Abstract
Caffeine and orexin can affect awakening, neuroendocrine, and sympathetic nerve function. Our previous study has reported that caffeine intake can increase human body temperature. However, little is known about the combined effects of thermotherapy and caffeine intake on human serum orexin concentrations. Forty-two healthy male subjects with age of 26.72 ± 5.05 years, height of 174.10 ± 7.09 cm, and body weight of 74.68 ± 8.91 kg participated in this study. They were randomly assigned to a control (CON) group (n = 21) and a caffeine (CAFF) group (n = 21). After thermotherapy (half-body immersion in a hot water bath at 42 ± 0.5 °C, circulating orexin level increased more (p < 0.05) in the CAFF group than in the CON group. Positive relationships between mean body temperature and orexin were observed before and after heat stimulation (p < 0.001). Caffeine intake boosted the upregulation of serum orexin concentrations in subjects undergoing thermotherapy.
Keywords: Caffeine, Orexin, Thermotherapy, Neuroendocrine, Sympathetic nervous system
Introduction
Caffeine is one of the most widely consumed psychoactive substances in the world regardless of gender. It is consumed most in the form of coffee (van Dam et al., 2020). Well-known action of caffeine is to reduce somnolence by reversibly blocking the action of adenosine on its receptors. As a nervous system stimulant, caffeine can affect basic physiological processes such as cognition and memory (Nehlig et al., 1992). However, it can negatively affect sleep quality (Snel and Lorist, 2011).
Caffeine can act as a stimulator of sympathetic activity, resulting in short-term increases in blood pressure and heart rate (Zimmermann-Viehoff et al., 2016). It can also increase sudomotor activity and sensitivity (Kim et al., 2011). The potential for detrimental effects of caffeine via the sympathetic nervous system such as changes in glucose metabolism and increased blood pressure has been suggested (Gonzalez de Mejia and Ramirez-Mares, 2014). However, it has also been reported that there is no increase in cardiovascular risk when an appropriate amount of caffeine up to 600 mg per day is ingested (Turnbull et al., 2017).
Orexin is an excitatory neuropeptide hormone first identified in 1998 (Sakurai et al., 1998). It has been confirmed that orexin is involved in sleep-arousal and feeding as well as autonomic function and neurohormonal regulation (Date et al., 1999; Girault et al., 2012; Shirasaka et al., 1999). According to a previous study, the autonomic nervous system-related fight-or-flight response such as respiration and increased blood pressure is reduced in orexin-deficient mice, suggesting that the orexin system is an essential regulator of autonomic function and emotional control (Kuwaki, 2011).
It is known that caffeine and orexin have a similar function in sleep regulation and sympathetic activation (Scheme 1). In addition, orexins play an important role in thermoregulation and energy metabolism by activating brown adipose tissue (BAT) (Liu et al., 2019). Interestingly, caffeine also stimulates thermogenesis of BAT, suggesting that it might be involved in the central nervous system and metabolic pathways related to thermogenesis and regulation (Velickovic et al., 2019). Greene et al. (2016) have reported that orexin expression is related to stress in an experiment with avian animals and explained the molecular mechanism of orexin response by heat and oxidative stress. Parsons et al. (2012) have reported that an increase in body temperature can induce the inhibition of orexin neurons through an experiment using rat brains. However, the effect of orexin on heat and oxidative stress in human body is not yet known.
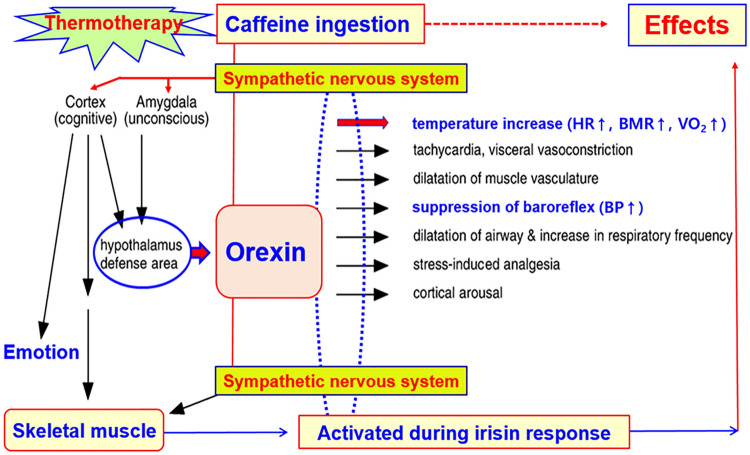
Scheme 1.
Orexin as a master switch of orchestrated sympathetic response and thermoregulation. HR heart rate; BMR basal metabolic rate; VO2 oxygen consumption; BP blood pressure. Via the sympathetic nervous system, caffeine ingestion and thermotherapy can activate hypothalamus defense area to release orexin and skeletal muscle to initiate irisin response. Orexin is thought to be involved in the sympathetic pathway
Our recent studies have focused on short- and long-term effects of thermal stimulation on body temperature regulation in an attempt to investigate the complicated relationship between orexin and caffeine in terms of sympathetic activation and oxidative stress induced by thermal stress (Lee and Kim, 2014; Park et al., 2021). The objective of the present study was to determine the combined effect of caffeine intake and hyperthermia on blood orexin concentration in the human body compared to heat stress alone.
Materials and methods
Subjects
Forty-two healthy Korean male subjects participated in this study. All subjects lived in Cheonan, Republic of Korea for at least 3 months before and during the experimental period (January 2020–March 2020). Cheonan extends from southwestern Korea (126° 52′ N, 33.38′ E) to northeast (130° 4′ N, 43.0′ E). The mean annual ambient temperature during October 2019–March 2020 was 5.3 °C with a relative humidity of 73.0%.
Table 1 shows general physical characteristics of our subjects. Inclusion criteria were no previous use of caffeine, and no history of smoking. Exclusion criteria included hypertension, cardiovascular disease, and history of any kind of syncope. Subjects were instructed to abstain from alcohol for at least 48 h and fast for 6 h before the test.
Table 1.
Physical characteristics of subjects
| Groups | Age (year) | Height (cm) | Weight (kg) | BSA (m2) | % fat | BMI (kg/m2) |
|---|---|---|---|---|---|---|
| CON | 26.38 ± 4.92 | 173.94 ± 6.24 | 74.21 ± 9.13 | 1.89 ± 0.17 | 23.28 ± 3.17 | 24.53 ± 2.64 |
| CAFF | 27.06 ± 5.18 | 174.26 ± 7.95 | 75.14 ± 8.68 | 1.90 ± 0.18 | 23.75 ± 3.42 | 24.74 ± 2.57 |
Forty-two male volunteers participated in this study. BSA was calculated using the Du Bois formula (Du and Du, 1916). Body fat percentage was measured using the bio-impedance method (InBody 520, Seoul, Korea). There was no statistical difference in physical characteristics of subjects between the CON and CAFF groups
CON control subjects (n = 21, placebo and 200 mL water); CAFF caffeine ingestion subjects (n = 21, 3 mg/kg body weight caffeine and 200 mL water); BSA body surface area; BMI body mass index
After being thoroughly explained the purpose of the study, procedures of the experiment, and potential risks, each subject submitted a written informed consent to voluntarily participate in this study. All procedures involving human participants complied with the 2013 Helsinki Declaration of the World Medical Association. They were approved by the Institutional Review Board on Human Subjects Research and Ethics Committees, Soonchunhyang University, Cheonan, Korea (Approval No. 2016R1D1A3B02015394).
Measurement and experimental procedure
Experiments were conducted in a climate chamber with the following conditions: temperature, 25.0 ± 0.5 °C; relative humidity, 60.0 ± 3.0%; air velocity, 1 m/s (Scheme 2). The experiment was conducted between 2:00 and 5:00 PM to reduce the effect of circadian rhythm on body temperature (Lee et al., 2015). To prevent dehydration during heat loading, all subjects were asked to drink 5–7 mL/kg body weight water 4 h before the test.
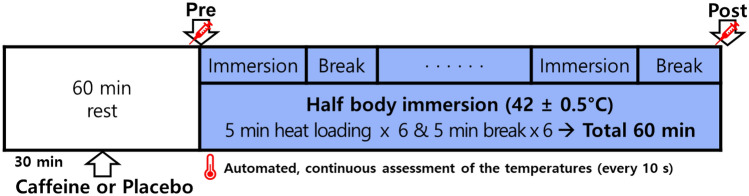
Scheme 2.
Protocol of experiments. Subjects in the control group (placebo, 200 mL water) and the caffeine group (3 mg/kg body weight caffeine and 200 mL water) drank each solution at 30 min before heat loading (HL). HL was carried out by immersing half body into a hot water bath (42 ± 0.5 °C) for a total of 30 min with breaks for a total of 30 min. Automatic tympanic and local skin temperature measurements were conducted continuously at 10-s intervals for 60 min and then used to calculate mean body temperature. Pre, blood sampling at start of half body immersion; Post, blood sampling immediately after a total 60 min of heat loading procedure
All forty-two subjects were randomly assigned to two groups: an experimental group (n = 21) and a control group (n = 21). Thirty minutes before the application of hyperthermia, subjects in the control group drank 200 mL of placebo water (CON) while those in the experimental group drank water containing 3 mg/kg body weight caffeine (CAFF). This is a dose showing adequate ergogenic effect but not reaching the recommended maximum daily intake (400 mg/day of caffeine) by the European Food Safety Authority (2015), Korean Ministry of Food and Drug Safety (2020), US Dietary Guidelines Advisory Committee (2015). Caffeine at 3 mg/kg body weight is roughly equivalent to two cups of coffee, a dose frequently applied in our previous studies (Kim and Lee, 2013; Kim et al., 2011; Lee et al., 2019). Caffeine used in this study was 99.9% pure caffeine powder produced by Scientific Fitness (Oakmont, PA, USA).
Thermal stimulation applied in this study was achieved by performing a half-body immersion to the waist in a hot tub maintained at 42 ± 0.5 °C, similar to the setting used in our previous experiments (Lee and Kim 2018; Park et al., 2021). When the subject arrived, urine specific gravity was obtained with a urine dipstick (Uriscan, Seoul, Korea) to assess hydration status. Subjects were guided to rest in chairs 60 min before the heat loading. They were instructed to drink the assigned solution or placebo 30 min before the procedure. Heat loading was performed for a total of 30 min and a 5-min break was given every 5 min, making the total experimental time 60 min (Scheme 2).
Mean body temperature (mTb) measurements
The parameter mTb was calculated using the formula of Sugenoya and Ogawa (1985) with tympanic temperature (Tty) and mean skin temperature (mTs): mTb = (0.9⋅Tty + 0.1⋅mTs). The Ramanathan equation was used to calculate mTs (Ramanathan, 1964). Tympanic temperature, skin temperatures of the chest, upper arm, thigh, and leg were assessed continuously at 10-s intervals for 60 min in the left ear by inserting a thermistor probe (TSK7+1, Songkitopia, Incheon, Republic of Korea) with a small spring (K923, Takara, Yokohama, Japan) connected to a CF-T1 personal computer (Panasonic, Tokyo, Japan) and a K-720 data logger (Technol Seven, Yokohama, Japan). When the thermistor probe contacted the tympanic membrane, the subject felt slight discomfort and could hear a scratching noise. The inner pinna was filled with small cotton balls to fix the probe in the ear (Lee et al., 2019).
Blood analysis
Blood sampling was performed from antecubital veins. Samples were collected right before and after the heat loading (Scheme 2) and transferred to a serum-separation tube. Samples were then immediately centrifuged at 1000 RCF for 10 min at 4 °C (Comb-514R, Hani science medical, Daejeon, Republic of Korea). The serum was subsequently removed and stored in 1 mL aliquots at − 80 °C until analyses using commercially available enzyme-linked immunosorbent assay kits (Orexin A EIA Kit, Phoenix Pharmaceuticals, Burlingame, CA, USA). Values below this limit were assumed to be zero for statistical analysis. Inter- and intra-assay coefficients of variance were < 10%.
Statistical analysis
Values are expressed as mean ± standard deviation (SD) using a commercially available computer software SPSS for Windows, version 21.0 (SPSS Inc., Chicago, IL, USA). Normality of data were verified via Shapiro–Wilk normality test followed by calculating skewness and kurtosis of the statistics. Statistical significance was determined using a paired Student’s t-test to conduct a comparison between before (Pre-HL, pre-heat loading) and after (post-heat loading, Post-HL) heat loading. Linear regression analysis and Pearson correlation coefficient were used to assess correlations between variables. Significant differences were considered at p < 0.05.
Results and discussion
Thermoregulation extends from central to peripheral along the hypothalamic–pituitary–adrenal axis. Autonomic nerves are involved in the process. In the process, orexins can promote thermogenic neurotransmission via periodic release and increase body temperature (Madden et al., 2012). Thermogenesis of BAT contributes to optimizing metabolic function. Excitatory neurotransmitters driving thermogenesis mechanisms through BAT play a central role in maintaining energy homeostasis.
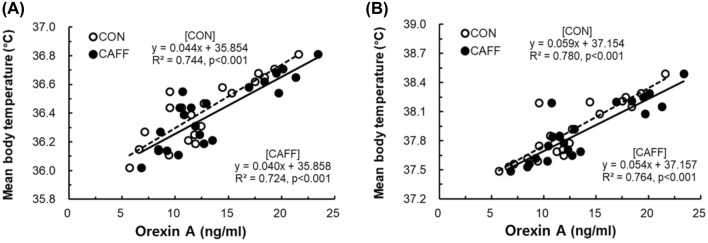
After heat loading, orexin levels showed significant increases in both the CAFF and CON group. Compared with the CON group, circulating orexin concentrations were significantly higher (p < 0.05) in the CAFF group both before and after heat loading (Fig. 1). From Pre-HL to Post-HL, the rate of increase of orexin level was 40.33% in the CAFF group and 30.77% in the CON group. Correlations between mTb and orexin before and after heat loading are shown in Fig. 2. Positive relationships between mTb and orexin before (CON, R2 = 0.744, p < 0.001; CAFF, R2 = 0.724, p < 0.001) and after (CON, R2 = 0.780, p < 0.001; CAFF, R2 = 0.764, p < 0.001) hot water immersion were observed.
Fig. 1.
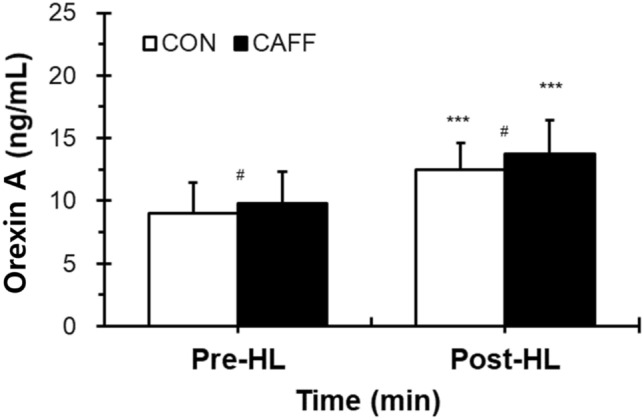
Changes in circulating orexin concentrations before and after heat loading. Values are presented as mean + standard deviation. CON control subjects (open bar, n = 21, placebo and 200 mL); CAFF caffeine ingestion subjects (closed bar, n = 21, 3 mg/kg body weight caffeine and 200 mL water). Pre-HL, before HL; Post-HL, immediately after HL procedure for a total 60 min. HL was carried out by half body immersion into a hot water bath (42 ± 0.5 °C) for a total of 30 min and breaks for a total of 30 min. Significance of difference between values is shown (***p < 0.001, Pre-HL versus Post-HL; #p < 0.05, between groups)
Fig. 2.
Correlation between mTb and orexin before and after heat loading. CON control subjects (n = 21, placebo and 200 mL); CAFF caffeine ingestion subjects (n = 21, 3 mg/kg body weight caffeine and 200 mL water); HL heat loading. (A) Pre-HL, results from samples collected immediately before HL; (B) Post-HL, results from samples collected immediately after HL procedure for a total 60 min
Looking at previous studies, no research has been conducted so far on the mechanism of increasing blood orexin by caffeine along with thermal therapy in humans. However, as previous animal experiments have shown, caffeine activates orexin neurons in rat brain tissue (Murphy et al., 2003), and the activity of orexin by such caffeine is sufficient to activate BAT by engaging in exothermic stimulation in the control of BAT (Van Schaik et al., 2021). This study was conducted based on the hypothesis and mechanism, and as a result, it was found that it was consistent with the existing research results.
Results of this study were consistent with previous findings showing that caffeine intake is associated with the fight-or-flight response of the autonomic nervous system in blood orexin levels (Johnson et al., 2012). They were also compatible with results of a previous study showing that caffeine intake before a heat loading (e.g., hyperthermia) had more metabolic efficiency than heat loading alone, resulting in higher levels of leptin and free fatty acids (Kim and Lee, 2013). Previous animal studies reported that orexin is downregulated by heat stimulation in both central and peripheral regions, which is in the opposite direction to our results (Nguyen et al., 2017; Parsons et al., 2012). However, considering that orexin is involved in complicated neurophysiological pathways including the sympathetic nervous system, it is best explained by in vivo experiment. In addition, previous in vivo experiment was conducted with a temperature lower than the mTb of the target animal. Therefore, in vivo experiments with hyperthermia stimulation have not been reported before, making our results in the human body are of great significance. However, the mechanism behind this result is still an area that needs to be further explored.
Orexin can promote arousal and energy expenditure. It can increase food intake (Tsujino and Sakurai, 2013). Orexin-A and orexin-B are formed from a single prepro-orexin precursor, with the former playing a more important role in regulating energy release than in food intake (Liu et al., 2019; Sakurai et al., 1998). In a mouse experiment, Tsuneki et al. (2010) have found that hyperglycemia can aggravate peripheral insulin resistance by inducing a decline in orexin expression in the hypothalamus. This mechanism is related to oxidative stress. One study has shown that orexin-A has protective effect on human endothelial cells against high-glucose induced oxidative stress and inflammation (Zhang et al., 2019). However, some animal experiments have been reported that sleep disorders such as insomnia can be induced by orexin excess (Prober et al., 2006; Willie et al., 2011).
The recent COVID-19 pandemic has resulted in a deterioration of human immunity. Caffeine can activate the immunity against various diseases (Romero-Martínez et al., 2021). It can be an effective anti-inflammatory and immunomodulatory agent to improve exercise performance, reduce fatigue, and increase awareness in people who have adapted to an indoor lifestyle (Lee et al., 2019). In our study, caffeine intake increased body temperature and serum orexin levels in subjects undergoing thermotherapy. Our results suggest that caffeine intake could be used as a novel form of therapy with potential to treat neurological and cardiovascular diseases. It might have versatile applications in a variety of health fields.
Acknowledgements
The authors extend their gratitude to subjects whose participation made this research possible. This work was supported by Soonchunhyang University Research Fund. This study was also supported by a Grant (No. 2016R1D1A3B02015394) of the Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Education, Republic of Korea.
Declarations
Conflict of interest
The authors declare that this study has been conducted without commercial or financial relations that can be interpreted as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tae-Hwan Park, Email: jess830@naver.com.
Hye-Jin Lee, Email: jin282@sch.ac.kr.
Ryeo-Won Kwon, Email: ryeowon@sch.ac.kr.
In-Ho Lee, Email: ihlee2225@naver.com.
Seung-Jea Lee, Email: ace0929@naver.com.
Jong-In Park, Email: g1733@naver.com.
Eon-Ah Choo, Email: cndjsdk@naver.com.
Jeong-Beom Lee, Email: leejb@sch.ac.kr.
References
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Bois D, Du Bois EF. Clinical calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Archives of Internal Medicine. 17(6_2): 863–871 (1916)
- European Food Safety Authority (2015) Scientific Opinion on the safety of caffeine. Available from: https://www.efsa.europa.eu/en/efsajournal/pub/4102. Accessed 8 May 2022
- Gonzalez de Mejia E, Ramirez-Mares MV. Impact of caffeine and coffee on our health. Trends in Endocrinology and Metabolism. 25: 489-492 (2014) [DOI] [PubMed]
- Greene E, Khaldi S, Ishola P, Bottje W, Ohkubo T, Anthony N, Dridi S. Heat and oxidative stress alter the expression of orexin and its related receptors in avian liver cells. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 191: 18-24 (2016) [DOI] [PubMed]
- Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A. Orexin, stress, and anxiety/panic states. Progress in Brain Research. 2012;198:133–161. doi: 10.1016/B978-0-444-59489-1.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Lee JB. The effects of caffeine ingestion before passive heat loading on serum leptin levels in humans. Applied Biochemistry and Biotechnology. 2013;171:1253–1261. doi: 10.1007/s12010-013-0296-x. [DOI] [PubMed] [Google Scholar]
- Kim TW, Shin YO, Lee JB, Min YK, Yang HM. Caffeine increases sweating sensitivity via changes in sudomotor activity during physical loading. Journal of Medicinal Food. 2011;14:1448–1455. doi: 10.1089/jmf.2010.1534. [DOI] [PubMed] [Google Scholar]
- Korean Ministry of Food and Drug Safety (2020) Presentation materials for the 1st Open Forum on Food and Drug Safety 2020. Available from: https://www.mfds.go.kr/brd/m_59/view.do?seq=404. Accessed 8 May 2022
- Kuwaki T. Orexin links emotional stress to autonomic functions. Autonomic Neuroscience: Basic & Clinical. 2011;161:20–27. doi: 10.1016/j.autneu.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Lee JB, Kim TW. Passive heat loading links lipolysis and regulation of fibroblast growth factor-21 in humans. Journal of Thermal Biology. 2014;45:163–167. doi: 10.1016/j.jtherbio.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Lee JB, Kim JH. Decreased thermal sweating of central sudomotor mechanism in African and Korean men. American Journal of Human Biology. 2018;30:e23091. doi: 10.1002/ajhb.23091. [DOI] [PubMed] [Google Scholar]
- Lee JB, Kim TW, Min YK, Yang HM. Seasonal acclimatization in summer versus winter to changes in the sweating response during passive heating in Korean young adult men. The Korean Journal of Physiology & Pharmacology. 2015;19(1):9–14. doi: 10.4196/kjpp.2015.19.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JB, Lee HJ, Lee SJ, Kim TW. Blood dopamine level enhanced by caffeine in men after treadmill running. Chinese Journal of Physiology. 2019;62:279. doi: 10.4103/CJP.CJP_10_18. [DOI] [PubMed] [Google Scholar]
- Liu L, Wang Q, Liu A, Lan X, Huang Y, Zhao Z, Jie H, Chen J, Zhao Y. Physiological implications of orexins/hypocretins on energy metabolism and adipose tissue development. ACS Omega. 2019;5:547–555. doi: 10.1021/acsomega.9b03106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Tupone D, Morrison SF. Orexin modulates brown adipose tissue thermogenesis. Biomolecular Concepts. 2012;3:381–386. doi: 10.1515/bmc-2011-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JA, Deurveilher S, Semba K. Stimulant doses of caffeine induce c-FOS activation in orexin/hypocretin-containing neurons in rat. Neuroscience. 2003;121:269–275. doi: 10.1016/S0306-4522(03)00461-5. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Research Reviews. 1992;17:139–170. doi: 10.1016/0165-0173(92)90012-B. [DOI] [PubMed] [Google Scholar]
- Nguyen PH, Greene E, Kong BW, Bottje W, Anthony N, Dridi S. Acute heat stress alters the expression of orexin system in quail muscle. Frontiers in Physiology. 2017;8:1079. doi: 10.3389/fphys.2017.01079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TH, Lee HJ, Lee JB. Effect of heat stimulation on circulating irisin in humans. Frontiers in Physiology. 2021;12:1004. doi: 10.3389/fphys.2021.675377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MP, Belanger-Willoughby N, Linehan V, Hirasawa M. ATP-sensitive potassium channels mediate the thermosensory response of orexin neurons. The Journal of Physiology. 2012;590:4707–4715. doi: 10.1113/jphysiol.2012.236497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober DA, Rihel J, Onah AA, Sung RJ, Schier AF. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. Journal of Neuroscience. 2006;26:13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan NL. A new weighting system for mean surface temperature of the human body. Journal of Applied Physiology. 1964;19:531–533. doi: 10.1152/jappl.1964.19.3.531. [DOI] [PubMed] [Google Scholar]
- Romero-Martínez BS, Montaño LM, Solís-Chagoyán H, Sommer B, Ramírez-Salinas GL, Pérez-Figueroa GE, Flores-Soto E. Possible beneficial actions of caffeine in SARS-CoV-2. International Journal of Molecular Sciences. 2021;22:5460. doi: 10.3390/ijms22115460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/S0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. The American Journal of Physiology. 1999;277:R1780–R1785. doi: 10.1152/ajpregu.1999.277.6.R1780. [DOI] [PubMed] [Google Scholar]
- Snel J, Lorist MM. Effects of caffeine on sleep and cognition. Progress in Brain Research. 2011;190:105–117. doi: 10.1016/B978-0-444-53817-8.00006-2. [DOI] [PubMed] [Google Scholar]
- Sugenoya J, Ogawa T. Characteristics of central sudomotor mechanism estimated by frequency of sweat expulsions. The Japanese Journal of Physiology. 1985;35:783–794. doi: 10.2170/jjphysiol.35.783. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Frontiers in Behavioral Neuroscience. 2013;7:28. doi: 10.3389/fnbeh.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneki H, Wada T, Sasaoka T. Role of orexin in the regulation of glucose homeostasis. Acta Physiologica (Oxford, England). 2010;198:335–348. doi: 10.1111/j.1748-1716.2009.02008.x. [DOI] [PubMed] [Google Scholar]
- Turnbull D, Rodricks JV, Mariano GF, Chowdhury F. Caffeine and cardiovascular health. Regulatory Toxicology and Pharmacology. 2017;89:165–185. doi: 10.1016/j.yrtph.2017.07.025. [DOI] [PubMed] [Google Scholar]
- US Dietary Guidelines Advisory Committee (2015) Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Available from: https://health.gov/sites/default/files/2019-09/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf. Accessed 8 May 2022
- van Dam RM, Hu FB, Willett WC. Coffee, caffeine, and health. The New England Journal of Medicine. 2020;383:369–378. doi: 10.1056/NEJMra1816604. [DOI] [PubMed] [Google Scholar]
- Van Schaik L, Kettle C, Green R, Sievers W, Hale MW, Irving HR, Whelan DR, Rathner JA. Stimulatory, but not anxiogenic, doses of caffeine act centrally to activate interscapular brown adipose tissue thermogenesis in anesthetized male rats. Scientific Reports. 11: 113 (2021) [DOI] [PMC free article] [PubMed]
- Velickovic K, Wayne D, Leija HAL, Bloor I, Morris DE, Law J, Budge H, Sacks H, Symonds ME, Sottile V. Caffeine exposure induces browning features in adipose tissue in vitro and in vivo. Scientific Reports. 2019;9:9104. doi: 10.1038/s41598-019-45540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie JT, Takahira H, Shibahara M, Hara J, Nomiyama M, Yanagisawa M, Sakurai T. Ectopic overexpression of orexin alters sleep/wakefulness states and muscle tone regulation during REM sleep in mice. Journal of Molecular Neuroscience. 2011;43:155–161. doi: 10.1007/s12031-010-9437-7. [DOI] [PubMed] [Google Scholar]
- Zhang C, Abdukerim M, Abilailieti M, Tang L, Ling Y, Pan S. The protective effects of orexin a against high glucose-induced activation of NLRP3 inflammasome in human vascular endothelial cells. Archives of Biochemistry and Biophysics. 2019;672:108052. doi: 10.1016/j.abb.2019.07.017. [DOI] [PubMed] [Google Scholar]
- Zimmermann-Viehoff F, Thayer J, Koenig J, Herrmann C, Weber CS, Deter HC. Short-term effects of espresso coffee on heart rate variability and blood pressure in habitual and non-habitual coffee consumers–a randomized crossover study. Nutritional Neuroscience. 2016;19:169–175. doi: 10.1179/1476830515Y.0000000018. [DOI] [PubMed] [Google Scholar]