Abstract
Controlling bacterial biofouling is desirable for almost every human enterprise in which solid surfaces are introduced into nonsterile aqueous environments. One approach that is used to decrease contamination of manufactured devices by microorganisms is using materials that easily slough off accumulated material (i.e., fouling release surfaces). The compounds currently used for this purpose rely on low surface energy to inhibit strong attachment of organisms. In this study, we examined the possible use of environmentally responsive (or “smart”) polymers as a new class of fouling release agents; a surface-grafted thermally responsive polymer, poly(N-isopropylacrylamide) (PNIPAAM), was used as a model compound. PNIPAAM is known to have a lower critical solubility temperature of ∼32°C (i.e., it is insoluble in water at temperatures above 32°C and is soluble at temperatures below 32°C). Under experimental conditions, >90% of cultured microorganisms (Staphylococcus epidermidis, Halomonas marina) and naturally occurring marine microorganisms that attached to grafted PNIPAAM surfaces during 2-, 18-, 36-, and 72-h incubations were removed when the hydration state of the polymer was changed from a wettability that was favorable for attachment to a wettability that was less favorable. Of particular significance is the observation that an organism known to attach in the greatest numbers to hydrophobic substrata (i.e., H. marina) was removed when transition of PNIPAAM to a more hydrated state occurred, whereas an organism that attaches in the greatest numbers to hydrophilic substrata (i.e., S. epidermidis) was removed when the opposite transition occurred. Neither solvated nor desolvated PNIPAAM exhibited intrinsic fouling release properties, indicating that the phase transition was the important factor in removal of organisms. Based on our observations of the behavior of this model system, we suggest that environmentally responsive polymers represent a new approach for controlling biofouling release.
Biofilms result from the accumulation of organic molecules, microorganisms, and the metabolites of microorganisms at solid-liquid interfaces (6). The formation of these structures represents a major survival strategy for the organisms involved. These structures provide a means of concentrating nutrients, increase the chance of genetic exchange, and protect organisms from desiccation, predators, and toxins (6, 20). Biofilms are ubiquitous on surfaces submerged in natural aqueous environments. While advantageous to the bacteria and algae involved, biofilms on manufactured devices or structures can adversely affect the lifetime and operation of a device or structure, resulting in increased cost of operation and maintenance and, in some cases, endangerment of human health. Biofilm research in recent years has emphasized obtaining information that suggests ways to prevent or control unwanted biofilm formation or biofouling (5, 6).
The most common commercially used technique to control biofouling in marine and freshwater environments is the use of paints and other coatings containing copper or other metal organocomplexes (5). While these compounds do efficiently reduce the amount of biological material that accumulates on a surface, they can also severely damage nontarget organisms and are therefore environmentally undesirable. Furthermore, these compounds are toxic to humans; this limits their utility for implanted prosthetics and other medical equipment, water treatment facilities, and many types of industrial bioconversion processes in which biofouling is deleterious and also endangers the health of people who apply the compounds to equipment. In recent years, there has been an emphasis on developing nontoxic artificial surfaces that are resistant to the formation of biofilms or from which fouling can be readily removed (fouling release surfaces) (5). Surfaces of the latter type, generally silicon elastomers and fluoropolymers, have been shown to be useful in controlling not only bacterial and algal fouling (soft fouling) but also the accumulation of barnacles and other mollusks (hard fouling) (5).
In a previous paper (18), we explored the possibility that environmentally responsive (“smart”) polymers may represent a new class of compounds that can be exploited for their fouling release properties. Some examples of these compounds are the polymers that exhibit dramatic changes in aqueous solubility in response to environmental stimuli, such as temperature, pH, electrical potential, or light (12, 15). In order to determine whether rapid changes in hydration resulted in fouling release, we studied a model system composed of dip-coated poly(N-isopropylacrylamide) (PNIPAAM) (18). PNIPAAM has a lower critical solubility temperature (LCST) of 32°C (13). At temperatures above the LCST the polymer is insoluble in water, and at temperatures below the LCST PNIPAAM is water soluble. We showed that organisms that attached to PNIPAAM at temperatures above the LCST were removed upon solvation of the coating when samples were rinsed with cold water. A major drawback of this method was that the coatings had to be reapplied after each release. Workers in other laboratories have successfully used PNIPAAM grafted onto polystyrene petri dishes to reversibly release and pattern cultured mammalian cells (25), which led us to attempt to use similarly grafted PNIPAAM to release bacteria.
In this study, we (i) developed a simple method for covalently grafting ultrathin layers of PNIPAAM onto polystyrene surfaces, (ii) found that the fouling release properties of PNIPAAM are preserved when the polymer is grafted onto a surface, and (iii) found that such a surface is capable of undergoing a limited number of repetitive fouling and release cycles. We also included in this study a controlled examination of attachment and release of two bacterial species, Halomonas marina and Staphylococcus epidermidis, which differ in the degree to which they attach to hydrophilic and hydrophobic surfaces, as we demonstrated previously (17). By modulating the hydration properties of the solid polymeric surfaces through the LCST transition, we demonstrated the release of biofilms of both types of organisms. We also determined the release properties of PNIPAAM for biofilms composed of organisms from complex natural marine communities (unfiltered seawater).
MATERIALS AND METHODS
Media and buffers.
All media and buffers were prepared with deionized water (d-H2O) generated by a system in which tap water was subjected sequentially to water softening, reverse osmosis, and ion exchange (Barnstead-Thermolyne RoPure/Nanopure system). The final resistivity of the processed water was greater than 18 MΩ cm−1. Marine broth 2216 (MB) (Difco) was prepared according to the manufacturer’s instructions. Marine agar was prepared by adding 1.5% Bacto Agar (Difco) to MB. Artificial seawater (ASW) contained 400 mM NaCl, 100 mM MgSO4, 20 mM KCl, and 10 mM CaCl2 (19). Modified basic marine medium supplemented with glycerol (MBMMG) contained 0.5× ASW, 19 mM NH4Cl, 0.33 mM K2HPO4, 0.1 mM FeSO4 · 7H2O, 5 mM Tris HCl (pH 7), and 2 mM glycerol (19). The concentration of Tris HCl was reduced from the concentration in the original recipe (100 mM) when we found that H. marina could utilize this buffer as a carbon source when it was grown in the medium described above (MBMMG) without glycerol.
Nutrient broth (NB) (Difco) was mixed according to the manufacturer’s directions. Nutrient agar was made by adding 1.5% Bacto Agar to NB. Staphylococcus basal medium supplemented with glycerol contained 6 mM (NH4)2SO4, 0.5 mM MgSO4 · 7H2O, 13.5 mM KCl, 28 mM KH2PO4, 72 mM Na2HPO4, 1 μg of thiamine per ml, 0.5 μg of biotin per ml, 0.5% Bacto Peptamin (Difco), and 1 mM glycerol (10). Dulbecco’s phosphate-buffered saline (PBS) (Sigma Chemical Co.) was mixed according to the manufacturer’s instructions.
Bacterial strains.
H. marina (basonym, Deleya marina Baumann et al. 1983) ATCC 25374 (3, 9, 33) was revived from the original lyophilized culture and was stored frozen stock aliquots in MB containing 20% glycerol at −70°C. Experimental stock preparations were maintained on marine agar slants and were stored at 4°C for up to 2 weeks. Prior to inoculation into a chemostat, a single colony from a slant was inoculated into 50 ml of MB and grown overnight with shaking at 25°C. A chemostat culture was established by inoculating 3 ml of the overnight culture into MBMMG. The chemostat was maintained at a flow rate of 1 ml min−1 (dilution rate, 0.16 h−1) with constant stirring. The titer of the subsequent culture was 107 cells ml−1.
S. epidermidis ATCC 14990 was revived from its original lyophilized culture in NB. Aliquots were stored in NB containing 20% glycerol at −70°C. Experimental stock preparations were maintained on nutrient agar slants and were stored at 4°C for up to 2 weeks. Prior to inoculation into a chemostat, a single colony was transferred into 50 ml of NB and grown overnight at 37°C with shaking. Five milliliters of this culture was inoculated into 500 ml of the chemostat medium (Staphylococcus basal medium supplemented with glycerol). The flow rate through the chemostat was 1.5 ml min−1, and the dilution rate was 0.16 h−1. The bacterial concentration was 4.6 × 107 ± 7.4 × 106 cells ml−1.
Bay water (Puget Sound water [PSW]) was collected in the southern part of Tulalip Bay in Puget Sound near Marysville, Wash. Samples were collected during ebb tide in early August 1997. The collection vessels were clean plastic 1-gallon jugs. After collection the containers were sealed and shipped to our laboratory by express delivery. Upon receipt, the samples were stored at 4°C until they were used.
Preparation of grafted PNIPAAM.
N-isopropylacrylamide (NIPAAM) (Aldrich Chemicals) was recrystallized from hexane and dissolved in 2-propanol in order to prepare a 10-mg ml−1 solution. This solution was degassed by repeated freeze-thaw cycles under a vacuum and was kept under argon until it was used. Polystyrene coupons were cut from the lids of standard laboratory petri dishes (VWR) soaked in ethanol, rinsed in d-H2O, and dried under nitrogen. These coupons were then subjected to an argon plasma treatment (pressure, 75 μm of Hg) for 5 min with in a Harrick model RDC-32G plasma cleaner-sterilizer. Exposure of the polystyrene samples to the argon plasma resulted in the formation of free radicals on the surfaces of the samples. The free radicals initiated polymerization upon addition of the NIPAAM monomer. With the preparations under argon, we added the NIPAAM solution to the plasma-treated polystyrene in the plasma cleaner, and the system was sealed. Polymerization was allowed to continue for 1 h, after which the NIPAAM solution was removed. The samples were then rinsed with three 25-ml washes of 2-propanol to remove the residual monomer and then with d-H2O, and then they were dried with a stream of N2. Samples were stored in the dark until they were used (usually no more than 1 week). Polystyrene samples that were plasma treated but were not used for grafting were kept as control samples and were designated plasma-cleaned polystyrene (PCPS).
Characterization of surfaces.
The wettability of the samples was determined by measuring advancing water contact angles (θAW) with a Ráme-Hart goniometer. When contact angles of PNIPAAM were determined at temperatures above room temperature, the stage of the goniometer was equipped with a variable-temperature heating strip (Cole Parmer). A silver-coated microscope slide was placed between the sample and the heating strip to ensure even distribution of heat and to provide a level surface. The temperature of the silver surface was monitored with a microthermocouple (Cole Parmer). θAW was measured when the surface temperature was 23 to 25 and 45°C. Before PNIPAAM samples were tested, they were soaked in d-H2O at the temperature at which θAW was to be measured for at least 15 min. This step prevented aberrant measurements caused by the polymer changing hydration while θAW was being measured.
PNIPAAM samples were studied to determine their abilities to endure several transitions through the LCST. A single experimental cycle consisted of (i) soaking a sample in 4°C d-H2O for 30 min, (ii) drying the sample under N2 and measuring θAW at 23°C, (iii) soaking the sample in 45°C d-H2O for 30 min, and (iv) drying the sample under N2 and measuring θAW at 45°C. The duration of the incubation reflected the amount of time required for the silver slide on the goniometer to reach the required temperature. For each measurement the θAW was determined at three different randomly chosen locations on each sample. The cycle was repeated until the wettability did not change after a temperature shift for several rounds.
For X-ray photoelectron spectroscopy (XPS) analyses of polystyrene, PCPS, and grafted PNIPAAM we used a model SSX-100 spectrometer (Surface Science Instruments, Mountain View, Calif.) at the National ESCA and Surface Analysis Center for Biomedical Problems at the University of Washington. The system used analyzed an elliptical area whose short axis was adjusted to 1,000 μm. An A1 Kα1,2 monochromatized X-ray source (hυ = 1,486.6 eV) was used to stimulate photoemission. The energy of the emitted photoelectrons was measured with a hemispherical analyzer. Survey scans ranging from 0 to 1,000 eV of binding energy (with a pass energy of 150 eV) were performed to examine the elemental composition of the surfaces. At this pass energy, the transmission function of the spectrometer can be assumed to be constant (34). The peak areas were normalized by the number of scans, the number of points per electron volt, the Scofield photoemission cross sections (32), and the sampling depth. The sampling depth was assumed to vary as KE0.7 varied, where KE is the kinetic energy of the photoelectrons (34). XPS data were acquired at a photoelectron take-off angle of 55°; the take-off angle was defined as the angle between the surface normal and the axis of the analyzer lens. High-resolution scans were also recorded at a pass energy of 50 eV. Peak positions were assigned by using the hydrocarbon (CHx) peak at 285.0 eV as a reference.
Static secondary-ion mass spectroscopy (SIMS) analyses were performed with a model 7200 time-of-flight instrument from Physical Electronics (Eden Prairie, Minn.) equipped with an 8-keV Cs+ ion source and a two-stage reflectron time-of-flight analyzer. The total accumulated ion dose per sample was kept below 1013 ions cm−2 to ensure that static SIMS conditions were met (4). Positive and negative ion spectra were recorded, and the positions of the peaks were calibrated by using the CH3+, C2H3+, and C3H3+ peaks as references for the positive spectra and the CH−, OH−, and C2H− peaks as references for the negative spectra.
Detachment studies.
In the initial detachment experiments we used surface-grafted PNIPAAM samples placed in glass petri dishes containing either (i) preequilibrated (37°C) ASW containing 5 × 106 H. marina cells ml−1, (ii) 37°C PBS containing 107 S. epidermidis cells ml−1, or (iii) unfiltered 37°C PSW. After incubation at 37°C for 2, 18, or 36 h, the samples were rinsed with 37°C d-H2O to remove salts, dried under nitrogen, and examined with a phase-contrast microscope (Nikon Labophot). Images of 10 randomly chosen fields of view were captured with an ×60 objective by using a digital camera interfaced with Image Tool image-processing software (35). Image capture and analysis were done as previously described (18). For each field of view, one in-focus frame and one out-of-focus frame were recorded and saved for processing. After the initial images were taken, the samples were rinsed at 4°C with 60-ml portions of the appropriate solutions (ASW for H. marina and organisms from PSW, PBS for S. epidermidis) delivered with a 60-ml syringe. The samples were then rinsed with d-H2O to remove the salts and dried, and again 10 randomly selected fields of view were captured by Image Tool image-processing software with the ×60 phase-contrast objective. In all cases, control samples consisting of PCPS and plasma-cleaned glass coverslips were processed at the same time.
The cellular densities of bacteria attached were assessed as described previously (18). For samples in which the bacteria were clumped in biofilms or otherwise not well separated, the numbers of pixels representing the areas covered by biofilms were counted. In the latter cases, the numbers of cells were estimated by allowing for 71 pixels per cell for H. marina and 51 pixels per cell for S. epidermidis values. These were determined experimentally by counting the numbers of pixels represented in fields of images containing less than 10 cells that were well separated (18). The numbers of cells in 10 fields of view were then averaged, and the results were divided by the area of one field of view (0.24 mm2) to determine the cell density. For each combination of organism and incubation time, a minimum of six replicate experiments were performed.
Studies were also performed to determine whether the hydrated form of PNIPAAM (i.e., the form at temperatures below the LCST) was able to release attached organisms or to resist bacterial adhesion. Samples were incubated in ASW containing H. marina, as described above; however, the incubation temperature was 25°C, which was below the LCST. The subsequent processing was identical to the processing described above.
Long-term incubation.
The integrity and performance of the polymer under more vigorous biofouling conditions were examined by incubating samples for longer periods of time and under conditions that permitted not only cellular attachment but also growth on the surface. Slightly different strategies were used to promote fouling by each of the organisms. Samples challenged with H. marina were incubated for 72 h at 37°C in fresh MBMMG inoculated with 5 × 106 cells from the chemostat per ml; additional glycerol (10 mM) was added on a daily basis, and after 36 h an additional aliquot of cells was added. Samples challenged with S. epidermidis were incubated at 25°C in 25 ml of MB inoculated with 107 cells from an overnight culture in MB per ml. No additional medium was added during the rest of the 72-h incubation. The samples were processed as described above.
Repetitive release of organisms.
Tests were also performed to determine whether repetitive fouling-release cycles affected the ability of PNIPAAM to release bacteria. The experiments were performed as described above for the basic detachment experiments, but the same sample was used for five cycles. Freshly prepared organisms were used for each incubation stage.
RESULTS
Surface analysis.
PCPS had a θAW of less than 10°, as did plasma-cleaned glass. The θAW of PNIPAAM was 60 ± 0.3° when it was measured at 45°C and 47 ± 1° when it was measured at 23°C.
XPS provides elemental composition information (for all elements except hydrogen) for the near-surface region (∼100 Å from the surface) of solid materials (21). The XPS spectra of PCPS and PNIPAAM differed significantly from the XPS spectrum of untreated polystyrene. The spectrum of polystyrene was dominated by the carbon 1s peak and had only trace signals (corresponding to ∼2 atomic percent) due to oxygen. In contrast, the spectra of PCPS and PNIPAAM had significant contributions from oxygen (corresponding to ∼19% and 15%, respectively). Trace amounts of nitrogen were also detected on the PCPS and PNIPAAM surfaces. Plasma treatment of polymers often results in the introduction of oxygen and nitrogen onto the surface as atmospheric components quench free radical and ionic species created on the surface during the treatment (29). It was not possible to verify the presence of PNIPAAM polymer chains on a PNIPAAM sample by XPS because (i) the substrate upon which the PNIPAAM was polymerized was treated with plasma initially, (ii) the spectra of both the PNIPAAM sample and PCPS had oxygen and nitrogen peaks, in addition to carbon peaks, that had similar intensities, and (iii) the low levels of nitrogen detected (∼1%) indicated that a very small amount of PNIPAAM (near the detection limit of XPS) was grafted onto the sample.
Static SIMS is an extremely sensitive method for obtaining structural information concerning the near-surface regions (∼top 15 Å) of polymeric materials (22). Static SIMS of the grafted PNIPAAM surfaces revealed peaks that were consistent with the peaks found in the spectrum of dip-coated, commercially prepared polymer (Polysciences Inc.) (27). Peaks characteristic of polystyrene were also present, suggesting that the surface-grafted PNIPAAM was not a complete overlayer or that the grafted polymer layer was extremely thin. The XPS and static SIMS data, together with the contact angle and bacterial attachment data that indicated that there was an LCST characteristic of PNIPAAM, strongly suggested that a very thin surface layer of PNIPAAM polymer chains was created by the plasma-induced grafting procedure. The XPS and static SIMS spectra described above and the results of a detailed analysis of the chemical structure of the layers will be published elsewhere.
Detachment studies.
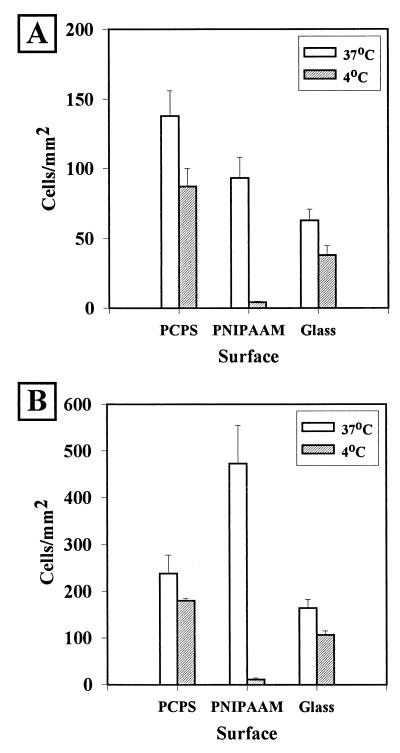
The results of detachment studies are summarized in Fig. 1 through 5. All of the data are presented as averages ± standard errors. As Fig. 1 shows, bacteria that attached during short-term incubations (2 h) exhibited different responses to surfaces and to detachment conditions. H. marina (a gram-negative marine organism) incubated in ASW at 37°C attached to all surfaces, adhering in the greatest numbers to PCPS (139 ± 16 cells mm−2), at intermediate levels to PNIPAAM (90 ± 11 cells mm−2), and at the lowest cellular density to glass (38 ± 8 cells mm−2). For all samples, the differences among the levels attachment to the surfaces were significant, as determined by the Student t test (P < 0.05). While statistically significant numbers of H. marina cells were released from all surfaces when they were rinsed with 4°C ASW, the greatest percentage of cells (95 ± 1%) was released from the PNIPAAM surface, which also had the lowest final concentration of cells (4.5 ± 0.6 cells mm−2).
FIG. 1.
Attachment and detachment of H. marina to solid surfaces. After incubation for 2 h (A) or 18 h (B) at 37°C in ASW containing 106 cells ml−1, the average number of adhering cells was determined (37°C). The samples were then rinsed in 60 ml of ASW at 4°C, and the remaining cells were counted (4°C). The average number of cells per field of view was calculated by using 10 fields of view per sample per experiment. Each graph represents the data from 10 such experiments.
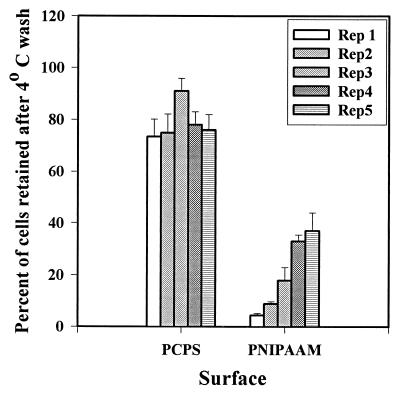
FIG. 5.
Repetitive release of organisms from PNIPAAM surfaces. Surfaces were incubated in the presence of a preparation containing 106 H. marina cells ml−1 for 2 h at 37°C, and the cells were counted. The samples were then washed in 4°C ASW, and the cells were counted again. The percentage of cellular retention in 10 fields of view was calculated for each sample. The attachment-release process was repeated five times to determine whether the release properties of PNIPAAM were retained. The data are the averages of the data for three sets of experiments. Rep, repetition.
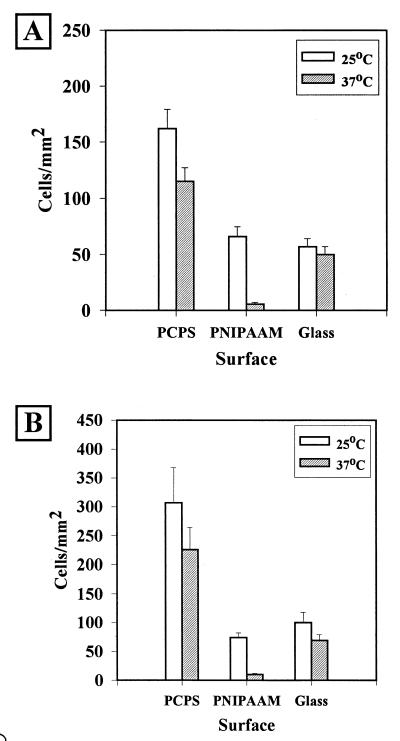
We also incubated preparations initially at 25°C to determine whether the solvated form of PNIPAAM was intrinsically capable of fouling release or exhibited fouling-resistant properties. After H. marina was incubated with samples at 25°C, there was not a significant difference between the number of cells attached to PCPS and the number of cells attached to PNIPAAM (58 ± 4 and 56 ± 4 cells mm−2, respectively), and the values obtained were only slightly greater than the number of cells attached to the glass sample (43 ± 3.5 cells mm−2). After the 4°C ASW rinse, the densities of cells remaining on the surfaces were virtually identical (for PCPS, 37 ± 1 cells mm−2; for PNIPAAM, 38 ± 4 cells mm−2; for glass, 34 ± 4 cells mm−2). Both before and after the 4°C wash, the numbers of cells attached to PCPS and PNIPAAM at 25°C were less than the numbers of cells attached at 37°C for similar samples; however, the levels of release for PCPS and for glass after incubation at 25°C (37% ± 4% and 20% ± 6%, respectively) were not significantly different from the levels of release after incubation at 37°C (38% ± 3% and 37% ± 4%, respectively). The level of release for PNIPAAM at 25°C, however, was very different than the level of release observed when the bacteria were attached at 37°C; the level of release obtained after incubation at 37°C (94.5% ± 0.6%) was much higher than the level of release after initial incubation at 25°C (23% ± 6.2%). Taken together, these data suggest that the solvated form of PNIPAAM is neither intrinsically capable of fouling release nor fouling resistant.
The results obtained in similar studies performed with S. epidermidis, a gram-positive skin bacterium, were quite different. After incubation at 37°C, cells attached to all samples, and the PCPS sample had the highest cellular density. None of the samples exhibited appreciable release capabilities when they were rinsed with 60 ml of 4°C PBS. However, previous studies performed in our laboratory and other laboratories (11, 17) indicated that S. epidermidis attaches in the greatest numbers to hydrophilic surfaces; thus, it might be predicted that a transition from a hydrophobic surface energy to a hydrophilic surface energy would not result in a net loss of cells. We therefore attempted the reverse experiment; we allowed initial attachment at a temperature below the LCST of PNIPAAM (25°C) and rinsed with PBS at a temperature above the LCST (37°C). Figure 2A shows the results of these experiments. Under these conditions, more S. epidermidis cells attached to PCPS than to other surfaces. Washing with 37°C PBS resulted in statistically significant detachment from PCPS and PNIPAAM but not from glass. Once again, however, the average percentage of cells removed from PNIPAAM (93% ± 1.6%) far exceeded that the average percentage of cells removed from either PCPS (22% ± 8%) or glass (13% ± 6%).
FIG. 2.
Attachment and detachment of S. epidermidis. Cells were incubated for 2 h (A) or 18 h (B) at 25°C in PBS containing 107 cells ml−1, and the numbers of attached cells were determined (25°C). The samples were then each rinsed in 60 ml of 37°C PBS, and the remaining cells were counted (37°C).
Overnight experiments (Fig. 1B and 2B) yielded detachment results similar to the results of the 2-h experiments. Incubation of samples overnight in the presence of H. marina at 37°C resulted in significantly more attachment to all surfaces tested than that observed at 25°C (Fig. 1B). Bacteria were released from control surfaces; however, the fewest cells remaining (11 ± 9 cells mm−2) and the greatest percentage of cells released were observed with the PNIPAAM samples (96% ± 1%, compared with 20% ± 4% for PCPS and 28% ± 7% for glass). Overnight incubation of the samples with S. epidermidis at 25°C followed by a rinse at 37°C yielded a similar pattern of cell detachment (Fig. 2B). Under these conditions, the cells again attached in the largest numbers to PCPS. The average percentage of cells released from PNIPAAM (86% ± 2.1%), however, far exceeded the average percentage of cells released from either of the two control surfaces, PCPS (20% ± 7%) and glass (26% ± 5%).
Natural organisms from PSW were allowed to attach at 37°C for 36 h before the preparations were counted, rinsed with 4°C ASW, and recounted. Because of the morphologic diversity among the organisms living in PSW, estimating the average number of pixels per cell was difficult, and, therefore, the total numbers of pixels covered by the organisms are reported below. The total field of view (area, 0.24 mm2) was composed of 307,200 pixels. Organisms attached to all surfaces, but slightly more cells attached to PCPS (Fig. 3). All three surfaces released cells when they were rinsed with 4°C ASW; however, PNIPAAM released a higher average percentage (95% ± 1.5%) than glass (34% ± 8%) or PCPS (25% ± 7%).
FIG. 3.
Attachment and detachment of PSW organisms after 36 h of incubation. Samples were incubated in PSW for 36 h at 37°C before the numbers of pixels covered in images of the surfaces were determined (37°C). The surfaces were each washed with 60 ml of ASW at 4°C, and the pixels were counted again. The data are averages of the data for 10 fields of view. Each data point represents seven repetitions of the experiment.
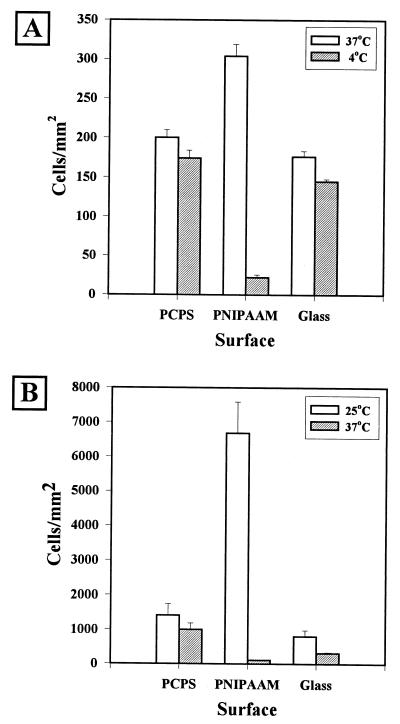
Long-term incubation.
The results of detachment studies performed with organisms that were attached after 3 days of incubation are shown in Fig. 4. H. marina yielded relatively low attachment densities over the 3-day attachment period. As observed for the 18-h incubation, cells attached in the greatest densities to PNIPAAM (304 ± 25 cells mm−2). The cellular density was not increased significantly by longer incubation (up to 6 days) (data not shown). PNIPAAM also exhibited the greatest reduction in cellular density after rinsing with 4°C ASW; the final density was 21 ± 6 cells mm−2.
FIG. 4.
Release of organisms after 72 h of incubation. Surfaces were incubated with either H. marina (A) or S. epidermidis (B) under conditions that promoted growth as well as attachment to the surfaces. The cellular densities were determined after incubation (open bars) and after rinsing (cross-hatched bars). The data are averages of the data for 10 fields of view. Each data point represents three repetitions of the experiment.
S. epidermidis formed much more dense bacterial films on all surfaces. The most densely covered surface was the PNIPAAM surface, which had a two-dimensional density of 6,680 ± 913 cells mm−2. This number did not take into account the three-dimensional character of the cellular aggregates and, therefore, was probably a low estimate. However, cells were still removed when the preparations were washed with PBS at a temperature above the LCST; the final density of cells on the PNIPAAM surface was 109 ± 18 cells mm−2, a 98% reduction.
Repetitions.
In order to test the durability of the PNIPAAM films with regard to their release properties, we challenged the surfaces with a series of fouling and release cycles consisting of 2-h incubations with H. marina. The data obtained are summarized in Fig. 5. We observed that there was a slow accumulation of nonremovable material during each repetition. By the fifth repetition, more than 25% of the initially attached cells were not removed by the 4°C rinse. It should be noted that this was still less total accumulation than the total accumulation observed for the PCPS controls.
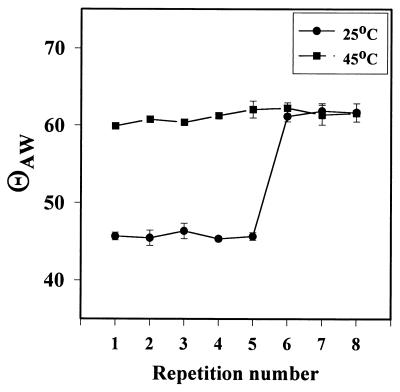
The question of whether the decrease in detachment efficiency was due to a loss of the resilience of the polymer was tested by cycling similar samples through a series of alternating incubations in d-H2O at 4 and 45°C and measuring the resultant contact angles with the goniometer at the appropriate temperature. The results are summarized in Fig. 6. The θAW of the polymer changed in response to temperature through five cycles, after which the more hydrophilic form of the polymer, indicated by a θAW of 45°, could not be regenerated.
FIG. 6.
θAW of PNIPAAM samples during repetitive cycling through the LCST. The data are the averages of the data obtained for three different PNIPAAM samples.
DISCUSSION
Based on our results obtained with the PNIPAAM model, we suggest that a rapid, dramatic change in the hydrophobicity of the substratum can result in detachment of biofilms from solid surfaces. That the surface energy and, therefore, wettability of the solid surface play a major role in both attachment and detachment is well-recognized (1, 5). The idea of modulating this property of the substratum in order to remove attached bacteria is, however, somewhat new and may be an additional approach that can be considered when fouling release surfaces are designed. Since there are a variety of environmentally sensitive polymers that respond to stimuli other than temperature (including light, pH, or electricity [12]), the utility of this class of materials in a number of microbiological applications is implied by the results obtained with our PNIPAAM model. Future investigations in our laboratory will include investigations of the fouling release properties of some of these compounds.
The finding that the fouling release properties were observed with samples on which attachment and growth were allowed to continue for several days is both interesting and encouraging. In situ examination of this process should prove to be interesting. We currently know that the vast majority of the cells are removed upon shearing; we do not know the process by which this occurs (i.e., whether the attached organisms are removed in one large mass). To address this question, observation in a flow cell is planned. Using a flow cell should also increase the extent of biofilm formation since larger numbers of cells come into contact with the samples per unit of time and longer incubation times are possible with such an apparatus. This technology also offers the possibility of examining the difference between biofilms formed by de novo attachment and biofilms formed by growth. We are currently modifying our experimental protocols to allow these studies to be performed.
The results obtained in this study may also give us some additional insight into how microorganisms interact with the supporting surface in a biofilm. It has been suggested that some bacteria employ different strategies for adhering to substrata having different hydrophobicities (20). For example, Paul and Jeffrey (26) found that enzymatic and detergent treatment of Vibrio proteolytica altered both the cell surface hydrophobicity and the adhesion of cells to polystyrene but not glass substrata, whereas Dalton et al. (7) found that for a marine pseudomonad, the morphology of entire colonies formed on hydrophobic surfaces differed greatly from the morphology of colonies formed on hydrophilic surfaces. In the present investigation, while H. marina cells were removed nearly completely when incubated with PNIPAAM at a temperature above the LCST and rinsed at a temperature below the LCST, they were not removed when we attempted to release cells that were first attached when the polymer was in the hydrophilic state (i.e., at a temperature below the LCST), shifted to the hydrophobic state, and then shifted back again to a more wettable surface (data not shown). Under the growth conditions used in these experiments, H. marina attaches in greater numbers to hydrophobic organic surfaces (17). These results imply that when H. marina attaches to a hydrophilic (or disfavored) surface, it does so in a different manner than it attaches to a more hydrophobic surface. Silverman et al. have postulated that different adhesion mechanisms are used under different environmental conditions and that a single organism may have a variety of different adhesins (23), based on studies of the expression of genes involved in the production lateral flagella in Vibrio parahaemolyticus in response to cellular contact with a surface. Similarly, Shea et al. (33) have suggested that a putative phase variation from gliding motility to flagellar motility in H. marina could be the result of environmental cues from the surface.
All of the organisms tested, whether they were cultured or indigenous, attached in the greatest numbers to PCPS. This finding was without regard to the general patterns of attachment to hydrophilic or hydrophobic surfaces. H. marina, for example, attached in the greatest numbers to PCPS surfaces after 2 h of incubation at 37°C than to the more hydrophobic PNIPAAM surfaces, and, although more cells adhering to a hydrophilic surface was not unexpected for S. epidermidis, the number of cells of this organism that attached to PCPS was far more than the number of cells that attached to glass, even though the surfaces had similar θAW values. It is known that plasma modification of polymers generates a number of different chemical moieties on the surfaces of treated materials, particularly organic materials. It has been suggested that some of the plethora of functional groups generated by plasma treatment of organic polymers serve as specific receptors for proteins and cells, which may increase the overall adhesion (28, 30). Furthermore, it is known that submersion of plasma-treated polystyrene in aqueous media can result in modification of the surface chemistry of the polymer (24). This may in part explain why large numbers of H. marina cells did not attach to PCPS after 18 h of incubation. Possible changes in the compositions of the surfaces of the cells themselves must also be considered. Prolonged incubation of H. marina under nutrient deprivation conditions and at temperatures near the high end of the growth temperature range is almost certainly stressful to the cells, and it is known that stressed bacteria can alter their cell surfaces and, concomitantly, their adherence properties, often dramatically (20). We are currently investigating surface changes of the solid surfaces and H. marina that occur during prolonged incubation at 37°C.
Although the layer of PNIPAAM grafted onto the polystyrene was thick enough to both affect the contact angle and function efficiently as a fouling release coating, XPS and static SIMS analyses indicated that this layer was nonetheless thin and possibly not uniform. Other researchers have noted that plasma-initiated polymerization leads to less-than-full coverage of a surface but have obtained high-molecular-weight polymers on surfaces (14, 16). One major difference between the technique used in the present work and the technique used more successfully by other workers is that it has been found that this sort of polymerization is optimal at temperatures around 60°C (14). The design of our equipment was such that we were not able to heat the polymerization chamber to temperatures above room temperature, and this may well have had a deleterious effect on the total amount of polymerization which we obtained.
The lack of uniform and complete coverage may have had a deleterious effect on the reversibility of PNIPAAM hydration. We observed a loss of the ability to cycle through two hydration states, as shown by the failure to return to a θAW of 45° upon repeated alternating exposure to 25 and 45°C d-H2O and by the decrease in the ability to release organisms after repeated cycles at temperatures above and below the LCST. The slight accumulation of organisms observed before the loss of the LCST behavior suggests that alterations in the polymer occurred while they were still undetectable by the θAW technique. Since the characteristic size of bacteria is 3 orders of magnitude smaller than the characteristic size of the water droplets used to measure the θAW, this observation is not surprising.
The source of the loss of resilience is not known. There are a number of possible reasons that such a loss might occur. (i) It is known that surface-grafted polymers can undergo rearrangements upon transition from aqueous media to air (21, 22). Specifically, the hydrophilic sectors of a polymer tend to bury themselves below the surface when they are removed from water. If this is coupled with a change in the actual hydration state of the hydrophilic regions of PNIPAAM during an increase in the temperature above the LCST, it is possible that a proportion of the chains get “locked” in the buried state and are less accessible to water when the temperature is decreased. (ii) The same molecular interactions that are responsible for the collapse of PNIPAAM chains at temperatures above the LCST (13) may also occur between different polymer chains. This may result in entanglement of the chains that may not be resolved when the temperature is decreased. (iii) In our experiments, the samples were repeatedly drawn through air-water interfaces and exposed to air, which may have resulted in contamination of the surfaces by dissolved molecules at the interfaces or by volatile chemicals in the air. We are currently investigating each of these possibilities. Recent results have indicated that samples cycled without exposure to the air-water interface do indeed retain their LCST activity for longer periods of time. We are continuing to investigate the molecular origin of this observation.
Determining the relative contribution of each of the processes described above to the loss of fouling release is necessary. In addition, it should also be worthwhile to examine the contribution of biological modifications to the polymer generated by the adhesion and release of the bacteria. In this context, it will be especially important to determine to what extent biologically derived macromolecules can be released from PNIPAAM. It is likely that the cycle-dependent loss of both contact angle resilience and fouling release capability is due to the cumulative effects of all of the factors mentioned above (molecular rearrangement of the polymer, environmental contamination, and biologically derived deposits).
To overcome the various difficulties encountered as a result of our grafting procedure, we are currently exploring methods for forming self-assembled monolayers (SAMs) of PNIPAAM-terminated alkane thiolates on gold (31). Such SAMs would be desirable experimental surfaces because they would have consistent surface chemistry and topology. They can be formed on gold films of different thicknesses, which would allow either transmission or reflective optical analysis techniques to be performed (2). SAM technology should also make generation of mixed or patterned stimulus-responsive surfaces possible. The SAMs should also be useful in flow chamber experiments, which the thickness of the polystyrene used in the current work precluded. Thus, we should be able to change the temperature of the surfaces without a transition through air and should be able to observe bacterial attachment and detachment in situ.
ACKNOWLEDGMENTS
We thank Deborah Leach-Scampavia for her help in acquiring the XPS and time-of-flight SIMS data.
This research was supported by ONR grants N00014-95-1-1315 and N00014-95-1-0901. The National ESCA and Surface Analysis Center for Biomedical Problems at the University of Washington is funded through NIH grant RR01296.
REFERENCES
- 1.Absolom D R, Alberti F V, Policova Z, Zingg W, Van Oss C J, Neumann A W. Surface thermodynamics of bacterial adhesion. Appl Environ Microbiol. 1983;46:90–99. doi: 10.1128/aem.46.1.90-97.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bain C D, Troughton E B, Tao Y-T, Evall J, Whitesides G M. Formation of monolayer films by spontaneous assembly of organic thiols from solution onto gold. J Am Chem Soc. 1989;111:323–335. [Google Scholar]
- 3.Baumann L, Bowditch R D, Baumann P. Description of Deleya gen. nov. created to accommodate the marine species Alcaligenes aestus, A. pacificus, A. cupidis, and Pseudomonas marina. Int J Syst Bacteriol. 1983;33:793–802. [Google Scholar]
- 4.Briggs D, Hearn M J. Interaction of ion-beams with polymers, with particular reference to SIMS. Vacuum. 1986;36:1005–1010. [Google Scholar]
- 5.Callow M E, Fletcher R L. The influence of low surface energy materials on bioadhesion—a review. Int Biodeteriorat Biodegrad. 1994;34:333–348. [Google Scholar]
- 6.Costerton J W, Lewandowski Z, Caldwell D E, Korber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 7.Dalton H M, Poulsen L K, Halasz P, Angles M L, Goodman A E, Marshall K C. Substratum-induced morphological changes in a marine bacterium and their relevance to biofilm structure. J Bacteriol. 1994;176:6900–6906. doi: 10.1128/jb.176.22.6900-6906.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies M C, Lynn R A P. Static secondary ion mass-spectrometry of polymeric biomaterials. Crit Rev Biocompat. 1990;5:297. doi: 10.1016/0267-6605(90)90010-s. [DOI] [PubMed] [Google Scholar]
- 9.Dobson S J, Franzmann P D. Unification of the genera Deleya (Baumann et al. 1983) and Halovibrio (Fendich 1988) and the species Paracoccus halodenitrificans (Robinson and Gibbons 1952) into a single genus, Halomonas, and placement of the genus, and placement of the genus Zymobacter in the family Halomonadaceae. Int J Syst Bacteriol. 1996;46:550–558. [Google Scholar]
- 10.Evans E, Brown M R W, Gilbert P. Iron chelator, exopolysaccharide and protease production in Staphylococcus epidermidis: a comparative study of the effects of specific growth-rate in biofilm and continuous culture. Microbiology. 1994;140:153–157. doi: 10.1099/13500872-140-1-153. [DOI] [PubMed] [Google Scholar]
- 11.Ferreirós C M, Carballo J, Criado N T, del Rio M C. Surface free energy and interaction of Staphylococcus epidermidis with biomaterials. FEMS Microbiol Lett. 1989;60:89–94. doi: 10.1016/0378-1097(89)90083-9. [DOI] [PubMed] [Google Scholar]
- 12.Galaev I Y. “Smart” polymers in biotechnology and medicine. Russ Chem Rev. 1995;65:471–489. [Google Scholar]
- 13.Heskins M, Guillet J E. Solution properties of poly(N-isopropylacrylamide) J Macromol Sci Chem A. 1968;2:1441–1455. [Google Scholar]
- 14.Hirosuto T. Effects of oxygen exposure on plasma graft-polymerization of some hydrophilic monomers onto polypropylene films. J Macromol Sci Chem A. 1996;33:1663–1674. [Google Scholar]
- 15.Hoffman A S. Environmentally sensitive polymers and hydrogels—“smart” biomaterials. Materials Res Soc Bull. 1991;16:42–46. [Google Scholar]
- 16.Inagaki N, Tasaka S, Inoue T. Surface modification of aromatic polyamide film by plasma graft-copolymerization of glycidylmethacrylate for epoxy adhesion. J Appl Polym Sci. 1998;69:1179–1185. [Google Scholar]
- 17.Ista L K, Fan H, Baca O, López G P. Attachment of bacteria to solid model surfaces: oligo(ethylene glycol) surfaces inhibit bacterial attachment. FEMS Microbiol Lett. 1996;142:59–63. doi: 10.1111/j.1574-6968.1996.tb08408.x. [DOI] [PubMed] [Google Scholar]
- 18.Ista L K, López G P. Lower critical solubility temperature materials as biofouling release agents. J Ind Microbiol Biotechnol. 1998;20:121–125. [Google Scholar]
- 19.Kersters K. The genus Deleya. In: Balows A, Trüper H G, Dworkin M, Harder W, Schliefer K-H, editors. The prokaryotes. Berlin, Germany: Springer-Verlag; 1992. pp. 3189–3197. [Google Scholar]
- 20.Lawrence J R, Korber D R, Wolfaardt G M, Caldwell D E. Behavioral strategies of surface colonizing bacteria. Adv Microb Ecol. 1995;14:1–75. [Google Scholar]
- 21.Lewis K B, Ratner B D. Observation of surface rearrangement of polymers using ESCA. J Colloid Interface Sci. 1993;159:77–85. [Google Scholar]
- 22.Magnani A, Barbucci R, Lewis K B, Leach-Scampavia D, Ratner B D. Surface properties and restructuring of a cross-linked polyurethane-poly(amido-amine) network. J Mater Chem. 1995;5:1321–1330. [Google Scholar]
- 23.McCarter L L, Showalter R E, Silverman M R. Genetic analysis of surface sensing in Vibrio parahaemolyticus. Biofouling. 1992;5:163–175. [Google Scholar]
- 24.Murakami T, Kuroda S, Osawa Z. Dynamics of polymeric solid-surfaces treated with oxygen plasma: effect of aging media after plasma treatment. J Colloid Interface Sci. 1998;202:37–44. [Google Scholar]
- 25.Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery-system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide) J Biomed Mater Res. 1993;27:1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 26.Paul J H, Jeffrey W H. Evidence for separate adhesion mechanisms for hydrophilic and hydrophobic surfaces in Vibrio proteolytica. Appl Environ Microbiol. 1985;50:431–437. doi: 10.1128/aem.50.2.431-437.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez-Luna, V. H., L. K. Ista, and G. P. López. Unpublished data.
- 28.Ratner B D. Surface modification of polymers: chemical, biological and surface analytical challenges. Biosensors Bioelectronics. 1995;10:797–804. doi: 10.1016/0956-5663(95)99218-a. [DOI] [PubMed] [Google Scholar]
- 29.Ratner B D, Chilkoti A, López G P. Plasma deposition and treatment for biomaterial applications. In: D’Agostino R, editor. Plasma deposition, treatment and etching of polymers. San Diego, Calif: Academic Press; 1990. pp. 463–516. [Google Scholar]
- 30.Ratner B D, Mateo N B, Ertel S I, Horbett T A. Abstracts of the American Chemical Society. Vol. 199. Washington, D.C: American Chemical Society; 1990. Interactions of plasma-deposited films with living cells, abstr. no. 51-pmse. [Google Scholar]
- 31.Rühe J. Polymers grafted from solid surfaces. Macromol Symp. 1998;128:215–222. [Google Scholar]
- 32.Scofield J H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J Electron Spectrosc Relat Phenom. 1976;8:129–137. [Google Scholar]
- 33.Shea C, Lovelace L J, Smith-Somerville H E. Deleya marina as a model organism for studies of bacterial colonization and biofilm formation. J Ind Microbiol. 1995;15:290–296. [Google Scholar]
- 34.Surface Science Instruments Company. Application note. Mountain View, Calif: Surface Science Instruments Company; 1987. [Google Scholar]
- 35.University of Texas Health Science Center at San Antonio, Tex. 2 February 1996, posting date of last revision. [Online.] Image Tool image processing program, version 1.27. http://ddsdx.uthscsa.edu/dig/itdesc.html. [17 January 1997, last date accessed.]