Abstract
Background
SARS-CoV-2 vaccination is the most effective strategy to protect older residents of long-term care facilities (LTCF) against severe COVID-19, but primary vaccine responses are less effective in older adults. Here, we characterised the humoral responses of institutionalised seniors 3 months after they had received the mRNA/BNT162b2 vaccine.
Methods
plasma levels of SARS-CoV-2-specific total IgG, IgM and IgA antibodies were measured before and 3 months after vaccination in older residents of LTCF. Neutralisation capacity was assessed in a pseudovirus neutralisation assay against the original WH1 and later B.1.617.2/Delta variants. A group of younger adults was used as a reference group.
Results
three months after vaccination, uninfected older adults presented reduced SARS-CoV-2-specific IgG levels and a significantly lower neutralisation capacity against the WH1 and Delta variants compared with vaccinated uninfected younger individuals. In contrast, COVID-19-recovered older adults showed significantly higher SARS-CoV-2-specific IgG levels after vaccination than their younger counterparts, whereas showing similar neutralisation activity against the WH1 virus and an increased neutralisation capacity against the Delta variant. Although, similarly to younger individuals, previously infected older adults elicit potent cross-reactive immune responses, higher quantities of SARS-CoV-2-specific IgG antibodies are required to reach the same neutralisation levels.
Conclusions
although hybrid immunity seems to be active in previously infected older adults 3 months after mRNA/BNT162b2 vaccination, humoral immune responses are diminished in COVID-19 uninfected but vaccinated older residents of LTCF. These results suggest that a vaccine booster dose should be prioritised for this particularly vulnerable population.
Keywords: older people, BNT162b2 vaccine, nursing homes, long-term care facilities, humoral immune response, SARS-CoV-2
Key Points
Previously infected and vaccinated older adults living in long-term care facilities (LTCF) had neutralising antibody levels comparable to those of younger individuals.
Vaccinated uninfected residents showed limited neutralisation capacity against both original and delta variants.
Hybrid immunity seems to be active in older adults and this may be relevant to the design of vaccine boosting campaigns.
Introduction
Older adults have been disproportionately affected by the coronavirus disease 2019 (COVID-19) pandemic. Older individuals are more prone to severe infection and over 95% of COVID-19-related deaths have occurred in the population > 60 years [1]. Among them, residents of long-term care facilities (LTCF), who live in a congregate setting and thus are at increased risk of transmission and infection, have shown higher mortality rates than the general population of the same age [2, 3]. Therefore, SARS-CoV-2 vaccination of LTCF residents has been a priority in most countries. SARS-CoV-2 vaccines are currently widely available in Europe, but we are still facing several months of continuous new infections and new circulating variants of concern (VOC). In late 2021, virus outbreaks involving the B.1.617.2/Delta variant were reported in nursing homes, despite the high vaccination rates among residents [4].
Currently available COVID-19 vaccines have proven to be safe and generate effective humoral and cellular immunity [5–8], but these studies mainly involved young or middle-aged healthy adults. Relatively few studies have evaluated the immune responses generated in older adults [9–13], and most of them studied short-term (few weeks) immune responses after vaccination. Ageing is associated with an immunosenescent phenotype characterised by a progressively proinflammatory state, and a diminished immune response to pathogens and vaccines [14]. Therefore, there is an urgent need to determine the quality and duration of immune responses in the older population to adapt SARS-CoV-2 vaccination calendars to their immune needs.
We performed a prospective study in LTCF residents to evaluate the anti-SARS-CoV-2 humoral response elicited before and 3 months after vaccination with the BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech). The study included individuals uninfected (N = 16) and recovered from a previous SARS-CoV-2 infection (N = 82). Humoral responses of these older participants were also compared with a younger population, and a functional neutralisation assay was performed against the original WH1 and Delta of variants.
Material and methods
Study participants
A total of 98 residents of three LTCF located in the area of Barcelona, Spain, were included in this prospective observational study (CoronAVI@S study, Table 1). Plasma samples were collected after 6 months of SARS-CoV-2 outbreaks before vaccination (September–November 2020, N = 98) and 3 months after full vaccination schedule (April–May 2021, N = 91). Participants were recruited irrespective of their SARS-CoV-2 infection status and SARS-CoV-2 serology was performed to subclassify participants into infected and uninfected groups, depending on their polymerase chain reaction (PCR) and serology status (Supplementary Figure 1). Both study groups were vaccinated with the BNT16b2 mRNA vaccine (Pfizer-BioNTech) between January and February 2021 in accordance with the Spanish government’s COVID-19 vaccination strategy. An additional sample was collected at a median of 2.8 interquartile range (IQR) [2.4–3.1] months after vaccination. We performed an additional serology test against nucleoprotein (NP)-protein in uninfected residents after vaccination to verify the absence of anti-NP antibodies and confirm their SARS-CoV-2-naïve status (data not shown).
Table 1.
Descriptive data for CoronAVI@S participants
| Previously SARS-CoV-2 infected group | Uninfected group | P-value | |
|---|---|---|---|
| N = 82 | N = 16 | ||
| Age, years, median [IQR] | 87 [81–90] | 79 [74–90] | 0.26 |
| Female, n (%) | 66 (80) | 8 (50) | 0.021 |
| AGM levela, n (%) | 0.11 | ||
| 1 | 0 (0) | 0 (0) | |
| 2 | 4 (5) | 3 (20) | |
| 3 | 38 (46) | 6 (40) | |
| 4 | 41 (49) | 6 (40) | |
| Pre-vaccine visit | N = 82 | N = 16 | |
| PCR positive, n (%) | 61 (73) | 0 (0) | <0.0001 |
| Time from PCR positive to sample extraction, months, median [IQR] | 6.4 [6–6.9] | NA | NA |
| Detection of SARS-CoV-2-specific antibodies, n (%) | 81 (98)b | 0 (0) | <0.0001 |
| Medical assistance, n (%) | 5 (6) | NA | |
| Post-vaccine visit | N = 76 | N = 15 | |
| Vaccine received | |||
| BNT16b2 (2 doses), n (%) | 76 (100) | 15 (100) | 1.0 |
| Time between vaccine doses, days, median [IQR] | 21 [21–23] | 21 [21–26] | 0.14 |
| Time between PCR positive and 1st vaccine dose, months, median [IQR] | 9.3 [9.1–9.6] | NA | |
| Time from full vaccine schedule to sample extraction, months, median [IQR] | 2.4 [2.36–2.95] | 2.8 [2.6–3.1] | 0.05 |
| PCR positive after vaccination, n (%) | 0 (0) | 1 (7) |
aAGM level: Stratum of Adjusted Morbidity ranging from 1 to 4 according to the number of comorbidities and their need for health care
bOne individual did not seroconvert despite Positive PCR.
Significant p-values are indicated in bold.
For comparison purposes, we selected younger and middle-aged adults (22–64 years) from the KING cohort extension study: 64 infected individuals (Table 2) for pre-vaccine visit and 51 individuals (32 infected and 16 uninfected, Table 3) for post-vaccine timepoint. Pre-vaccine blood samples from these groups were matched with those from the older CoronAVI@S group by time elapsed since SARS-CoV-2 infection (6 months). All matched individuals had experienced mild cases of the disease. Post-vaccine samples were selected from among individuals in the younger group who had been vaccinated with BNT16b2 (Pfizer-BioNTech), and blood samples were obtained a median of 3 IQR [2.5–3.4] months after completing the full vaccination schedule.
Table 2.
Descriptive data for younger and older participants, pre-vaccine
| CoronAVI@S | KING Cohort extension | P-value | |
|---|---|---|---|
| Infected | Infected | ||
| N = 82 | n = 64 | ||
| Age, years, median [IQR] | 87 [81–90] | 44 [38–51] | <0.001 |
| Age, years, n (%) | |||
| <45 | 0 (0) | 36 (56) | |
| 45–65 | 0 (0) | 27 (42) | |
| 65–85 | 37 (45) | 0 (0) | |
| >85 | 45 (55) | 0 (0) | |
| Female, n (%) | 66 (80) | 47 (73) | 0.33 |
| AGM levela,b, n (%) | |||
| 1 | 0 (0) | 32 (50) | <0.001 |
| 2 | 4 (5) | 17 (27) | <0.001 |
| 3 | 38 (46) | 11 (17) | <0.001 |
| 4 | 41 (49) | 0 (0) | <0.001 |
| History of SARS-CoV-2 infection, n (%) | 82 (100) | 64 (100) | 1 |
| Pre-vaccine visit | |||
| Time from PCR positive/symptoms to sample extraction, months, median [IQR] | 6.5 [6.0–6.9] | 6.6 [6.1–7.4] | 0.04 |
| Detection of SARS-CoV-2-specific antibodies, n (%) | 81 (99) | 52 (81) | 0.0002 |
| Medical assistance, n (%) | 5 (6) | 0 (0) | 0.25 |
aAGM: Stratum of Adjusted Morbidity ranging from 1 to 4 according to the number of comorbidities and their need for health care. AGM level was not available for four individuals from the KING Cohort extension bAGM level was not available for four individuals from the KING Cohort extension. Significant p-values are indicated in bold.
Table 3.
Descriptive data for younger and older participants, post-vaccine
| CoronAVI@S | KING Cohort extension | P-values between younger and older | ||||
|---|---|---|---|---|---|---|
| Infected | Uninfected | Infected | Uninfected | Infected | Uninfected | |
| n = 76 | n = 14 | n = 32 | n = 19 | |||
| Age, years, median [IQR] | 87 [81–90] | 81 [74–90] | 43 [38–50] | 39 [28–48] | <0.001 | <0.001 |
| Age, years, n (%) | ||||||
| ≤45 | 0 (0) | 0 (0) | 19 (59) | 12 (63) | ||
| 46–65 | 0 (0) | 0 (0) | 13 (41) | 7 (37) | ||
| 65–85 | 33 (43) | 9 (64) | 0 (0) | 0 (0) | ||
| >85 | 43 (57) | 5 (36) | 0 (0) | 0 (0) | ||
| Female, n (%) | 62 (82) | 8 (57) | 22 (69) | 14 (74) | 0.20 | 0.46 |
| AGM level a, n (%) | ||||||
| 1 | 0 (0) | 0 (0) | 19 (59) | 13 (69) | <0.001 | <0.001 |
| 2 | 4 (4) | 3 (21) | 6 (19) | 5 (26) | 0.06 | 1.0 |
| 3 | 36 (48) | 5 (37) | 4 (13) | 1 (5) | <0.001 | 0.06 |
| 4 | 36 (48) | 6 (42) | 0 (0) | 0 (0) | <0.001 | <0.001 |
| Medical assistance, n (%) | 4 (5) | NA | 2 (6) | NA | 1.0 | NA |
| Vaccine received, n (%) | ||||||
| BNT16b2, 1 doses | 76 (100) | 14 (100) | 32 (100) | 19 (100) | 1.0 | 1.0 |
| BNT16b2, 2 doses | 76 (100) | 14 (100) | 30 (94) | 19 (100) | 0.08 | 1.0 |
| Time between vaccine doses, days, median [IQR] | 21 [21–23] | 21 [23–26] | 22 [21–22] | 22 [21–22] | 0.34 | 0.23 |
| Time between PCR positive/symptoms and complete vaccine schedule, months, median [IQR] | 12.9 [12.7–13.3] |
NA | 10.6 [10.4–11.6] |
NA | <0.001 | NA |
| Time from complete vaccine schedule to sample extraction, months, median [IQR] | 2.4 [2.4–3.0] |
2.4 [2.3–2.9] |
2.0 [1.9–3.1] |
3.1 [3.0–3.4] |
0.53 | <0.001 |
aAGM level: Stratum of Adjusted Morbidity ranging from 1 to 4 according to the number of comorbidities and their need for health care. AGM level was not available for three individuals from the KING cohort extension. Significant p-values are indicated in bold.
The CoronAVI@S study and KING cohort extension were approved by the Ethics Boards of the Institut Universitari d’Investigació en Atenció Primària Jordi Gol and the Hospital Universitari Germans Trias i Pujol, respectively (20-116P and PI-20-217). All participants provided written informed consent before inclusion.
Determination of anti-SARS-CoV-2 antibodies
The presence of anti-SARS-CoV-2 antibodies against Spike S2 domain (S2) + Receptor Binding Domain (RBD) or NP in plasma samples was evaluated using an in-house sandwich-enzyme-linked immunosorbent assay (ELISA) [15]. The specific signal for each antigen was calculated after subtracting the background signal obtained for each sample in antigen-free wells. Values were plotted into the standard curve, calculated by plotting and fitting the log of standard dilution (in arbitrary units) versus response to a four-parameter equation in Prism 8.4.3 (GraphPad Software) (Supplementary data).
Pseudovirus neutralisation assay
Neutralisation assay was performed using SARS-CoV-2.Sct∆19 WH1 and B.1.617.2/Delta pseudovirus [16, 17]. Upper and lower limits of detection were 60 and 14,580 (reciprocal dilution). The ID50 (the reciprocal dilution inhibiting 50% of the infection) was calculated by plotting and fitting the log of plasma dilution against response to a four-parameter equation (Supplementary data).
Statistics
Continuous variables were described using medians and the IQR(25th and 75th percentiles), whereas categorical factors were reported as percentages. Quantitative variables were compared using the Mann–Whitney or Wilcoxon tests for unpaired and paired data, respectively; age ranges were compared using the Kruskal–Wallis (K–W) test. Percentages between groups were compared using the Chi-squared test. Analyses were performed with Prism 9.1.2 (GraphPad Software). To assess the impact of several variables (age, sex, Adjusted Morbidity Group [AMG] level and hospitalisation) on neutralisation levels post-vaccination, univariate and multivariate linear models were generated using R (version 4.1.2), analysing infected and uninfected individuals separately. The multivariate models were built using variables which proved to be significant in the univariate analysis, with a P-value cut-off at ≤0.1. AMG level was excluded from the multivariate models due to its correlation with age. The quality of the models was evaluated via residual analysis.
Results
Characterisation of older participants
The 98 older participants in this study were > 65 years, with 90% of them belonging to moderate- to high-risk groups using the AMG classification system, a measure of the general health status [18]. The most common chronic pathologies included hypertension (62%), arthritis (40%), dementia (38%) or diabetes (33%). Individuals were tested by PCR during SARS-CoV-2 outbreaks at their respective LTCFs between March and June 2020.
Serologic and PCR tests before vaccination revealed that 84% of the LTCF residents had experienced SARS-CoV-2 infection before testing. Previously infected residents (n = 82) had a median age of 87 years and were 80% female, whereas uninfected residents before vaccination (n = 16) had a median age of 79 years and were 50% female (Table 1). Among infected LTCF residents, only three required hospitalisation due to acute infection.
We took advantage of the Spanish national vaccination plan for LTCFs to evaluate the immune responses generated by the BNT16b2 vaccine. We confirmed that 14 out of the 15 uninfected residents remained seronegative after vaccination (testing negative for antibodies against the NP-protein by ELISA). The infected resident after vaccination developed a mild case and was removed from post-vaccine analysis.
Humoral responses 3 months after vaccination
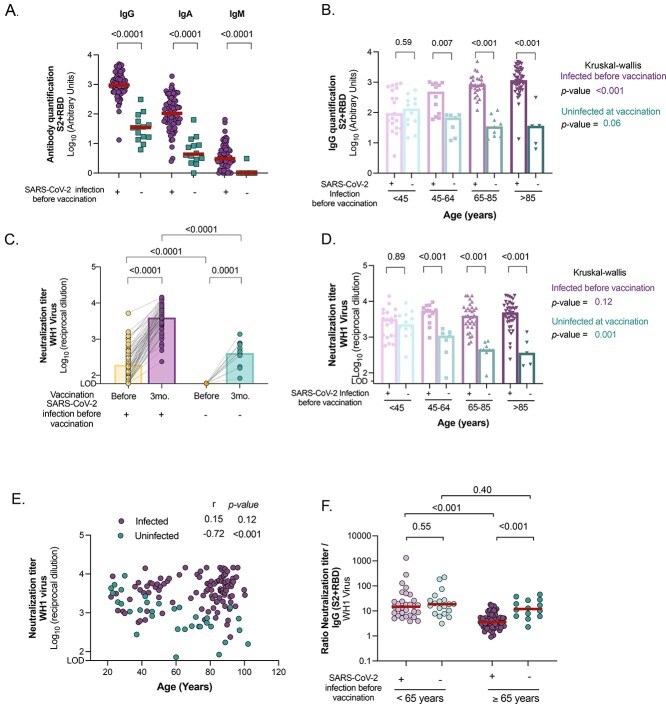
The levels of SARS-CoV-2-specific IgG, IgA and IgM antibodies were significantly higher in individuals who were infected before vaccination compared with their uninfected counterparts (P < 0.0001 in all cases, Figure 1A). There was a significant increase in all isotypes of immunoglobulins between pre- and post-vaccine samples in infected participants (Supplementary 2A), whereas uninfected individuals maintained detectable levels of SARS-CoV-2-specific IgGs and IgAs, but no IgMs, 3 months after vaccination (Supplementary Figure 2B). The levels of SARS-CoV-2-specific IgGs induced after vaccination across age groups revealed higher levels in older participants (≥65 years) who had been previously infected compared with the reference group of younger adults (Figure 1B, K-WP < 0.001, and r = 0.41, P < 0.001, Supplementary Figure 2C). To determine whether those higher levels were related to the vaccine or to the natural infection, we evaluated SARS-CoV-2-specific IgGs before vaccination across ages (Figure 2D). We determined that 6 months after infection (and prior to vaccination), older participants already showed higher levels of SARS-CoV-2-specific IgGs compared with the younger reference group (K–W, P < 0.001).
Figure 1.
Comparison of humoral response and neutralising activity between uninfected and infected individuals 3 months after BNT162b2 mRNA COVID-19 vaccine. (A) Levels of SARS-CoV-2-specific immunoglobulins (IgG, IgA and IgM) against S2 + RBD proteins quantified in plasma from uninfected and infected older participants by ELISA. (B) SARS-CoV-2-specific IgG antibody levels (against S2 + RBD proteins) after vaccination across ages in infected and uninfected participants. (C) Neutralising activity against WH1 virus before and after 3 months of vaccine administration in infected and uninfected older residents of LTCF. (D) Neutralising activity against WH1 after vaccination across ages in infected and uninfected participants. (E) Correlation of neutralising activity after vaccination with age in infected and uninfected participants. Correlation coefficient and P-values were obtained from Spearman correlation. (F) Ratio of plasma neutralisation titre per total SARS-CoV-2-specific IgG antibodies in younger and older participants, sub-grouped by previous SARS-CoV-2 infection history. Median values are indicated; P-values were obtained from Mann–Whitney test for comparison between groups A, B, C, D and F), Wilcoxon test for paired tests ( C) and Kruskal–Wallis test for comparison between age ranges for infected and uninfected groups (B and D). In all panels, uninfected and infected participants at vaccination are indicated in turquoise and purple, respectively.
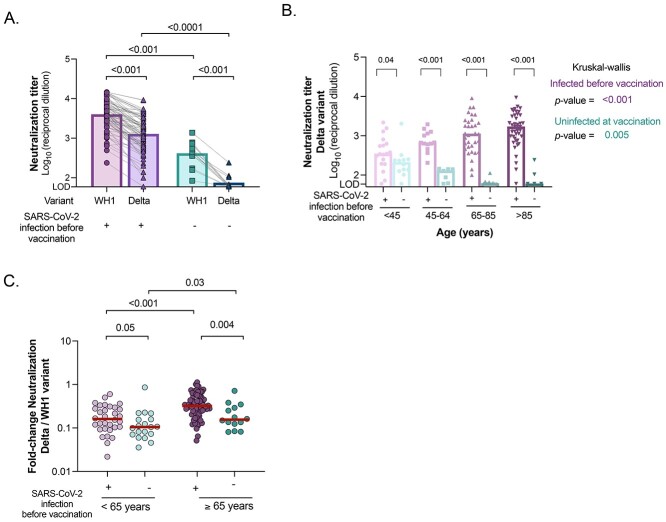
Figure 2.
Neutralising activity against B.1.617.2/Delta variant between uninfected and infected participants 3 months after BNT162b2 mRNA Covid-19 vaccine. (A) Comparison of plasma neutralising activity against WH1 and B.1.617.2/Delta variant in uninfected and infected older participants after vaccination. (B) Neutralising activity against Delta variant after vaccination across ages in infected and uninfected, younger and older participants, sub-grouped by previous SARS-CoV-2 infection history. (C) Fold change ratios between neutralisation titres against Delta variant and WH1 (reference spike) virus in infected and uninfected participants, sub-grouped by age (<65 years and ≥ 65 years). Median values are indicated. P-values were obtained from Mann–Whitney test for comparison between groups (all panels), Wilcoxon test for paired tests (A and C) and Kruskal–Wallis test for each group (B). In all panels, uninfected and infected participants at vaccination are indicated in turquoise and purple, respectively.
The levels of circulating SARS-CoV-2-specific IgGs in uninfected vaccinated participants tended to decrease across both age groups (K–W P = 0.06, Figure 1B) and were negatively correlated with age (r = −0.73, P < 0.001, Supplementary Figure 2C). When infected and uninfected groups were compared after vaccination, the levels of SARS-CoV-2-specific IgGs were already significantly lower in individuals between 45 and 64 years (P = 0.007), a difference maintained in the older age group (P < 0.001 in all cases, Figure 1B). These results highlighted some differences in antibody production between infected and uninfected individuals, only the uninfected younger group being able to produce levels of SARS-CoV-2 antibodies similar to that produced by their infected counterparts.
Neutralisation capacity against original WH1 virus
SARS-CoV-2 vaccination increased the titres of antibodies with neutralising capacity in infected and uninfected participants (P < 0.0001 in both cases, Figure 1C), being significantly higher in individuals with a previous history of SARS-CoV-2 infection compared with the uninfected counterparts (P < 0.0001). For the infected population, there was a median fold increase of 18.7 [9.3–44.8] from pre- to post-vaccination. Previously infected subjects had similar levels of neutralisation independently of age (K–W P = 0.12, Figure 1D and Supplementary Figure 2E). We hypothesised that the neutralisation response after natural infection reached a plateau in recovered older participants similar to that seen in younger participants, therefore similar levels of neutralisation were reached in both groups after vaccination. To test this hypothesis, we compared plasma neutralisation capacity before vaccination for older and younger individuals 6 months after SARS-CoV-2 infection. Surprisingly, the neutralisation titres were significantly lower in older compared with younger (45–64 years) participants 6 months after infection (K–W P = 0.01, Supplementary Figure 2F). These results suggest a greater boost of humoral responses in infected older adults after vaccination compared with the younger group. In contrast, uninfected vaccinated subgroups showed a sequential decrease in neutralisation titres across ages (K–W P = 0.001 Figure 1D and Supplementary Figure 2G). Indeed, age was only negatively correlated with plasma neutralisation capacity in the uninfected group (r = −0.63, P < 0.001; Figure 1E). Overall, these results suggest that uninfected older adults show lower titres of neutralising antibodies and might be at higher risk of infection than previously infected individuals of the same age.
The proportion of effective neutralising antibodies among the total SARS-CoV-2-specific IgG (ratio of plasma neutralisation titre to total SARS-CoV-2 IgGs) was reduced in infected older participants compared with their younger counterparts (Figure 1F, P < 0.001), suggesting low functionality of antibodies in older participants. In contrast, no differences were found between both uninfected and vaccinated participants (Figure 1C). These results suggest that mRNA vaccines induce effective neutralising antibodies in older uninfected individuals, but at lower levels. Levels of anti-S2 + RBD IgGs positively correlated with neutralisation capacity in all groups (Supplementary Figure 2H).
Lastly, we performed an exploratory analysis to understand the potential impact of different parameters (sex, age, AMG level and hospitalisation) in neutralising immune response by linear regression models. In the univariate analysis, age was significantly associated with lower neutralising capacity in the uninfected group, and AMG level had a similar tendency (Supplementary Table 1A). In the infected group, we observed a tendency for a positive association between neutralisation levels with age, AMG level and sex, there being higher responses in males (Supplementary Table 1B). In the multivariate analysis, sex remained the only significant predictor, but we also observed a tendency related to age and hospitalisation.
Neutralisation capacity against the Delta variant
Given the lower neutralisation responses against WH1 in uninfected vaccinated LTCF residents, we hypothesised that this particular group might experience lower neutralising responses against the VOC B.1.617.2/Delta (circulating variant in Spain since July–December 2021). Indeed, we found a significant decrease in the plasma neutralisation capacity of Delta VOC compared with WH1 in both older and younger participants, regardless of their previous infection status (P < 0.001 in both cases, Figure 2A and Supplementary Figure 3A-C). Similarly to what was observed for WH1, we found a progressive decrease in the levels of neutralisation across ages in vaccinated uninfected individuals (Figure 2B and Supplementary Figure 3D, K–WP = 0.005), being barely detected in uninfected vaccinated older individuals. Surprisingly, neutralisation capacity against the Delta variant increased significantly across ages in individuals with prior SARS-CoV-2 infection (Figure 2B and Supplementary Figure 3E, K–WP < 0.001). Next, we compared cross-neutralisation capacities by determining fold change ratios between Delta and WH1 (reference spike) variants, as a measure of the loss of neutralising capacity. Median fold-change of all groups was <1, highlighting the greater difficulty in neutralising the Delta variant compared with WH1 (Figure 2C). Among all groups, previously infected older participants after vaccination showed significantly higher ratios compared with all other groups, demonstrating the better cross-neutralisation of the Delta variant.
A linear regression model was also built for neutralisation of the Delta variant revealing that older age was a strong predictor of a lower neutralisation capacity in the uninfected group (Supplementary Table 2A). In contrast, univariate analysis demonstrated that higher levels of neutralising antibodies were associated with hospitalisation, higher age and AMG level in the infected group (Supplementary Table 2B). Both age and hospitalisation remained significant predictors in the multivariate analysis.
Discussion
LTCF residents have been prioritised for COVID-19 vaccination in most countries due to their increased risk of morbidity and mortality. Although the effectiveness of COVID-19 vaccines is very high, SARS-CoV-2 infections among fully vaccinated individuals (i.e. breakthrough infections) are expected, especially with the appearance of VOC with higher transmissibility and immune resistance compared with the original WH1 variant. The reduced ability of older adults to elicit effective immune responses to vaccines and pathogens means that this population is one of the most vulnerable populations.
Here we demonstrate that, 3 months after COVID-19 vaccination, uninfected LTCF residents showed limited neutralisation responses compared with their younger counterparts, indicating that the uninfected older population is at higher risk. Vaccination of uninfected LTCF residents with two doses of the BNT162b2 vaccine reduced the risk of infection by 90% and by >95% COVID-19-related hospital admissions and mortality for up to 5 months [19]. Overall, uninfected older adults may develop a memory response which will protect them in the event of a new infection. However, the low levels of circulating neutralising antibodies after 3 months highlight the need for further studies to assess the long-term effectiveness of SARS-CoV-2 vaccines, and suggest that this specific subgroup will particularly benefit from receiving a booster vaccination [20].
Importantly, a potent immune responses to COVID-19 vaccines is developed in previously infected individuals independently of age [10, 12, 13, 21]. Our results suggest that the combination of natural and vaccine-generated immunity (hybrid immunity [22]) elicits a larger than expected immune response even in the older population, despite a high degree of immunosenescence and increased comorbidities. It has been recently shown that hybrid immunity improves B cells and antibodies against SARS-CoV-2 variants [23]. Older adults show a higher capacity to neutralise more resistant variants (i.e. B.1.617.2/Delta), such hybrid immunity implying a great benefit for this recovered population. In fact, hospitalisation and older age were identified as predictors of higher neutralisation levels, which could be associated with more severe disease.
Our results demonstrate that natural infection (pre-vaccine) generates higher virus-specific IgG antibodies in older adults compared with the younger population. In contrast, in uninfected older adults, vaccination generates lower SARS-CoV-2-specific IgGs, similarly to other studies [9, 10, 12, 13], and these antibodies show a lower neutralisation capacity in the older population. On the other hand, previously infected older persons after vaccine show higher levels of total SARS-CoV-2-specific IgG compared with younger adults, with similar levels of neutralising antibodies. Therefore, the measurement of SARS-CoV-2-specific IgGs might not be a good surrogate marker of protection in older adults, and functional assays, such as virus/pseudovirus-based neutralisation tests, are required.
Some studies showed lower levels of neutralising titters in uninfected older adults than in younger adults 2–3 weeks after BNT162b2 vaccination [11, 12], suggesting that older adults have a more limited initial immune response after vaccine administration. In another study, serum from older adults 3 weeks after vaccination showed a lower albeit still detectable neutralisation capacity relative to different VOC (Alpha, Beta or Gamma) compared with younger adults [11]. Here we showed that plasma from uninfected LTCF residents was unable to neutralise the Delta variant 3 months after vaccination, suggesting a greater resistance of this variant [24] and/or a lower durability of neutralising antibodies in older adults (3 weeks vs. 3 months) [9]. Nonetheless, the low levels of neutralising antibodies exhibited by uninfected LTCF residents against the original WH1 variant 3 months after vaccination, and even lower levels against the Delta variant, suggest that this specific population may require a third dose to boost their humoral response. In fact, the administration of a third dose to the Israeli population over age 60 reduced the number and severity of COVID-19 cases [20, 25], supporting our recommendation. On the other hand, it has been demonstrated that mRNA vaccination boosts cross-variant antibodies elicited by natural infection in the general population [26, 27]. Our results are in line with these findings and clearly reveal that previously infected older adults develop the same cross-neutralisation capacity against the Delta variant as the younger population. Therefore, previously infected older adults would not benefit from an additional vaccine dose at this time.
Our study has some limitations. First, the number of participants, especially for the uninfected group of LTCF residents, was unexpectedly low. Although 64% of the cohort tested positive by PCR, SARS-CoV-2 serologic test revealed that in fact 84% of residents had already experienced COVID-19. In addition, we did not assess specific SARS-CoV-2 cellular responses before and after vaccination, which could also be used as a correlate of protection [28].
Larger longitudinal studies targeting the older population are required to evaluate immune responses, as well as to monitor breakthrough infections and reinfection rates in vaccinated LTCF residents. For this purpose, determination of the quality of humoral immune responses—using functional neutralisation assays—as well as evaluation of the features of cellular responses to SARS-CoV-2 vaccines is necessary to better define the immune correlates of protection and to properly carry out tailored booster campaigns in this frail and vulnerable population. Taken together, our results suggest that only uninfected LTCF residents, who do not develop adequate immune responses, will clearly benefit from a booster vaccine dose; an adapted vaccination calendar would be necessary to respond to their immune needs. Importantly, hybrid immunity seems to be active in older adults and is likely to be relevant to the design of future vaccine boosting campaigns.
Supplementary Material
Acknowledgements
We are deeply grateful to all participants and their families, to the LTCF who participated in this study. We also thank the technical staff of Direcció d’Atenció Primària de la Metropolitana Nord for sample collection in LTCF (S. Reyes Carrión, N. Salarich Solà, A. Vidal, R. Alvarez Viñallonga, J. Tornero, E. Vilamala, C. Suarez, T. Gonzalo, L. Perez, D. Sans, A. Blancas Loras, A. Garcia Archer, J. Borràs, S. Cervelló) and staff of IrsiCaixa for sample processing (L. Ruiz, R. Ayen, L. Gomez, C. Ramirez, M. Martinez, T Puig). We thank CERCA Programme/Generalitat de Catalunya for institutional support and the Foundation Dormeur.
Alternative contact: Nuria Prat, Direcció d’Atenció Primària – Metropolitana Nord, Carretera de Barcelona, 473, 08203 Sabadell, Catalonia, Spain. Email: nprat@gencat.cat
Contributor Information
on behalf of the CoronAVI@S and the KING cohort extension studies:
S Reyes Carrión, N Salarich Solà, A Vidal, R Alvarez Viñallonga, J Tornero, E Vilamala, C Suarez, T Gonzalo, L Perez, D Sans, A Blancas Loras, A Garcia Archer, J Borràs, S Cervelló, G Llados, S España, J R Santos, C Loste, C López, I Casafont, C Estany, C Rodriguez, J Moreno-Muñoz, A Prats, C Herrero, A Garcia, M Montero, P Tornero, N Gonzalez Palomares, A Grajea, L Ortiz, C Miranda, E Abad, D Figueroa, A Mancera, S Gonzalez Alonso, M Perez, L Esteban, M Ortiz, L Valls, L Ceron, T Baena, C Puig, M Cucurell, and J Puig
Acknowledgement of Collaborative Authors
We wish to acknowledge the two collaborative group authors:
1. The CoronAVI@S collaborative group: S. Reyes Carrión, N. Salarich Solà, A. Vidal, R. Alvarez Viñallonga, J. Tornero, E. Vilamala, C. Suarez, T. Gonzalo, L. Perez, D. Sans, A. Blancas Loras, A. Garcia Archer, J. Borràs, S. Cervelló.
2. The KING cohort extension collaborative group: G. Llados, S. España, JR Santos, C. Loste, C. López, I. Casafont, C. Estany, C. Rodriguez, J Moreno-Muñoz, A Prats.
C. Herrero, A. Garcia, M. Montero, P. Tornero, N. Gonzalez Palomares, A. Grajea, L. Ortiz, C. Miranda, E. Abad, D. Figueroa, A. Mancera, S. Gonzalez Alonso, M. Perez, L. Esteban, M. Ortiz, L. Valls, L. Ceron, T. Baena, C. Puig, M. Cucurell, J. Puig.
Declaration of Conflicts of Interest
J.B. and J.C. reports personal fees from Albajuna Therapeutics, outside the submitted work.
Declaration of Sources of Funding
Gloria Soler Foundation, Grifols, the Departament de Salut of the Generalitat de Catalunya (Grant DSL0016 to J.B. and Grant DSL015 to J.C.), the Spanish Health Institute Carlos III (Grant PI17/01518 and PI18/01332 to J.C.) and the crowdfunding initiatives ‘https://www.yomecorono.com’, BonPreu/Esclat and Correos. M.T. and C.A.N. were supported by the Early Stage Research Staff Fellowship (FI21-B00327 and FI20-B00742) from the Catalan Agency for Management of University and Research Grants (AGAUR) and the European Social Fund. M.T. was then supported by a doctoral fellowship from the Departament de Salut from Generalitat de Catalunya (SLT017/20/000095). E.P. was supported by a doctoral grant from ANID, Chile: Grant 72,180,406.
References
- 1. Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol 2020; 35: 1123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prieto-Alhambra D, Balló E, Coma Eet al. . Filling the gaps in the characterization of the clinical management of COVID-19: 30-day hospital admission and fatality rates in a cohort of 118 150 cases diagnosed in outpatient settings in Spain. Int J Epidemiol 2020; 49: 1930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meis-Pinheiro U, Lopez-Segui F, Walsh S. et al. Clinical characteristics of COVID-19 in older adults. A retrospective study in long-term nursing homes in Catalonia. PLoS One 2021; 16: e0255141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Report ET . Data collection on COVID-19 outbreaks in closed settings with a completed vaccination programme : long-term care facilities purpose, aim and scope of this activity changes in version 2 primary objectives methods inclusion criteria for long-term care faci. 2021.
- 5. Folegatti PM, Ewer KJ, Aley PKet al. . Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020; 396: 467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baden LR, El Sahly HM, Essink Bet al. . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2020; 384: 403–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xia S, Zhang Y, Wang Yet al. . Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis 2020; 21: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Polack FP, Thomas SJ, Kitchin Net al. . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383: 2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Praet JT, Vandecasteele S, De Roo A, Vynck M, De Vriese AS, Reynders M. Dynamics of the cellular and humoral immune response after BNT162b2 messenger ribonucleic acid Coronavirus Disease 2019 (COVID-19) vaccination in COVID-19-naive nursing home residents.. J Infect Dis 2021; 224: 1690–3. [DOI] [PubMed] [Google Scholar]
- 10. Van Praet JT, Vandecasteele S, De Roo A, De Vriese AS, Reynders M. Humoral and cellular immunogenicity of the BNT162b2 messenger RNA coronavirus disease 2019 vaccine in nursing home residents. Clin Infect Dis 2021; 73: 2145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Collier DA, Ferreira IATM, Kotagiri Pet al. . Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021; 596: 417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Canaday DH, Carias L, Oyebanji OAet al. . Reduced BNT162b2 messenger RNA vaccine response in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)–naive nursing home residents. Clin Infect Dis 2021; 73: 2112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tut G, Lancaster T, Krutikov Met al. . Profile of humoral and cellular immune responses to single doses of BNT162b2 or ChAdOx1 nCoV-19 vaccines in residents and staff within residential care homes (VIVALDI): an observational study. Lancet Heal Longev 2021; 7568: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Del Giudice G, Goronzy JJ, Grubeck-Loebenstein Bet al. . Fighting against a protean enemy: immunosenescence, vaccines, and healthy aging. npj Aging Mech Dis 2018; 4: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Massanella M, Martin-Urda A, Mateu Let al. . Critical presentation of a severe acute respiratory syndrome coronavirus 2 reinfection: a case report. Open Forum Infect Dis 2021; 8: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trinité B, Tarrés-Freixas F, Rodon Jet al. . SARS-CoV-2 infection elicits a rapid neutralizing antibody response that correlates with disease severity. Sci Rep 2021; 11: 2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pradenas E, Trinité B, Urrea Vet al. . Clinical course impacts early kinetics and long-term magnitude and amplitude of SARS-CoV-2 neutralizing antibodies beyond one year after infection Edwards . medRxiv. 2021. [DOI] [PMC free article] [PubMed]
- 18. Espieén ID, Vela E, Pauws Set al. . Proposals for enhanced health risk assessment and stratification in an integrated care scenario. BMJ Open 2016; 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cabezas C, Coma E, Mora-Fernandez Net al. . Associations of BNT162b2 vaccination with SARS-CoV-2 infection and hospital admission and death with covid-19 in nursing homes and healthcare workers in Catalonia: prospective cohort study. BMJ 2021; 374: n1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bar-On YM, Goldberg Y, Mandel Met al. . Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 2021; 385: 1393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frieman M, Harris AD, Herati RSet al. . SARS-CoV-2 vaccines for all but a single dose for COVID-19 survivors. EBioMedicine 2021; 68: 103401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crotty S. Hybrid immunity. Science 2021; 372: 1392–3. [Google Scholar]
- 23. Andreano E, Paciello I, Piccini Get al. . Hybrid immunity improves B cells and antibodies against SARS-CoV-2 variants. Nature 2021; 600: 530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Planas D, Veyer D, Baidaliuk Aet al. . Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021; 596: 276–80. [DOI] [PubMed] [Google Scholar]
- 25. Barda N, Dagan N, Cohen Cet al. . Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 2021; 6736: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stamatatos L, Czartoski J, Wan Y-Het al. . mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science 2021; 372: 1413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reynolds CJ, Pade C, Gibbons JMet al. . Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science 2021; 372: 1418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. San Segundo D, Comins A, Lamadrid Pet al. . COVID-19 mRNA based vaccine immune-response assessment for public health decision. SSRN Electron J 2021; 11: 204–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.