of the inclusion or exclusion of participants who attended less than six MBSR classes (n = 2) or participants who practiced for less than 2 hours outside of class (n = 6), including when we add an additional covariate to control for the time between scans. We also confirmed that there were no significant differences between groups at baseline.
Whole-brain analysis
There were no significant group differences for change in brain structure (GMV, GMD, or CT) for MBSR compared to the Health Enhancement Program (HEP) active control group, or the WL control group, in the whole-brain analysis (including when controlling for the timing between scans). This is consistent with a prior whole-brain analysis of GMV conducted with sample one (37). Significant within-group increases in CT were present for MBSR in the left lingual gyrus, for HEP in the left rostral middle frontal gyrus, and for WL in the bilateral precuneus and the right superior parietal cortex. The WL group also had a significant increase in the left rostral middle frontal gyrus volume. See table S1 for detailed cluster information. There were no significant interactions between MBSR and HEP practice time and change in GMV in the whole-brain analysis. Unthresholded statistical maps are available at NeuroVault (https://neurovault.org/collections/7634/) (38).
ROI analysis
There were no significant group differences for change in brain structure for MBSR compared to HEP or WL for any of the ROIs (P > 0.10) (including when controlling for the timing between scans). The nonsignificant result for right amygdala is depicted in Fig. 1A. See table S2 for results of statistical tests of change in GMV for all ROIs. Results are consistent regardless of the inclusion or exclusion of influential outliers. Nonsignificant results of sensitivity analysis using multiple imputation to account for missing data, as well as analysis of GMD from SPM12 and GMV from SPM-CAT12, are presented in tables S4 to S9. We also completed a small volume–corrected, voxel/vertex-wise analysis of the four anatomically defined ROIs (insula, hippocampus, amygdala, and caudate), as in prior work (17), and found no significant group differences for change in GMV. Last, there were no significant within-group changes in brain structure across time (P > 0.05).
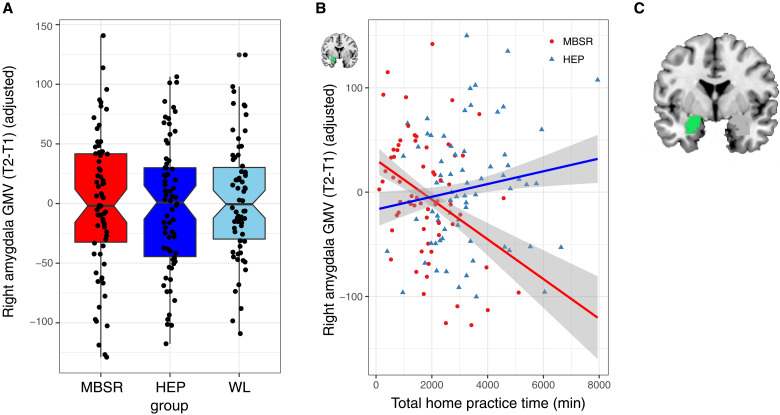
Fig. 1. Change in right amygdala GMV and MBSR practice time.
(A) There were no significant differences between groups in change in right amygdala GMV from baseline (T1) to postintervention period (T2). (B) MBSR practice time was associated with reduced right amygdala GMV significantly more than practice in the HEP active control. (C) FreeSurfer anatomical label from aseg for right amygdala (in green). Error envelopes represent 1 SE above and below the point estimates of the means, the dependent variables are adjusted for covariates (i.e., age, gender, sample, and total brain GMV), and adjusted data points are overlaid.
MBSR practice time
MBSR participants practiced at home an average of 32 hours (SD, 20 hours; range, 2 to 85 hours), and HEP participants practiced at home an average of 56 hours (SD, 33 hours; range, 7 to 255 hours). MBSR participants attended 8.14 of 9 possible classes, on average (range, 4 to 9 classes), and HEP participants attended 8.44 of 9 possible classes, on average (range, 2 to 9 classes). Significant effects of MBSR practice time were limited to the amygdala, and relationships with the other eight ROIs were nonsignificant (P > 0.05). MBSR practice time was associated with reduction in right amygdala volume significantly more than HEP practice [t(128) = −3.30, P = 0.001, P* = 0.01, Pη2 = 0.08; Fig. 1B]. See table S3 for results of all statistical tests examining the impact of practice time on change in GMV. However, the group by practice time interaction was trend level or nonsignificant in the sensitivity analyses (see tables S5, S7, and S9).
Mindfulness
We examined self-reported mindfulness based on the Five Facet Mindfulness Questionnaire (FFMQ) to gauge the effectiveness of the MBSR intervention. A prior study reported on the results for sample one, whereby MBSR was associated with increased mindfulness (P < 0.05, within-group) that marginally differed from WL (P = 0.09) but did not differ from HEP (P = 0.33) (39). When collapsing across both studies, results were consistent with the prior report, whereby MBSR differed significantly from WL [t(208) = −2.70, P = 0.01], but not from HEP [t(208) = −1.01, P = 0.31, Pη2 = 0.05]. Across both samples, mindfulness increased following MBSR [t(70) = 3.86, P < 0.001, Pη2 = 0.18] and HEP [t(65) = 3.39, P = 0.001, Pη2 = 0.15].
DISCUSSION
The current study sought to conceptually replicate and extend the significance of prior work demonstrating increased GMD following mindfulness meditation training in the hippocampus, posterior cingulate, cerebellum, brainstem, and TPJ (17) and increased GMV in the caudate (18). We combined two datasets to yield sample sizes of 70 or more participants per group. Both datasets were collected with the same rigorous methods and three-arm RCT design, using MBSR, a well-matched active control (HEP), and a WL control. We expected to find increased GMD following short-term MBSR training in the hippocampus, caudate, TPJ, and PCC and reduced volume for the amygdala, in line with prior work. We also hypothesized that these effects would be larger for participants who spent more time practicing mindfulness meditation. We failed to find any group differences in GMV, GMD, or CT in support of these hypotheses.
It is unlikely that the failure to detect structural brain changes following MBSR was due to ineffective training. The MBSR intervention was effective regarding expected changes in neural function and connectivity, as well as psychological and cognitive outcomes: MBSR reduced amygdala reactivity and increased amygdala–ventromedial prefrontal cortex functional connectivity to emotional stimuli in sample one (8), increased PCC resting functional connectivity with dorsolateral prefrontal cortex in sample two (35), and increased self-reported mindfulness [reported in (39) for sample one, in addition to the results presented here]. The active control intervention, HEP, also increased self-reported mindfulness, and MBSR participants did not differ significantly from HEP on this measure. The current study lends evidence that MBSR-related improvements in self-reported mindfulness may not be specific to mindfulness meditation practice but rather related to other aspects of the course that are common to similar interventions [e.g., benefits from learning well-being skills from experts in HEP; for an in-depth discussion, see (40)]. Participants in the current study were at least as engaged with the MBSR coursework as in the prior research, if not more engaged, based on the time they reported practicing meditation at home (which ranged from 2 to 85 hours with a mean of 32 hours in the current study, compared to a range of 7 to 42 hours and a mean of 23 hours in prior work) (36).
Despite the lack of group differences in change in regional brain structure, we observed a significant interaction of group (MBSR versus HEP) and practice time on change in right amygdala GMV. The more time participants spent practicing MBSR outside of class, the larger their reduction in right amygdala volume following the intervention compared to practice with HEP, the active control intervention. On average, participants with less than 27 hours of total MBSR practice time had no change in amygdala volume, and the lower bound of the confidence interval at this point was 20 hours of total MBSR practice time (or an average of about 22 min/day). Therefore, practicing mindfulness meditation for less than 22 min/day for a few months is unlikely to lead to structural change in the amygdala. In addition, changes in the early stages of mindfulness meditation training, such as during MBSR training in previously untrained individuals, may be different from the changes in later stages or for longer interventions. Along these lines, a recent, well-powered study assessing meditation training similar to mindfulness meditation, but with a 50% longer duration than MBSR, found significant increases in CT relative to two active control interventions in prefrontal cortex extending to anterior cingulate and in bilateral occipital cortex extending to inferior temporal cortex (41). While the current study thus provides initial evidence that MBSR-related reductions in amygdala volume may depend on the degree of engagement with practice, the effect was small and failed to survive the sensitivity analyses. Thus, it should be interpreted with caution and warrants attempts to replicate in future work.
The results of the current study failed to support the hypothesis that short-term training in mindfulness meditation is associated with significant group differences in change in regional brain structure compared to a well-matched active control intervention or a WL control group in an adequately powered, rigorous RCT design. Despite previous research suggesting that short-term mindfulness meditation training affects the structure of the brain, results of the present study failed to detect these group differences. While this highlights the importance of conceptual replications, it also raises new questions and highlights limitations of conceptual replications relative to direct replications. There were important differences between the current study and prior work, including the populations from which participants were drawn and differences in the study design and methods. Prior work recruited participants who elected to participate in an MBSR course (17, 18) and were thus not randomly assigned, while the current study used a rigorous RCT design. The participants in prior studies may have had more “room for improvement,” because they sought out a course for stress reduction, with some samples recruited specifically on the basis of the presence of high stress in participants the month before study participation (17). In contrast, the current set of RCTs used a relatively long list of inclusion/exclusion criteria, including exclusion for use of psychotropic medication or psychiatric diagnosis in the past year, resulting in unusually healthy samples with, e.g., very low levels (or absence) of baseline anxiety and negative affect. While the RCT design used here provides the most rigorous experimental control, this increased rigor likely comes at the expense of ecological validity—the simple act of choosing to enroll in MBSR may be associated with increased benefit.
It is notable that the current study also had sample sizes over three times that of prior work (e.g., n = 75 MBSR participants in our final sample compared to n = 20 or less participants per group in prior work) (17, 18). Given the low sample sizes of prior work and the larger samples and lack of replication in the current study, there is a possibility that prior results suffered from inflated effect sizes and low positive predictive value (24). For example, we found medium effect sizes (ranging from Cohen’s d = 0.39 to d = 0.43) for the significant group differences between MBSR and WL in self-reported mindfulness (from pre- to postintervention), whereas the prior research found large effect sizes (ranging from Cohen’s d = 0.77 to d = 1.48) (17, 18). Moreover, the very small magnitude of standard effect size estimates for the nonsignificant group differences in change in regional GMV in the current study can be interpreted as “no effect” (e.g., partial η2 = 0.01 for the difference between MBSR and HEP for change in the left hippocampus GMV). These null effects, in conjunction with our large sample size and rigorous matched comparison condition, allow us to conclude that MBSR does not differentially affect brain structure.
Various forms of psychopathology have been associated with alterations in brain structure (42). In addition, exposure to trauma has also been associated with structural alterations in the brain (43). It is this body of work that initially led investigators including ourselves to hypothesize that MBSR, an intervention that has been found to elevate well-being, may also alter the structure of the brain in a direction opposite to that found in psychopathology and trauma.
Alterations in brain structure are present after other types of training, including aerobic exercise and balance training (14, 15). The latter study entailed a 12-week intervention, along a similar time scale as the current study. These forms of training involved specific, repetitive actions within a physical domain. Conversely, MBSR training spans varied meditative and educational practices across multiple psychological domains (e.g., attention, compassion, and emotion). MBSR also requires higher-order cognitive skills that engage a complex, distributed network of brain regions relative to work in other domains. If MBSR induced structural neuroplasticity, then it would likely occur in a similarly distributed pattern and with potentially higher interindividual spatial variability relative to training that involves a more localized brain region (e.g., balance training and vestibular cortex) (15). Detecting MBSR-induced changes in brain structure may thus be difficult. Moreover, variation in the location of brain activation with MBSR practice may preclude the repetitive, localized activation theorized to underlie training-induced structural neuroplasticity (16). MBSR is similar to other types of meditation training in that practitioners are often taught a range of different methods, each addressing somewhat different aspects of well-being. While such training may be optimally effective in producing psychological change, because of its multifaceted nature, it is unlikely to induce discrete focal changes in brain structure. It may be that only with much longer duration of training and/or training explicitly focused on a single form of practice, that structural alterations will be identified.
As more research is conducted on this topic, the importance of reporting results of conceptual and direct replication attempts should be emphasized considering known publication bias for positive findings (44). Likewise, it is important for future research to examine individual differences in engagement and efficacy of MBSR, as well as the optimum length and duration of daily practice for a mindfulness meditation intervention to confer benefits. The lack of significant group differences between MBSR and control groups in the current study suggests that interventions lasting longer than the standard 8-week MBSR course and/or singularly focused on one specific meditation practice might be required to produce changes in brain structure.
MATERIALS AND METHODS
Experimental design
The present study was registered with ClinicalTrials.gov and combined data across two clinical trials (NCT01057368 and NCT02157766), which started approximately 5 years apart. Baseline data collection (T1) for sample 1 occurred between February 2010 and May 2011, and data collection following the intervention period (T2) occurred between June 2010 and October 2011. For sample 2, baseline data (T1) collection occurred between November 2014 and March 2017, and data collection following the intervention period (T2) occurred between March 2015 and July 2017. While the average time between scans (T1 to T2) ranged from 62 to 238 days (mean, 120.6 days; SD, 28.0 days), most of this time gap was due to a lag between the T1 scan and the start of the intervention period. We prioritized scanning participants as close to the end of the intervention as possible for T2, to capture potential intervention-related changes. Thus, the average time between the last day of the intervention and the T2 scan was 19.4 days for MBSR participants (SD, 11.7 days; range, 1 to 46 days) and 15.6 days for controls (SD, 13.4 days; range, 0 to 93 days). Results are consistent with those reported when a covariate is included to control for the time between scans. The experimental design was comparable across both datasets (see figs. S1 and S2 for the CONSORT diagrams from each trial). Both clinical trials ended upon completion.
Participants
Data were combined for meditation-naïve participants (MNPs) from sample 1 (n = 124; average age, 48.1 ± 10.7 years; 79 female) and sample 2 (n = 139; average age, 44.1 ± 12.7 years; 82 female). Sample size for each dataset was determined with a power analysis. We recruited healthy human participants within the Madison, WI, community using flyers, online advertisements, and advertisements in local media for a study of “health and well-being” or the “benefits of health wellness classes.” Participants were included if they were adults between 18 and 65 years old with no prior training or formal practice in meditation or mind-body techniques (e.g., Tai chi) or expertise in physical activity, music, or nutrition. Participants were excluded from enrollment if they had used psychotropic or nervous system altering medication, a current diagnosis of sleep disorder, a psychiatric diagnosis in the past year, any history of bipolar or schizophrenic disorders, history of brain damage or seizures, or a medical condition that would affect the participants ability to safely participate in study procedures. There were no differences in socioeconomic status between MBSR and either control group based on the Hollingshead index (P > 0.10) (45).
Following baseline data collection, participants were randomized to one of three groups using a stratified block assignment procedure to ensure age- and gender-balanced groups: MBSR (n = 90; average age, 46.6 ± 11.8 years; 53 female), WL (n = 84; average age, 46.0 ± 11.7 years; 53 female), or the HEP active control intervention (n = 90; average age, 45.4 ± 12.5 years; 55 female), which has been validated in a separate study in which the intervention procedures are described in further detail (46). (See Table 1 for detailed demographic information for each group.) All study staff, except the participant coordinator and project manager (and their undergraduate assistants), were blind to group assignment. Blinded group indicators were used, and only the participant coordinator had access to the key.
Table 1. Detailed demographic information.
| Dataset 1 | Dataset 2 | ||||||
| MBSR (n = 35) | HEP (n = 36) | WL (n = 35) | MBSR (n = 40) | HEP (n = 37) | WL (n = 35) | ||
| Age | Mean | 50.2 | 47.9 | 48.4 | 44.8 | 44.2 | 45.2 |
| SD | 9.4 | 12.5 | 10.5 | 13.3 | 12.8 | 12.5 | |
| Minimum | 26 | 26 | 26 | 25 | 25 | 25 | |
| Maximum | 65 | 66 | 65 | 64 | 65 | 65 | |
| Sex | Female | 23 | 20 | 23 | 20 | 23 | 23 |
| Male | 12 | 16 | 12 | 20 | 14 | 12 | |
| Race | American Indian/ Alaska Native |
1 | 0 | 0 | 1 | 1 | 1 |
| Asian | 1 | 1 | 0 | 5 | 1 | 6 | |
| Black/African American | 0 | 0 | 0 | 0 | 2 | 0 | |
| Native Hawaiian/ Pacific Islander |
0 | 0 | 0 | 0 | 0 | 1 | |
| Multiracial | 1 | 1 | 1 | 2 | 1 | 3 | |
| White | 32 | 34 | 32 | 30 | 31 | 24 | |
| Declined response | 0 | 0 | 2 | 2 | 1 | 0 | |
| Ethnicity | Hispanic | 1 | 1 | 6 | 3 | 3 | 1 |
| Not Hispanic | 34 | 35 | 29 | 37 | 33 | 34 | |
Six participants were excluded because of brain abnormalities, and two participants were missing structural data because of technical difficulties, resulting in 256 participants (average age, 45.7 ± 12.0 years; 156 female) with baseline (T1) structural magnetic resonance imaging (MRI) data. Eighteen participants withdrew before T2 data collection (eight MBSR, one HEP, and nine WL), 13 participants were excluded because they failed to attend a minimum of two classes for the assigned intervention (nine HEP and four MBSR), and 7 participants were missing T2 structural MRI data because of technical difficulties, resulting in 75 MBSR (average age, 47.3 ± 11.9 years; 43 female), 73 HEP (average age, 46.0 ± 12.7 years; 43 female), and 70 WL (average age, 46.8 ± 11.6 years; 46 female) participants with T2 structural MRI data.
The MBSR courses were delivered by experienced and certified MBSR instructors and consisted of practices and teachings aimed at increasing mindfulness, including yoga, meditation, and body awareness. The HEP course served as an active control, which was matched to MBSR and consisted of exercise, music therapy, and nutrition education and practices. The intervention and randomization procedures were identical to those detailed by MacCoon et al. (46). MBSR and HEP participants recorded logs of the minutes they spent each day on the respective practices at home (i.e., outside of class), which were summed to calculate a variable for total minutes of practice for each participant (except those in the WL group). Classes for both interventions (MBSR and HEP) were taught through the Integrative Health program at the University of Wisconsin School of Medicine and Public Health in Madison, WI.
Data collection
Participants completed a baseline data collection visit before randomization and a second visit following the 8-week intervention period. Both visits took place at the Waisman Laboratory for Brain Imaging and Behavior at the University of Wisconsin–Madison. The second sample of MNP also completed a third, long-term follow-up session approximately 6 months after the second visit that was not included in the current analysis. At each visit, participants attended a 24-hour laboratory session that included an MRI scan and the FFMQ (47), among other measures as part of a larger multisession, multiproject study. We examined the FFMQ to gauge the efficacy of MBSR for improving mindfulness, given its use in the prior studies that we attempt to replicate here (17) and despite its apparent limitations (21, 39, 47). Experimenters were blind to the group assignment during data collection. University of Wisconsin–Madison’s Health Sciences Institutional Review Board approved the study (protocol numbers H-2009-0017 and 2014-0116), and all participants provided consent and were given monetary compensation for their participation.
Image acquisition
Anatomical images for sample one were acquired on a GE X750-3.0 Tesla MRI scanner device with an eight-channel head coil and consisted of a high-resolution three-dimensional T1-weighted inversion recovery fast gradient echo image (inversion time, 450 ms; in-plane resolution, 256 by 256; field of view, 256 mm; axial slices, 124 mm by 1.0 mm). Anatomical images for sample two were acquired on the same scanner using a 32-channel head coil with the same scan sequence, except with axial slices of 192 mm by 1.0 mm.
Image processing
Image processing was conducted in FreeSurfer using the automated longitudinal pipeline (stable release version 6.0), which included skull stripping, registration, intensity normalization, Talairach transformation, tissue segmentation, and surface tessellation (48, 49). Hand edits were conducted to correct errors in the automated processing, primarily to the base, and, if needed, to the subsequently generated longitudinal images. Manual edits in the base included editing the Talairach registration, wm.mgz, and brainmask.mgz volumes. Edits to the Talairach registration occurred when the initial registration was a poor fit to the participant brain. In both the base and longitudinal phases, control points were added to correct intensity normalization errors and white matter omissions. In addition, if the white and pial surfaces did not follow white and gray matter boundaries, then voxel edits were made on the wm.mgz and brainmask.mgz volumes, respectively. Images were resampled to fsaverage space using the FreeSurfer program mris_preproc, and difference maps were generated for each participant (T2-T1) using the “paired-diff” option. The resulting difference maps were then smoothed to 8-mm full width at half maximum with mri_surf2surf and used as inputs for subsequent group analysis.
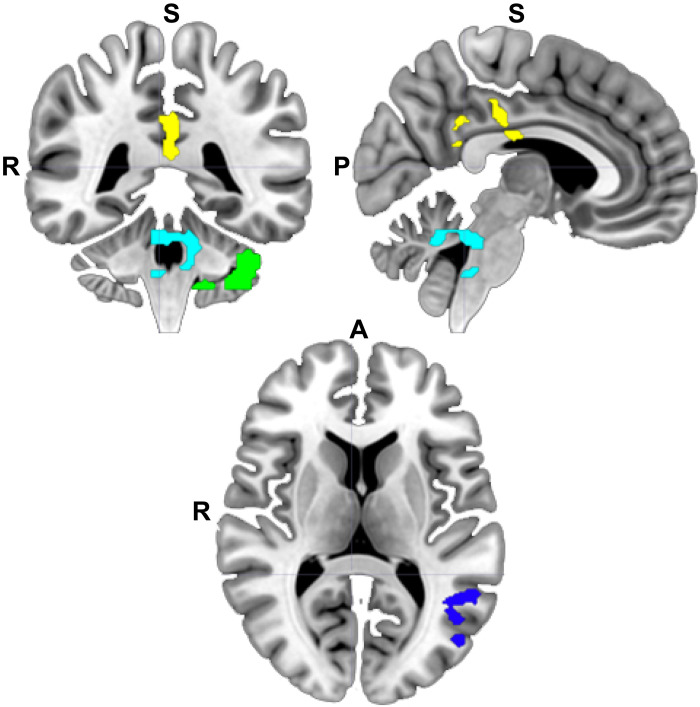
FreeSurfer’s automated brain segmentation tool (aseg) was used to extract measures of GMV from subcortical regions (see Fig. 1C for depiction of the right amygdala ROI), and the Desikan-Killiany atlas was used to extract GMV for the insula (50). A mask of clusters with significant change in MBSR participants was provided by Hölzel et al. (17), which was registered to individual participant space using the FreeSurfer command mri_label2vol using transformation matrices generated for each participant with the FreeSurfer command mni152reg. The resultant TPJ and PCC and two cerebellar masks (depicted in the MNI152 template space in Fig. 2) were then used to extract GMV measures for each participant using the FreeSurfer program mris_segstats. We masked each of these cortical ROIs to exclude white matter based on each participant white matter segmentation, as generated in the FreeSurfer processing pipeline with the program recon-all. The significance of results does not change using the original ROIs, without additional masking.
Fig. 2. Masks defined from prior research.
ROIs for posterior cingulate (yellow), left TPJ junction (dark blue), cerebellum (green), and cerebellum/brainstem (teal) were defined on the basis of a thresholded statistical map provided by Hölzel et al. (17) in which increased GMV was previously reported following MBSR. White matter was masked from the cortical ROIs for each participant before extraction of GMV. R, right; A, anterior; P, posterior; S, superior.
We also completed sensitivity analysis with multiple imputation for ROI analyses and with data processed using Statistical Parametric Mapping (SPM) software to verify that the absence of significant results was not due to differences in software or the specific structural measures used (e.g., GMV versus GMD). Details for multiple imputation and SPM pipelines are available in the Supplementary Materials and included both standard SPM12 and SPM12 Computational Anatomy Toolbox (CAT12) longitudinal pipelines, the latter being calibrated to detect smaller changes in brain structure than earlier software. Full results of sensitivity analysis are presented in tables S4 to S9.
Statistical analysis—Voxel-wise
Whole-brain analysis was performed on GMV and CT difference maps (T2-T1) using the FreeSurfer program mri_glmfit with a single, three-level variable of interest to model group (MBSR, HEP, or WL) with covariates to control for age, gender, total (whole-brain) GMV, and sample. The FreeSurfer program mri_glmfit-sim was then used to correct for multiple comparisons with Z Monte Carlo simulation (voxel/vertex-wise threshold, P < 0.001; cluster-forming threshold, P < 0.05), including an additional correction to control for comparisons across the two hemispheres (51). Analysis of the effect of home practice included an additional regressor with total home practice minutes and modeled a contrast for the interaction of group × practice. Small volume–corrected analyses were also conducted for the anatomical ROIs using FreeSurfer for the insula cortical ROI and the Wake Forest University PickAtlas in SPM for subcortical ROIs (amygdala, caudate, and hippocampus) (52, 53).
Statistical analysis—ROI
All ROI analyses (with the exception of voxel-wise analysis) were performed using the lm function in R statistics software (54), and P value computation used the modelSummary function of the lmSupport package (55). The GMV difference (T2-T1) for each ROI was regressed (separately) on group with covariates to control for participant age, gender, sample (i.e., 1 versus 2), and total whole-brain GMV. Analysis of the impact of home practice time on GMV included the addition of total home practice minutes and its interaction with group. Outliers were identified on the basis of Cook’s D using a cutoff threshold of 4/(N − P), where N and P correspond to the sample size and number of model parameters, respectively, and removed from analysis. The number of outliers per group ranged from two to eight for MBSR, from three to six for HEP, and from one to six for WL. We used a false discovery rate correction to control for multiple comparisons across all 12 ROIs using the p.adjust function. Corrected P values are indicated by P* in the Results. A summary of descriptive statistics for average GMV for all ROIs is presented in Table 2, and the results of all statistical tests are presented in tables S2 to S9.
Table 2. Descriptive statistics.
Mean (M), SD, minimum (Min), and maximum (Max) for all measures. LTM, long-term meditators.
| Measure | Left amygdala GMV (mm3) | Right amygdala GMV (mm3) | Left TPJ GMV (mm3) | |||||||||
| Group | M | SD | Min | Max | M | SD | Min | Max | M | SD | Min | Max |
| T1 MBSR | 1541 | 191 | 1113 | 1882 | 1846 | 234 | 1272 | 2631 | 1916 | 196 | 1452 | 2438 |
| T1 HEP | 1594 | 215 | 1134 | 2277 | 1873 | 226 | 1427 | 2511 | 1914 | 219 | 1479 | 2572 |
| T1 WL | 1565 | 216 | 1107 | 2101 | 1839 | 240 | 1237 | 2578 | 1928 | 194 | 1375 | 2390 |
| T2 MBSR | 1549 | 202 | 1103 | 1987 | 1843 | 239 | 1299 | 2577 | 1910 | 216 | 1420 | 2384 |
| T2 HEP | 1591 | 222 | 1179 | 2430 | 1876 | 227 | 1437 | 2492 | 1899 | 186 | 1550 | 2319 |
| T2 WL | 1565 | 217 | 1059 | 2087 | 1847 | 221 | 1402 | 2439 | 1922 | 190 | 1430 | 2396 |
| Measure | Left hippocampus GMV (mm3) | Right hippocampus GMV (mm3) | Posterior cingulate GMV (mm3) | |||||||||
| Group | M | SD | Min | Max | M | SD | Min | Max | M | SD | Min | Max |
| T1 MBSR | 4212 | 465 | 3131 | 5162 | 4294 | 418 | 3283 | 5225 | 2604 | 291 | 1993 | 3373 |
| T1 HEP | 4284 | 433 | 3574 | 5367 | 4340 | 447 | 3546 | 5720 | 2628 | 332 | 1984 | 3661 |
| T1 WL | 4233 | 436 | 3320 | 5431 | 4282 | 420 | 3261 | 5407 | 2663 | 305 | 1819 | 3408 |
| T2 MBSR | 4190 | 484 | 3006 | 5190 | 4270 | 427 | 3288 | 5172 | 2591 | 302 | 1860 | 3327 |
| T2 HEP | 4273 | 439 | 3600 | 5440 | 4338 | 457 | 3470 | 5717 | 2579 | 301 | 1985 | 3305 |
| T2 WL | 4218 | 429 | 3234 | 5614 | 4263 | 418 | 3255 | 5491 | 2640 | 302 | 1843 | 3368 |
| Measure | Left caudate GMV (mm3) | Right caudate GMV (mm3) | Cerebellum GMV (mm3) | |||||||||
| Group | M | SD | Min | Max | M | SD | Min | Max | M | SD | Min | Max |
| T1 MBSR | 3561 | 483 | 3472 | 5275 | 3728 | 518 | 2563 | 5373 | 2225 | 225 | 1730 | 2704 |
| T1 HEP | 3572 | 479 | 2516 | 4794 | 3731 | 502 | 2905 | 5043 | 2220 | 245 | 1701 | 2966 |
| T1 WL | 3596 | 434 | 2731 | 4561 | 3741 | 428 | 2758 | 4897 | 2230 | 221 | 1676 | 2762 |
| T2 MBSR | 3560 | 490 | 2516 | 5242 | 3734 | 521 | 2518 | 5416 | 2233 | 223 | 1750 | 2747 |
| T2 HEP | 3554 | 490 | 2512 | 4864 | 3730 | 505 | 2912 | 5155 | 2231 | 245 | 1737 | 2950 |
| T2 WL | 3594 | 435 | 2718 | 4601 | 3758 | 433 | 2734 | 4886 | 2239 | 218 | 1666 | 2825 |
| Measure | Left insula GMV (mm3) | Right insula GMV (mm3) | Cerebellum/brainstem GMV (mm3) | |||||||||
| Group | M | SD | Min | Max | M | SD | Min | Max | M | SD | Min | Max |
| T1 MBSR | 7074 | 783 | 5335 | 8542 | 6997 | 750 | 5191 | 8512 | 3375 | 348 | 2607 | 4056 |
| T1 HEP | 7189 | 816 | 5959 | 9221 | 7012 | 842 | 5656 | 10023 | 3374 | 374 | 2620 | 4482 |
| T1 WL | 7017 | 744 | 5509 | 8834 | 6962 | 854 | 5390 | 9269 | 3387 | 341 | 2567 | 4177 |
| T2 MBSR | 7103 | 823 | 5290 | 8856 | 6998 | 749 | 5140 | 8598 | 3382 | 341 | 2621 | 4056 |
| T2 HEP | 7199 | 834 | 5801 | 9348 | 7066 | 880 | 5795 | 10565 | 3377 | 373 | 2633 | 4499 |
| T2 WL | 7024 | 756 | 5515 | 8943 | 6933 | 847 | 5349 | 9170 | 3401 | 328 | 2548 | 4185 |
Acknowledgments
We would like to thank M. Anderle, R. Fisher, L. Angelos, H. Hessenthaler, T. Nelson, A. Cayo, M. Kruepke, J. Brumbaugh, S. Christens, J. Sachs, J. Harris, M. Brown, E. Nord, G. Bednarek, K. Chung, P. Lhamo, D. Bachhuber, A. Cayo, C. Harty, S. Kindy, and D. Dewitz for assistance with recruitment and/or data collection. We would also like to thank K. Bonus, D. Coogan, B. Gillespie, D. Grove, L. Gustafson, M. Hirshberg, P. Kalscheur, C. McGehee, V. Minichiello, L. Pinger, L. T. Prince, K. Rietz, S. Shatz, C. Smith, H. Sorensen, J. Sullivan, J. Thurlow, M. Waupoose, S. Wojtal-Weber, and P. Young for teaching and/or coordinating the interventions and J. Koger, T. Christian, D. Thompson, J. Ollinger, M. Ly, N. Vack, N. Adluru, and A. Schoen for technical assistance.
Funding: This work was supported by National Institutes of Health, National Center for Complementary and Alternative Medicine (NCCAM) grant P01AT004952 (to R.J.D.); National Institutes of Health, National Institute of Mental Health (NIMH) grants R01-MH43454 (to R.J.D.), P50-MH084051 (to R.J.D.), and T32MH018931 (to R.J.D. and T.R.A.K.); Fetzer Institute grant (to R.J.D.); John Templeton Foundation grant (to R.J.D.); National Institutes of Health, National Institute of Child Health and Human Development grants P30 HD003352-449015 and U54 HD090256; and National Academy of Education/Spencer Foundation postdoctoral fellowship (to M.J.H.).
Author contributions: Conceptualization: T.R.A.K., R.I.G., M.A.R., A.L., and R.J.D. Methodology: T.R.A.K., K.D., C.K., M.J.H., R.H., and L.Y.T. Investigation: T.R.A.K., C.K., and M.J.H. Visualization: T.R.A.K. Supervision: R.J.D. Writing—original draft: T.R.A.K., K.D., R.H., and L.Y.T. Writing—review and editing: T.R.A.K., K.D., C.K., M.J.H., R.I.G., M.A.R., A.L., and R.J.D.
Competing interests: R.J.D. is the founder and president and serves on the board of directors for the nonprofit organization, Healthy Minds Innovations Inc. No donors, either anonymous or identified, have participated in the design, conduct, or reporting of research results in this manuscript. All other authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. All neuroimaging data, meta-data, and code needed to evaluate the conclusions of the paper are available at the Open Science Framework (https://osf.io/jrpnq/). Unthresholded statistical maps are available at NeuroVault (https://identifiers.org/neurovault.collection:7634).
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 and S2
Tables S1 to S9
References
REFERENCES AND NOTES
- 1.C. Congleton, B. K. Hölzel, S. W. Lazar, Mindfulness Can Literally Change Your Brain (Harvard Business Review, 2015). [Google Scholar]
- 2.B. Schulte, Harvard Neuroscientist: Meditation Not Only Reduces Stress, Here’s How it Changes Your Brain (Washington Post, 2015). [Google Scholar]
- 3.Goldberg S. B., Tucker R. P., Green P. A., Davidson R. J., Wampold B. E., Kearney D. J., Simpson T. L., Mindfulness-based interventions for psychiatric disorders: A systematic review and meta-analysis. Clin. Psychol. Rev. 59, 52–60 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabat-Zinn J., An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. Gen. Hosp. Psychiatry 4, 33–47 (1982). [DOI] [PubMed] [Google Scholar]
- 5.Wielgosz J., Goldberg S. B., Kral T. R. A., Dunne J. D., Davidson R. J., Mindfulness meditation and psychopathology. Annu. Rev. Clin. Psychol. 15, 285–316 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiesa A., Serretti A., Mindfulness-based stress reduction for stress management in healthy people: A review and meta-analysis. J. Altern. Complement. Med. 15, 593–600 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Desbordes G., Negi L. T., Pace T. W. W., Wallace B. A., Raison C. L., Schwartz E. L., Effects of mindful-attention and compassion meditation training on amygdala response to emotional stimuli in an ordinary, non-meditative state. Front. Hum. Neurosci. 6, 292 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kral T. R. A., Schuyler B. S., Mumford J. A., Rosenkranz M. A., Lutz A., Davidson R. J., Impact of short- and long-term mindfulness meditation training on amygdala reactivity to emotional stimuli. Neuroimage 181, 301–313 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiesa A., Calati R., Serretti A., Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clin. Psychol. Rev. 31, 449–464 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Gallant S. N., Mindfulness meditation practice and executive functioning: Breaking down the benefit. Conscious. Cogn. 40, 116–130 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Gard T., Hölzel B. K., Sack A. T., Hempel H., Lazar S. W., Vaitl D., Ott U., Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb. Cortex 22, 2692–2702 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeidan F., Vago D. R., Mindfulness meditation–based pain relief: A mechanistic account. Ann. N. Y. Acad. Sci. 1373, 114–127 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox K. C. R., Nijeboer S., Dixon M. L., Floman J. L., Ellamil M., Rumak S. P., Sedlmeier P., Christoff K., Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci. Biobehav. Rev. 43, 48–73 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Colcombe S. J., Erickson K. I., Scalf P. E., Kim J. S., Prakash R., McAuley E., Elavsky S., Marquez D. X., Hu L., Kramer A. F., Aerobic Exercise training increases brain volume in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 61, 1166–1170 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Rogge A., Röder B., Zech A., Hötting K., Exercise-induced neuroplasticity: Balance training increases cortical thickness in visual and vestibular cortical regions. Neuroimage 179, 471–479 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Ilg R., Wohlschläger A. M., Gaser C., Liebau Y., Dauner R., Wöller A., Zimmer C., Zihl J., Mühlau M., Gray matter increase induced by practice correlates with task-specific activation: A combined functional and morphometric magnetic resonance imaging study. J. Neurosci. 28, 4210–4215 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hölzel B. K., Carmody J., Vangel M., Congleton C., Yerramsetti S. M., Lazar S. W., Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 191, 36–43 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farb N. A. S., Segal Z. V., Anderson A. K., Mindfulness meditation training alters cortical representations of interoceptive attention. Soc. Cogn. Affect. Neurosci. 8, 15–26 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang C.-C., Barrós-Loscertales A., Li M., Pinazo D., Borchardt V., Ávila C., Walter M., Alterations in brain structure and amplitude of low-frequency after 8 weeks of mindfulness meditation training in meditation-naïve subjects. Sci. Rep. 9, 10977 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kriegeskorte N., Simmons W. K., Bellgowan P. S., Baker C. I., Circular analysis in systems neuroscience: The dangers of double dipping. Nat. Neurosci. 12, 535–540 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidson R. J., Kaszniak A. W., Conceptual and methodological issues in research on mindfulness and meditation. Am. Psychol. 70, 581–592 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ioannidis J. P. A., Why most published research findings are false. PLOS Med. 2, e124 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moonesinghe R., Khoury M. J., Janssens A. C. J. W., Most published research findings are false—But a Little replication goes a long way. PLOS Med. 4, e28 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Button K. S., Ioannidis J. P. A., Mokrysz C., Nosek B. A., Flint J., Robinson E. S. J., Munafò M. R., Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Zatorre R. J., Fields R. D., Johansen-Berg H., Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat. Neurosci. 15, 528–536 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dewey J., Hana G., Russell T., Price J., McCaffrey D., Harezlak J., Sem E., Anyanwu J. C., Guttman C. R., Navia B., Cohen R., Tate D. F.; HIV Neuroimaging Consortium , Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. Neuroimage 51, 1334–1344 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarkson M. J., Cardoso M. J., Ridgway G. R., Modat M., Leung K. K., Rohrer J. D., Fox N. C., Ourselin S., A comparison of voxel and surface based cortical thickness estimation methods. Neuroimage 57, 856–865 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Lutz A., Jha A. P., Dunne J. D., Saron C. D., Investigating the phenomenological matrix of mindfulness-related practices from a neurocognitive perspective. Am. Psychol. 70, 632–658 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeley W. W., Menon V., Schatzberg A. F., Keller J., Glover G. H., Kenna H., Reiss A. L., Greicius M. D., Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox K. C. R., Spreng R. N., Ellamil M., Andrews-Hanna J. R., Christoff K., The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage 111, 611–621 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Spreng R. N., Mar R. A., Kim A. S. N., The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. J. Cogn. Neurosci. 21, 489–510 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Jain S., Shapiro S. L., Swanick S., Roesch S. C., Mills P. J., Bell I., Schwartz G. E. R., A randomized controlled trial of mindfulness meditation versus relaxation training: Effects on distress, positive states of mind, rumination, and distraction. Ann. Behav. Med. 33, 11–21 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Creswell J. D., Taren A. A., Lindsay E. K., Greco C. M., Gianaros P. J., Fairgrieve A., Marsland A. L., Brown K. W., Way B. M., Rosen R. K., Ferris J. L., Alterations in resting-state functional connectivity link mindfulness meditation with reduced interleukin-6: A randomized controlled trial. Biol. Psychiatry 80, 53–61 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Fox K. C. R., Dixon M. L., Nijeboer S., Girn M., Floman J. L., Lifshitz M., Ellamil M., Sedlmeier P., Christoff K., Functional neuroanatomy of meditation: A review and meta-analysis of 78 functional neuroimaging investigations. Neurosci. Biobehav. Rev. 65, 208–228 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Kral T. R. A., Imhoff-Smith T., Dean D. C., Grupe D., Adluru N., Patsenko E., Mumford J. A., Goldman R., Rosenkranz M. A., Davidson R. J., Mindfulness-based stress reduction-related changes in posterior cingulate resting brain connectivity. Soc. Cogn. Affect. Neurosci. 14, 777–787 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hölzel B. K., Carmody J., Evans K. C., Hoge E. A., Dusek J. A., Morgan L., Pitman R. K., Lazar S. W., Stress reduction correlates with structural changes in the amygdala. Soc. Cogn. Affect. Neurosci. 5, 11–17 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korponay C., Dentico D., Kral T. R. A., Ly M., Kruis A., Davis K., Goldman R., Lutz A., Davidson R. J., The effect of mindfulness meditation on impulsivity and its neurobiological correlates in healthy adults. Sci. Rep. 9, 11963 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorgolewski K. J., Varoquaux G., Rivera G., Schwarz Y., Ghosh S. S., Maumet C., Soschat V. V., Nichols T. E., Poldrack R. A., Poline J., Yarkoni T., Margulies D. S., NeuroVault.org: A web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Front. Neuroinform. 9, 8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldberg S. B., Wielgosz J., Dahl C., Schuyler B., MacCoon D. S., Rosenkranz M., Lutz A., Sebranek C. A., Davidson R. J., Does the five facet mindfulness questionnaire measure what we think it does? Construct validity evidence from an active controlled randomized clinical trial. Psychol. Assess. 8, 1009–1014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenkranz M. A., Dunne J. D., Davidson R. J., The next generation of mindfulness-based intervention research: What have we learned and where are we headed? Curr. Opin. Psychol. 28, 179–183 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valk S. L., Bernhardt B. C., Trautwein F.-M., Böckler A., Kanske P., Guizard N., Collins D. L., Singer T., Structural plasticity of the social brain: Differential change after socio-affective and cognitive mental training. Sci. Adv. 3, e1700489 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gray J. P., Müller V. I., Eickhoff S. B., Fox P. T., Multimodal abnormalities of brain structure and function in major depressive disorder: A meta-analysis of neuroimaging studies. Am. J. Psychiatry 177, 422–434 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cassiers L. L. M., Sabbe B. G. C., Schmaal L., Veltman D. J., Penninx B. W. J. H., Van Den Eede F., Structural and functional brain abnormalities associated with exposure to different childhood trauma subtypes: A systematic review of neuroimaging findings. Front. Psychiatry 9, 329 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coronado-Montoya S., Levis A. W., Kwakkenbos L., Steele R. J., Turner E. H., Thombs B. D., Reporting of positive results in randomized controlled trials of mindfulness-based mental health interventions. PLOS ONE 11, e0153220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.A. Hollingshead, Four Factor Index of Social Status (Yale University, 1975). [Google Scholar]
- 46.MacCoon D. G., Imel Z. E., Rosenkranz M. A., Sheftel J. G., Weng H. Y., Sullivan J. C., Bonus K. A., Stoney C. M., Salomons T. V., Davidson R. J., Lutz A., The validation of an active control intervention for Mindfulness Based Stress Reduction (MBSR). Behav. Res. Ther. 50, 3–12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baer R. A., Smith G. T., Lykins E., Button D., Krietemeyer J., Sauer S., Walsh E., Duggan D., Williams J. M. G., Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment 15, 329–342 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Fischl B., Dale A. M., Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. 97, 11050–11055 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reuter M., Schmansky N. J., Rosas H. D., Fischl B., Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61, 1402–1418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desikan R. S., Ségonne F., Fischl B., Quinn B. T., Dickerson B. C., Blacker D., Buckner R. L., Dale A. M., Maguire R. P., Hyman B. T., Albert M. S., Killiany R. J., An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Hagler D. J. Jr., Saygin A. P., Sereno M. I., Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage 33, 1093–1103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maldjian J. A., Laurienti P. J., Kraft R. A., Burdette J. H., An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239 (2003). [DOI] [PubMed] [Google Scholar]
- 53.Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M., Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 (2002). [DOI] [PubMed] [Google Scholar]
- 54.R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2013).
- 55.J. Curtin, lmSupport: Support for Linear Models (R package version 2.9.13, 2015).
- 56.White I. R., Horton N. J., Carpenter J., Pocock S. J., Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 342, d40 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Buuren S., Groothuis-Oudshoorn K., MICE: Multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2011). [Google Scholar]
- 58.D. B. Rubin, Multiple Imputation for Nonresponse in Surveys (John Wiley & Sons, 2004). [Google Scholar]
- 59.Ashburner J., Friston K. J., Voxel-based morphometry—The methods. Neuroimage 11, 805–821 (2000). [DOI] [PubMed] [Google Scholar]
- 60.Ashburner J., Ridgway G. R., Symmetric diffeomorphic modeling of longitudinal structural MRI. Front. Neurosci. 6, 197 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaser C., Dahnke R., CAT - A computational anatomy toolbox for the analysis of structural MRI data. HBM 2016, 336–348 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 and S2
Tables S1 to S9
References