Abstract
Functional alterations after ischemic stroke have been described with Magnetic Resonance Imaging (MRI) and perfusion Positron Emission Tomography (PET), but no data on in vivo synaptic changes exist. Recently, imaging of synaptic density became available by targeting synaptic vesicle protein 2 A, a protein ubiquitously expressed in all presynaptic nerve terminals. We hypothesized that in subacute ischemic stroke loss of synaptic density can be evaluated with 11C-UCB-J PET in the ischemic tissue and that alterations in synaptic density can be present in brain regions beyond the ischemic core. We recruited ischemic stroke patients to undergo 11C-UCB-J PET/MR imaging 21 ± 8 days after stroke onset to investigate regional 11C-UCB-J SUVR (standardized uptake value ratio). There was a decrease (but residual signal) of 11C-UCB-J SUVR within the lesion of 16 stroke patients compared to 40 healthy controls (ratiolesion/controls = 0.67 ± 0.28, p = 0.00023). Moreover, 11C-UCB-J SUVR was lower in the non-lesioned tissue of the affected hemisphere compared to the unaffected hemisphere (ΔSUVR = −0.17, p = 0.0035). The contralesional cerebellar hemisphere showed a lower 11C-UCB-J SUVR compared to the ipsilesional cerebellar hemisphere (ΔSUVR = −0.14, p = 0.0048). In 8 out of 16 patients, the asymmetry index suggested crossed cerebellar diaschisis. Future research is required to longitudinally study these changes in synaptic density and their association with outcome.
Keywords: Ischemic stroke, molecular imaging, positron emission tomography, synaptic density, SV2A
Introduction
Predicting outcome in patients with subacute ischemic stroke is challenging. Most of the developed prediction tools focus on motor recovery and use baseline characteristics obtained in the acute phase that are associated with outcome. Age and stroke severity, measured by a variety of clinical scales, are the most frequently used clinical characteristics and a number of Magnetic Resonance Imaging (MRI) parameters and results of Transcranial Magnetic Stimulation (TMS) have been investigated. MRI and TMS are mostly used to assess the integrity of the corticospinal tract and if the corticospinal tract is too severely damaged, the proportional recovery rule (which states that 3 months after stroke, patients will have regained about 70% of their maximum recovery potential) is not valid anymore. 1 For most of these prediction tools, however, external validation and implementation in clinical practice is lacking.2–4 Therefore, there is a need to identify subclinical, preferentially modifiable biomarkers of motor recovery during the acute and subacute phase to improve prognostication and potentially evaluate the response to treatment aimed at improving recovery. 5 However, as long as the processes underlying recovery remain obscure, identification of such a biomarker is hard. Preclinical studies investigating the mechanisms underlying stroke recovery identified changes of synaptic density in the peri-ischemic area and remote regions, suggesting plasticity of the brain.6–8 Functional MRI and perfusion Positron Emission Tomography (PET) in humans during the recovery period revealed alterations in activity with evidence of both ipsilesional as well as contralesional and bilateral cerebral (over)activation. 9 Functional changes also occur in the cerebellum after supratentorial stroke, known as crossed cerebellar diaschisis. Crossed cerebellar diaschisis is characterized by a loss of function of the contralesional cerebellum due to disruption of cerebro-cerebellar pathways.10–15 Recently, imaging of synaptic density has become available by targeting synaptic vesicle protein 2 A (SV2A), a protein ubiquitously expressed in presynaptic nerve terminals.16–19 This novel PET tracer offers a unique opportunity to gain insight in the spatial and temporal dynamics of synaptic alterations after ischemic stroke in humans, which may be a key factor in unraveling the processes underlying recovery.
In this study, we investigated the feasibility of SV2A PET imaging in patients with subacute ischemic stroke. We hypothesized that ischemic brain tissue is characterized by loss of synaptic density.20,21 Furthermore, we investigated if SV2A imaging could also identify alterations in synaptic density beyond the ischemic core possibly in the context of plasticity or injury beyond the ischemic lesion.6–9 In addition, we explored if crossed cerebellar diaschisis could be detected by SV2A PET as disruption of cerebro-cerebellar connections could translate into decrease of presynaptic vesicles.
Methods
This study was approved by the local University Hospital Ethics committee (UZ Leuven/KU Leuven) and was conducted in accordance with the latest version of the Declaration of Helsinki. All participants (or relatives in case the patient was unable to give written informed consent) signed written informed consent prior to participation. This study is part of a larger, multimodal, multitracer, longitudinal imaging study with different patient populations (ClinicalTrials.gov: NCT03514524).
Participants
We recruited patients between 18–85 years with a first ischemic stroke. Inclusion criteria were: first ischemic stroke within 2 weeks prior to inclusion; significant motor deficit of the upper extremity, defined as MRC (Medical Research Council) 22 grade 3 or less for finger extension and/or elbow flexion and/or shoulder abduction and sufficient cooperation/comprehension to cooperate with clinical evaluation. Major exclusion criteria were: pre-stroke Barthel Index <19 23 ; history of traumatic brain injury, Alzheimer disease or other important neurological disorders; contra-indications for MRI; use of levetiracetam/brivaracetam and/or previous participation in other research studies involving ionizing radiation with >1 mSv over the past 12 months. Patients underwent an extensive test battery at inclusion, including NIHSS (National Institutes of Health Stroke Scale),24,25 Fugl-Meyer upper extremity (FMUE) and lower extremity (FMLE) 26 and Barthel Index (BI). 23 Patients underwent 11C-UCB-J PET/MR imaging within the first month after the event.
We also included healthy controls between 18–85 years, with main exclusion criteria: history of important neurological, psychiatric or internal medical disorder; chronic use of medication with effects on the central nervous system; pregnancy; history or active use of alcohol or other drug abuse; contra-indications for MR; brain white matter disease Fazekas 3 (for subjects ≥60 years) or other relevant MR abnormalities and previous participation in other research studies involving ionizing radiation with >1 mSv over the past 12 months.
Image acquisition and reconstruction
All participants underwent simultaneous 11C-UCB-J PET/MR imaging 60–90 minutes post tracer injection on a General Electric (GE, Milwaukee, WI, USA) Signa PET/MR (time-of-flight (TOF) PET, 3-Tesla MR) scanner. Median injected 11C-UCB-J activity was 214 MBq (IQR: 204-231 MBq) with a mean specific activity of 244 ± 147 GBq/µmol for patients. Median injected 11C-UCB-J activity for healthy controls was 246 MBq (IQR: 216-294 MBq) with a median specific activity of 222 GBq/µmol (IQR: 159–318 GBq/µmol). PET acquisitions were performed in list mode, rebinned into 6 frames of 5 minutes and reconstructed using vendor software. As reconstruction algorithm, an ordered subset expectation maximization (28 subsets, 4 iterations) was used including TOF information and decay, scatter, attenuation, deadtime and random correction. Isotropic Gaussian post smoothing was performed to reduce the noise (full width at half maximum (FWHM) of 4 mm; final image resolution 5 mm). Individual attenuation correction was performed using a validated zero echo time (ZTE) approach. 27
We acquired 3 D T1-weighted MR images with an 8-channel head coil (plane: sagittal; TE: 3.2 ms; TR: 8.5 ms; TI: 450 ms; flip angle: 12; receiver bandwidth: 31.25 kHz; NEX: 1; voxelsize: 1 × 1 × 1 mm) as well as 3 D T2-weighted FLAIR images (plane: sagittal; TE: 136/137 ms; TR: 8500 ms; TI: 2298 ms; receiver bandwidth: 31.25 kHz; NEX: 1; voxelsize: 0.7 × 1 × 1 mm).
Image processing
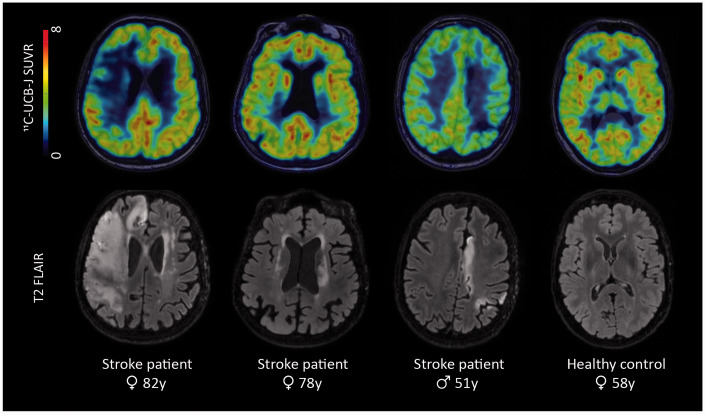
We preprocessed the 11C-UCB-J PET images using PMODv3.9 (PMOD technologies, Zurich, Switzerland): first, motion correction was applied by rigidly realigning each frame to the first frame, then averaging all frames. Second, we co-registered the PET images to the corresponding T1-weighted MRI data and calculated standardized uptake value ratios (SUVR) using the centrum semiovale (CS) as reference region (see Supplementary Methods for detailed description). Representative images of 3 patients and 1 healthy control are shown in Figure 1.
Figure 1.
Representative images of different stroke patients and one healthy control, shown in radiological convention. Top row: 11C-UCB-J SUVR images overlaid on T1-weighted MR images; bottom row: T2 FLAIR images showing the lesions of the patients. Patient 1 was a 82-year old female patient with a large ischemic stroke in the right middle cerebral artery territory. Patient 2 was a 78-year old female patient with a subcortical stroke in the left corona radiata, internal capsule and lentiform nucleus. Patient 3, a 51-year old male, suffered an ischemic stroke in the watershed area of the left hemisphere. The last column shows a 11C-UCB-J SUVR image and T2 FLAIR image of a 58-year-old, female healthy control. SUVR = standardized uptake value ratio.
For volume-of-interest (VOI)-based analyses, we delineated VOI’s with FreeSurfer (Laboratory for Computational Neuroimaging v6.0, Boston, USA; cortical parcellation based on the Desikan-Killiany atlas,28,29 subcortical structures as described in Fischl et al. 30 ). As the large cortical stroke lesions could interfere with proper VOI delineation, we used virtual brain grafting (version 4) for all patients with cortical involvement of the stroke. Virtual brain grafting is a processing pipeline that replaces a delineated lesion by healthy tissue, based on the contralesional hemisphere. This healthy T1-weighted image is then used as input for VOI delineation in FreeSurfer. 31
To correct for partial volume effects (PVE), we applied a region-based voxel-wise (RBV) partial volume correction (point spread function 5 mm).32,33 For optimization of this partial volume correction, the lesion itself was not considered as a homogeneous entity, but subdivision in grey and white matter regions was preserved as SV2A uptake is different in grey and white matter. The peri-ischemic area was not handled as separate region for RBV as this area is not homogeneous either. However, in contrast to the lesion, subdividing the peri-ischemic area in grey and white matter regions would have created too many too small regions resulting in less robust RBV estimation.
We defined the stroke lesions semi-automatically using the 3 D T2-weighted FLAIR data and created a peri-ischemic area by applying a 4 mm simple binary dilation of the lesion mask. We adapted the peri-ischemic area manually to exclude voxels not consisting of brain parenchyma. Representative images of these VOI’s are shown in Figure 2. To be able to compare values of the stroke lesions and peri-ischemic areas with healthy controls, we transformed all VOI’s of the lesions and peri-ischemic areas to MNI space (Montreal Neurological Institute; see Supplementary Methods for more information about the non-linear deformation fields). We created an average 11C-UCB-J SUVR image of the healthy controls in MNI space (=AV HC) to extract an average normal value for all lesions and peri-ischemic areas. Moreover, to compare the values of the lesions and peri-ischemic areas to the homologous areas in the unaffected hemisphere (=mirror regions), we flipped the 11C-UCB-J SUVR images of all patients in MNI space over the sagittal axis and we co-registered them to the original MNI space. We extracted the average values of the lesion/peri-ischemic mirror areas.
Figure 2.
Representative images of four patients with stroke, shown in radiological convention. Top row: Volumes-of-interest, overlaid on T1-weighted MR images. The lesion is visualized in red, the peri-ischemic area in yellow, the non-lesioned tissue of the affected hemisphere in blue and the non-lesioned tissue of the unaffected hemisphere in green. Bottom row: corresponding T2 FLAIR images. VOI = volume-of-interest.
We defined the non-lesioned tissue as all grey matter brain tissue of the affected and unaffected hemisphere that was not part of the ischemic lesion or peri-ischemic area. For each hemisphere, we extracted an average 11C-UCB-J SUVR value of the non-lesioned tissue (see Figure 2 for visualization of the non-lesioned tissue).
Simulation study
To assess the impact of changes in blood flow on SUVR quantification, we performed a simulation study using the 11C-UCB-J input function of one healthy volunteer (historical data), by applying a one-tissue compartment model. 34 We simulated region-specific changes in tracer delivery by altering K1 and adapting k2 accordingly to preserve the K1/k2 ratio (=VT (volume of distribution)) constant (PMODv3.9).35,36 We applied following baseline values: K1 =0.45 ml.cm−³.min−1 and k2 = 0.015 min−1 (VT = 30.0 ml.cm−³) for the cerebral cortex, K1 = 0.45 ml.cm−³.min−1 and k2 = 0.020 min−1 (VT = 22.5 ml.cm−³) for the cerebellum and K1 = 0.123 ml.cm−³.min−1 and k2 = 0.023 min−1 (VT = 5.3 ml.cm−³) for the CS. We used the simulation of the cerebral cortex relative to the CS to assess the impact of perfusion asymmetries in non-lesioned tissue and the simulation of the cerebellum relative to the CS to assess crossed cerebellar diaschisis.
Based on available literature data, we assumed regional perfusion asymmetry in the non-lesioned tissue ranging from −15% to +15%37–39 and for crossed cerebellar diaschisis, we assumed asymmetry in perfusion ranging from −35% to +15%.11,40
We assessed the possible role of perfusion changes on ΔSUVR by reporting the results of the simulation and the measured values of the non-lesioned tissue (measured ΔSUVRnon-lesioned) and cerebellar hemispheres (measured ΔSUVRcerebellum).
Statistics
We performed general statistics in RStudio (v1.1.463. RStudio, Inc., Boston, MA) and we presented the data as mean ± standard deviation if normally distributed and as median (interquartile range (IQR)) if not normally distributed. We verified normality of the distributions with Shapiro-Wilk test (α = 0.05).
We tested differences in baseline characteristics with tests for proportions or Wilcoxon rank sum tests as appropriate.
To assess the changes in synaptic density within the lesion and the peri-ischemic area, we calculated the ratios for the lesion and peri-ischemic area for each patient compared to the set of healthy controls:
We conducted one sample t-tests to assess whether the ratios were different from 1.
As the normal inter-subject variation of 11C-UCB-J SUVR appeared rather high, we calculated the ratios for the lesions and peri-ischemic areas compared to the lesion/peri-ischemic mirror as well, as this may be more sensitive to detect a decrease:
We conducted one sample t-tests to assess whether the ratios were different from 1. We also conducted a one sample t-tests to assess whether the mean SUVR in the lesion area was different from 1.
We investigated the relationship between ratiolesion/controls and ratiolesion/mirror with the timing of scan (= days after stroke) with a Pearson correlation.
To detect differences in the non-lesioned tissue, we used Welch’s t-tests for group comparisons of VOI’s between patients and healthy controls. We conducted a paired t-test to detect differences between both hemispheres within the patients with ischemic stroke.
To investigate crossed cerebellar diaschisis on a group level, we used Welch’s t-test. We performed a paired t-test between ipsilesional and contralesional cerebellar hemispheres for the patients. We calculated individual asymmetry indices (AI’s) for all subjects to define crossed cerebellar diaschisis, as described in literature:11–13
AI’s of patients were considered significantly asymmetric if they exceeded the mean ± 2 standard deviation boundaries (= z-score >2 of <-2) of the AI’s of the controls. A significant AI in disfavor of the contralesional cerebellar hemisphere (= positive AI value) is the hallmark of crossed cerebellar diaschisis.
We also explored the effect of lesion size on 11C-UCB-J SUVR by correlating lesion size with SUVR, with lesion ratios and peri-ischemic ratios and with cerebellar AI (Spearman correlation).
For all group level analyses with healthy controls, the right hemisphere was assigned as the equivalent of the affected hemisphere and the left hemisphere the equivalent of the unaffected hemisphere as the majority of the patients had a stroke lesion in the right hemisphere.
As this study was exploratory, significance level for all statistical analyses was set to α = 0.05 without correction for multiple comparisons. 95% confidence intervals (95% CI) are reported as well.
We performed all analyses without and with correction for PVE. All reported results are without correction for PVE unless otherwise specified. All PVE-corrected results are shown in the Supplementary Results (including Supplementary Table 1). In addition, we decided to analyze the data in the subgroup of patients without vascular lesions in the reference region (CS, n = 9) considering the possibility that variability in nonspecific uptake could increase as a result of white matter pathology. We reported these results only if they differed in reaching statistical significance from the main analysis. The full results of the subanalysis are provided in Supplementary Table 2.
Results
Demographics
Baseline characteristics are shown in Table 1 (and Supplementary Table 3). We included 20 patients with a first ischemic stroke of whom 19 underwent 11C-UCB-J PET/MR imaging. We excluded 3 patients due to missing or insufficient quality of the MR data resulting in a dataset of 16 patients (5 male, 11 female). Median age was 74 years (IQR: 66–82 years) and patients were scanned at a mean interval of 21 ± 8 days after the event. Mean NIHSS at inclusion was 12 ± 5, median FMUE was 7.5 (IQR: 4–13), mean FMLE was 12 ± 8 and mean Barthel Index was 4 ± 3. Median stroke volume was 26 ml (IQR: 7.1–105 ml) which was present in the right hemisphere in 11 out of 16 patients.
Table 1.
Demographics and clinical characteristics.
| Patients with stroke | Healthy controls | p-value | |
|---|---|---|---|
| Number of subjects (n) | 16 | 40 | |
| Male / Female (n) | 5/11 | 19/21 | 0.25 |
| Age (years) | 74 (66–82) | 59.5 (43–72) | 0.0085* |
| Injected 11C-UCB-J activity (MBq; median (IQR)) | 214 (204–231) | 246 (216–294) | 0.016* |
| Interval between event and scan (days; mean ± SD) | 21 ± 8 | NA | |
| NIHSS (mean ± SD) | 12 ± 5 | NA | |
| Fugl-Meyer – upper extremity (median (IQR)) | 7.5 (4–13) | NA | |
| Fugl-Meyer – lower extremity (mean ± SD) | 12 ± 8 | NA | |
| Barthel index (mean ± SD) | 4 ± 3 | NA | |
| Lesioned hemisphere (n) | 11 right/5 left | NA | |
| Lesion volume (ml; median (IQR)) | 26 (7.1–105) | NA |
Significant p-values are marked with *. For NIHSS, higher values represent more severe stroke (maximum score 42); for Barthel index, higher values represent more independence in activities of daily living (maximum score 20); for Fugl-Meyer, higher values represent better performance (maximum score upper extremity 66, maximum score lower extremity 34). IQR = interquartile range; NA = not applicable; NIHSS = National Institutes of Health Stroke Scale; SD = standard deviation.
The set of healthy controls consisted of 40 subjects (19 male, 21 female). These controls were younger compared to the patients (median age 59.5 years (IQR 43–72 years); 95% CI = 3 – 23, p = 0.0085).
11C-UCB-J SUVR within the lesion and correlation with time of imaging
The mean 11C-UCB-J SUVR within the lesion was 1.94 ± 0.71, which is significantly higher than 1 (95% CI = 1.6 – 2.3, p = 9.3*10−5). The ratioslesion/controls of 11C-UCB-J SUVR in the lesion were lower than 1 (mean = 0.67 ± 0.28, 95% CI = 0.52 – 0.82, p = 0.00023). The ratioslesion/mirror also differed from 1 (mean = 0.66 ± 0.24, 95% CI = 0.53 – 0.78, p = 3.3*10−5; Table 2). There was no correlation between the decrease of SUVR (expressed as ratioslesion/controls or ratioslesion/mirror) and time between the index event and imaging (ratioslesion/controls: rP = −0.30, 95% CI = −0.69 – 0.23, p = 0.26; ratioslesion/mirror: rP = −0.35, 95% CI = −0.72 − 0.18, p = 0.19; Supplementary Figure 1).
Table 2.
Results of 11C-UCB-J SUVR in the different regions for patients and healthy controls.
| Patients with stroke | Healthy controls | One sample t-test (µ=1) | |
|---|---|---|---|
| Lesion | 1.94 ± 0.71 | NA | p = 9.3*10-5 * |
| ratiolesion/controls | 0.67 ± 0.28 | p = 0.00023 * | |
| ratiolesion/mirror | 0.66 ± 0.24 | p = 3.3*10-5 * | |
| Peri-ischemic area | 2.66 ± 0.58 | NA | |
| ratioperi/controls | 0.86 ± 0.18 | p = 0.0076 * | |
| ratioperi/mirror | 0.85 ± 0.13 | p = 0.00035 * | |
| Two sample t-test | |||
| Non-lesioned tissue | |||
| affected hemisphere | 4.44 ± 0.81 | 4.61 ± 0.41 | p = 0.43 |
| unaffected hemisphere | 4.61 ± 0.85 | 4.59 ± 0.41 | p = 0.95 |
| paired t-test | p = 0.0035 * | ||
| Cerebellar hemisphere | |||
| ipsilesional | 3.98 ± 0.77 | 3.83 ± 0.37 | p = 0.44 |
| contralesional | 3.84 ± 0.77 | 3.82 ± 0.38 | p = 0.91 |
| paired t-test | p = 0.0048 * |
Data are presented as mean ± standard deviation. Significant p-values are marked with *. NA = not applicable; SUVR = Standardized Uptake Value Ratio.
11C-UCB-J SUVR within the peri-ischemic area
The mean 11C-UCB-J SUVR within the peri-ischemic area was 2.66 ± 0.58. The ratiosperi/controls of 11C-UCB-J SUVR in the peri-ischemic area were lower than 1 (mean = 0.86 ± 0.18, 95% CI = 0.76 – 0.96, p = 0.0076) as were the ratiosperi/mirror (mean = 0.85 ± 0.13, 95% CI = 0.79 – 0.92, p = 0.00035; Table 2).
We performed partial volume correction (PVC) as this region is located at the border between the uninjured brain parenchyma and the ischemic lesion with decreased uptake. After PVC the ratiosperi/controls were no longer different from 1 (mean = 0.93 ± 0.19; 95% CI = 0.83 – 1.0; p = 0.16), but the ratiosperi/mirror of 11C-UCB-J SUVR still differed from 1 (mean = 0.92 ± 0.12, 95% CI = 0.86 – 0.99; p = 0.026).
11C-UCB-J SUVR in the non-lesioned tissue
11C-UCB-J SUVR in the non-lesioned grey matter part of the affected hemisphere (mean = 4.44 ± 0.81) did not differ from 11C-UCB-J SUVR of healthy controls in the right hemisphere (mean = 4.61 ± 0.41, 95% CI = −0.61 – 0.27, p = 0.43). 11C-UCB-J SUVR of the grey matter of the unaffected hemisphere (mean = 4.61 ± 0.85) did not differ from 11C-UCB-J SUVR of healthy controls in the left hemisphere (mean = 4.59 ± 0.41, 95% CI = −0.45 – 0.48, p = 0.95). In an intra-individual analysis, we did identify decreased 11C-UCB-J SUVR when comparing the non-lesioned tissue grey matter of the affected hemisphere with the unaffected hemisphere (mean difference = −0.17, 95% CI = −0.27 – −0.064, p = 0.0035; Table 2).
In the subgroup analysis of patients without vascular lesions in the CS (n = 9), we also detected a decrease of 11C-UCB-J SUVR in the non-lesioned grey matter of the affected hemisphere compared to the hemispheric grey matter of healthy controls (mean patients = 4.04 ± 0.63, 95% CI = −1.1 – −0.073, p = 0.029).
11C-UCB-J SUVR in the cerebellar hemispheres
On a group level, 11C-UCB-J SUVR did not differ in the ipsilesional (mean = 3.98 ± 0.77), nor contralesional cerebellar hemisphere (mean = 3.84 ± 0.77) of the patients with stroke compared to healthy controls (right: mean = 3.83 ± 0.37; left: mean = 3.82 ± 0.38; ipsilesional vs right: 95% CI = −0.27 – 0.58, p = 0.44; contralesional vs left: 95% CI = −0.40 – 0.45, p = 0.91). In stroke patients we assessed the presence of an asymmetry by comparing the ipsilesional cerebellar hemisphere with the contralesional cerebellar hemisphere. The contralesional cerebellar hemisphere showed a decrease of 11C-UCB-J SUVR compared to the ipsilesional cerebellar hemisphere (mean difference = −0.14, 95% CI = −0.24 – −0.051, p = 0.0048; Table 2).
The mean AI in the cerebellar hemispheres of healthy controls was 0.18 ± 1.6. The mean AI of patients was 3.6 ± 4.9 and in 9 patients (56%) the AI exceeded the 2 standard deviation boundaries of the AI in healthy controls, indicative of cerebellar diaschisis. In most of those patients (8; 89%) the asymmetry was in disfavor of the contralesional cerebellum.
Effect of lesion size on 11C-UCB-J SUVR
There was no correlation between lesion size and SUVR in any of the investigated regions (lesion, peri-ischemic area, non-lesioned tissue and cerebellar hemispheres). There was no correlation between lesion size and lesion ratios or peri-ischemic ratios, nor between lesion size and cerebellar AI.
Simulation of perfusion effects
The results of the simulation study are shown in Supplementary Figure 2 and Supplementary Table 4. The measured ΔSUVRnon-lesioned ranged from -13% to +5.5% (mean = −3.6% ± 4.1%), whereas the simulated ΔSUVR was −4.3% for ΔK1 −15% and +2.5% for ΔK1 +15% for cerebral cortical regions. The measured ΔSUVRcerebellum ranged from −16% to +6.6% (mean = −4.0% ± 5.4%), whereas the simulated ΔSUVR was −7.2% for ΔK1 −35% and −0.27% for ΔK1 +15% for the cerebellum.
Discussion
In this study, we identified a decrease, but no total loss, of synaptic density with 11C-UCB-J PET within the ischemic stroke lesion several weeks after the index event. Evaluating synaptic density in the non-ischemic brain parenchyma revealed a reduction in the ipsilesional hemisphere compared to the contralesional hemisphere and we detected crossed cerebellar diaschisis.
The strong decrease of synaptic density signal within the FLAIR-based ischemic lesion is a largely expected finding as ischemia results in cell death and tissue loss. However, we did not observe a total loss of synaptic density in the first weeks after the ischemic stroke, as reflected by values above 1 in most lesions. Potentially, synaptic loss could progress over weeks which we studied by analyzing the correlation between time after onset and synaptic density. Likely due to the low sample size, rather narrow range of time after stroke onset and imaging between patients and heterogeneous population we could not detect this correlation.
In the peri-ischemic area we could not demonstrate any increases in synaptic density in the tissue surrounding the lesion. This is in contrast to preclinical research that described increases in synaptic density and metabolism in the peri-ischemic tissue.6,41 Heterogeneity within the peri-ischemic area, the early time window between the index event and imaging as well as insufficient resolution of PET to detect subtle changes, may account for the lack of identifying this in our cohort. However, dissimilarities between different species or non-representative preclinical stroke models may also contribute to the contrasting results. 42 Our results are in line with a 18 F-flumazenil study, showing a decreased signal in the peri-infarct cortex compared to contralesional cortex. 43 18F-flumazenil binds to postsynaptic GABAA (gamma-aminobutyric acid) receptors and is therefore considered as a proxy for neuronal density.
In the non-lesioned tissue of the affected hemisphere, we found a lower synaptic density compared to the unaffected hemisphere. Stroke lesions and underlying white matter disease were quite heterogeneous as reflected in the wide range in SUVR, which may have hampered detection of changes in the total cohort of patients compared to controls. In the subgroup of patients without white matter pathology in the CS, we did observe a reduction in the ipsilesional hemisphere compared to controls. This may suggest reduced synaptic density to exist in the ipsilesional hemisphere outside the ischemic lesion. This reduction may be explained by Wallerian degeneration, a phenomenon in which distal axons of damaged neurons degenerate and therefore cause a decrease of presynaptic vesicles. Although most studies investigating Wallerian degeneration after stroke focused on the corticospinal tract,44,45 reduced intrahemispheric connections are also described.46,47 Likewise, transcallosal Wallerian degeneration leading to reduced interhemispheric connections has been reported as well,48,49 but this was not (yet) seen in our population. Follow-up on outcome of this cohort and further research in larger samples will have to explore if this reduced synaptic density within and/or outside the ischemic lesion is linked to outcome and may have a predictive value.
Neural plasticity, including reorganization and strengthening of existing connections, could translate into functional recovery. Underlying processes such as axonal sprouting and synaptic alterations may be at play. Functional compensation after ischemic stroke has been explored in patients by measuring relative cerebral blood flow using 15O tracers and resulted in a variety of patterns of ipsilesional, contralesional or bilateral (over)activation.50–56 The populations studied previously were always relatively small and heterogeneous, both in terms of included patients as well as timing of scans, possibly responsible for rather ambiguous results. We explored changes in synaptic density by measuring SV2A, which is a marker of structural presynaptic vesicles, as a potential biomarker of neuronal loss or neural plasticity. So far, 11C-UCB-J PET has only been used to detect losses of synaptic density57–64 and we have no data on the relationship between changes in SV2A density and synaptic function, making it uncertain if upregulation of SV2A is related to plasticity or recovery. It is unclear which time window is most optimal to identify loss of synapses, axonal reorganization or strengthening of existing connections. Most studies exploring functional recovery after stroke so far, focused on later time windows. Considering the short time interval between the ischemic insult and PET imaging in the presented data, we assumed to be able to capture synaptic loss but possibly not yet increases in the SUVR, as potential indicator of synaptic plasticity. In the first month after ischemic stroke, we did not detect any increases in synaptic density. It is possible that the neural plasticity has only been initiated and will further develop over weeks and months which may become more apparent during the follow-up of our cohort with imaging at 6 months and 2 years after the index event.
Crossed cerebellar diaschisis is a known phenomenon in the first weeks after ischemic stroke and reflects a decrease in function, metabolism or perfusion of the contralesional cerebellum caused by a supratentorial, remotely connected lesion. Perfusion PET/SPECT (single photon emission computed tomography),10–13,40,65 FDG (fluorodeoxyglucose) PET14 and ASL (arterial spin labeled) MRI 15 have revealed these changes. We demonstrated that crossed cerebellar diaschisis can be assessed with 11C-UCB-J PET and the rate as measured with 11C-UCB-J in our population (50%) was in line with previously reported percentages as measured with perfusion SPECT/PET in patients with supratentorial lesions (46-62.5%).11,40,65 One patient had a significant cerebellar asymmetry reversed to the expected crossed cerebellar diaschisis, which is previously described as well. 11 Thus far, the exact interpretation of the crossed cerebellar diaschisis that we found is unclear. Although it can reflect a loss of cortical input connections, it is not excluded that the results are (partially) driven by changes in perfusion as quantification with SUVR may be influenced by perfusion effects. However, the results of the simulation study suggests that perfusion changes are likely not sufficient to fully explain our findings as some individual differences in cerebellar asymmetry were more pronounced than could be explained by perfusion changes alone. A 123I-iomazenil SPECT study could not identify crossed cerebellar diaschisis in two patients scanned at an earlier time point. 66 This might suggest that the presynaptic SV2A tracer and postsynaptic GABAA receptor tracer behave differently, but differences in time window after the event and the fact that not all stroke patients manifest crossed cerebellar diaschisis, may be other explanations.
Our study has some limitations. The first relates to the quantification method which made use of a reference tissue normalization with 60-90 minutes SUVR data normalized on the CS as proxy to the distribution volume. Although this reference region is validated by different research groups19,34,67,68 and used in several patient populations,57–59,61–64 validation in a cohort of ischemic stroke patients is lacking. The main concern is the potential influence of white matter disease in the CS as it is unsure whether the nonspecific binding in the CS is influenced by white matter pathology. There was no difference in SUV of the CS between patients and healthy controls in our study (See Supplementary Material), but since the role of white matter changes on the SUV in the CS is unknown, we conducted a subgroup analysis in patients without white matter pathology in the CS (n = 9). This approach reduced the variation in SUVR compared to the total cohort which is suggestive of a potential influence of white matter disease in the CS. This needs further follow-up and study in a larger cohort of patients with white matter pathology to ensure the validity of the CS as a reference region or evaluate a different reference region. However, the reproducibility of the results in the within subjects analyses, which are not influenced by the reference region, suggests that our findings are valid, even despite the potential influence of white matter pathology. Moreover, the results of a post hoc sensitivity analysis with pons as normalization region were similar to the results of the subanalysis with CS as reference region (data not shown). The pons is no validated reference region for 11C-UCB-J PET quantification, but the similarities between all different methods strengthen the validity of our results. Another limitation of the quantification method is the possible bias in SUVR that is introduced by perfusion changes and/or changes in blood brain barrier permeability due to blood brain barrier breakdown in the subacute phase after ischemic stroke. Based on our simulation study we cannot exclude that perfusion changes after stroke may have influenced the SUVR. This could have been further explored by dynamic scan acquisition with full kinetic modelling which however was considered unfeasible in this patient population. Another limitation is the difference in age between the two groups as the patients were older compared to the controls. We have previously demonstrated that there is no major influence of age on synaptic density with SUVR as measure and therefore we included the full set of healthy controls instead of limiting the control cohort to age-matched subjects as this would decrease the power of the study. 69 To confirm this hypothesis we conducted a post hoc analysis with an age-matched control cohort (data not shown) which revealed comparable results. Another difference we observed between patients and controls was the lower injected 11C-UCB-J activity which was inevitable as patients will receive follow-up scans, necessitating a lower injected dose to not exceed the legally allowed yearly radiation dose (as this study is part of a multimodal, multitracer imaging study). Last, 11C-UCB-J PET is a marker of presynaptic SV2A vesicles and the approximation of SV2A PET as proxy for synaptic density is under the assumption that there is a correlation between density of SV2A vesicles and synaptic density and that this correlation is not influenced by the pathology under investigation. Moreover, although there is no evidence of off-target binding to inflammatory cells so far, this possibility should be kept in mind as subacute stroke is accompanied by an inflammatory process. Future in vitro assays should assess the possibility of off-target binding to inflammatory or other cells.
A strength of our study is the inclusion of healthy controls. Neuroimaging studies of patients with stroke frequently define the contralateral hemisphere as control region instead of hemispheres of healthy controls. This hampers the detection of potential changes occurring in the unaffected hemisphere. This may become of even greater relevance in the longitudinal follow-up of patients which will enable making comparisons with the contralateral hemisphere within each subject, but also with healthy controls. With this follow-up we intend to provide more insights in changes in synaptic density during the recovery phase after an ischemic insult.
In conclusion, 11C-UCB-J PET was able to demonstrate a decrease, but not total loss, of synaptic density in ischemic stroke lesions in the first month after the index event. When comparing non-lesioned tissue in the two cerebral hemispheres, an asymmetry in synaptic density was present likely caused by a decrease in the ipsilesional hemisphere. 11C-UCB-J PET was able to detect crossed cerebellar diaschisis in a proportion of patients with ischemic stroke. Further follow-up of patients with 11C-UCB-J PET is needed to gain more insight in the temporal and spatial changes of synaptic density after ischemic stroke and their relation to outcome parameters.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211047759 for Changes in synaptic density in the subacute phase after ischemic stroke: A 11C-UCB-J PET/MR study by Laura Michiels, Nathalie Mertens, Liselot Thijs, Ahmed Radwan, Stefan Sunaert, Mathieu Vandenbulcke, Geert Verheyden, Michel Koole, Koen Van Laere and Robin Lemmens in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
We thank all the participants for their willingness to participate in these studies. The authors are also grateful to Kwinten Porters and Jef Van Loock for their contribution to the scanning and data handling and the PET radiopharmacy team, the MR radiology team and nuclear medicine medical physics team of UZ Leuven for their skilled contributions.
Data availability: The data that support the findings of this study are not publicly available due to privacy/ethical restrictions. Upon reasonable request, the anonymized data could be shared on approval by the local Ethics Committee.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by an FWO grant (FWO/G093218N) and KU Leuven internal C2 funding (C24-17-063).
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LM, AR, SS, MV and GV report no disclosures. NM is a predoctoral fellow of FWO (= Research Foundation Flanders). LT reports no conflict of interest and is supported by the grants EU Eurostars project funding, Promobilia and internal KU Leuven funding (internal EU Horizon 2020 runner-up funding). MK has performed contract research through KU Leuven for UCB and Merck. KVL has performed contract research through KU Leuven for Merck, Janssen Pharmaceuticals, UCB, Cerveau, Syndesi, Eikonizo, GE Healthcare and Curasen; he has received speaker fees from GE Healthcare. RL is a Senior Clinical Investigator of FWO.
Authors’ contributions: LM, GV, MK, KVL and RL were responsible for conceptualization of the study. LM, NM, AR, SS, MK, KVL and RL contributed to design the methodology. Data collection and analyses was done by LM, NM and LT, supervised by MV, GV, MK, KVL and RL. LM and RL prepared and wrote the first draft of the manuscript. All authors critically revised the intellectual content of the manuscript.
ORCID iDs: Laura Michiels https://orcid.org/0000-0002-1208-5334
Nathalie Mertens https://orcid.org/0000-0002-2409-7586
Stefan Sunaert https://orcid.org/0000-0002-1177-4680
Supplemental material: Supplemental material for this article is available online.
References
- 1.Byblow WD, Stinear CM, Barber PA, et al. Proportional recovery after stroke depends on corticomotor integrity. Ann Neurol 2015; 78: 848–859. [DOI] [PubMed] [Google Scholar]
- 2.Harvey RL. Predictors of functional outcome following stroke. Phys Med Rehabil Clin N Am 2015; 26: 583–598. [DOI] [PubMed] [Google Scholar]
- 3.Stinear CM, Smith M-C, Byblow WD. Prediction tools for stroke rehabilitation. Stroke 2019; 50: 3314–3322. [DOI] [PubMed] [Google Scholar]
- 4.Kim B, Winstein C. Can neurological biomarkers of brain impairment be used to predict poststroke motor recovery? A systematic review. Neurorehabil Neural Repair 2017; 31: 3–24. [DOI] [PubMed] [Google Scholar]
- 5.Ward NS, Carmichael ST. Blowing up neural repair for stroke recovery: preclinical and clinical trial considerations. Stroke 2020; 51: 3169–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stroemer RP, Kent TA, Hulsebosch CE. Increase in synaptophysin immunoreactivity following cortical infarction. Neurosci Lett 1992; 147: 21–24. [DOI] [PubMed] [Google Scholar]
- 7.Mostany R, Chowdhury TG, Johnston DG, et al. Local hemodynamics dictate long-term dendritic plasticity in peri-infarct cortex. J Neurosci 2010; 30: 14116–14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarkson AN, Lopez-Valdes HE, Overman JJ, et al. Multimodal examination of structural and functional remapping in the mouse photothrombotic stroke model. J Cereb Blood Flow Metab 2013; 33: 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke 2003; 34: 1553–1566. [DOI] [PubMed] [Google Scholar]
- 10.Baron JC, Bousser MG, Comar D, et al. “ Crossed cerebellar diaschisis” in human supratentorial brain infarction. Trans Am Neurol Assoc 1981; 105: 459–461. [PubMed] [Google Scholar]
- 11.Kim SE, Choi CW, Yoon BW, et al. Crossed-Cerebellar diaschisis in cerebral infarction: Technetium-99m-HMPAO SPECT and MRI. J Nucl Med 1997; 38: 14–19. [PubMed] [Google Scholar]
- 12.Takasawa M, Watanabe M, Yamamoto S, et al. Prognostic value of subacute crossed cerebellar diaschisis: single-photon emission CT study in patients with Middle cerebral artery territory infarct. Am J Neuroradiol 2002; 23: 189–193. [PMC free article] [PubMed] [Google Scholar]
- 13.Sobesky J, Thiel A, Ghaemi M, et al. Crossed cerebellar diaschisis in acute human stroke: a PET study of serial changes and response to supratentorial reperfusion. J Cereb Blood Flow Metab 2005; 25: 1685–1691. [DOI] [PubMed] [Google Scholar]
- 14.Tien RD, Ashdown BC. Crossed cerebellar diaschisis and crossed cerebellar atrophy: correlation of MR findings, clinical symptoms, and supratentorial diseases in 26 patients. AJR Am J Roentgenol 1992; 158: 1155–1159. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Guan M, Lian HJ, et al. Crossed cerebellar diaschisis detected by arterial spin-labeled perfusion magnetic resonance imaging in subacute ischemic stroke. J Stroke Cerebrovasc Dis 2014; 23: 2378–2383. [DOI] [PubMed] [Google Scholar]
- 16.Nabulsi NB, Mercier J, Holden D, et al. Synthesis and preclinical evaluation of 11C-UCB-J as a PET tracer for imaging the synaptic vesicle glycoprotein 2A in the brain. J Nucl Med 2016; 57: 777–784. [DOI] [PubMed] [Google Scholar]
- 17.Finnema SJ, Nabulsi NB, Eid T, et al. Imaging synaptic density in the living human brain. Sci Transl Med 2016; 8: 348ra96. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Cai Z, Zhang W, et al. Synthesis and in vivo evaluation of [18F]UCB-J for PET imaging of synaptic vesicle glycoprotein 2A (SV2A). Eur J Nucl Med Mol Imaging 2019; 46: 1952–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finnema SJ, Nabulsi NB, Mercier J, et al. Kinetic evaluation and test–retest reproducibility of [11 C]UCB-J, a novel radioligand for positron emission tomography imaging of synaptic vesicle glycoprotein 2A in humans. J Cereb Blood Flow Metab 2018; 38: 2041–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmeijer J, van Putten M. Ischemic cerebral damage: an appraisal of synaptic failure. Stroke 2012; 43: 607–615. [DOI] [PubMed] [Google Scholar]
- 21.Brown CE, Wong C, Murphy TH. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke 2008; 39: 1286–1291. [DOI] [PubMed] [Google Scholar]
- 22.Medical Research Council. Aids to examination of the peripheral nervous system. Memorandum no. 45. London: Her Majesty’s Stationery Office, 1976, pp.1–62.
- 23.Mahoney F, Barthel DW. Functional evaluation: the Barthel index. M State Med J 1965; 14: 61–65. [PubMed] [Google Scholar]
- 24.Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20: 864–870. [DOI] [PubMed] [Google Scholar]
- 25.Lyden P, Brott T, Tilley B, et al. Improved reliability of the NIH stroke scale using video training. Stroke 1994; 25: 2220–2226. [DOI] [PubMed] [Google Scholar]
- 26.Fugl-Meyer A, Jääskö L, Leyman I, et al. The post stroke hemiplegic patient. I. A method for evaluation of physical performance. Scand J Rehabil Med 1975; 7: 13–31. [PubMed] [Google Scholar]
- 27.Schramm G, Koole M, Willekens SMA, et al. Regional accuracy of ZTE-based attenuation correction in static and dynamic brain PET/MR. Front Phys 2019; 7: 211. [Google Scholar]
- 28.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex 2004; 14: 11–22. [DOI] [PubMed] [Google Scholar]
- 29.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuron 2006; 33: 341–355. [DOI] [PubMed] [Google Scholar]
- 30.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33: 341–355. [DOI] [PubMed] [Google Scholar]
- 31.Radwan AM, Emsell L, Blommaert J, et al. Virtual brain grafting: enabling whole brain parcellation in the presence of large lesions. Neuroimage 2021; 229: 117731. [DOI] [PubMed] [Google Scholar]
- 32.Thomas BA, Erlandsson K, Modat M, et al. The importance of appropriate partial volume correction for PET quantification in Alzheimer’s disease. Eur J Nucl Med Mol Imaging 2011; 38: 1104–1119. [DOI] [PubMed] [Google Scholar]
- 33.Greve DN, Salat DH, Bowen SL, et al. Different partial volume correction methods lead to different conclusions: an 18 F-FDG-PET study of aging. Neuroimage 2016; 132: 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koole M, van Aalst J, Devrome M, et al. Quantifying SV2A density and drug occupancy in the human brain using [11C]UCB-J PET imaging and subcortical white matter as reference tissue. Eur J Nucl Med Mol Imaging 2019; 46: 396–406. [DOI] [PubMed] [Google Scholar]
- 35.Kolinger GD, Vállez García D, Lohith TG, et al. A dual-time-window protocol to reduce acquisition time of dynamic tau PET imaging using [18F]MK-6240. EJNMMI Res 2021; 11: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Berckel BNM, Ossenkoppele R, Tolboom N, et al. Longitudinal amyloid imaging using 11C-PiB: Methodologic considerations. J Nucl Med 2013; 54: 1570–1576. [DOI] [PubMed] [Google Scholar]
- 37.Richardson JD, Baker JM, Morgan PS, et al. Cerebral perfusion in chronic stroke: implications for lesion-symptom mapping and functional MRI. Behav Neurol 2011; 24: 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C, Miao P, Liu J, et al. Cerebral blood flow features in chronic subcortical stroke: lesion location-dependent study. Brain Res 2019; 1706: 177–183. [DOI] [PubMed] [Google Scholar]
- 39.Miao P, Wang C, Li P, et al. Altered gray matter volume, cerebral blood flow and functional connectivity in chronic stroke patients. Neurosci Lett 2018; 662: 331–338. [DOI] [PubMed] [Google Scholar]
- 40.Yamauchi H, Fukuyama H, Yamaguchi S, et al. Crossed cerebellar hypoperfusion in unilateral major cerebral artery occlusive disorders. J Nucl Med 1992; 33: 1637–1641. [PubMed] [Google Scholar]
- 41.Jablonka JA, Burnat K, Witte OW, et al. Remapping of the somatosensory cortex after a photothrombotic stroke: dynamics of the compensatory reorganization. Neuroscience 2010; 165: 90–100. [DOI] [PubMed] [Google Scholar]
- 42.Sommer CJ. Ischemic stroke: experimental models and reality. Acta Neuropathol 2017; 133: 245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Funck T, Al-Kuwaiti M, Lepage C, et al. Assessing neuronal density in peri-infarct cortex with PET: effects of cortical topology and partial volume correction. Hum Brain Mapp 2017; 38: 326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puig J, Pedraza S, Blasco G, et al. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR Am J Neuroradiol 2010; 31: 1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puig J, Blasco G, Schlaug G, et al. Diffusion tensor imaging as a prognostic biomarker for motor recovery and rehabilitation after stroke. Neuroradiology 2017; 59: 343–351. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Zhang Y, Xing S, et al. Secondary neurodegeneration in remote regions after focal cerebral infarction: a new target for stroke management? Stroke 2012; 43: 1700–1705. [DOI] [PubMed] [Google Scholar]
- 47.Haque ME, Gabr RE, Hasan KM, et al. Ongoing secondary degeneration of the limbic system in patients with ischemic stroke: a longitudinal MRI study. Front Neurol 2019; 10: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta RK, Saksena S, Hasan KM, et al. Focal Wallerian degeneration of the corpus callosum in large middle cerebral artery stroke: Serial diffusion tensor imaging. J Magn Reson Imaging 2006; 24: 549–555. [DOI] [PubMed] [Google Scholar]
- 49.Wang LE, Tittgemeyer M, Imperati D, et al. Degeneration of corpus callosum and recovery of motor function after stroke: a multimodal magnetic resonance imaging study. Hum Brain Mapp 2012; 33: 2941–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelles G, Spiekermann G, Jueptner M, et al. Reorganization of sensory and motor systems in hemiplegic stroke patients. Stroke 1999; 30: 1510–1516. [DOI] [PubMed] [Google Scholar]
- 51.Weiller C, Ramsay SC, Wise RJS, et al. Individual patterns of functional reorganization in the human cerebral cortex after capsular infraction. Ann Neurol 1993; 33: 181–189. [DOI] [PubMed] [Google Scholar]
- 52.Weiller C, Chollet F, Friston KJ, et al. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol 1992; 31: 463–472. [DOI] [PubMed] [Google Scholar]
- 53.Chollet F, DiPiero V, Wise RJS, et al. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol 1991; 29: 63–71. [DOI] [PubMed] [Google Scholar]
- 54.Calautti C, Leroy F, Guincestre JY, et al. Dynamics of motor network overactivation after striatocapsular stroke: a longitudinal PET study using a Fixed-Performance paradigm. Stroke 2001; 32: 2534–2542. [DOI] [PubMed] [Google Scholar]
- 55.Carey LM, Abbott DF, Egan GF, et al. Motor impairment and recovery in the upper limb after stroke. Stroke 2005; 36: 625–629. [DOI] [PubMed] [Google Scholar]
- 56.Lewis DH, Toney LK, Baron JC. Nuclear medicine in cerebrovascular disease. Semin Nucl Med 2012; 42: 387–405. [DOI] [PubMed] [Google Scholar]
- 57.Chen MK, Mecca AP, Naganawa M, et al. Assessing synaptic density in Alzheimer disease with synaptic vesicle glycoprotein 2A positron emission tomographic imaging. JAMA Neurol 2018; 75: 1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mecca AP, Chen M-K, O'Dell RS, et al. In vivo measurement of widespread synaptic loss in Alzheimer’s disease with SV2A PET. Alzheimers Dement 2020; 16: 974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vanhaute H, Ceccarini J, Michiels L, et al. In vivo synaptic density loss is related to tau deposition in amnestic mild cognitive impairment. Neurology 2020; 95: e545–e553. [DOI] [PubMed] [Google Scholar]
- 60.Holmes SE, Scheinost D, Finnema SJ, et al. Lower synaptic density is associated with depression severity and network alterations. Nat Commun 2019; 10: 1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delva A, Van Weehaeghe D, Koole M, et al. Loss of presynaptic terminal integrity in substantia Nigra in early Parkinson’s disease. Mov Disord 2020; 35: 1977–1986. [DOI] [PubMed] [Google Scholar]
- 62.Matuskey D, Tinaz S, Wilcox KC, et al. Synaptic changes in Parkinson disease assessed with in vivo imaging. Ann Neurol 2020; 87: 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holland N, Jones PS, Savulich G, et al. Synaptic loss in primary tauopathies revealed by [11C]UCB-J positron emission tomography. Mov Disord 2020; 35: 1834–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finnema SJ, Toyonaga T, Detyniecki K, et al. Reduced synaptic vesicle protein 2A binding in temporal lobe epilepsy: A [11C]UCB‐J positron emission tomography study. Epilepsia 2020; 61: 2183–2193. [DOI] [PubMed] [Google Scholar]
- 65.Kushner M, Alavi A, Reivich M, et al. Contralateral cerebellar hypometabolism following cerebral insult: a positron emission tomographic study. Ann Neurol 1984; 15: 425–434. [DOI] [PubMed] [Google Scholar]
- 66.Hashimoto J, Sasaki T, Itoh Y, et al. Brain SPECT imaging using three different tracers in subacute cerebral infarction. Cinical Nucl Med 1998; 23: 275–277. [DOI] [PubMed] [Google Scholar]
- 67.Rossano S, Toyonaga T, Finnema SJ, et al. Assessment of a white matter reference region for 11 C-UCB-J PET quantification. J Cereb Blood Flow Metab 2020; 40: 1890–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mertens N, Maguire RP, Serdons K, et al. Validation of parametric methods for [11C]UCB-J PET imaging using subcortical white matter as reference tissue. Mol Imaging Biol 2020; 22: 444–452. [DOI] [PubMed] [Google Scholar]
- 69.Michiels L, Delva A, van Aalst J, et al. Synaptic density in healthy human aging is not influenced by age or sex: a 11C-UCB-J PET study. Neuroimage 2021; 232: 117877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X211047759 for Changes in synaptic density in the subacute phase after ischemic stroke: A 11C-UCB-J PET/MR study by Laura Michiels, Nathalie Mertens, Liselot Thijs, Ahmed Radwan, Stefan Sunaert, Mathieu Vandenbulcke, Geert Verheyden, Michel Koole, Koen Van Laere and Robin Lemmens in Journal of Cerebral Blood Flow & Metabolism