Abstract
The role of tenascin-C (TNC) in ischemic stroke pathology is not known despite its prognostic association with cerebrovascular diseases. Here, we investigated the effect of TNC knockdown on post-stroke brain damage and its putative mechanism of action in adult mice of both sexes. Male and female C57BL/6 mice were subjected to transient middle cerebral artery occlusion and injected (i.v.) with either TNC siRNA or a negative (non-targeting) siRNA at 5 min after reperfusion. Motor function (beam walk and rotarod tests) was assessed between days 1 and 14 of reperfusion. Infarct volume (T2-MRI), BBB damage (T1-MRI with contrast), and inflammatory markers were measured at 3 days of reperfusion. The TNC siRNA treated cohort showed significantly curtailed post-stroke TNC protein expression, motor dysfunction, infarction, BBB damage, and inflammation compared to the sex-matched negative siRNA treated cohort. These results demonstrate that the induction of TNC during the acute period after stroke might be a mediator of post-ischemic inflammation and secondary brain damage independent of sex.
Keywords: Matricellular protein, ischemia-reperfusion, blood-brain barrier, neuroprotection, inflammation
Introduction
Ischemic stroke induces significant time-dependent changes in extracellular matrix (ECM) composition, which contributes to BBB disruption, inflammation, and remodeling of the brain parenchyma. 1 Tenascin-C (TNC) is a major ECM glycoprotein that regulates diverse cellular functions in developing and adult brains in health and disease.2–4 TNC was shown to be highly induced in response to CNS injury and promote neuroinflammation. 3
TNC induction is also an early prognostic biomarker of cerebrovascular and neurodegenerative diseases like Alzheimer’s disease, subarachnoid hemorrhage (SAH), temporal lobe epilepsy, spinal cord injury, and large artery atherosclerotic stroke.3,5,6 Significantly higher TNC levels are positively correlated with poor outcomes in hemorrhagic stroke patients. 7 Furthermore, TNC levels are positively correlated with c-reactive protein levels and inflammation in patients with atrial fibrillation. 8 In addition, elevated circulating TNC levels are associated with large artery atherosclerotic stroke and post-stroke chronic inflammation in humans. 5
In the brain, TNC is expressed by astrocytes, microglia and neurons. 9 TNC is also expressed by circulating macrophages and neutrophils9,10 that enter the brain after stroke. TNC induces proinflammatory cytokines by activating toll-like receptor 4 (TLR-4) 11 as well via integrin receptors, the epidermal growth factor receptor, complement receptor 3, and interleukin (IL) 1 receptor like-1. 12 TNC modulates the microglial function through TLR dependent/independent signaling.4,9
Recent studies demonstrated the involvement of TNC in promoting thrombosis, BBB disruption, neuronal apoptosis, and inflammation after CNS diseases.13,14 Further, TNC deletion showed anti-inflammatory effects, primarily due to a reduction in microglial activation and TLR-4 dependent effects in SAH. 15 Acute induction of TNC after traumatic brain injury was shown to be region-specific and linked to inflammation and brain damage. 6 Despite existing clinical and preclinical evidence on the significance of TNC in brain diseases, its pathologic role after ischemic stroke has not yet been explored. To fill that void, we currently tested the impact of TNC knockdown on post-stroke brain damage and motor function recovery in adult mice of both sexes.
Methods and methods
All animal procedures were approved by the University of Wisconsin Research Animal Resources and Care Committee. Animals were cared for in compliance with the Guide for the Care and Use of Laboratory Animals [U.S. Department of Health and Human Services publication no. 86–23 (revised)]. All procedures were conducted in compliance with the “Animal Research: Reporting of In Vivo Experiments” guidelines. 16 Mice were randomly assigned to experimental groups. When mice were subjected to 1 h transient MCAO, adult females showed relatively less neurological deficits compared to adult males. Hence, we used 4 exclusion criteria. (1) absence of CBF lowering during MCAO, (2) no noticeable neurological deficits on day 1 of reperfusion, (3) no sign of infarction in MRI on day 1 of reperfusion and (4) an intracerebral hemorrhage after euthanasia.
Focal cerebral ischemia
Adult C57BL/6 mice of both sexes (12 weeks old; Jackson Laboratories USA) were subjected to 1 h of transient middle cerebral artery occlusion (MCAO) under isoflurane anesthesia followed by 1 to 14 days of reperfusion as described earlier. 17 Sham-operated animals served as the control. Regional cerebral blood flow (rCBF) and physiological parameters (blood pressure, pH, PO2, PCO2, hemoglobin, and glucose) were monitored, and rectal temperature was maintained at 37 ± 0.5°C during surgery and recovery from anesthesia (data were given in supplementary figure 4 and table 1). Post-surgical care and monitoring were provided as specified in the IMPROVE guidelines. 18
siRNA treatment
Mice were injected via a retroorbital route with 25 nmol (100 µl) of Ambion® silencer select in vivo grade TNC siRNA or negative siRNA (Neg siRNA; cat# 4404020; siRNA ID#: s813) (a cocktail of 3 siRNAs in each case; Thermo-Fisher USA) mixed with 50 µl polyethylene glycol-liposome in vivo transfection reagent and 10 µl transfection enhancer (Altogen Biosystems USA). The sequences (5′->3′) of the TNC siRNAs used as a cocktail are (cat# 4457308; siRNA IDs# s75239-s775241) sense: CUG AGA UAG AUG UUC CAA Att, CAA UGA CUG CAG CAA CCA Att, and GGG AGA UCA UUU UCC GAA Att, and antisense: UUU GGA ACA UCU AUC UCA Gca, UUG GUU GCU GCA GUC AUU Ggg, and UUU CGG AAA AUG AUC UCC Cat. The siRNA cocktails were injected at 5 min of reperfusion (siRNA delivery to brain data were given in supplementary figure 4 C).
MRI
At 3 days of reperfusion following transient MCAO, mice were anesthetized with isoflurane and subjected to T1- and T2-weighted MRI using a 4.7‐T small animal system with 205/120/HD/S gradient 210 mm bore Varian magnet (Agilent Technologies USA). Respiration rate was monitored during the imaging. T1-MRI scans were acquired 25 min after the administration of 0.3 ml of the contrast agent gadobenate dimeglumine (Gd; 529 mg/ml; i.p.). 8-10 equidistant coronal slices/mice were acquired with a slice thickness of 1.0 mm. None of the mice showed any distress or mortality during or after scanning. MRI scans were analyzed using NIH ImageJ software with an FDF plugin by a person blinded to the study groups to estimate infarct volume (T2-MRI) and BBB breakdown (T1-MRI). Mice that underwent MRI scanning with a contrast agent were not used for any further analysis.
Motor function analysis
Motor function was evaluated by the rotarod test (4 min on a cylinder rotating at 8 rpm) and beam walk test (number of foot faults while crossing a 120-cm long beam) at days 1, 7, and 14 of reperfusion by a person blinded to the groups, as described earlier.17,19 Mice were trained in the tasks for 3 days before MCAO. (detailed methodology was given in supplementary methods)
Real-time PCR, Western blotting, and immunostaining
Proteins and RNA were extracted from the ipsilateral cortex of mice. Real-time PCR was performed in triplicate with the SYBR-green method. Primers sequence (5′ to 3′) for amplifying TNC, TNR, TNW/N, TNX, TLR-4, TNF-α, IL-6, IL-1β, MCP-1, TRAIL, and 18S were given in supplementary table 2. Relative quantification of gene expression was normalized to 18S and calibrated to the appropriate control by the comparative CT method (2−ΔΔCt). RT2 Profiler PCR array (Qiagen) detailed methodology was given in supplementary methods and data were given in supplementary material 2.
Protein samples (40 μg) were electrophoresed, transferred to nitrocellulose membranes, and probed with antibodies against TNC, claudin 5, occludin and zona occludens 1 (1:1000; ThermoFisher Scientific), TLR-4, myeloperoxidase (MPO), intercellular adhesion molecule-1 (ICAM-1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:2000 each; all from Santa Cruz) followed by appropriate HRP-conjugated secondary antibodies (1:3000; Santa Cruz/ABCAM). Blots were developed using enhanced chemiluminescence (Life Technologies USA) (data were given in supplementary figure 3) and quantified with Image Studio software (LI-COR Biotechnology USA). Brains were embedded in paraffin, sectioned (coronal; 5 µm) and immunostained with antibodies against TNC (1:200; ThermoFisher Scientific), NeuN (1:300; Millipore), GFAP (1:300; ABCAM), TMEM119 (1:500; Synaptic Systems), cleaved caspase-3 (1:200; Cell Signaling Technology), Ly-6G (1:200; R&D systems), and TLR-4 (1:200; Santa Cruz) followed by Alexa Fluor or Discovery UltraMap secondary antibodies (1:1000; ThermoFisher Scientific). In some experiments, sections were counterstained with Discovery RED detection kit followed by hematoxylin or DAPI. Brain coordinates and microscopic navigation were used to ensure the accurate location of the images across the groups (image location and individual image sets data were given in supplementary figures 1 and 2). Negative control (without primary or secondary antibody) and BlockAid™ Blocking Solution (Life Technologies USA) were used to decrease false positives or cross staining.
Statistical analysis
Power analysis was used to determine the sample size that detect the significant differences. Data is presented as mean ± SD. After the Shapiro-Wilk normality test, the data distribution, two groups were compared with the Mann-Whitney U test and multiple groups with one-way ANOVA with Tukey’s multiple comparisons test. The data collected repeatedly from the same set of subjects at different time points (such as rotarod and beam-walk tests) were analyzed by two-way repeated-measures ANOVA with Sidak's multiple-comparisons test.
Results
TNC siRNA prevented post-ischemic TNC protein induction
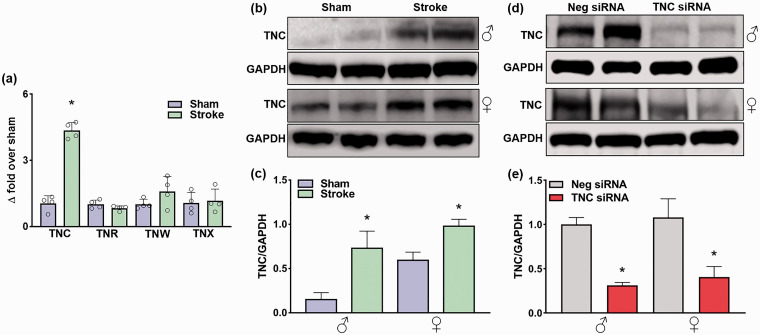
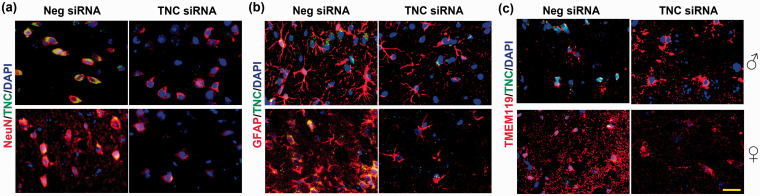
In adult male mice, transient MCAO led to a significant upregulation of TNC mRNA, but not other tenascins (R/W/X) mRNAs in the peri-infarct cortex at 1 day of reperfusion compared with sham (n = 4/group, *p < 0.05) (Figure 1(a)). TNC protein levels also significantly increased at 1 day of reperfusion in both sexes compared with sex-matched shams (n = 5/group, *p < 0.05) (Figure 1(b) and (c)). In both males and females, the TNC siRNA treatment led to a significant knockdown of TNC protein levels in the peri-infarct cortex at 3 day of reperfusion compared with the Neg siRNA treatment (n = 5/group, *p < 0.05) (Figure 1(d) and (e)). In the peri-infarct cortex of both male and female mice treated with Neg siRNA, TNC protein was observed to be mainly localized in neurons (NeuN+) (Figure 2(a)), while astroglia (GFAP+) and microglia (TMEM119+) showed low TNC staining (n = 4/group, coordinates matched locations) (Figure 2(b) and (c)). The TNC siRNA treated cohort showed very little TNC protein in all of the cell types evaluated (Figure 2(a) to (c)).
Figure 1.
Focal ischemia significantly increased TNC protein expression, which was inhibited by TNC siRNA treatment. (a) Real-time PCR analysis shows the expression of tenascins at 24 h of reperfusion in the ipsilateral cerebral cortex of adult male mice subjected to 1 h MCAO. Values are mean ± SD; n = 4/group, *p < 0.05 compared with sham by using the Mann-Whitney U test. (b) & (c) TNC protein expression in adult male and female mice at 1 day of reperfusion following transient ischemia. (d) & (e) The TNC siRNA treated cohort showed significant knockdown compared with the non-targeting Neg siRNA treated cohort at 3 days of reperfusion following transient ischemia. The values in the histograms are means ± SD (n = 5/group). *p < 0.05 compared with the respective sham in (b) & (c) and *p < 0.05 compared with the respective Neg siRNA in (d) & (e) by using the Mann-Whitney U test.
Figure 2.
TNC protein was expressed in neurons, astrocytes, and microglia after focal ischemia. Neurons, astrocytes, and microglia were immunostained with NeuN, GFAP, and TMEM119, respectively. Brain sections were from representative male and female mice of each cohort treated with TNC siRNA or Neg siRNA following 1 h of transient MCAO and 3 days of reperfusion. The scale bar is 50 μm. Similar results were observed in n = 4/group.
Post-stroke TNC siRNA treatment decreased brain damage and improved motor function in both sexes
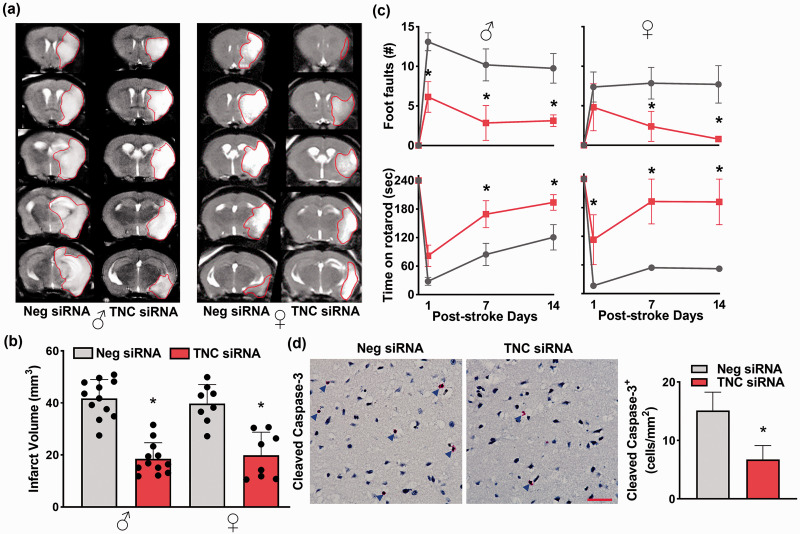
The TNC siRNA treated male and female mice showed significantly reduced infarct volume compared with the sex-matched Neg siRNA treated mice (by 56% in males and by 48% in females; n = 12 for male and 8 for female/group; p < 0.05) at 3 days of reperfusion (Figure 3(a) and (b)). Post-stroke motor function recovery (rotarod test and beam walk test) was also significantly higher in the TNC siRNA cohorts between days 7 and 14 of reperfusion compared with the sex-matched Neg siRNA cohorts (n = 7/group for both sexes; *p < 0.05) (Figure 3(c)). Post-stroke cleaved caspase-3+ cell number in the peri-infarct cortex was significantly lower in the TNC siRNA treated cohort compared with the Neg siRNA treated cohort at 3 days of reperfusion (n = 4/group, coordinates matched locations; *p < 0.05) (Figure 3(d)).
Figure 3.
Post-stroke TNC siRNA treatment decreased brain damage and improved motor function in adult male and female mice. (a) & (b) The TNC siRNA cohort showed significantly smaller infarcts than the Neg siRNA cohort in both male and female mice subjected to 3 days of reperfusion after transient MCAO. T2 MRI scans (a) are from representative mice of the 2 groups. Bar graphs (b) values are mean ± SD (n = 12 and 8 per group for male and female, respectively). (c) Both male and female TNC siRNA cohorts (red color) showed significantly improved post-stroke motor function between days 1 and 14 of reperfusion compared with sex-matched Neg siRNA cohorts (grey color) studied by beam walk test and rotarod test. Values in the line graphs are n = 7/group for both sexes. (d) The male TNC siRNA cohort also showed a reduced number of cleaved caspase-3+ cells at 3 days of reperfusion following transient MCAO. Immunostained images are from representative mice of the 2 groups. Blue arrows indicate the cleaved caspase-3+ cells. Images are from the peri-infarct areas of the ipsilateral cortex. Values in the histogram are mean ± SD; n = 4/group. Y-axis of males and females combined (b); *p < 0.05 compared with the respective Neg siRNA cohort by using the Mann-Whitney U test (b) and (d) and two-way repeated-measures ANOVA followed by Sidak’s multiple comparisons posttest (c). The scale bar is 100 μm.
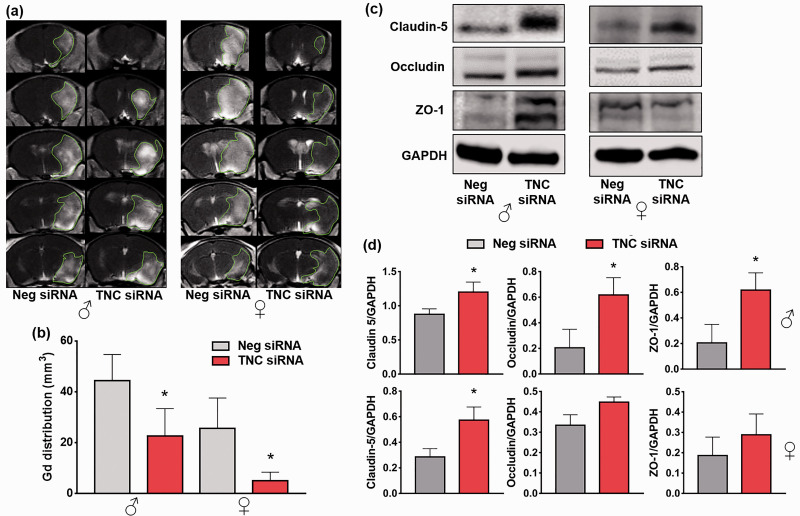
TNC siRNA treatment prevented post-stroke BBB disruption
TNC siRNA treated male and female mice showed a significant reduction in post-stroke Gd contrast agent uptake (T1-MRI) into the brain (by 49% in males and by 80% in females; n = 4–5/group/sex; *p < 0.05) at 3 days of reperfusion compared with the Neg siRNA treated sex-matched cohorts (Figure 4(a) and (b)). In addition, TNC knockdown prevented the tight junction proteins degradation in males (claudin 5, occludin and zona occludens 1) and females (only claudin 5) at 3 days of reperfusion (n = 4/group, *p < 0.05) (Figure 4(c) and (d)).
Figure 4.
Post-stroke TNC siRNA treatment prevented BBB disruption. (a) & (b) T1-MRI scans and bar graphs show a reduction in Gd brain uptake in the TNC siRNA treated cohorts at 3 days reperfusion after 1 h of transient MCAO compared with the Neg siRNA cohort. Values are mean ± SD (n = 4-5/group/sex); *p < 0.05 compared with the Neg siRNA cohort by the Mann-Whitney U test. (c) & (d) Western blots and bar graphs show tight junction protein (claudin-5, occludin, and zona occludens-1) expression in the TNC siRNA or Neg siRNA treated cohorts at 3 days of reperfusion after 1 h of transient ischemia. Values are mean ± SD; Y-axis of males and females combined (b); n = 4/group, *p < 0.05, compared with the Neg siRNA by using the Mann-Whitney U test.
TNC-knockdown reduced post-stroke neutrophil infiltration and inflammation
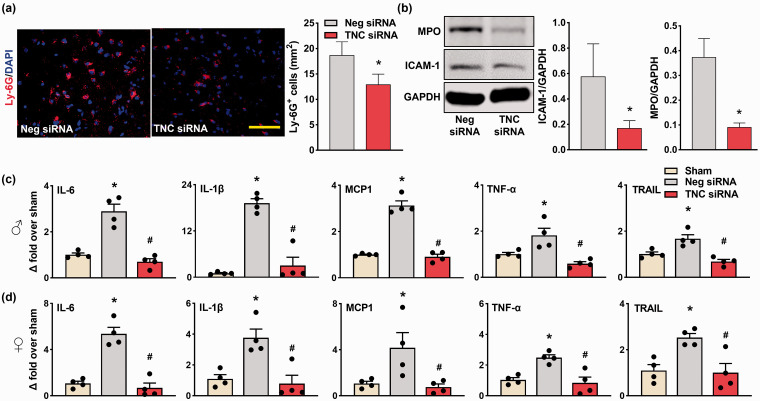
In the peri-infarct area, TNC siRNA treated male mice showed significantly less Ly-6G+ cells compared to Neg siRNA treated cohort at 3 days of reperfusion (n = 4/group, coordinates matched location; *p < 0.05) (Figure 5(a)). TNC knockdown also reduced the MPO and ICAM-1 protein induction compared with Neg siRNA treated control at 3 days of reperfusion (n = 4/group, *p < 0.05) (Figure 5(b)). TNC siRNA treatment also significantly curtailed the post-stroke mRNA expression of the inflammatory molecules IL-6, IL-1β, MCP-1, TNF-α, and TRAIL compared with the Neg siRNA treated cohort at 3 days of reperfusion in both male and female adult mice (n = 4/group, *p < 0.05) (Figure 5(c) and (d)).
Figure 5.
TNC knockdown prevented post-stroke inflammation. (a) The male TNC siRNA cohort showed reduced infiltration of Ly-6G+ microglia/macrophages compared with the Neg siRNA cohort. Similar staining was observed in n = 4/group. Scale bar: 100 μm. (b) The male TNC siRNA cohort showed reduced protein expression of MPO and ICAM-1 compared with the Neg siRNA cohort. (c & d) RNA levels of the inflammatory molecules (IL-6, IL-1β, MCP-1, TNF-α, and TRAIL) were significantly lower in both the male and female TNC siRNA cohorts compared with the Neg siRNA cohorts at 3 days reperfusion following transient MCAO. All parameters were estimated in the peri-infarct cortex at 3 days of reperfusion after transient MCAO. Values are mean ± SD; n = 4/group, *p < 0.05 compared with the Neg siRNA by using the Mann-Whitney U test, and */#p < 0.05 compared with the sham/Neg siRNA by one-way ANOVA with Tukey’s multiple comparisons test.
TNC-knockdown reduced the TLR-dependent post-stroke neuroinflammation
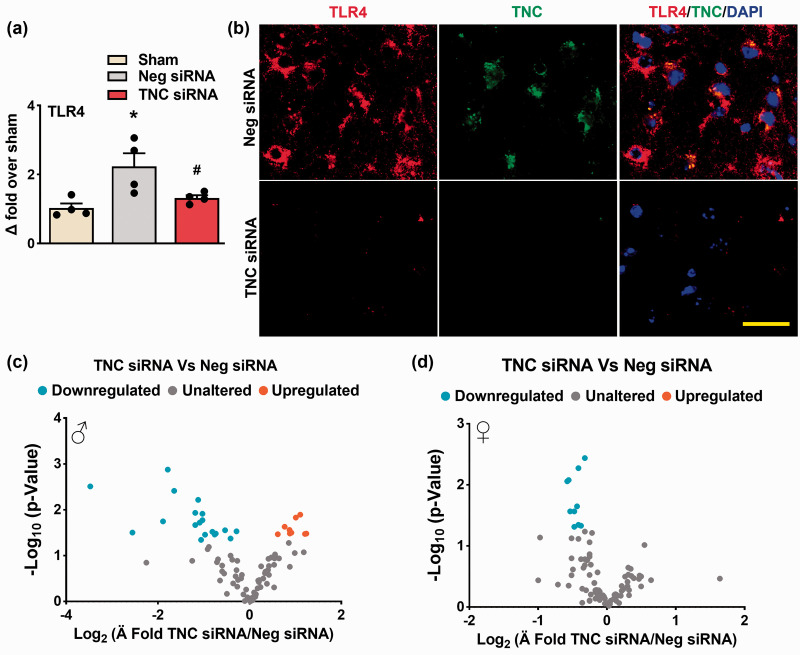
As TNC knockdown reduced the neutrophil infiltration and proinflammatory cytokine expression after ischemia, we investigated TLR downstream effectors. The TNC siRNA treated male mice showed a significant reduction in TLR-4 mRNA expression in the peri-infarct cortex compared with the Neg siRNA treatment at 3 days of reperfusion following transient MCAO (n = 4/group, *p < 0.05) (Figure 6(a)). Furthermore, TNC and TLR-4 colocalized in the peri-infarct cortex of the Neg siRNA treated male mice at 3 days reperfusion (Figure 6(b)). This effect was mitigated by TNC knockdown (n = 4/group, coordinates matched locations; *p < 0.05) (Figure 6(a) and (b)). In addition, TNC knockdown curtailed TLR signaling predominantly in males (Figure 6(c)) over females (Figure 6(d)) compared with the corresponding Neg siRNA treatments at 3 days of reperfusion following transient MCAO (n = 4/group).
Figure 6.
TNC knockdown reduced TLR-dependent post-stroke neuroinflammation. (a) TLR-4 mRNA levels were lower in the male TNC siRNA cohort compared to the Neg siRNA cohort at 3 days of reperfusion following transient ischemia. Values are mean ± SD; n = 4/group, *p < 0.05 compared with the Neg siRNA group by using the Mann-Whitney U test. (b) TNC/TLR-4 coexpression was curtailed in the male TNC siRNA treated cohort compared with the Neg siRNA cohort at 3 days reperfusion following transient MCAO. Similar results were observed with n = 4/group. Scale bar: 30 μm. (c & d) TLR signaling was curtailed by TNC knockdown after stroke in both male and female mice. n = 4/group.
Discussion
TNC expression in the normal adult brain is low and restricted to the neurogenic niche, but it highly upregulates in response to injury or inflammation.3,20,21 Brain injury induces TNC in as early as 15 minutes and it lasts up to 2 weeks in the cerebral cortex, thalamus, and hippocampus. 6 In the brain, several factors including proinflammatory cytokines, oxidative stress, and mechanical stress can induce TNC expression.2,21 Increased TNC expression is known to mediate early brain injury and cerebral vasospasm after SAH both in humans and rodents.7,22 In a rat model of SAH, both TNC induction in the cerebral cortex (at 24-72 h) and recombinant TNC injection (at 24 h) promoted neuronal apoptosis by activation of platelet-derived growth factor (PDGF) and mitogen-activated protein kinases (MAPKs), extracellular signal-regulated kinase (ERK) 1/2 and p38. 23 PDGF activation in neurons and astrocytes upregulates TNC. 24 In another study, TNC knockout mice showed decreased expression of MAPK, matrix metalloproteinase-9 (MMP-9), and decreased tight junction protein degradation at 24 h after SAH, which was reversed with an intracerebroventricular injection of TNC. 13 In addition, in tumor tissues, TNC activates MMP-9 that subsequently cleaves TNC.25,26 The role of MMP-9 is well-documented in the stroke pathogenesis, and its acute levels correlate with the infarct growth and hemorrhagic transformation in both rodents and humans.27,28 Further, as a positive feedback mechanism, TNC induces its own expression in neurons and astrocytes by the activation of MAPK, leading to aggravation of caspase‐dependent neuronal apoptosis.23,24 Further, a cisternal TNC injection to healthy/injured rats constricts cerebral arteries by activating TLR-4, c-Jun NH (2)-terminal kinase, p38 and also induced TNC expression in the arteries by positive feedback resulting in prolonged vasoconstriction.29,30 In the present study, we observed that TNC knockdown after ischemic stroke reduced the TNC induction in neurons, astrocytes, and microglia. Further, TNC knockdown prevented cell death and tight junction proteins degradation thereby prevented the BBB disruption and brain damage. BBB integrity is sex specific as estrogen protects and testosterone damages the BBB.31–35 Estrogen protects the BBB by inhibiting stroke-induced MMP2 and MMP9 and cyclooxygenase-2 activation. 36 As anticipated, we observed relatively less post-stroke degradation of tight junction proteins in females than males.
Post-stroke neutrophil infiltration is a hallmark of increased brain damage and inflammation in both rodents and humans.37,38 We identified that post-stroke neutrophil infiltration was reduced with TNC knockdown. TNC promotes the macrophage translation of proinflammatory cytokines and migration.4,39 In tandem, TNC upregulates the vascular endothelial growth factor in macrophages by interacting with annexin II and promotes the migration of macrophages. 40 TNC also promotes T‐cell activation and polarization during inflammation.41–43 TNC knockout mice showed restricted microglial surveillance and increased neutrophil infiltration into the ischemic core with no significant impact on post-ischemic cell death and early neurological dysfunction. 44 In contrast, autoimmune glaucoma in TNC knockout showed the absence of reactive gliosis and an increase in anti-inflammatory cytokine expression. 15 Further, TNC‐knockout mice showed less inflammatory cell infiltration in the periarterial subarachnoid space after SAH due to the inactivation of MAPKs in the smooth muscle cell layers of the cerebral arteries. 45 Similarly, inhibition of TNC expression after hepatic injury showed decreased neutrophil/leukocyte recruitment and also a reduction in the levels of pro-inflammatory cytokines. 46 Further, TNC increases MPO activity in the neutrophils and ICAM-1 levels which is crucial for neutrophil extravasation into the brain after stroke.46,47 Reduction in the protein expression of MPO and ICAM-1 with TNC knockdown observed presently suggests that a lack of TNC limits the migration of neutrophils from the periphery and prevents the chemotaxis entry to the brain parenchyma.
TNC mediates neuroinflammation by activating TLR-4.48,49 TLR-4 exacerbates the post-stroke pathogenesis and inflammation.50,51 TNC, as an endogenous activator of TLR-4, co-expresses in the injured brain and triggers cytokine synthesis. 48 In addition, the inflammatory role of TNC was found to be reduced in the absence of TLR-4. 9 A fibrinogen-like globe of TNC domain was shown to be important for the TLR-4 activation, and TLR-4 dependent expression of proinflammatory cytokines TNF-α, IL-6, and IL-8 in primary human fibroblasts.48,49 In addition, experimental autoimmune myocarditis in TNC knockout mice showed a reduction in IL-6 dependent Th17 differentiation. 52 Recent studies identified that TNC regulates MAPKs, phosphoinositide 3-kinase/Akt, protein kinase C, and ERK/NF-kB signaling pathways through its binding to cell surface receptors like TLR-4.23,53,54 In the current study, TNC knockdown after stroke showed downregulation of TLR signaling. Males showed predominant downregulation of the overall TLR signaling. On the other hand, in females, TNC knockdown downregulated post-stroke TLR signaling relatively less compared to males. In females, estrogen disrupts myeloid differentiation primary response protein 88 interaction with a methylated estrogen receptor-α which is crucial for NF-κB transcriptional activity and proinflammatory cytokine production. 55 Collectively, these observations suggest that TLR signaling is crucial for TNC to promote post-stroke brain damage and inflammation. In addition, TNC alters post-stroke inflammation in both males and females.
Further, disparities exist in the post-stroke outcomes between knockdown and knockout of a specific gene/its product as the later develops compensatory mechanisms. CNS injury studies with TNC knockout mice consistently showed reduced brain damage and improved neurological function at an early phase after an insult due to less inflammatory response and better BBB integrity brain damage.9,14,56 TNC knockout mice showed poor sensorimotor coordination and hyperactivity. 57 In CNS injuries, TNC upregulation correlates with astrocyte activation and glial scar formation.58,59 In addition, conditional TNC overexpression in mouse heart did not lead to any functional abnormalities, but increased expression of proinflammatory cytokines and mortality at the acute stage after myocardial infarction. 60 Interestingly, a recent study did not find a significant change in post-ischemic neurological deficits in TNC knockout mice. 44 This suggests a diverse role of TNC deletion in brain development and its induction after CNS diseases. This also suggests that the time to target TNC is crucial in CNS diseases and that TNC-KO might have compensatory mechanisms. In the current study, we observed improved motor functions with TNC knockdown after stroke suggesting that TNC induced after stroke promotes the post-stroke neurological deficits by exacerbating the ischemic pathogenesis.
Our study concludes that post-stroke TNC induction mediates ischemic pathogenesis, contributing to BBB disruption and brain damage in both males and females. TNC knockdown prevented post-stroke neutrophil infiltration and neuroinflammation, which suggests a considerable scope to expand our findings to understand the TNC molecular mechanisms that offer neuroprotection after stroke.
Supplementary Material
Acknowledgements
The authors thank the University of Wisconsin Translational Research Initiatives in Pathology laboratory, supported by the UW Department of Pathology and Laboratory Medicine, UWCCC (P30 CA014520), and the University of Wisconsin small animal imaging facility.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partially supported by NIH grants NS099531, NS109459 and NS101960, and the Department of Neurological Surgery, University of Wisconsin-Madison.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: BC and RV conceived and designed the study. BC and AKC contributed to the acquisition and analysis of data. SLM, SB, and JSP contributed to the blind evaluation of MRI and behavioral outcomes. AKC, KCM and SS contributed to arrays. BC and RV drafted the manuscript.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs: Bharath Chelluboina https://orcid.org/0000-0001-8834-6484
Anil K Chokkalla https://orcid.org/0000-0002-0101-0417
Raghu Vemuganti https://orcid.org/0000-0002-7915-2810
References
- 1.Baeten KM, Akassoglou K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol 2011; 71: 1018–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiquet-Ehrismann R, Tucker RP. Tenascins and the importance of adhesion modulation. Cold Spring Harb Perspect Biol 2011; 3: a004960–a004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Midwood KS, Chiquet M, Tucker RP, et al. Tenascin-C at a glance. J Cell Sci 2016; 129: 4321–4327. [DOI] [PubMed] [Google Scholar]
- 4.Marzeda AM, Midwood KS. Internal affairs: Tenascin-C as a clinically relevant, endogenous driver of innate immunity. J Histochem Cytochem 2018; 66: 289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clancy P, Lincz LF, Maguire J, et al. Tenascin-C is increased in atherothrombotic stroke patients and has an anti-inflammatory effect in the human carotid artery. Biofactors 2014; 40: 448–457. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths DR, Jenkins TM, Addington CP, et al. Extracellular matrix proteins are time-dependent and regional-specific markers in experimental diffuse brain injury. Brain Behav 2020; 10: e01767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki H, Kanamaru K, Suzuki Y, et al. Tenascin-C is induced in cerebral vasospasm after subarachnoid hemorrhage in rats and humans: a pilot study. Neurol Res 2010; 32: 179–184. [DOI] [PubMed] [Google Scholar]
- 8.Shiomi Y, Yokokawa M, Uzui H, et al. Serum tenascin-C levels in atrium predict atrial structural remodeling processes in patients with atrial fibrillation. J Interv Card Electrophysiol 2020; 59: 401–406. [DOI] [PubMed] [Google Scholar]
- 9.Haage V, Elmadany N, Roll L, et al. Tenascin C regulates multiple microglial functions involving TLR4 signaling and HDAC1. Brain Behav Immun 2019; 81: 470–483. [DOI] [PubMed] [Google Scholar]
- 10.Smith GM, Hale JH. Macrophage/microglia regulation of astrocytic tenascin: synergistic action of transforming growth factor-beta and basic fibroblast growth factor. J Neurosci 1997; 17: 9624–9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dzaye OD, Hu F, Derkow K, et al. Glioma stem cells but not bulk glioma cells upregulate IL-6 secretion in microglia/brain macrophages via toll-like receptor 4 signaling. J Neuropathol Exp Neurol 2016; 75: 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McQuitty CE, Williams R, Chokshi S, et al. Immunomodulatory role of the extracellular matrix within the liver disease microenvironment. Front Immunol 2020; 11: 574276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujimoto M, Shiba M, Kawakita F, et al. Deficiency of tenascin-C and attenuation of blood-brain barrier disruption following experimental subarachnoid hemorrhage in mice. J Neurosurg 2016; 124: 1693–1702. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Fujimoto M, Nakano F, et al. Deficiency of Tenascin-C alleviates neuronal apoptosis and neuroinflammation after experimental subarachnoid hemorrhage in mice. Mol Neurobiol 2018; 55: 8346–8354. [DOI] [PubMed] [Google Scholar]
- 15.Wiemann S, Reinhard J, Faissner A. Immunomodulatory role of the extracellular matrix protein tenascin-C in neuroinflammation. Biochem Soc Trans 2019; 47: 1651–1660. [DOI] [PubMed] [Google Scholar]
- 16.Percie Du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab 2020; 40: 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chelluboina B, Kim T, Mehta SL, et al. Impact of age and sex on α-Syn (α-Synuclein) knockdown-mediated poststroke recovery. Stroke 2020; 51: 3138–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Percie Du Sert N, Alfieri A, Allan SM, et al. The IMPROVE guidelines (ischaemia models: procedural refinements of in vivo experiments). J Cereb Blood Flow Metab 2017; 37: 3488–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim T, Mehta SL, Morris-Blanco KC, et al. The microRNA miR-7a-5p ameliorates ischemic brain damage by repressing alpha-synuclein. Sci Signal 2018; 11: eaat4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faissner A, Roll L, Theocharidis U. Tenascin-C in the matrisome of neural stem and progenitor cells. Mol Cell Neurosci 2017; 81: 22–31. [DOI] [PubMed] [Google Scholar]
- 21.Midwood KS, Hussenet T, Langlois B, et al. Advances in tenascin-C biology. Cell Mol Life Sci 2011; 68: 3175–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki H, Kanamaru K, Suzuki Y, et al. Possible role of tenascin-C in cerebral vasospasm after aneurysmal subarachnoid haemorrhage. Cerebral Vasospasm 2008; 104: 179–182. [Google Scholar]
- 23.Shiba M, Fujimoto M, Imanaka-Yoshida K, et al. Tenascin-C causes neuronal apoptosis after subarachnoid hemorrhage in rats. Transl Stroke Res 2014; 5: 238–247. [DOI] [PubMed] [Google Scholar]
- 24.Shiba M, Suzuki H, Fujimoto M, et al. Imatinib mesylate prevents cerebral vasospasm after subarachnoid hemorrhage via inhibiting tenascin-C expression in rats. Neurobiol Dis 2012; 46: 172–179. [DOI] [PubMed] [Google Scholar]
- 25.Cai J, Du S, Wang H, et al. Tenascin-C induces migration and invasion through JNK/c-Jun signalling in pancreatic cancer. Oncotarget 2017; 8: 74406–74422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giblin SP, Midwood KS. Tenascin-C: form versus function. Cell Adh Migr 2015; 9: 48–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mechtouff L, Bochaton T, Paccalet A, et al. Matrix metalloproteinase-9 relationship with infarct growth and hemorrhagic transformation in the era of thrombectomy. Front Neurol 2020; 11: 473–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong X, Song Y-N, Liu W-G, et al. Mmp-9, a potential target for cerebral ischemic treatment. Curr Neuropharmacol 2009; 7: 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujimoto M, Shiba M, Kawakita F, et al. Vasoconstrictive effect of tenascin-C on cerebral arteries in rats. Acta Neurochir Suppl 2015; 120: 99–103. [DOI] [PubMed] [Google Scholar]
- 30.Fujimoto M, Suzuki H, Shiba M, et al. Tenascin-C induces prolonged constriction of cerebral arteries in rats. Neurobiol Dis 2013; 55: 104–109. [DOI] [PubMed] [Google Scholar]
- 31.Jiang M, Ma C, Li H, et al. Sex dimorphisms in ischemic stroke: from experimental studies to clinic. Front Neurol 2020; 11: 504–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Donnell ME, Lam TI, Tran LQ, et al. Estradiol reduces activity of the blood–brain barrier Na–K–Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab 2006; 26: 1234–1249. [DOI] [PubMed] [Google Scholar]
- 33.Cheng J, Alkayed NJ, Hurn PD. Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J Cereb Blood Flow Metab 2007; 27: 1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cipolla MJ, Godfrey JA, Wiegman MJ. The effect of ovariectomy and estrogen on penetrating brain arterioles and blood-brain barrier permeability. Microcirculation 2009; 16: 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parrado-Fernández C, Blennow K, Hansson M, et al. Evidence for sex difference in the CSF/plasma albumin ratio in ∼20 000 patients and 335 healthy volunteers. J Cell Mol Med 2018; 22: 5151–5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu R, Wen Y, Perez E, et al. 17β-Estradiol attenuates blood–brain barrier disruption induced by cerebral ischemia–reperfusion injury in female rats. Brain Res 2005; 1060: 55–61. [DOI] [PubMed] [Google Scholar]
- 37.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 2010; 87: 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manda-Handzlik A, Demkow U. The brain entangled: the contribution of neutrophil extracellular traps to the diseases of the central nervous system. Cells 2019; 8: 1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piccinini AM, Midwood KS. Endogenous control of immunity against infection: tenascin-C regulates TLR4-mediated inflammation via microRNA-155. Cell Rep 2012; 2: 914–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Wei Q, Han L, et al. Tenascin‐c renders a proangiogenic phenotype in macrophage via annexin II. J Cell Mol Med 2018; 22: 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanayama M, Morimoto J, Matsui Y, et al. α9β1 integrin-mediated signaling serves as an intrinsic regulator of pathogenic Th17 cell generation. J Immunol 2011; 187: 5851–5864. [DOI] [PubMed] [Google Scholar]
- 42.Ruhmann M, Piccinini AM, Kong PL, et al. Endogenous activation of adaptive immunity: tenascin‐C drives interleukin‐17 synthesis in murine arthritic joint disease. Arthritis Rheum 2012; 64: 2179–2190. [DOI] [PubMed] [Google Scholar]
- 43.Nakahara H, Gabazza EC, Fujimoto H, et al. Deficiency of tenascin C attenuates allergen-induced bronchial asthma in the mouse. Eur J Immunol 2006; 36: 3334–3345. [DOI] [PubMed] [Google Scholar]
- 44.Manrique-Castano D, Dzyubenko E, Borbor M, et al. Tenascin-C preserves microglia surveillance and restricts leukocyte and, more specifically, T cell infiltration of the ischemic brain. Brain Behav Immun 2021; 91: 639–648. [DOI] [PubMed] [Google Scholar]
- 45.Fujimoto M, Shiba M, Kawakita F, et al. Effects of Tenascin-C knockout on cerebral vasospasm after experimental subarachnoid hemorrhage in mice. Mol Neurobiol 2018; 55: 1951–1958. [DOI] [PubMed] [Google Scholar]
- 46.Kato H, Duarte S, Miller MG, et al. Overproduction of Tenascin-C driven by lipid accumulation in the liver aggravates hepatic ischemia/reperfusion injury in steatotic mice. Liver Transpl 2019; 25: 288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindsberg PJ, Carpén O, Paetau A, et al. Endothelial ICAM-1 expression associated with inflammatory cell response in human ischemic stroke. Circulation 1996; 94: 939–945. [DOI] [PubMed] [Google Scholar]
- 48.Zuliani-Alvarez L, Marzeda AM, Deligne C, et al. Mapping tenascin-C interaction with toll-like receptor 4 reveals a new subset of endogenous inflammatory triggers. Nat Commun 2017; 8: 1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Midwood K, Sacre S, Piccinini AM, et al. Tenascin-C is an endogenous activator of toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med 2009; 15: 774–780. [DOI] [PubMed] [Google Scholar]
- 50.Gesuete R, Kohama SG, Stenzel-Poore MP. Toll-like receptors and ischemic brain injury. J Neuropathol Exp Neurol 2014; 73: 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caso JR, Pradillo JM, Hurtado O, et al. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation 2007; 115: 1599–1608. [DOI] [PubMed] [Google Scholar]
- 52.Machino-Ohtsuka T, Tajiri K, Kimura T, et al. Tenascin‐C aggravates autoimmune myocarditis via dendritic cell activation and Th17 cell differentiation. J Am Heart Assoc 2014; 3: e001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jinnin M, Ihn H, Asano Y, et al. Upregulation of tenascin-C expression by IL-13 in human dermal fibroblasts via the phosphoinositide 3-kinase/akt and the protein kinase C signaling pathways. J Invest Dermatol 2006; 126: 551–560. [DOI] [PubMed] [Google Scholar]
- 54.Shi M, He X, Wei W, et al. Tenascin-C induces resistance to apoptosis in pancreatic cancer cell through activation of ERK/NF-κB pathway. Apoptosis 2015; 20: 843–857. [DOI] [PubMed] [Google Scholar]
- 55.El Sabeh R, Bonnet M, Le Corf K, et al. A gender-dependent molecular switch of inflammation via MyD88/estrogen receptor-alpha interaction. J Inflamm Res 2021; 14: 2149–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu L, Kawakita F, Fujimoto M, et al. Role of periostin in early brain injury after subarachnoid hemorrhage in mice. Stroke 2017; 48: 1108–1111. [DOI] [PubMed] [Google Scholar]
- 57.Morellini F, Schachner M. Enhanced novelty-induced activity, reduced anxiety, delayed resynchronization to daylight reversal and weaker muscle strength in tenascin-C-deficient mice. Eur J Neurosci 2006; 23: 1255–1268. [DOI] [PubMed] [Google Scholar]
- 58.Bijelić D, Adžić M, Perić M, et al. Different functions of recombinantly expressed domains of Tenascin-C in glial scar formation. Front Immunol 2020; 11: 624612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okada T, Suzuki H. The role of Tenascin-C in tissue injury and repair after stroke. Front Immunol 2020; 11: 607587–607587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yonebayashi S, Tajiri K, Hara M, et al. Generation of transgenic mice that conditionally overexpress Tenascin-C. Front Immunol 2021; 12: 620541–620541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.