Abstract
Background
Canine transmissible venereal tumour (CTVT) is a naturally occurring neoplasia affecting dogs worldwide. Previous CTVT studies in Grenada were limited to case records of dogs with neoplastic conditions at a veterinary diagnostic laboratory.
Objectives
The present retrospective study aimed to determine the occurrence and risk factors of CTVT in a wider population of owned dogs presented to a university‐affiliated veterinary hospital between 2008 and 2018.
Methods
Data on the age, breed, gender, and gonadectomy status were retrieved from an electronic database and analyzed using logistic regression.
Results
Of the 7180 dogs presented during the period, 102 dogs (1.4%) were diagnosed with CTVT. A higher predisposition was observed in Grenadian pothounds (odds ratio [OR] = 22.8, 95% confidence interval [CI] 10.3–50.4; p < 0.001) and mixed‐breed dogs (OR = 9.2, 95% CI 4.1–20.7; p < 0.001) in comparison to the purebreds. Neutered dogs (OR = 2.2, 95% CI 1.4–3.3; p < 0.001) were at an increased risk of CTVT than intact dogs. Age and gender were not identified as significant risk factors.
Conclusions
The percentage of dogs with CTVT in this study represents a crude estimate of the CTVT prevalence in the owned dog population in Grenada. Further studies including both owned and free‐roaming dogs are required for a more accurate estimation of the CTVT prevalence in the region. Our results indicate that breed and gonadectomy status are significant risk factors for the occurrence of CTVT in Grenada.
Keywords: breed, canine, Grenada, neuter, transmissible venereal tumour
Canine transmissible venereal tumor (CTVT) is a naturally occurring neoplasia affecting female and male dogs worldwide. This study aimed to determine the occurrence and risk factors of CTVT in Grenada. Our results indicate that breed and gonadectomy status are significant risk factors for the occurrence of CTVT in Grenada.
1. INTRODUCTION
Canine transmissible venereal tumour (CTVT) is an understudied naturally occurring neoplasia affecting dogs worldwide. Previous studies have reported CTVT as enzootic throughout 90 countries, with the highest prevalence in South America, Central America, and some parts of Asia and Africa (Ganguly et al., 2016; Strakova & Murchison, 2014). With no evidence of a specific gender predisposition, CTVT appears to predominate in communities with free‐roaming and community‐owned dogs due to less strict leash laws, unrestricted sexual activity, and lack of owner awareness (Batamuzi et al., 1992; Strakova & Murchison, 2014). Transmission occurs through coitus with a CTVT‐affected dog or through licking, biting, sniffing, or scratching the tumor‐affected areas (Ganguly et al., 2016). Although commonly described as benign tumours, metastatic CTVT has also been reported in some cases (Ferreira et al., 2000; Oduye et al., 1973; Park et al., 2006; Rogers et al., 1998; Yang, 1987). Grossly, these round cell tumours appear cauliflower‐like and are more commonly seen on the male prepuce and the female vestibule and vagina (Ganguly et al., 2016).
Limited information exists on the occurrence and risk factors of CTVT in Grenada. In a retrospective study of 462 cases of tumours in dogs, CTVT accounted for 7.6% of the tumour cases (Bhaiyat et al., 2013). Distribution of the CTVT cases showed that 54% were male and 46% were female. In another retrospective study involving 420 tumour cases, CTVT comprised 18% of the tumour cases with almost equal representation of both genders (51% male and 49% female; Chikweto et al., 2013). Affected dogs in the two studies ranged in age between 1 year and 12 years or older. A case–control study involving 38 CTVT cases and 114 controls identified breed and age as significant risk factors for the occurrence of CTVT in Grenada (Kabuusu et al., 2010). Grenadian pothounds were more likely to be affected compared to other breeds and dogs with age range categories 1–7 years and >7 years were more likely to be affected than dogs <1 year. These studies were based on a narrow population of neoplasia cases presented to a veterinary diagnostic laboratory, which makes them less representative of the canine population in Grenada. Therefore, the present study was conducted to determine the occurrence and risk factors of CTVT in a wider population of owned dogs presented to a veterinary hospital over a period of 10 years.
2. MATERIALS AND METHODS
2.1. Study design and data collection
Using a retrospective observational case series study design, clinical case records of dogs presented to a university‐affiliated veterinary hospital in Grenada, West Indies, between 2008 and 2018 were reviewed. The dogs were classified into CTVT and non‐CTVT groups based on the diagnosis listed in their clinical records. The presumptive diagnosis of CTVT in the dogs was based on history, clinical signs, and visual inspection of the characteristic lesions associated with the disease. The diagnoses were confirmed by a clinical pathologist after cytological examination of impression smears or fine‐needle aspirates. The CTVT group included dogs with a confirmed diagnosis of the disease. Data on age, breed, gender, and gonadectomy status of the dogs were retrieved from the veterinary practice management software used at the clinic (AVImark®, Coventrus Software Solutions, Oshkosh, WI, USA). Data retrieval was conducted using the information search option and specific keywords (age, breed, and sex name) available within the software for the variables of interest. The search was limited to dates between 1 January 2008 and 31 December 2018 to exclude patients that presented outside the study period. Identifying information about the dogs and their owners was coded to maintain confidentiality throughout the study.
2.2. Statistical analysis
Data were analyzed using SPSS Version 16.0 (SPSS Inc., Chicago, IL, USA). Age was analyzed as a numerical variable, whereas breed (purebred, Grenadian pothound and mixed‐breed), gender (male and female), and gonadectomy status (intact and neutered) were analyzed as categorical variables. Descriptive statistics were calculated to evaluate the distribution of CTVT cases by age, breed, gender, and gonadectomy status. Univariate analysis was initially conducted to examine the potential association between the individual predictor variables and the occurrence of CTVT. Dogs with missing information on an individual predictor variable were excluded from the univariate analysis for that variable. Variables with p < 0.20 were entered into a multivariate logistic regression model to predict the occurrence of CTVT. Hosmer–Lemeshow test was used to evaluate the goodness of fit of the regression model. Dogs with missing information on any of the variables were excluded from the analysis. Results are presented as odds ratios (ORs) and 95% confidence interval (95% CIs). p‐Values less than 0.05 were considered significant.
3. RESULTS
A total of 7180 dogs were presented to the clinic during the period. Of the 7180 dogs presented during the period, 102 dogs (1.4%) were diagnosed with CTVT. The descriptive information of the dogs that were diagnosed with CTVT is presented in Table S1. The median age of dogs affected with CTVT was 7 years (range 2–18 years; interquartile range = 4). Distribution of the CTVT cases by breed showed a predominant representation of Grenadian pothounds and mixed‐breed dogs (Table 1). Both genders were represented almost equally (Table 1). Similarly, the distribution of the CTVT cases by gonadectomy status was almost equal (Table 1).
TABLE 1.
Descriptive statistics for age, breed, gender, and gonadectomy status of the dogs presented to the hospital between 2008 and 2018
| Parameter | CTVT | Non‐CTVT |
|---|---|---|
| Age (years) | Median = 7, Q1 = 5, Q3 = 9 (n = 102) | Median = 8, Q1 = 5, Q3 = 11 (n = 7074) |
| Breed | ||
| Purebred | 7 (7%) | 3562 (50%) |
| Grenadian pothound | 54 (53%) | 1064 (15%) |
| Mixed‐breed | 37 (36%) | 2092 (30%) |
| Unspecified | 4 (4%) | 360 (5%) |
| Gender | ||
| Male | 49 (48%) | 3400 (48%) |
| Female | 53 (52%) | 3530 (50%) |
| Unspecified | 0 (0%) | 148 (2%) |
| Gonadectomy status | ||
| Intact | 50 (49%) | 4835 (68%) |
| Neutered | 52 (51%) | 2094 (30%) |
| Unspecified | 0 (0%) | 149 (2%) |
Note: Values listed represent the number and percentage of dogs unless otherwise stated.
Abbreviations: CTVT, canine transmissible venereal tumour; Q1, first quartile; Q3, third quartile.
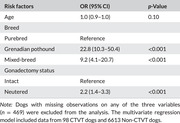
Univariate analysis revealed that age, breed, and gonadectomy status were significantly associated with the occurrence of CTVT (Table 2). A multivariate regression model including the three variables was built to predict the occurrence of CTVT. Hosmer–Lemeshow test indicated a good model fit to the data (p = 0.28). The multivariate analysis indicated that breed and gonadectomy status were significant risk factors for the occurrence of CTVT (Table 3). In comparison to purebred dogs, Grenadian pothounds and mixed‐breed dogs were at a higher risk of CTVT occurrence. Neutered dogs were at a higher risk of CTVT than intact dogs.
TABLE 2.
Univariate odds ratios (ORs) and 95% confidence intervals (95% CIs) of risk factors for canine transmissible venereal tumour (CTVT) occurrence in Grenada, West Indies
| Risk Factors | CTVT | Non‐CTVT | OR (95% CI) | p‐Value |
|---|---|---|---|---|
| Age (Median) | 7.0 | 8.0 | 1.0 (0.9–1.0) | 0.02 |
| Breed | ||||
| Purebred | 7 | 3562 | Reference | |
| Grenadian pothound | 54 | 1064 | 25.8 (11.7–56.9) | <0.001 |
| Mixed‐breed | 37 | 2092 | 9.0 (4.0‐20.2) | <0.001 |
| Gender | ||||
| Male | 49 | 3400 | Reference | |
| Female | 53 | 3530 | 1.0 (0.6–1.4) | 0.84 |
| Gonadectomy status | ||||
| Intact | 50 | 4835 | Reference | |
| Neutered | 52 | 2094 | 2.4 (1.6–3.6) | <0.001 |
Note: Dogs with missing observations on an individual predictor variable were excluded from the univariate analysis for that variable. The total number of missing observations for age, breed, gender, and gonadectomy status were 4, 364, 148, and 149, respectively.
TABLE 3.
Multivariate odds ratios (ORs) and 95% confidence intervals (95% CIs) of risk factors for canine transmissible venereal tumour (CTVT) occurrence in Grenada, West Indies
| Risk factors | OR (95% CI) | p‐Value |
|---|---|---|
| Age | 1.0 (0.9–1.0) | 0.10 |
| Breed | ||
| Purebred | Reference | |
| Grenadian pothound | 22.8 (10.3–50.4) | <0.001 |
| Mixed‐breed | 9.2 (4.1–20.7) | <0.001 |
| Gonadectomy status | ||
| Intact | Reference | |
| Neutered | 2.2 (1.4–3.3) | <0.001 |
Note: Dogs with missing observations on any of the three variables (n = 469) were excluded from the analysis. The multivariate regression model included data from 98 CTVT dogs and 6613 Non‐CTVT dogs.
4. DISCUSSION
The present study represents the first scientific attempt at quantifying the prevalence of CTVT in the owned canine population of Grenada. The percentage of dogs with CTVT observed in this study is much lower than the estimated prevalence (10%–20%) reported by Strakova and Murchison (Strakova & Murchison, 2014). The previously estimated prevalence should be interpreted with caution because it was based on the individual perception of two survey respondents rather than any data. The percentage of dogs with CTVT in the present study represents a crude estimate of CTVT prevalence in the owned dog population in Grenada. The estimated prevalence of CTVT in Grenada is similar to the prevalence reported in Jamaica (0.8%), another island nation in the Caribbean region (Thorburn et al., 1968). Since the present study was conducted in a hospital setting, free‐roaming dogs were not appropriately represented. Therefore, further studies including both owned and free‐roaming dogs are required for a more accurate estimation of the CTVT prevalence in the region.
The higher likelihood of CTVT occurrence in Grenadian pothounds confirms the findings of a previous case–control study that reported a greater predisposition of this breed to CTVT (Kabuusu et al., 2010). Besides, the present study showed that mixed‐breed dogs are at a higher risk of CTVT occurrence than purebred dogs. The greater likelihood of CTVT in Grenadian pothounds and mixed‐breed dogs could be attributed to a potential genetic predisposition of these breeds to CTVT. Ganguly and colleagues (2016) have previously suggested that the molecular interplay of a transposable element within the c‐myc oncogene might represent a genetic predisposition to CTVT (Amariglio et al., 1991; Murgia et al., 2006). Since there is no published information on the dog leukocyte antigen (DLA) haplotypes of Grenadian pothounds, further studies are required to investigate the possibility of a genetic predisposition for CTVT in this breed. A more plausible reason for the greater likelihood in Grenadian pothounds and mixed‐breed dogs could be the difference in the management of these dogs compared to the management of purebred dogs. A case–control study from Tanzania showed that management methods that allow frequent contact with other dogs increase the likelihood of contracting CTVT (Batamuzi et al., 1992). Grenadian pothounds and mixed‐breed dogs are mostly community‐owned and more likely to be left to roam off‐leash in comparison to purebreds that are expensive to acquire and, therefore, more likely to be leashed on the property or kept inside the home. Moreover, these dogs mostly get a basic pet care with relatively limited access to vaccinations and deworming.
The finding that neutered dogs in Grenada are at a higher risk of contracting CTVT than intact dogs does not concur with the reported greater occurrence in intact dogs elsewhere (Setthawongsin et al., 2016; Strakova & Murchison, 2014). Transmission of CTVT can also occur through non‐sexual modes such as licking, biting, and scratching of tumour‐affected areas (Ganguly et al., 2016). Besides, a majority of the dogs in Grenada are neutered after they have attained puberty and it has been demonstrated previously that copulatory behaviours persist after gonadectomy in postpubertal dogs (Beach, 1970; Garde et al., 2016). Due to a general lack of awareness about non‐sexual modes of transmission and persistence of learned behaviours after gonadectomy, owners are more likely to allow their neutered dogs to roam the community as there is no fear of mismating, thereby, increasing their chances of contracting CTVT. Further studies including additional variables such as management and roaming are required to better understand the factors affecting CTVT transmission in Grenada.
The finding that age is not a significant risk factor for the occurrence of CTVT in Grenada agrees with previous studies that reported CTVT occurrence in all age categories >1 year (Bhaiyat et al., 2013; Chikweto et al., 2013). The median age of dogs with CTVT in the present study was higher than the average age reported in some of the previous studies (Chikweto et al., 2011; DEN Otter et al., 2015; Setthawongsin et al., 2019). This may be due to a variable duration from CTVT occurrence to its detection in different settings. Since the majority of dogs with CTVT in this study were either Grenadian pothounds or mixed‐breed dogs, it is possible that the dogs could have had CTVT for a longer period before being noticed by the owners and presented to the clinic.
The absence of gender bias for the occurrence of CTVT has been shown in some of the previous studies (Kabuusu et al., 2010; Strakova & Murchison, 2014). Our findings further confirm the result of these studies and contradict the older notion that females are more likely to be infected than males because an infected male often mates with more than one female (Ganguly et al., 2016; Singh et al., 1996). The bias towards female occurrence might have been because the studies involved data collected on more females than males. It seems more logical that both genders are equally likely to contract CTVT as it can be contracted through both sexual and non‐sexual modes.
A limitation of the present study, owing to its hospital‐based nature, is that stray dogs were not included, which means that the results do not completely represent the whole canine population of Grenada. The study also has other limitations inherent to retrospective studies, including being subject to missing observations and selection or recall bias. Despite these limitations, distinctive advantages associated with our study include a very large sample size that allowed us to address a disease of low prevalence. Moreover, the canine population included in the present study was much wider than the subsets of dogs with neoplasia investigated in the previous studies on CTVT in the region.
5. CONCLUSIONS
In summary, this study provided a hospital‐based estimate of the CTVT prevalence in Grenada. A breed predisposition was observed indicating that Grenadian pothounds and mixed‐breed dogs are at a greater risk of contracting CTVT in the region. Interestingly, neutered dogs were more predisposed to CTVT in comparison to intact dogs. These findings suggest a need to educate dog owners about the persistence of learned mating behaviours in dogs neutered postpubertally and the transmission of CTVT through both sexual and non‐sexual modes. There is also a need to evaluate and reform the lack of leash laws and normalization of community‐owned dogs to reduce the occurrence of CTVT in the region.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
Data curation, Investigation, Visualization, Writing original draft: Sara Schectman. Conceptualization, Data curation, Investigation, Methodology, Writing review & editing: Afroza Khanam. Resources, Writing review & editing: Mellisa Walters. Data curation, Writing review & editing: Elliot Kirwan. Resources, Writing review & editing: Wayne Sylvester. Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Visualization, Writing review & editing: Firdous Khan.
ETHICS STATEMENT
Since the study did not involve any live animals but was rather based on archived clinical case records, ethical review and approval were not required.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
The first author is thankful to the Boehringer Ingelheim Veterinary Scholars Program for the scholarship she received during the present study.
Schectman, S. J. , Khanam, A. , Walters, M. N. D. , Kirwan, E. , Sylvester, W. R. , & Khan, F. A. (2022). A retrospective study of canine transmissible venereal tumour in Grenada, West Indies. Veterinary Medicine and Science, 8, 1008–1012. 10.1002/vms3.778
Authors Sara J. Schectman and Afroza Khanam contributed equally to this work.
Funding information
None.
DATA AVAILABILITY STATEMENT
The datasets generated for this study are available on request to the corresponding author.
REFERENCES
- Amariglio, E. N. , Hakim, I. , Brok‐Simoni, F. , Grossman, Z. , Katzir, N. , Harmelin, A. , Ramot, B. , & Rechavi, G. (1991).Identity of rearranged LINE/c‐MYC junction sequences specific for the canine transmissible venereal tumor. PNAS, 88(18), 8136–8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batamuzi, E. K. , Kassuku, A. A. , & Agger, J. F. (1992). Risk factors associated with canine transmissible venereal tumour in Tanzania. Preventive Veterinary Medicine, 13(1), 13–17. [Google Scholar]
- Beach, F. A. (1970).Coital behavior in dogs. VI. Long‐term effects of castration upon mating in the male. Journal of Comparative and Physiological Psychology, 70(3), 1–32. [PubMed] [Google Scholar]
- Bhaiyat, M. I. , Chikweto, A. , Tiwari, K. P. , DeAllie, C. , Pawaiya, R. S. , Inga, A. , Hegamin‐Younger, C. , & Sharma, R. N. (2013). A retrospective study of canine tumors in Grenada, West Indies. Advances in Animal and Veterinary Sciences, 1(5), 134–139. [Google Scholar]
- Chikweto, A. , Kumthekar, S. , Larkin, H. , Deallie, C. , Tiwari, K. P. , Sharma, R. N. , & Bhaiyat, M. I. (2013).Genital and extragenital canine transmissible venereal tumor in dogs in Grenada, West Indies. Open J Vet Med, 3, 111–114. [Google Scholar]
- Chikweto, A. , McNeil, P. , Bhaiyat, M. I. , Stone, D. , & Sharma, R. N. (2011).Neoplastic and nonneoplastic cutaneous tumors of dogs in Grenada, West Indies. ISRN Vet Sci, 2011, 416435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEN Otter, W. , Hack, M. , Jacobs, J. J. , Tan, J. F. , Rozendaal, L. , & VAN Moorselaar, R. J. (2015).Effective treatment of transmissible venereal tumors in dogs with vincristine and IL2. Anticancer Research, 35(6), 3385–3391. [PubMed] [Google Scholar]
- Ferreira, A. J. A. , Jaggy, A. , Varejäo, A. P. , Ferreira, M. L. P. , Correia, J. M. J. , Mullas, J. M. , Almeida, O. , Oliveira, P. , & Prada, J. (2000).Brain and ocular metastases from a transmissible venereal tumour in a dog. Journal of Small Animal Practice, 41(4), 165–168. [DOI] [PubMed] [Google Scholar]
- Ganguly, B. , Das, U. , & Das, A. K. (2016).Canine transmissible venereal tumour: A review. Veterinary and comparative oncology, 14(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Garde, E. , Pérez, G. E. , Vanderstichel, R. , Dalla Villa, P. F. , & Serpell, J. A. (2016).Effects of surgical and chemical sterilization on the behavior of free‐roaming male dogs in Puerto Natales, Chile. Preventive Veterinary Medicine, 123, 106–120. [DOI] [PubMed] [Google Scholar]
- Kabuusu, R. M. , Stroup, D. F. , & Fernandez, C. (2010).Risk factors and characteristics of canine transmissible venereal tumours in Grenada, West Indies. Vet Comp Oncol, 8(1), 50–55. [DOI] [PubMed] [Google Scholar]
- Murgia, C. , Pritchard, J. K. , Kim, Su Y. , Fassati, A. , & Weiss, R. A. (2006).Clonal origin and evolution of a transmissible cancer. Cell, 126(3), 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oduye, O. O. , Ikede, B. O. , Esuruoso, G. O. , & Akpokodje, J. U. (1973). Metastatic transmissible venereal tumour in dogs. Journal of Small Animal Practice, 14(10), 625–649. [DOI] [PubMed] [Google Scholar]
- Park, Mi‐S. , Kim, Y. , Kang, M.‐S. , Oh, S.‐Y. , Cho, D.‐Y. , Shin, N.‐S. , & Kim, D.‐Y. (2006).Disseminated transmissible venereal tumor in a dog. Journal of Veterinary Diagnostic Investigation, 18(1), 130–133. [DOI] [PubMed] [Google Scholar]
- Rogers, Ks , Walker, Ma , & Dillon, Hb (1998).Transmissible venereal tumor: A retrospective study of 29 cases. Journal of the American Animal Hospital Association, 34(6), 463–470. [DOI] [PubMed] [Google Scholar]
- Setthawongsin, C. , Techangamsuwan, S. , Tangkawattana, S. , & Rungsipipat, A. (2016).Cell‐based polymerase chain reaction for canine transmissible venereal tumor (CTVT) diagnosis. Journal of Veterinary Medical Science, 78(7), 1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setthawongsin, C. , Teewasutrakul, P. , Tangkawattana, S. , Techangamsuwan, S. , & Rungsipipat, A. (2019).Conventional‐vincristine sulfate vs. modified protocol of vincristine sulfate and L‐asparaginase in canine transmissible venereal tumor. Frontiers in veterinary science, 6, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, J. , Rana, J. S. , Sood, N. , Pangawkar, G. R. , & Gupta, P. P. (1996).Clinico‐pathological studies on the effect of different anti‐neoplastic chemotherapy regimens on transmissible venereal tumours in dogs. Veterinary Research Communications, 20(1), 71–81. [DOI] [PubMed] [Google Scholar]
- Strakova, A. , & Murchison, E. P. (2014). The changing global distribution and prevalence of canine transmissible venereal tumour. BMC Vet Res., 10, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn, M. J. , Gwynn, R. V. R. , Ragbeer, M. S. , & Lee, B. I. (1968).Pathological and cytogenetic observations on the naturally occurring canine venereal tumour in Jamaica (Sticker's tumour). British Journal of Cancer, 22(4), 720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, T. J. (1987).Metastatic transmissible venereal sarcoma in a dog. Journal of the American Veterinary Medical Association, 190(5), 555–556. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.


