Abstract
The present study was conducted to investigate the protective action of Salvia officinalis flowers aqueous extract (SOFAE) against combined gastro-intestinal (GI) disorders-induced by ethanol and castor oil administration in rats. Adult male Wistar rats were divided into seven groups of ten each and various doses of SOFAE (50, 100, and 200 mg kg−1, b.w., p.o.) and sulfasalazine (100 mg kg−1, b.w., p.o.) were daily administrated during 15 days. After, animals were intoxicated with a single oral administration of ethanol (4 g kg−1, b.w., p.o.) and castor oil (5 mL kg−1, b.w., p.o.). We found that SOFAE contains several phytoactive compounds with a strong ABTS scavenging ability. In vivo, we showed that SOFAE protected against EtOH/CO-induced macroscopic and histological alterations in GI tract accompanied by intestinal fluid accumulation and gastric juice decrease. SOFAE significantly counteracted lipoperoxydation increase and reversed the depletion of both enzymatic and non-enzymatic antioxidants. More importantly, SOFAE significantly reduced the levels of inflammatory markers (CRP and ALP) in plasma and mucosal GI tract. In conclusion, our data clearly indicate that the SOFAE exerted a potential protective effect against EtOH-induced peptic ulcer combined with CO-induced diarrhea in rats. These effects could be associated with its antioxidant and anti-inflammatory properties.
Keywords: Salvia officinalis, phytoactive compounds, gastro-intestinal disorders, antioxidant, anti-inflammatory markers
Introduction
In gastrointestinal disorders, ulcers and diarrhea are common diseases with multiple etiologies. The ulcerative disease frequently affects more men than women between 50 and 70 years. This situation affects about 10–15% of the world’s population. 1
Peptic ulcer is distinguished by mucosal lesions and mostly by the bacterium Helicobacter pylori 2 and antiplatelet agents such as acetylsalicylic acid, 3 non-steroidal anti-inflammatory drugs (NSAIDs), potassium chloride and immunosuppressive drugs, 4 smoking and alcohol consumption. 5
Diarrheal disease is one of the most common causes of morbidity and mortality in many developing countries. 6 Anti-secretory, anti-inflammatory agents and some rehydration may be recommended. However, the majority of these drugs induce a complication in the gastrointestinal tract of severe diarrhea leading in some cases to colorectal cancer. 7 Intestinal transport of water, electrolytes, and nutrient substances maintain homeostasis for organisms and exert a nutritive role. The fluid secretion is required to solubilize complex foods, promote the digestion process and produce an isotonic absorbate consisting of small molecules by which nutrient absorption can take place. 8 The secretory mechanism is balanced by fluid absorption largely by the epithelial hump border. 9 In addition, bacterial infection can also cause diarrhea in the small intestine or colon. Enterotoxigenic bacteria, such as Escherichia coli, Clostridium perfringens, Campylobacter jejuni, Salmonella typhimurium, and Staphylococcus aureus bind and penetrate the mucous surface, release enterotoxins, and induce secretory diarrhea and ulcerations.10,11
The gastric and intestinal lesions induced by ethanol and castor oil are mainly related to an intense infiltration in the mucosa and the submucosa which promotes the formation of reactive oxygen species (ROS), an alteration of the mucus, decreased levels of thiol groups, and decreased blood flow, leading to damage in the gastrointestinal mucosa. 12 ROS, in particular hydroxyl radicals, which are the most reactive species, play the major role in oxidative mucosal damage in all types of diarrhea and ulcers.13,14 To correct oxidative stress damage and prevent peptic ulcer and diarrhea, the use of plant-based remedies with antioxidant capacities is highly desirable.
Because of their accessibility and low cost, populations in developing countries largely used medicinal plants, by preference or necessity. However, the Salvia genus is recommended in traditional medicine and as a food ingredient. They are rich in polyphenolic compounds that can be involved in the prevention of illness and the deterioration of food due in part to their antioxidant activity. 15
Sage (Salvia officinalis L.) is a medicinal plant cultivated in Mediterranean countries. Flowers contain many bioactive substances such as dietary fiber, alkaloids, carbohydrates, fatty acids, glycosidic derivatives tannins, carotenoids, anthocyanins, and polyphenols.14,16 The beneficial health effects associated with the consumption of sage, rich in phenolic compounds are essentially related to their antioxidant activities. 17 This property has been previously reported in vivo and in vitro studies. 18 Recently, we discovered that Tunisian Salvia officinalis flowers and leaves extracts present some ameliorative effects against alcohol or castor oil-induced oxidative damages.14,19
Inflammation of the small intestine can cause the leak of fluids and electrolytes and also blood and protein into the lumen of the intestines leading to nutrient malabsorption and the onset of osmotic diarrhea. 20 Hence, the general objective of our study was to examine the therapeutic efficacy of Salvia officinalis flowers aqueous extract on ethanol-induced gastrointestinal ulcer combined with castor oil-induced diarrhea in male rats.
Materials and Methods
Reagents and Chemicals
2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS); bovine catalase; bovine serum albumin (BSA); butylated hydroxyl toluene (BHT); eosin; epinephrine; Folin-Ciocalteu; hydrochloric acid (HCl); hematoxylin; hydrogen peroxide (H2O2); methanol; paraffin; potassium dihydrogen phosphate (KH2PO4); dipotassium hydrogen phosphate (K2HPO4); rutin; sodium hydroxide (NaOH); sodium pentobarbital (C11H18N2O3); tannic acid; 2-thio-barbituric acid (TBA) and trichloroacetic acid (TCA) were purchased products from Sigma Chemical Co. (Sigma-Aldrich GmbH, Steinheim, Germany). Ethanol (EtOH); sulfasalazine; sodium chloride (NaCl); loperamide; and castor oil were supplied by the Central Pharmacy of Tunisia. All other reagents used were of analytical quality.
Preparation of Salvia officinalis Flowers Aqueous Extract (SOFAE)
The sage flowers were cultivated in the region of Tabarka (NW-Tunisia) in April 2019 and were identified by Dr Imen Bel Haj Ali, Associate Professor at the University of Jendouba. The Voucher specimens (No. SO.321) have been deposited with the herbarium of the Higher Institute of Biotechnology of Béja, Tunisia. After washing, the flowers were dried using an oven ventilated at 40°C and then crushed. The aqueous extract was obtained by maceration of 1 g of the powder in 20 mL of distilled water (1/20; w/v) for 24 h. The homogenate was filtered through a .45 μm Whatman #1 filter paper (Bärenstein, Germany). Finally, the filtrate was lyophilized and the obtained dry residues were weighed and subsequently used for the phytochemical analysis and the in vivo experiments.
Phytochemical Studies of SOFAE
Determination of Minerals and Parietal Constituents
The plant material was gathered and dried in an oven set at 60°C. One gram of sample was placed in a stainless steel capsule for calcination in a muffle furnace at 550°C for 4 hours. After cooling, the ash was combined with hydrochloric acid 2N. The solution is then filtered by Whatman filter paper, into a volumetric flask of 25 mL, and the magnesium, iron, and calcium concentrations in the samples were estimated by an atomic absorption flame spectrophotometer (SHIMADZU AA-6200).
The determination of constituents for plant cell walls such as cellulose, hemicelluloses, and lignin were determined according to the analytical model by Van Soest et al 21 from the neutral detergent-insoluble residue (NDF), the insoluble residue, and acid detergent (ADF).
Total tannins, Flavonols, Carotenoids, and Anthocyanins
Total tannins were determined using the Folin-Ciocalteu reagent. 22 In fact, 500 μL of Folin-Ciocalteu (50%) was added to .5 mL of extract followed by 1 mL of Na2CO3 (20%). The absorbance of the supernatant was measured using an ultraviolet (UV)-visible spectrophotometer (DU 640B, Beckman Coulter) at 730 nm.
The quantification of flavonols was assessed according to the method of Rigane et al. 23 Briefly, 1 mL AlCl3 (20%) was added to 1 mL of the extract followed and 3 mL of sodium acetate (50 mg/mL). After incubation for 2 hours and 30 minutes, the absorbance was read at 440 nm.
Total carotenoids were estimated according to Marina et al 24 by adding 1 mL of hexane extract and measuring the absorbance at 450 nm. The result was expressed in β-carotene using the absorbance coefficient of 2500.
Total anthocyanin compounds were estimated according to the absorbance differentiation and using two buffers: KCl at pH 1.0 (.025 M) and CH3COONa (.025 M) pH 4.5 (0.4 M). 400 μL of the extract were mixed with 3.6 mL of KCl, followed by 400 μL of CH3COONa. Then, the mixture was incubated during 30 minutes in the dark and the absorbance was read at 510 nm. 25
Characterization of Phenolic Compounds of SOFAE by Liquid Chromatography-High Resolution Electrospray Ionization Mass Spectrometry (LC-HRESIMS) Analysis
Analysis of the phenolic compounds of Salvia officinalis flowers aqueous extract (SOFAE) was carried out using a Shimadzu UFLC XR system (Kyoto, Japan), equipped with a SIL-20AXR autosampler, an oven with CTO-20 AC column, a LC-20ADXR binary pump, and a 2020 quadrupole detection system. This instrument was equipped with an Inertsil ODS-4 C18 column of 3 μm (L150 × 3.0 mm id). 20 mg of extract were dissolved in 1 mL of 10% methanol and filtered, and then the mixture was transferred to vials of LC-MS. The column temperature was adjusted to 40°C and the injection volume was 20 μL with a flow rate of .5 mL/min. 5% methanol +.2% acetic acid and 50% acetonitrile +.2% acetic acid were used as mobile phases A and B, respectively. The analysis was carried out using a linear gradient programmed as follows: .01–14 min, from 10% to 20% of B; 14–27 min, 0 from 20% to 55% B; 27 to 37 min, from 55% to 100% of B; 37–45 min, 100% B; 45–50 min 10% B. The temperature of the dissolution line was 275° C, the fogging gas flow rate was 1.50 L/min, the drying gas was adjusted to 15.00 L/min and the heating temperature block was 450° C. The LC-ESI (−) MS [M−H]− mass spectra were acquired using LabSolutions software. The phenolic compounds were identified by comparison with the retention time of the phenolic compound standards. The laboratory standards were LGC and Sigma Aldrich. 4
Free Radical-Scavenging Activities on ABTS
The antioxidant capacity of Salvia officinalis flowers aqueous extract was evaluated using the 2,2′-azino-bis [3-ethylbenzthiazoline-6-sulphonic acid] (ABTS) method. 26 Briefly, we associated 1 mL of a diluted extract with 3 mL of 7 mM ABTS radical solution (ABTS•+) and the mixture was kept in dark at room temperature for 60 min. The absorbance was evaluated at 734 nm and the scavenging capacity was calculated as ((1 − Ab/A0) × 100%). Ab and A0 are the absorbance of samples as well as the ABTS•+ solution at 734 nm.
Animals and Treatment
Healthy adult male Wistar rats (weighing 230 ± 18.54 g; housed 5 per cage) and adult male Swiss Albino mice (weighing approximately 25 g; housed ten per cage) were purchased from the Society of Pharmaceutical Industries of Tunisia (SIPHAT, Ben-Arours, TN). All animal procedures were performed in accordance with the Guidelines for Care and Use of Animals Laboratory and approved by the Animal Ethics Committee of the National Institute of Health. The test was performed in compliance with the Commission Directive 2000/32/EC and the OECD Guideline 474. They were provided with standard food (BADR, Utique, TN) and water ad libitum and maintained in an animal house under controlled temperature (22 ± 2°C) with a 12/12 h light–dark cycle.
Acute Toxicity
Salvia officinalis flowers aqueous extract in the dose range of 10, 50, 100, 250, 500, 1000, 1500, 2500, and 3500 mg/kg was orally administrated to different groups of mice (n = 10). The animals were examined every 30 min during 4 h and then, occasionally for an additional period of 8 h. 24 h after, the mortality was recorded. Mice were also observed for other signs of toxicity, such as motor co-ordination, righting reflex, and respiratory changes.
Gastro-Intestinal (GI) Disorders-Induced with Ethanol and Castor Oil
All the animals were pre-treated for 15 days with different doses at 8 a.m. Rats were divided into seven groups of ten each. Groups 1, 2, and 3 served as controls and received distilled water (5 mL/kg, b.w., p.o.) for 15 days. Groups 4, 5, and 6 were pre-treated with various doses of SOFAE (50, 100, and 200 mg/kg, b.w., p.o.). Preliminary experiment indicated that 50, 100, and 200 mg kg−1 SOFAE were the lowest doses that give a significant protective effect. Finally, group 7 was pre-treated with sulfasalazine (100 mg/kg, b.w., p.o.). All these treatments were given for 15 days by force-feeding. Rats were fasted for 18 h before the last administration of SOFAE and reference molecules. After 60 min, each animal, except group 1, received EtOH (4 g/kg, b.w., p.o.) by oral administration and after 30 minutes, each animal, except groups 1 and 2 was per orally treated with castor oil (5 mL/kg, bw., p.o.). Two hours later, rats were anaesthetized by intraperitoneal administration of sodium pentobarbital (40 mg kg−1, b.w.) and sacrificed by decapitation. The blood was collected and plasma processed for electrolytes (free iron and ionizable calcium), plasma scavenging activity, alkaline phosphatase activity, and C-reactive protein (CRP) determinations.
Fluid Accumulation-Induced by Ethanol and Castor Oil
The antidiarrheal activity of SOFAE was determined according to Dicarlo et al. 27 The fluid was harvested and centrifuged at 3000 × g for 5 min to remove insoluble materials. The small intestines were removed and weighed and the volume of their contents was determined using graduated tubes. For each animal, the intestine was reweighed and the difference between full and empty intestine was calculated.
Evaluation of Gastric and Intestinal Mucosal Damage
To test the gastric and intestinal mucosal damage, the stomach and small intestine of each animal were thrown out and opened along their greater curvature. Tissues were gently rinsed with NaCl .9%. The lesions in gastric mucosa were macroscopically examined and the photographs of hemorrhagic erosions were taken using Canon EOS1100 D (ISO 6400) digital camera. Ulcer indexes were evaluated as the sum of the lengths of the whole gastric lesions (in mm2). Two independent, blinded observers performed the measurements of lesion lengths.
Histopathological Analysis
Immediately after sacrifice, small pieces of the stomach and small bowel were gathered and washed with a solution of NaCl (.9%). Tissue fragments were then fixed in a 10% neutral buffered formalin solution, embedded in paraffin and used for histopathological examination. 5 μm thick sections were cut, deparaffinized, hydrated, and stained with hematoxylin and eosin (HE). Finally, gastric and small intestine sections were examined in a blind fashion for all treatments.
Biochemical Assessments
Lipid Peroxidation Measurement
Gastric and intestinal mucosa lipid peroxidation was performed by MDA measurement according to the double heating method. 28 Aliquots from gastric and intestinal mucosa homogenates were added to BHT-trichloroacetic acid (TCA) solution containing 1% BHT (w/v) dissolved in 20% TCA (w/v) and centrifuged at 1000g for 5 min at 4°C. Then, the supernatant was mixed with a solution containing (.5 N HCl, 120 mM TBA buffered in 26 mM Tris). The mixture was heated at 80°C for 10 min. After cooling, the absorbance of the resulting chromophore was estimated at 532 nm. MDA levels were calculated using an extinction coefficient for MDA-TBA complex of 1.56 × 105 M−1 cm−1.
H2O2 Determination
The gastric and intestinal mucosa H2O2 levels were determined according to Dingeon et al. 29 However, the hydrogen peroxide reacts with p-hydroxybenzoic acid and 4-aminoantipyrine in the presence of peroxidase leading to the formation of quinoneimine that has a pink color detected at 505 nm. Briefly, 1 mL of reaction mixture containing 100 μL of colonic mucosa and 900 μL of working reagent (100 mM tris buffer pH 7, .3 mM phenol, 10 000 U/L glucose oxidase, 1000 U/L peroxidase, and 2.6 mM amino 4-antipyrine) was incubated at 37°C for 15 min. The standard curve is made with hydrogen peroxide at different concentrations.
Plasma Scavenging Activity
The plasma scavenging activity in the different groups was evaluated using the DPPH radical according to the method of Brand-Williams et al. 30 We mixed 100 μL of plasma sample with 2 mL of 2,2-diphenyl-1-picrylhydrazyl (DPPH) in methanol solution (100 mM). Then, we added 1 mL of chloroform after incubation of the solution at 37°C for 30 min and the mixture was centrifuged at 3000 g for 10 min. The absorbance of clear supernatant was then determined at 517 nm using spectrophotometer (DU 640B; Beckman Coulter, Indianapolis, Indiana, USA). The DPPH solution was considered as control and the plasma scavenging activities (PSA), expressed in percentage, was calculated according to the following equation:
PSA (%) = 100 × (A517 (control) × A517 (sample)/A517 (control).
Antioxidant Enzyme Activity Assays
The SOD activity in gastric and intestinal mucosa was evaluated using modified epinephrine assays. 31 At alkaline pH, superoxide anion induces the autoxidation of epinephrine to adenochrome; while competing with this reaction, SOD decreased the adenochrome formation. One unit of SOD is considered as the amount of the extract that inhibits the rate of adenochrome formation by 50%. Enzyme extract was blended to 2 mL reaction mixture containing 10 μL of bovine catalase (CAT, .4 U/mL), 20 μL of epinephrine (5 mg/mL) and 62.5 mM of sodium carbonate/bicarbonate buffer (pH 10.2). Changes in absorbance were assessed at 480 nm.
The CAT activity in gastric and intestinal mucosa was registered by Aebi 32 by measuring the initial rate of H2O2 disappearance at 240 nm. The reaction mixture contained 33 mM H2O2 in 50 mM phosphate buffer (pH 7) then we calculated the activity of CAT using the extinction coefficient of 40 mM−1 cm−1 for H2O2.
The activity of glutathione peroxidase was quantified following the procedure of Flohé and Gunzler 33 We incubated 1 mL of reaction mixture containing .2 mL of gastric or intestinal mucosa supernatant to .2 mL of phosphate buffer .1 M pH 7.4, .2 mL of GSH (4 mM) and .4 mL of H2O2 (5 mM) at 37°C for 1 min and the reaction was stopped by the addition of .5 mL TCA (5%, w/v). After centrifugation at 1500g for 5 min, an aliquot (.2 mL) from the supernatant was accompanied by .5 mL of phosphate buffer .1 M pH 7.4 and .5 mL DTNB (10 mM) and the absorbance was read at 412 nm. The activity of GPx was expressed as nmol of GSH consumed/min/mg protein.
Non-Enzymatic Antioxidants Levels
The total concentrations of thiol groups (-SH) in the gastric and intestinal mucosa were evaluated following Ellman’s method. 34 Aliquots of gastric and intestinal mucosa were mixed with 800 μL of .25 M phosphate buffer (pH 8.2) and 100 μL of 20 mM EDTA. We measured the optical density at 412 nm (A1) and subsequently, we added 100 μL of 10 mM DTNB. Then, we determined a new value (A2) after incubating the reaction mixture at 37°C for 15 minutes. The thiol groups concentration was calculated by the difference between A2 to A1 using a molar extinction coefficient of 13.6×103 M−1 cm−1. The results are expressed in nmol of thiol groups per mg of total proteins.
The level of GSH was performed by the colorimetric method recorded by the method of Sedlak and Lindsay. 35 Briefly, 5 mL of supernatant were mixed with 4 mL of cold distilled water and 1 mL of 50% TCA. The samples were shaken using a vortex mixer and centrifuged at 1200g for 15 min. 2 mL of supernatant were mixed with 4 mL of .4 M Tris buffer (pH 8.9) and .1 mL of DTNB (.01 M) was added to the reaction medium. The absorbance was measured at 412 nm against a blank containing only the buffer.
Iron and Calcium Measurement
Free iron (Fe) and calcium (Ca) concentrations in plasma, gastric, and intestinal mucosa were performed using commercially available diagnostic kits (Biomaghreb, Ariana, TN, ISO 9001 certificate)
Protein Determination
Protein concentration was assessed according to Hartree 36 which is a slight change of the Lowry method. This method is based on the capacity of the protein-copper complex to reduce the Folin-Ciocalteu reagent inducing a blue coloration measured at 650 nm. We used serum albumin as a standard.
C-reactive Protein Determination and ALP Activity
C-reactive protein (CRP) and alkaline phosphatase (ALP) activity were performed using commercially available diagnostic kits (Biomaghreb, Ariana, TN, ISO 9001 certificate).
Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA), were expressed as means ± standard error of the mean (S.E.M.) and post hoc LSD using SAS (2009). The data are representative of 10 independent experiments. All statistical tests were two-tailed, and a P-value of .05 or less was considered significant.
Results
Phytochemical and In Vitro Antioxidant Properties of SOFAE
The data of the quantitative and qualitative evaluation of secondary metabolites, minerals, and parietal constituents are presented in Tables 1 and 2. We first showed that SOFAE is rich in calcium, magnesium, and iron. Our extract also contains a high levels of total tannins (51.30± 2.57 mg TAE/g DM), flavonols (2.01±.13 mg RE/g DM), anthocyanins (2.48 ± .04 mg CG/mL), and carotenoids (2.33 ± .29 μg/100 mL). In contrast, the flowers extract of Salvia officinalis presents a moderate fiber content (parietal constituents) such as neutral detergent fiber (29.16 ± 1.30% of DM), crude lignin (22.40 ± 4.74% of DM), cellulose (1.37 ± .49% of DM), hemicelluloses (5.69 ± 1.97% of DM), and soluble fraction (69.63 ± 1.31% of DM).
Table 1.
Phytochemical composition and IC50 value of the ABTS radical-scavenging activity of Salvia officinalis flowers aqueous extract (SOFAE) and butylated hydroxytoluene (BHT); IC50: the inhibitory concentration of sample that can decrease ABTS concentration by 50%.
| Parameters | Contents |
|---|---|
| Neutral detergent fiber (% of DM) | 29.16 ± 1.30 |
| Crude lignin (% of DM) | 22.40 ± 4.74 |
| True raw cellulose (% of DM) | 1.37 ± .49 |
| Hemicellulose (% of DM) | 5.69 ± 1.97 |
| Soluble fraction (% of DM) | 69.63 ± 1.31 |
| Iron (μmol/L) | 1.46 ± .33 |
| Magnesium (mmol/L) | 3.93 ± .02 |
| Calcium (mmol/L) | 6.16 ± .04 |
| Total tannins (mg TAE/g DM) | 51.30 ± 2.57 |
| Flavonols (mg RE/g DM) | 2.01 ±.13 |
| Total carotenoids (μg/mL) | 2.33 ±.29 |
| Total anthocyanins content (mg CG/g DM) | 6.67 ± .18 |
| ABTS (IC50, μg/mL) | 52.58 ± 4.13 |
| Butylated hydroxytoluene (IC50, μg/mL) | 33.17 ± 2.29 |
Data are expressed as mean ± SEM (n = 3); SEM: standard error of the mean; SOFAE: Salvia officinalis aqueous extract; DM: dry matter; TAE: tannic acid equivalent; CG: cyanidine glucosyl-3; RE: Rutin equivalent.
Table 2.
High-resolution liquid chromatography/electrospray ionization (LC-HRESIMS) identification of Salvia officinalis flowers aqueous extract (SOFAE).
| Identification a | Molecular formula | [M]- H m/z b | Retention time | Concentration (ppm) |
|---|---|---|---|---|
| Quinic acid | C7H12O6 | 191.00 | 2.154 | 119.779 |
| Catechin (+) | C15H14O6 | 289.00 | 11.067 | 24.218 |
| Protocatchuic acid | C7H6O4 | 153.00 | 6.825 | 15.249 |
| 1,3-di-O-caffeoyquinic acid | C25H24O12 | 515.00 | 16.951 | 23.938 |
| p-coumaric acid | C9H8O3 | 163.00 | 20.642 | 157.432 |
| Luteolin-7-O-glucoside | C21H20O11 | 447.00 | 24.496 | 236.518 |
| Naringin | C27H32O14 | 57.00 | 25.560 | 98.371 |
| Apigenin-7-O-glucoside | C21H20O10 | 431.00 | 26.775 | 22.828 |
| Trans cinnamic | C9H8O2 | 147.00 | 31.617 | 433.142 |
| Quercetin | C21H20O11 | 301.00 | 31.757 | 63.878 |
| Kampherol | C15H10O6 | 285.00 | 31.800 | 2.760 |
| Apigenin | C15H10O5 | 269.00 | 36.476 | 151.499 |
aThe compounds are suggested according to the dictionary of natural products and the characteristic fragmentation pattern.
bThe formulas were deduced from the quasi molecular ion peak [M + H]+.
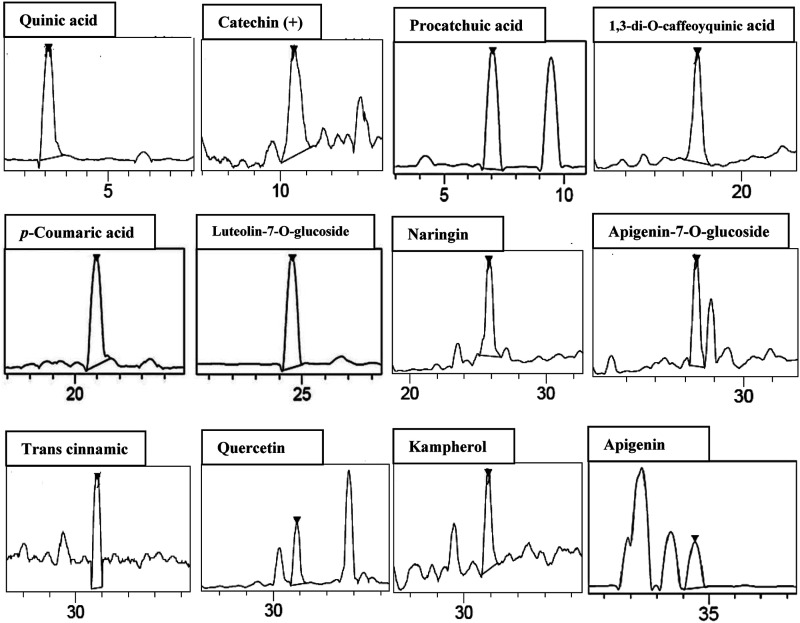
The use of the chromatographic technique for SOFAE characterization allowed to the identification of 12 phenolic compounds (Figure 1). We showed four phenolic acids such as quinic acid, protocatechuic acid, 1,3-di-O-caffeoyquinic acid, and p-coumaric acid. The chromatographic elution profile also revealed eight flavonoid compounds; the main ones are trans cinnamic, catechin (+), naringin, and quercetin (Table 2).
Figure 1.
Representative LC-HRESIMS of phenolic compounds of Salvia officinalis flowers aqueous extract (SOFAE) (assignments of peaks are given in Table 2).
Concerning the antioxidant capacity, we showed that the RSA of SOFAE and butylated hydroxyl toluene (BHT) against ABTS radical significantly increased in a dose-dependent manner. However, SOFAE revealed an important RSA (IC50 = 52.58 ± 4.13 μg/mL) but lesser than BHT (IC50 = 33.17 ± 2.29 μg/mL) used as reference antioxidant molecule (Table 1).
Acute Oral Toxicity of SOFAE
In the acute toxicity test, oral administration of SOFAE increasing doses (10, 50, 100, 250, 500, 1000, 1500, 2500, and 3500 mg/kg, p.o.) to mice, did not result in any significant alterations in behavior, breathing, sensory nervous system responses, or gastrointestinal impacts during the manipulation. Furthermore, there were no apparent changes in body weights and no changes in consumption of water and food. Finally, no mortality or any toxic reactions was observed in any group after 72 h of administration. Therefore, SOFAE is characterized by an LD50 greater than 3500 mg/kg.
Effects of SOFAE on Ethanol and Castor Oil-Induced Gastro-Intestinal Fluid Accumulation
We found that ethanol and castor oil significantly increased the volume and the weight of stomach and intestinal fluid accumulation when compared to the control group. In contrast, SOFAE and sulfasalazine pre-treatment significantly restricted the castor oil-induced enteropooling and gastric fluid accumulation in a dose-dependent manner (Table 3).
Table 3.
Effect of Salvia officinalis flowers aqueous extract (SOFAE) and sulfasalazine (SULF) on small bowel fluid accumulation (enteropooling) induced by ethanol (EtOH) and castor oil (CO): Weight and volume of content in small intestine and percentage of protection (%). Animals were pre-treated with various doses of SOFAE (50, 100 and 200 mg/kg, b.w., p.o.) and reference molecule (SULF, 100 mg/kg, b.w., p.o.) or vehicle (NaCl .9%). One hour after, animals received EtOH (4 g/kg, b.w.) and after 30 minutes, each animal, except groups 1 and 2 was received castor oil (5 mL/kg, bw., p.o.) by gavage for 2h.
| Groups | Weight of intestinal content (g) | Protection (%) | Intestinal fluid (mL) | Protection (%) |
|---|---|---|---|---|
| Control | .34 ± .37 | .61 ± .21 | ||
| EtOH | 1.13 ± .90* | 1.69 ± .31* | ||
| EtOH+ CO | 3.81 ± .11*# | 4.08 ± 1.16*# | ||
| EtOH + CO+ SOFAE-50 | 2.62 ± .39#a | 31.23 | 3.14 ± .39#a | 23.03 |
| EtOH + CO+ SOFAE-100 | 1.87 ± .20#a | 50.91 | 2.14 ± .20#a | 47.54 |
| EtOH + CO+ SOFAE-200 | 1.10 ± .19#a | 71.12 | 1.41 ± .85#a | 65.44 |
| EtOH + CO+ SULF | 1.40 ± .97#a | 63.25 | 2.62 ± .97#a | 35.78 |
The data are expressed as mean ± S.E.M. (n = 10). *: P < .05 compared to control group, # : P < .05 compared to ethanol group and a: P < .05 compared to ethanol and castor oil group.
Effects of SOFAE on Gastro-Intestinal Qualitative and Quantitative Macroscopic Evaluation
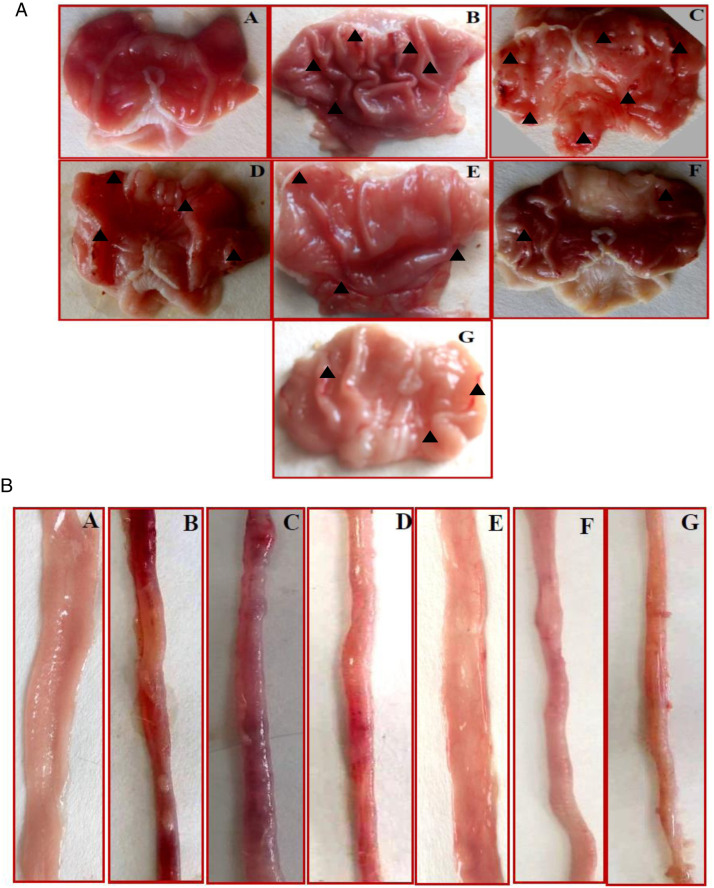
Qualitative and quantitative macroscopic evaluation of SOFAE anti-ulcer activities were achieved by macroscopic examination of the stomach and small intestine. Animals intoxicated with ethanol and castor oil had hemorrhagic lesions on the glandular part of the stomach, dark red and black (Figure 2). Moreover, we noticed the appearance of macroscopically clear lesions along the duodenum and the jejunum. However, SOFAE and sulfasalazine treatments significantly protected the gastric and intestinal mucosa from alcohol and castor oil-induced injuries (Table 4).
Figure 2.
Gastric (a) and intestinal (b) morphology showing the protective effects of Salvia officinalis flowers aqueous extract (SOFAE) and sulfasalazine (SULF) on ethanol (EtOH)-induced ulcer combined with castor oil (CO)-induced diarrhea. Animals were treated with various doses of SOFAE (50, 100, and 200 mg/kg, b.w., p.o.), SULF (100 mg/kg, b.w., p.o.) or vehicle (NaCl .9%). (A) H2O + NaCl; (B) H2O + EtOH; (C) H2O + EtOH+ CO; (D, E and F) SOFAE (50, 100, and 200 mg/kg, b.w., p.o., respectively) + EtOH+ CO; (G) SULF (100 mg/kg, b.w., p.o.) + EtOH+ CO.
Table 4.
Effect of Salvia officinalis flowers aqueous extract (SOFAE) and sulfasalazine (SULF) on gastric and small bowel macroscopic alterations induced by Ethanol (EtOH) and Castor oil (CO): Mucus volume, ulcer index, and percentage of protection (%). Animals were pre-treated with various doses of SOFAE (50, 100, and 200 mg/kg, b.w., p.o.) and reference molecule (SULF, 100 mg/kg, b.w., p.o.) or vehicle (NaCl .9%). One hour after, animals received EtOH (4 g/kg, b.w.) and after 30 minutes, each animal, except groups 1 and 2 was received castor oil (5 mL/kg, bw., p.o.) by gavage for 2h.
| Groups | Stomach | Small intestine | ||||
|---|---|---|---|---|---|---|
| Mucus volume (ml) | Protection (%) | Ulcer area (mm2) | Protection (%) | Ulcer area (mm2) | Protection (%) | |
| Control | 4.64 ± .48 | 0 | 0 | |||
| EtOH | 1.54 ± .37* | 73.60 ± 4.05* | 20.82 ± 3.01* | |||
| EtOH+ CO | 1.84 ± .23*# | 99.17 ± 6.97*# | 23.12 ± 2.56*# | |||
| EtOH + CO + SOFAE-50 | 2.12 ± .89#a | 45.69 | 61.48 ± 3.74#a | 37.18 | 14.43 ± 3.21#a | 37.59 |
| EtOH + CO + SOFAE-100 | 2.85 ± .51#a | 61.42 | 40.54 ± 2.41#a | 58.29 | 11.26 ± .98#a | 51.30 |
| EtOH + CO + SOFAE-200 | 3.69 ± .23#a | 79.53 | 20.58 ± 3.13#a | 78.42 | 7.87 ± 1.03#a | 65.96 |
| EtOH + CO + SULF | 2.99 ± .26#a | 64.44 | 35.83 ± 2.16#a | 63.04 | 8.41 ± 1.16#a | 63.62 |
The data are expressed as mean ± S.E.M. (n = 10). *: P < .05 compared to control group, # : P < .05 compared to ethanol group and a: P < .05 compared to ethanol and castor oil group.
Effects of SOFAE on Gastro-Intestinal Histopathological Evaluation
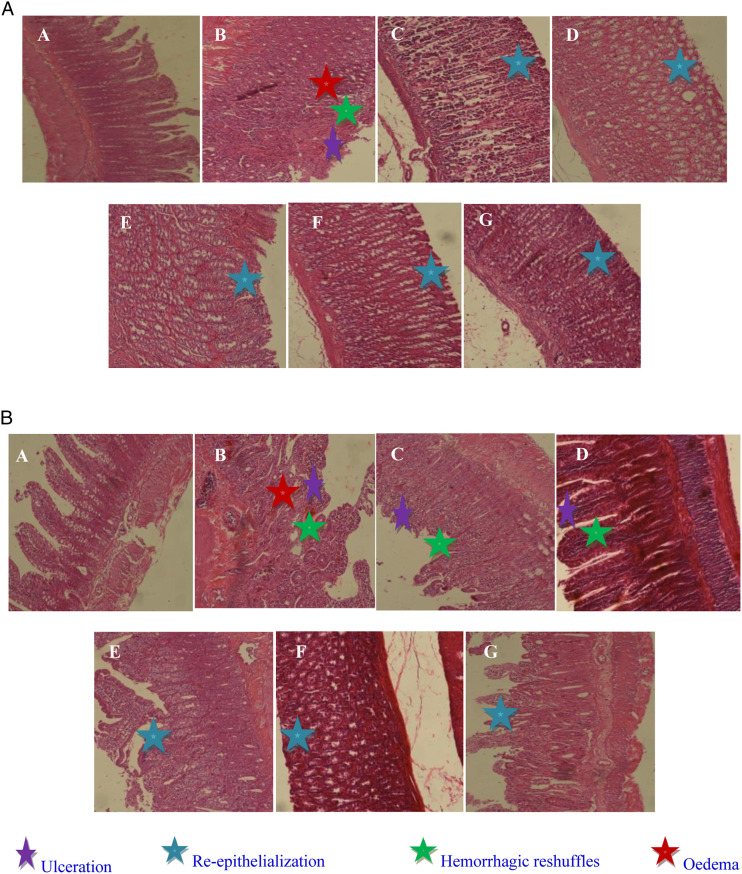
The histological observation of ethanol and castor oil-induced gastric and intestinal lesions in EtOH and EtOH + CO groups demonstrated a comparative extensive congestion, surface coating alteration, edema, necrotic lesions, epithelial, and vascular cells alteration. Also, a hemorrhage, hyperemia, inflammatory cell infiltration in the stomach and intestinal were observed in mucosa and submucosa (Figure 3).
Figure 3.
Gastric (a) and duodenal (b) histology showing the protective effects of Salvia officinalis flowers aqueous extract (SOFAE) and sulfasalazine (SULF) on ethanol (EtOH)-induced ulcer combined with castor oil (CO)-induced diarrhea. Animals were treated with various doses of SOFAE (50, 100, and 200 mg/kg, b.w., p.o.), SULF (100 mg/kg, b.w., p.o.) or vehicle (NaCl .9%). (A) H2O + NaCl; (B) H2O + EtOH; (C) H2O + EtOH+ CO; (D, E, and F) SOFAE (50, 100, and 200 mg/kg, b.w., p.o., respectively) + EtOH+ CO; (G) SULF (100 mg/kg, b.w., p.o.) + EtOH+ CO.
We proclaimed that SOFAE pretreatment recorded obvious dose-dependent protection of the gastric and intestine mucosa as assessed by the depletion of mucosal and submucosal edema as well as a clear leucocytes infiltration. The most important protection was detected in the group receiving the high dose of SOFAE. A similar protective effect had also observed in sulfasalazine pretreated rats.
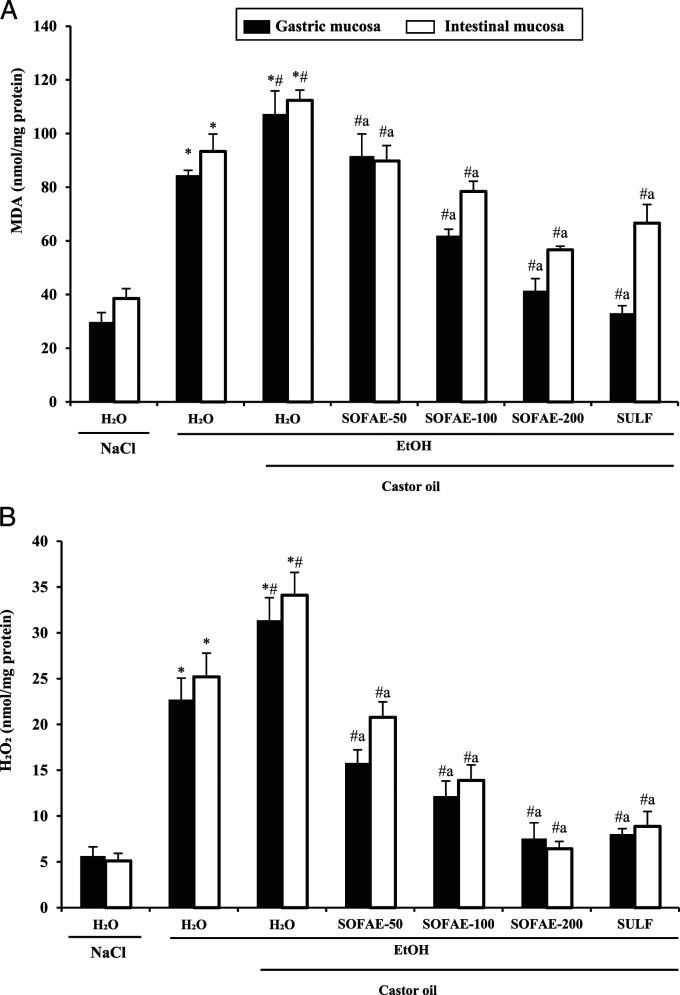
Effects of SOFAE on Ethanol and Castor Oil-Induced Gastro-Intestinal Lipoperoxidation and Hydrogen Peroxide Increase
In our study, we firstly studied the implication of oxidative stress in the gastrointestinal protective effects of SOFAE against combined gastro-intestinal disorders by estimating the levels of MDA and hydrogen peroxide. EtOH and/or castor oil administration per se significantly increased stomach and small bowel MDA levels. Alcohol and castor oil-induced lipoperoxidation was significantly overturned by SOFAE or sulfasalazine pre-treatment in a dose-dependent manner.
Our result also revealed a significant increase in H2O2 levels in gastric and intestinal mucosa in EtOH and/or castor oil groups when compared to the negative control group. SOFAE and sulfasalazine treatment significantly and dose-dependently reduced the EtOH/CO-induced hydrogen peroxide deregulation. The high dosage of Salvia officinalis extract registered a more significant therapeutic effect than sulfasalazine (Figure 4).
Figure 4.
Effect of Salvia officinalis flowers aqueous extract (SOFAE) and sulfasalazine (SULF) on ethanol (EtOH) and castror oil (CO)-induced changes in stomach and intestinal mucosa MDA (A) and H2O2 (B) levels. Animals were pre-treated with various doses of SOFAE (50, 100, and 200 mg/kg, b.w., p.o.) and reference molecule (SULF, 100 mg/kg, b.w., p.o.) or vehicle (NaCl .9%). One hour after, animals received EtOH (4 g/kg, b.w., p.o.) and after 30 minutes, each animal, except groups 1 and 2 was received castor oil (5 mL/kg, bw., p.o.) by gavage for 2 h. The data are expressed as mean ± S.E.M. (n = 10). *: P < .05 compared to control group, # : P < .05 compared to ethanol group and a: P < .05 compared to ethanol and castor oil group.
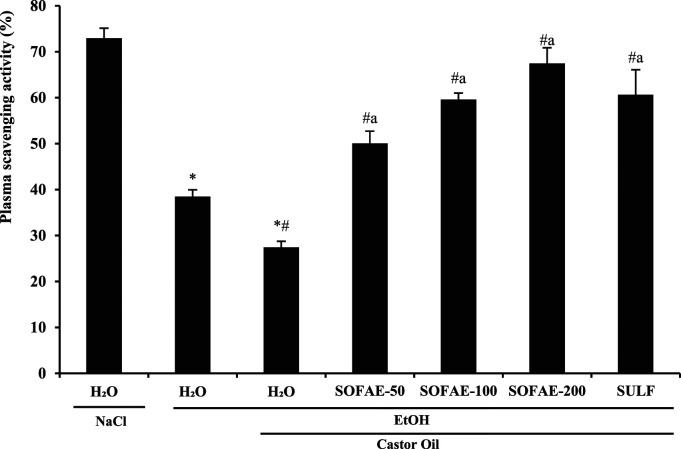
Effects on Plasma Scavenging Activity
Our results demonstrated a significant decrease in plasma scavenging activity induced by EtOH/CO treatment when compared to the control group (Figure 5). However, after SOFAE pre-treatment, the PSA percentage showed a significant augmentation in a dose-dependent manner. A similar effect was also noticed for sulfasalazine, used as a reference molecule, but lesser than the group that received the high dose of SOFAE.
Figure 5.
Effect of Salvia officinalis flowers aqueous extract (SOFAE) and sulfasalazine (SULF) on ethanol (EtOH) and castror oil (CO)-induced disturbances in plasma scavenging activity (PSA). Animals were pre-treated with various doses of SOFAE (50, 100 and 200 mg/kg, b.w., p.o.) and reference molecule (SULF, 100 mg/kg, b.w., p.o.) or vehicle (NaCl .9%). One hour after, animals received EtOH (4 g/kg b.w., p.o.) and after 30 minutes, each animal, except groups 1 and 2 was received castor oil (5 mL/kg, bw., p.o.) by gavage for 2 h. The data are expressed as mean ± S.E.M. (n = 10). *: P < .05 compared to control group, # : P < .05 compared to ethanol group and a: P < .05 compared to ethanol and castor oil group.
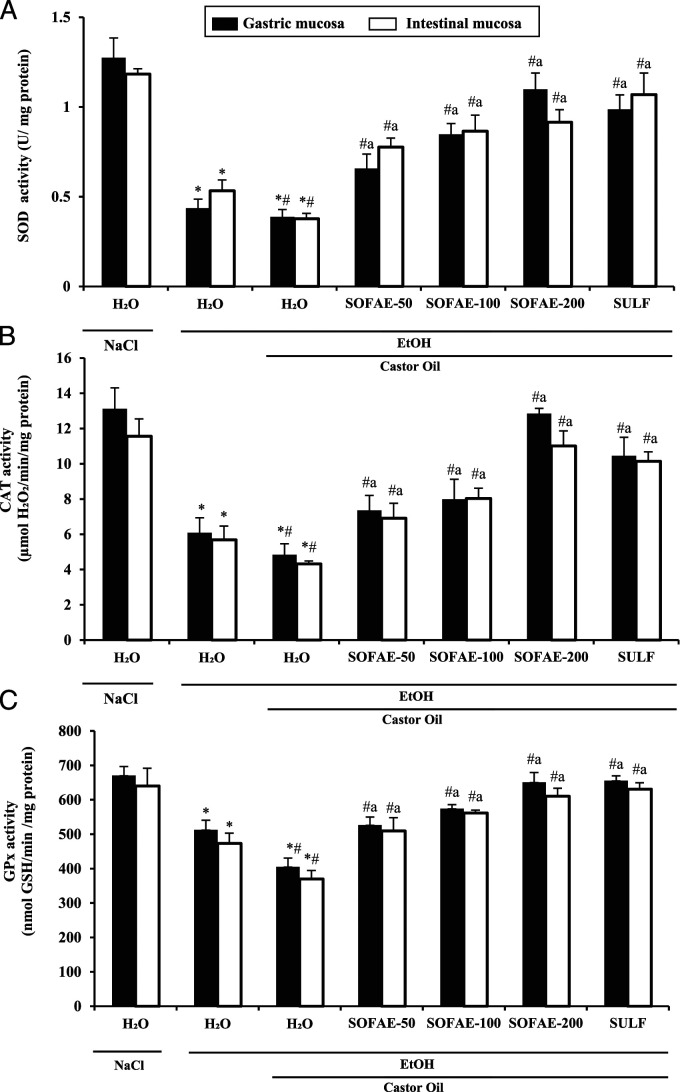
Effect of SOFAE and Sulfasalazine on EtOH and/or CO-Induced Antioxidant Enzyme Activities Depletion
On the other hand, we tested the effect of SOFAE, SULF, EtOH, and CO treatments on antioxidant enzyme activities (Figure 6). We observed that gastric and small bowel injuries are associated with significant depletion of superoxide dismutase (A), catalase (B), and glutathione peroxidase (C) activities. SOFAE treatment significantly corrected the depletion of enzyme activities in a dose-dependent manner. More importantly, SOFAE high dose had the greater effect than sulfasalazine.
Figure 6.
Effect of Salvia officinalis flowers aqueous extract (SOFAE) and sulfasalazine (SULF) on ethanol (EtOH) and castror oil (CO)-induced changes in stomach and intestinal mucosa antioxidant enzyme activities: SOD (A), CAT (B), and GPx (C). Animals were pre-treated with various doses of SOFAE (50, 100, and 200 mg/kg, b.w., p.o.) and reference molecule (SULF, 100 mg/kg, b.w., p.o.) or vehicle (NaCl .9%). One hour after, animals received EtOH (4 g/kg, b.w., p.o.) and after 30 minutes, each animal, except groups 1 and 2 was received castor oil (5 mL/kg, b.w., p.o.) by gavage for 2 h. The data are expressed as mean ± S.E.M. (n = 10). *: P < .05 compared to control group, # : P < .05 compared to ethanol group and a: P < .05 compared to ethanol and castor oil group.
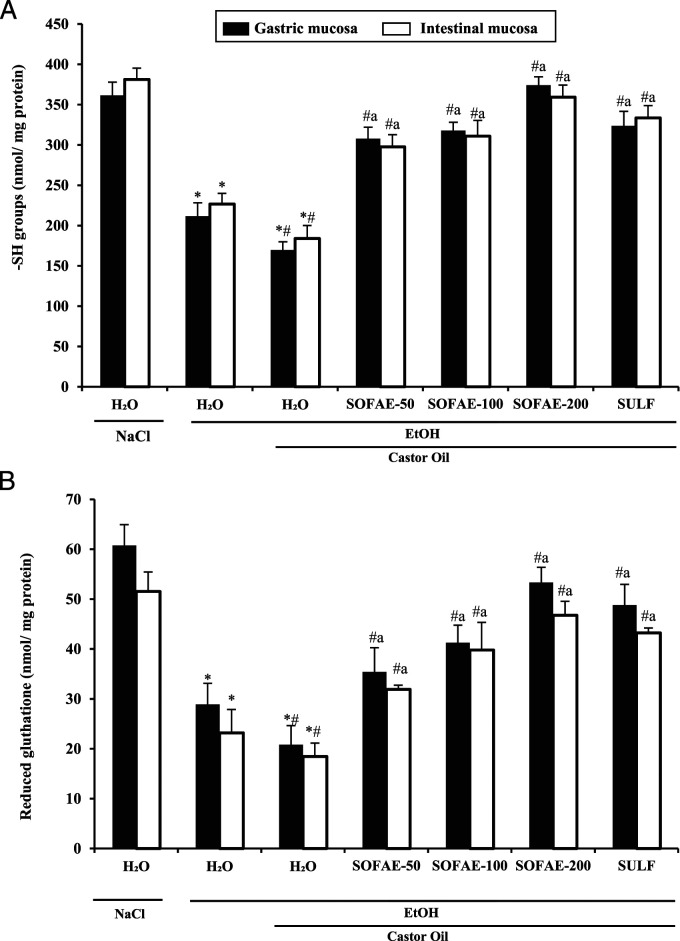
Effect of SOFAE and Sulfasalazine on EtOH and/or CO-Induced Non-enzymatic Antioxidants Levels
We also studied the gastric and small bowel non-enzymatic antioxidants levels (Figure 7). As expected, we showed that stomach and intestinal thiol groups (A) and glutathione (B) contents were significantly decreased after alcohol and/or castor oil intoxication. SOFAE exhibited a significant and dose-dependent regulation of all those parameters. Furthermore, we showed that the high dose of SOFAE exerts a more important effect than sulfasalazine.
Figure 7.
Effect of Salvia officinalis flowers aqueous extract (SOFAE) and sulfasalazine (SULF) on ethanol (EtOH) and castror oil (CO)-induced changes in stomach and intestinal mucosa sulfhydryl groups (A) and reduced glutathione (B). Animals were pre-treated with various doses of SOFAE (100, 200, and 400 mg/kg, b.w., p.o.) and reference molecule (SULF, 100 mg/kg, b.w., p.o.) or vehicle (NaCl .9%). One hour after, animals received EtOH (4 g/kg, b.w.) and after 30 minutes, each animal, except groups 1 and 2 was received castor oil (5 mL/kg, bw., p.o.) by gavage for 2 h. The data are expressed as mean ± S.E.M. (n =10). *: P < .05 compared to control group, # : P < .05 compared to ethanol group and a: P < .05 compared to ethanol and castor oil group.
Effect of SOFAE and Sulfasalazine on Free Iron and Calcium Levels
In our investigation, we also studied the effect of EtOH/CO and SOFAE on intracellular mediators such as calcium and free iron levels in plasma, gastric and intestinal mucosa (Table 5). EtOH and/or castor oil groups registered a significant increase of those intracellular mediators in all studied tissues when compared to the control group. However, SOFAE and sulfasalazine significantly reduced all studied parameters.
Table 5.
Subacute effect of Salvia officinalis flowers aqueous extract (SOFAE) and sulfasalazine (SULF) on free iron and calcium levels in plasma, gastric and intestinal mucosa induced by ethanol (EtOH) and castor oil (CO) in rats. Animals were pre-treated with various doses of SOFAE (50, 100 and 200 mg/kg, b.w., p.o.) and reference molecule (SULF, 100 mg/kg, b.w., p.o.) or vehicle (NaCl .9%). One hour after, animals received EtOH (4 g/kg, b.w.) and after 30 minutes, each animal, except groups 1 and 2 was received castor oil (5 mL/kg, bw., p.o.) by gavage for 2h.
| Groups | Free Iron | Calcium | ||||
|---|---|---|---|---|---|---|
| Gastric mucosa (μmol/mg protein) | Intestinal mucosa (μmol/mg protein) | Plasma (μmol/L) | Gastric mucosa (mmol/mg protein) | Intestinal mucosa (mmol/mg protein) | Plasma (mmol/L) | |
| Control | 27.29 ± 1.92 | 12.37 ± 1.28 | 1.40 ± .07 | .74 ± .06 | .89 ± .09 | .58 ± .04 |
| EtOH | 68.35 ± 4.07* | 28.60 ± .79* | 2.46 ± .11* | 2.63 ± .21* | 3.42 ± 0,54* | 1.08 ± .08* |
| EtOH + CO | 80.55 ± 6.67*# | 33.34 ± 3.31*# | 2.92 ± .08*# | 2.83 ± .31*# | 3.66 ± .36*# | 1.16 ± .06*# |
| EtOH + CO + SOFAE-50 | 60.63 ± 3.85#a | 24.57 ± 2.30#a | 2.14 ± .13#a | 2.56 ± .48#a | 3.25 ± .09#a | .94 ± .03#a |
| EtOH + CO + SOFAE-100 | 50.37 ± 1.48#a | 21.06 ± 1.36#a | 1.81 ± .05#a | 2.20 ± .31#a | 2.94 ± .18#a | .74 ± .08#a |
| EtOH + CO + SOFAE-200 | 40.89 ± 3.73#a | 16.93 ± 3.34#a | 1.43 ± .08#a | 1.77 ± .10#a | 2.57 ± .22#a | .36 ± .06#a |
| EtOH + CO + SULF | 36.77 ± 1.98#a | 15.44 ± 3.44#a | 1.54 ± .15#a | 1.93 ± .35#a | 2.08 ± .18#a | .79 ± .05#a |
The data are expressed as mean ± S.E.M. (n =10). *: P < .05 compared to control group, # : P < .05 compared to ethanol group and a: P < .05 compared to ethanol and castor oil group.
Effect of SOFAE on Inflammation Induced with Ethanol and Castor oil
We further looked at plasma CRP level and plasma, gastric and intestinal ALP activity and showed a significant increase after EtOH/CO intoxication when compared to the control group. Importantly, we showed important anti-inflammatory properties after SOFAE and SULF treatment (Table 6). In addition, we registered that the high dose of SOFAE recorded a more protective effect than sulfasalazine.
Table 6.
Subacute effect of Salvia officinalis flowers aqueous extract (SOFAE) and sulfasalazine (SULF) on CRP level in plasma and ALP activity in plasma, gastric and intestinal mucosa induced by ethanol (EtOH) and castor oil (CO) in rats. Animals were pre-treated with various doses of SOFAE (50, 100 and 200 mg/kg, b.w., p.o.) and reference molecule (SULF, 100 mg/kg, b.w., p.o.) or vehicle (NaCl .9%). One hour after, animals received EtOH (4 g/kg, b.w.) and after 30 minutes, each animal, except groups 1 and 2 was received castor oil (5 mL/kg, bw., p.o.) by gavage for 2h.
| Groups | CRP (μg/mL) | ALP (U/L) at 37°C | ||
|---|---|---|---|---|
| Plasma | Plasma | Gastric mucosa | Intestinal mucosa | |
| Control | .032 ± .02 | 57.29 ± 9.12 | 70.81 ± 6.41 | 79.29 ± 9.86 |
| EtOH | .152 ± .02* | 189.75 ± 5.65* | 192.04 ± 4.78* | 199.60 ± 7.57* |
| EtOH + CO | .172 ± .03*# | 205.05 ± 8.08*# | 241.72 ± 4.28*# | 252.08 ± 8.70*# |
| EtOH + CO + SOFAE-50 | .130 ± .04#a | 179.67 ± 10.23#a | 188.83 ± 6.13#a | 193.42 ± 9.67#a |
| EtOH + CO + SOFAE-100 | .110 ± .03#a | 123.75 ± 4.30#a | 132.46 ± 5.63#a | 141.85 ± 7.96#a |
| EtOH + CO + SOFAE-200 | .088 ± .06#a | 98.54 ± 5.05#a | 107.71 ± 6.72#a | 117.56 ± 2.41#a |
| EtOH + CO + SULF | .072 ± .05#a | 91.90 ± 7.93#a | 98.77 ± 8.93#a | 107.48 ± 8.48#a |
The data are expressed as mean ± S.E.M. (n = 10). *: P < .05 compared to control group, # : P < .05 compared to ethanol group and a: P < .05 compared to ethanol and castor oil group.
Discussion
In the present work, we evaluated the antioxidant potential of SOFAE phytoactive compounds as well as their protective action against gastroduodenal ulcer combined with diarrhea.
Our phytochemical screening showed that SOFAE exhibit an important scavenging action against ABTS radical. This antioxidant activity of SOFAE could be, in part, attributed to its high phenolic compounds levels such as total tannins, flavonols, anthocyanins, and carotenoids. These molecules are defined by their in vitro and in vivo strong antioxidant activity.37-39
The analysis of SOFAE using the HPLC-PDA/ESI‐MS method allowed to the identification of 12 phenolic compounds, especially phenolic acids and flavonoids such as quinic acid, protocatchuic acid, p-coumaric acid, trans cinnamic, catechin (+), naringin, and quercetin. These compounds have important antioxidant and anti-inflammatory properties and can protect the gastrointestinal environment against oxidative stress, inflammation, diarrhea, and peptic ulcer.14,40
In vivo, we firstly showed that LD50 was greater than 3500 mg/kg, b.w., p.o. However, during the period of observation, no mortality or behavioral changes were recorded. Other reports have shown that the LD50 of the aqueous leaf extract is greater than 3200 mg/kg b.w., p.o.19,41
We also demonstrated in the present study that simultaneous acute administration of ethanol and castor oil induced a significant diarrhea. As known, ricinolic acid is the active substance of castor oil, 42 which modifies electrolytes and water transport and stimulates the liberation of different mediators such as prostaglandins, nitric oxide, platelet-activating factor, cAMP, and tachykinins43,44 and produced an aqueous luminal content that circulates rapidly in small and large intestines. 45 According to Singh et al, 46 the inhibition of intestinal Na+, K+ -ATPase activity leads to the inhibitory effect on normal fluid absorption and restriction of the release of acetylcholine. The phytochemical screening also showed that SOFAE contains a low quantity of compounds responsible for laxative activity such as fibers and polysaccharide constituents, especially, crude lignin, true raw cellulose and hemicelluloses, which explains the inhibitory effect of the GIT. Our results are in line with those of Al-Qarawi et al. 47 We also found that SOFAE is rich in total tannins which has an astringent activity 48 and can denature proteins into tannate-protein complex that decreased the GIT leading to antidiarrheal activity. 49
It was also demonstrated that oral administration of ethanol alone or combined with castor oil leads to severe lesions in the gastric and intestinal mucosa. Our results are in line with many previous works. 44 Gastric and intestine diseases are associated to edema, surface coating and epithelial cells alterations, as well as leukocyte infiltration. However, prostaglandins deficiencies are known as the major pathogenic mechanism of alcohol and castor oil-induced digestive system disorders. Although it is well noted that insufficiency in endogenous prostaglandins does not directly contribute to the digestive lesions, it plays a central role in the pathogenic process by making the mucosa more sensitive to aggression. 50 Indeed, we have demonstrated that castor oil administration aggravates the ulceration surface in stomach and small intestine and reduces the mucus volume. Our results are fully in line with previous works showing the same result. 51
However, previous studies have shown a link between ulcers and diarrhea observed in humans. The results of upper and lower gastrointestinal endoscopy, in a 26-year-old man with a 6-month history of diarrhea and abdominal pain, showed mild erosive duodenitis and the patient was started on an inhibitor of the proton pump. 52 Additionally, Chan et al 53 revealed that, clinically, a peptic ulcer with multiple ulcerations observed in the duodenum can be associated with a large volume of watery diarrhea, exhaustion and electrolyte disturbance. Eventually, these two pathologies evolved into gastrinoma. The result of gastroscopy was also identified, in a 47-year-old woman with a large gastric ulcer with chronic diarrhea for two months, starchy material in the gastric body and the presence of a fistula tract at the site of the ulcer previous. 54 The results of histological studies by Rtibi et al 55 on rats showed ulcerations after castor oil-induced diarrhea
Interestingly, sub-acute pretreatment with SOFAE reduced ethanol and castor oil-induced histological changes. Previously, we showed that SOFAE contributed to the prevention of gastric and intestinal epithelium induced by ethanol 56 as well as diarrhea induced by castor oil. 57 In addition, Salvia officinalis extract showed a potent antibacterial capacity in infectious diarrhea. 58 The strong antiulcer and antidiarrheal activities may be partly explained by the richness of SOFAE in total tannins. 59 In addition, sulfasalazine acts directly by inhibiting inflammation. 60 However, for phenolic compounds many mechanisms might be involved such as intracellular mediators by chelation of metal ions (Fe2+, Cu2+), membrane stabilization and increased mucus production, 61 characterized by a film formed by the polymerization of glycoproteins and delay the penetration of endoluminal H+ ions. 62
Simultaneous acute administration of ethanol (4 g/kg, b.w. p.o.) and castor oil (5 mL/kg, b.w., p.o.) altered also the gastric and the small intestine redox balance, as assessed by an increase of lipoperoxidation, a depletion of antioxidant enzymes activity such as SOD, CAT, and GPx, as well as a strong decrease of sulfhydryl groups and GSH levels. The same effect was previously registered by Selmi et al 12 and Sebai et al 13 but for EtOH or CO, each alone. Indeed, reactive oxygen species have been shown to induce gastric and intestinal inflammation leading to mucosa lesions. 63 Also, Gerstgrasser et al 64 exposed a decrease in glutathione synthesis, a key molecule of GPx activity, which plays an important role with catalase in the degradation of hydrogen peroxide. Because of its richness in polyphenolic compounds, the subacute SOFAE administration showed a significant protective effect against ethanol and castor oil-induced oxidative stress. Indeed, we detected in our extract important levels of total tannins, anthocyanins, and carotenoids. The antioxidant activities of these molecules are studied and confirmed in many previous works.37-39
Moreover, administration of ethanol combined with castor oil has been associated to an augmentation of hydrogen peroxide (H2O2) and MDA in the gastric and intestinal mucosa. Because of their extreme reactivity and their capacity to attack all biological materials (DNA, proteins and lipids), hydroxyl radicals are considered the most damaging ROS of oxidative stress. These radicals lead to the initiation of lipid peroxidation chains by reacting with the polyunsaturated fatty acids of the membrane phospholipids and lipoproteins. 65 The superoxide radical’s toxicity seems rather be exercised, in an indirect manner, by reacting with H2O2 leading to OH• radicals generation. 66 In addition, the spontaneous dismutation of superoxide anion is sufficiently rapid, so that hydrogen peroxide production conducts to a deleterious phenomenon via the Fenton reaction. 67 However, all the radical damage induced through ethanol and castor oil seems to be restricted by the SOFAE administration. The Salvia officinalis aqueous extract is very rich in protocatechuic acid, apigenin-7-glucoside, quercetin, kampherol, and naringin. These antioxidant molecules can minimize the toxicity of ethanol and castor oil because of their structure that facilitates the free radicals scavenging. 68
Our results also showed an increase in ionizable calcium levels in plasma, gastric and intestinal mucosa in response to oxidative stress induced by ethanol combined with castor oil acute administration. However, SOFAE treatment for two weeks significantly restored calcium homeostasis. Moreover, bioactive molecules in plant extract cause inhibition of calcium influx and nuclease activity, since Ca2+ depends on nitric oxide synthase (NOS) by producing NO, which causes free radicals increasing the risk of ONOO− formation. 69
More importantly, we have reported that SOFAE prevents against ethanol and castor oil intoxication induced inflammation as assessed by a significant increase in plasma CRP and in plasma, in gastric and intestinal mucosa ALP. Indeed, several studies showed that ethanol and castor oil were accompanied by an inflammatory status via the expression of pro-inflammatory cytokines and biological markers. 70 In this context, Boghori et al 71 revealed that the enzymatic activity of alkaline phosphatase (ALP) significantly increased in patients with a peptic ulcer when compared to healthy control. Expression of alkaline phosphatase in the intestine was increased during inflammation. 72 Although, inflammatory state and oxidative stress were intimately linked, as reported by the constant elevation of oxidative stress markers during inflammatory stomach and bowel disease. 73 Moreover, 3,4-di-O-caffeoyquinic acid which was identified with an abandoned amount in SOFAE (23.938 ppm) possesses an important anti-inflammatory activity by inhibiting NO release, inducible nitric oxide synthase and cyclooxygenase-2 expression, as well as granulocyte-macrophage colony-stimulating factor overproduction.12,74,75 However, the antioxidant properties of polyphenols unquestionably contribute to their anti-inflammatory roles by interrupting the ROS inflammation cycle. These bioactive molecules are known for their anti-inflammatory responses by blocking many roots such as cyclo-oxygenase (COX), lipoxygenase (LOX) nitric oxide synthase (iNOS), cytokines, NF-kB and apoptosis distortion.76,77
Conclusion
These findings clearly indicate that Salvia officinalis flowers aqueous extract protected against ethanol-induced lesions in gastric and intestinal mucosa combined with castor oil-induced diarrhea. Our results also demonstrated that SOFAE protection might be related, at least in part, to its antioxidant and anti-inflammatory properties as well as opposite effects on some intracellular mediators such as free iron, hydrogen peroxide, and ionizable calcium. Therefore, it is interesting to use officinal sage flowers for patients with gastro-intestinal injuries.
Footnotes
Author Contribution: Saber Jedidi, Foued Aloui and Houcine Selmi performed the experiments, analyzed data and wrote the article. Kais Rtibi and Houcem Sammari participated in data processing and analyzed the data. Chaabane Abbes and Hichem Sebai contributed reagents and materials and participated in the data processing and design of experiments. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The financial support of the Tunisian Ministry of Higher Education and Scientific Research and the Institution of Agricultural Research and Higher Education (IRESA) Tunisia is appreciatively acknowledged.
Ethical Consideration: All animal procedures on animals of this study were approved with the National Institute of Health recommendations for the use and care of animals.
ORCID iDs
Saber Jedidi https://orcid.org/0000-0002-5405-8385
Selmi Houcine https://orcid.org/0000-0002-6974-6973
References
- 1.Paguigan ND, Castillo DHB, Chichioco-Hernandez CL. Anti-ulcer activity of leguminosae plants. Arq Gastroenterol. 2014;51(1):815-829. [DOI] [PubMed] [Google Scholar]
- 2.Bouvenot G, Devulder GL. Pathologie Médicale, Gastro-Entérologie, Hépatologie, Hématologie. Paris: Masson; 1995:27-42. [Google Scholar]
- 3.Yeomans ND, Hawkey CJ, Brailsford W, Næsdal J. Gastro-duodenal toxicity of low-dose acetylsalicylic acid: a comparison with non-steroidal anti-inflammatory drugs. Curr Med Res Opin. 2009;25(11):2785-2793. [DOI] [PubMed] [Google Scholar]
- 4.Sebai H, Jabri MA, Souli A, Hosni K, Selmi S, Tounsi H, et al. Protective effect of Artemisia campestris extract against aspirin-induced gastric lesions and oxidative stress in rat. RSC Adv. 2014;4(91):49831-49841. [Google Scholar]
- 5.Gimenez F, Brazier M, Calop J, Dine T, Tchiakpé L, Claerbout JF. Traitement de l’ulcère gastro-duodénal dans pharmacie clinique et thérapeutique. Paris: Edition Masson; 2000:1065. [Google Scholar]
- 6.Shoba FG, Thomas M. Study of antidiarrhoeal activity of four medicinal plants in castor oil induced diarrhea. J Ethnopharmacol. 2001;76(1):73-76. [DOI] [PubMed] [Google Scholar]
- 7.Tashiro N, Budhathoki S, Ohnaka K, Toyomura K, Kono S, Ueki T, et al. Constipation and colorectal cancer risk: the fukuoka colorectal cancer study. Asian Pac J Cancer Prev. 2011;12(8):2025-2030. [PubMed] [Google Scholar]
- 8.Kiela PR, Ghishan FK. Physiology of intestinal absorption and secretion. Best Practice Res Clin Gastroent. 2016;30(2):145-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farthing MJG, Casburn-Jones A, Banks MR. Getting control of intestinal secretion: thoughts for 2003. Dig Liver Dis. 2003;35(6):378-385. [DOI] [PubMed] [Google Scholar]
- 10.Guilford WG, Strombeck DR. Classification, pathophysiology, and symptomatic treatment of diarrheal diseases. In: Guilford WG, ed., et al. eds. Ed. 3; 1996:351-365.Strombeck's Small Animal Gastroenterology. [Google Scholar]
- 11.Toyin YM, Khadijat OF, Saoban SS, Olakunle AT, Abraham BF, Luqman QA. Antidiarrheal activity of aqueous leaf extract of Ceratotheca sesamoides in rats. Bangladesh J Pharmacol. 2012;7(1):14-20. [Google Scholar]
- 12.Selmi S, Rtibi K, Grami D, Sebai H, Marzouki L. Protective effects of orange (Citrus sinensis L.) peel aqueous extract and hesperidin onoxidative stress and peptic ulcer induced by alcohol in rat. Lipids Health Dis. 2017;16(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sebai H, Jabri MA, Souli A, Rtibi K, Selmi S, Tebourbi O, et al. Antidiarrheal and antioxidant activities of chamomile (Matricaria recutita L.) decoction extract in rats. J Ethnopharmacol. 2014;152(2):327-332. [DOI] [PubMed] [Google Scholar]
- 14.Jedidi S, Aloui F, Rtibi K, Sammari H, Selmi H, Rejeb A, et al. Individual and synergistic protective properties of Salvia officinalis decoction extract and sulfasalazine against ethanol-induced gastric and small bowel injuries. RSC Adv. 2020;59(10):35998-36013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roby MHH, Sarhan MA, Selim KAH, Khalel KI. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind Crops Prod. 2013;43:827-831. [Google Scholar]
- 16.Veličković DT, Ranđelović NV, Ristić MS, Veličković AS, Šmelcerović AA. Chemical constituents and antimicrobial activity of the ethanol extracts obtained from the flower, leaf and stem of Salvia officinalis L. J Serb Chem Soc. 2003;68(1):17-24. [Google Scholar]
- 17.Horváthová E, Srančíková A, Regendová-Sedláčková E, Melušová M, Meluš V, Netriová J, et al. Enriching the drinking water of rats with extracts of Salvia officinalis and Thymus vulgaris increases their resistance to oxidative stress. Mutagenesis. 2016;31(1):51-59. [DOI] [PubMed] [Google Scholar]
- 18.Lima CF, Andrade PB, Seabra RM, Fernandes-Ferreira M, Pereira-Wilson C. The drinking of a Salvia officinalis infusion improves liver antioxidant status in mice and rats. J Ethnopharmacol. 2005;97(2):383-389. [DOI] [PubMed] [Google Scholar]
- 19.Jedidi S, Rtibi K, Selmi S, Aloui F, Selmi H, Wannes D, et al. Phytochemical/antioxidant properties and individual/synergistic actions of Salvia officinalis L. aqueous extract and loperamide on gastrointestinal altering motor function. J Med Food. 2019;22(12):1235-1245. [DOI] [PubMed] [Google Scholar]
- 20.Peterson DA, Frank DN, Pace NR, Gordon JI. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe. 2008;3(6):417-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74(10):3583-3597. [DOI] [PubMed] [Google Scholar]
- 22.Kujala TS, Loponen JM, Klika KD, Pihlaja K. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: distribution and effect of cold storage on the content of total phenolics and three individual compounds. J Agric Food Chem. 2000;48(11):5338-5342. [DOI] [PubMed] [Google Scholar]
- 23.Rigane G, Ben Salem R, Sayadi S, Bouaziz M. Phenolic composition, isolation and structure of a new deoxyloganic acid derivative from Dhokar and Gemri-Dhokar olive cultivars. J Food Sci. 2011;76(7):965-973. [DOI] [PubMed] [Google Scholar]
- 24.Marina IH, Velumuttu O, Pekka EK. Carotenoids in finish foods: vegetables, fruits and berries. J Agr Food Chem. 1989;37:655-659. [Google Scholar]
- 25.Lee J, Durst RW, Wrolstad RE, Eisele T, Giusti MM, Hach J, et al. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the ph differential method: collaborative study. J AOAC Intern. 2005;88(5):1269-1278. [PubMed] [Google Scholar]
- 26.Siddhuraju P. The antioxidant activity and free radical-scavenging capacity of phenolics of raw and dry heated moth bean (Vigna aconitifolia) (Jacq.) Marechal seed extracts. Food Chem. 2006;99(1):149-157. [Google Scholar]
- 27.Dicarlo GD, Mascolo N, Izzo AA, Capasso F, Autore G. Effects of quercetin on gastrointestinal tract in rats and mice. Phytother Res. 1994;8(1):42-45. [Google Scholar]
- 28.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421-431. [DOI] [PubMed] [Google Scholar]
- 29.Dingeon B, Ferry JP, Roullet A. Automatic assay of blood sugar by Trinder's method. Ann Biol Clin. 1975;33:3-13. [PubMed] [Google Scholar]
- 30.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28(1):25-30. [Google Scholar]
- 31.Kakkar P, Das B, Viswanathan PN. Modified spectrophotometric assay of SOD. Indian J Biochem Biophys. 1984;2:130-132. [PubMed] [Google Scholar]
- 32.Aebi H. Catalase in vitro. Meth Enzymol. 1984;105:121-126. [DOI] [PubMed] [Google Scholar]
- 33.Flohé L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:14-121. [DOI] [PubMed] [Google Scholar]
- 34.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70-77. [DOI] [PubMed] [Google Scholar]
- 35.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and non-protein sulfhydryl groups in tissue with Ellman’s reagent. Analytical Biochem. 1968;25:192-205. [DOI] [PubMed] [Google Scholar]
- 36.Hartree EF. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972;48(2):422-427. [DOI] [PubMed] [Google Scholar]
- 37.Khennouf S, Benabdallah H, Gharzouli K, Amira S, Ito H, Kim T, et al. Effect of tannins from Quercus suber and Quercus coccifera leaves on ethanol-induced gastric lesions in mice. J Agric Food Chem. 2003;51(5):1469-1473. [DOI] [PubMed] [Google Scholar]
- 38.Shih PH, Yeh CT, Yen GC. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J Agric Food Chem. 2007;55(23):9427-9435. [DOI] [PubMed] [Google Scholar]
- 39.Liebler DC, Stratton SP, Kaysen KL. Antioxidant actions of beta-carotene in liposomal and microsomal membranes: role of carotenoid-membrane incorporation and alpha-tocopherol. Arch Biochem Biophys. 1997;338(2):244-250. [DOI] [PubMed] [Google Scholar]
- 40.Salaritabar A, Darvishi B, Hadjiakhoondi F, Manayi A, Sureda A, Nabavi SF, et al. Therapeutic potential of flavonoids in inflammatory bowel disease. Compreh Rev. 2017;23(28):5097-5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mills S. The Essential Guide to Herbal Safety. Missouri: Elsevier St Louis BK; 2005:558-559. [Google Scholar]
- 42.Burdock GA, Carabin IG, Griffiths JC. Toxicology and pharmacology of sodium ricinoleate. Food Chem Toxicol. 2006;44(10):1689-1698. [DOI] [PubMed] [Google Scholar]
- 43.Tan XD, Chang H, Qu XW, et Caplan M, Gonzalez‐Crussi F, Hsueh W. Platelet activating factor increases mucosal permeability in rat intestine via tyrosine phosphorylation of E-cadherin. Br J Pharmacol. 2000;129(7):1522-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sánchez-Mendoza ME, López-Lorenzo Y, Cruz-Antonio L, Matus-Meza AS, Sánchez-Mendoza Y, Arrieta J. Gastroprotection of Calein D against ethanol-induced gastric lesions in mice: role of prostaglandins, nitric oxide and sulfhydryls. Molecules. 2019;24(3):pii:E622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchida M, Kato Y, Matsuede K, Shode R, Muraoka A, Yemato S. Involvement of NO from nerves in diarrhea induced by castor oil in rats. Jpn J Pharmacol. 2000;82(2):168-170. [DOI] [PubMed] [Google Scholar]
- 46.Singh S, Rai AK, Sharma P, Barshiliya Y, Sihare M, Negi A. Antidiarrhoeal activity of Rotula aquatica in rats. Asian Pacific J Tropical Biomed. 2012;2(1):S175-S177. [Google Scholar]
- 47.Al-Qarawi AA, Ali BH, Al-Mougy SA, Mousa HM. Gastrointestinal transit in mice treated with various extracts of date (Phoenix dactylifera L.). Food Chem Toxicol. 2003;41(1):37-39. [DOI] [PubMed] [Google Scholar]
- 48.Ashok PK, Upadhyaya K. Tannins are astringent. J Pharmac Phytoch. 2012;1(3):45-50. [Google Scholar]
- 49.Kouitcheu MLB, Penlap BV, Kouam J, Ngadjui BT, Fomum ZT, Etoa FX. Evaluation of antidiarrhoeal activity of the stem bark of Cylicodiscus gabunensis (Mimosaceae). Afr J Biotechnol. 2006;5(11):1062-1066. [Google Scholar]
- 50.Halter F, Tarnawski A. Cyclooxygenase 2-implications on maintenance of gastric mucosal integrity and ulcer healing: controversial issues and perspectives. Gut. 2001;49(3):443-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vedøy OB, Hanevik K, Sakkestad ST, Sommerfelt H, Steinsland H. Proliferation of enterotoxigenic Escherichia coli strain TW11681in stools of experimentally infected human volunteers. Gut Pathog. 2018;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buchkremer J, Epple HJ, Tröger H, Heller F, Frericks B, Herpel E, et al. A young man with chronic diarrhea and epigastric pain. Med Klin. 2010;105(4):242-245. [DOI] [PubMed] [Google Scholar]
- 53.Chan DK, Simonetto DA, Hauser SC. 60-year-old man with chronic diarrhea and peptic ulcer disease. Mayo Clin Proc. 2015;90(1):e1-5. [DOI] [PubMed] [Google Scholar]
- 54.Sharma S, Bhatia R, Vasudevan A. Abdominal pain and diarrhea in peptic ulcer disease. Gastroenter. 2021;161(2):e48-e49. [DOI] [PubMed] [Google Scholar]
- 55.Rtibi K, Hammami I, Selmi S, Gramia D, Sebai H, Amri M, et al. Phytochemical properties and pharmacological effects of Quercus ilex L. aqueous extract on gastrointestinal physiological parameters in vitro and in vivo. Biomed Pharmacother. 2017;94:787-793. [DOI] [PubMed] [Google Scholar]
- 56.Fiorentin RT, De Mello MB, Aquino AMK, Rigo BA, Loss CG, Schwanz M, et al. Antiulcerogenic potential of Salvia officinalis L. extract in rats. J Appl Pharmac Sci. 2013;3(8):32-35. [Google Scholar]
- 57.Al-Maamouri JAI. Evaluation of the antimotility related diarrhoeal of the sage tea Salvia officinalis L. in laboratory mice. Res J Biol Sci. 2011;6(1):33-36. [Google Scholar]
- 58.Mosafa E, Yahyaabadi S, Doudi M. In vitro antibacterial properties of sage (Salvia officinalis) ethanol extracts against multidrug resistant Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumonia. Zahedan J Res Med Sci. 2014;16(10):42-46. [Google Scholar]
- 59.Demarque DP, Callejon DR, de Oliveira GG, Silva DB, Carollo CA, Lopes NP. The role of tannins as antiulcer agents: a fluorescence-imaging based study. Revista Bras Farmacog. 2018;28(4):425-432. [Google Scholar]
- 60.Brown JB, Lee G, Managlia E, Grimm GR, Dirisina R, Goretsky T, et al. Mesalamine inhibits epithelial β-catenin activation in chronic ulcerative colitis. Gastroenter. 2010;138(2):595-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.da Silva DM, Martins J, de Oliveira DR, Florentino IF, da Silva D, Dos Santos F, et al. Effect of allantoin on experimentally induced gastriculcers: pathways of gastroprotection. Eur J Pharmacol. 2018;821:68-78. [DOI] [PubMed] [Google Scholar]
- 62.Gufo Kamguia HF, Fokunang C, Ngameni B, Njinkio Nono B, Tembe-Fokunang E. Effet cytoprotecteur de l’extrait aqueux des racines de Dorstenia psilurus sur l’ulcère gastrique chez les rats males de la souche Wistar. Health Sci Dis. 2011;12(4). [Google Scholar]
- 63.Kwiecień S, Brzozowski T, Konturek SJ. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J Physiol Pharmacol. 2002;53(1):39-50. [PubMed] [Google Scholar]
- 64.Gerstgrasser A, Melhem H, Leonardi I, Atrott K, Schäfer M, Werner S, et al. Cell-specific activation of the Nrf2 antioxidant pathway increases mucosal inflammation in acute but not in chronic colitis. J Crohn's Colitis. 2017;11(4):485-499. [DOI] [PubMed] [Google Scholar]
- 65.Fang JG, Lu M, Chen ZH, Zhu HH, Li Y, Yang L, et al. Antioxidant effects of resveratrol and its analogues against the free-radical-induced peroxidation of linoleic acid in micelles. Inside Chem. 2002;8(18):4191-4198. [DOI] [PubMed] [Google Scholar]
- 66.Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem. 2006;290(1-2):87-96. Das SK, Roy C. The protective role of Aegle marmelos on aspirin–induced gastro-duodenal ulceration in albino rat model: a possible involvement of antioxidants. Saudi J Gastroenterol. 2019;18(3):188–194. [DOI] [PubMed] [Google Scholar]
- 67.Lindenmeier M, Burkon A, Somoza V. A novel method to measure both the reductive and the radical scavenging activity in a linoleic acid model system. Mol Nutr Food Res. 2007;51(12):1441-1446. [DOI] [PubMed] [Google Scholar]
- 68.Lü J-M, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med. 2010;14(4):840-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hernandez-Muñoz R, Montiel-Ruiz C, Vazquez-Martinez O. Gastric mucosal cell proliferation in ethanol induced chronic mucosal injuryis related to oxidative stress and lipid peroxidation in rats. Labo Invest. 2000;80(8):1161-1169. [DOI] [PubMed] [Google Scholar]
- 70.Kim DH, Kang SH, Jeong WS, Moon HS, Lee ES, Kim SH, et al. Serum C-reactive protein (CRP) levels in young adults can be used to discriminate between inflammatory and non-inflammatory diarrhea. Dig Dis Sci. 2013;58(2):504-508. [DOI] [PubMed] [Google Scholar]
- 71.Boghori M, Aghamaali M, Sariri R, Mohamadpour F, Ghafouri H. Salivary enzymes and flow rate: Markers of peptic ulcer. J Oral Biol Craniofac Res. 2014;4(1):24-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lalles JP. Intestinal alkaline phosphatase: novel functions and protective effects. Nutr Rev. 2014;72(2):82-94. [DOI] [PubMed] [Google Scholar]
- 73.Tian T, Wang Z, Zhang J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid Med Cell Longev. 2017;2017:4535194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karker M, Falleh H, Msaada K, Smaoui A, Abdelly C, Legault J, et al. Antioxidant, anti-inflammatory and anticancer activities of the medicinal halophyte Reaumuria vermiculata. EXCLI J. 2016;15:297-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weiss M, Moldawer LL, Schneider EM. Granulocyte colony-stimulating factor to prevent the progression of systemic nonresponsiveness in systemic inflammatory response syndrome and sepsis. Blood J. 2019;93(2):425-439. [PubMed] [Google Scholar]
- 76.Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10(11):1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ren S, Wei Y, Wang R, Wei S, Wen J, Yang T, et al. Rutaecarpine ameliorates ethanol- induced gastric mucosal injury in mice by modulating genes related to inflammation, oxidative stress and apoptosis. Front Pharmacol. 2020;11:600295. [DOI] [PMC free article] [PubMed] [Google Scholar]