Abstract
Delirium is associated with cognitive decline and subsequent dementia, and rises in plasma total tau (tTau) and neurofilament light (NfL), providing links to Amyloid-Tau-Neurodegeneration (ATN) pathophysiology. We investigated whether changes in delirium severity after surgery correlated with changes in cerebrospinal fluid (CSF) ATN biomarkers. Thirty-two thoracic vascular surgical patients were recruited into a prospective biomarker cohort study with assessment of delirium severity and incidence (NCT02926417). CSF (n = 54) and plasma (n = 118) samples were sent for biomarker analysis for tTau, phosphorylated tau-181 (pTau) (plasma n = 53), NfL, and amyloid-β 42/40 ratio (Ab42/40-ratio). The primary outcome was the correlation of preoperative to postoperative change in ATN biomarkers with the highest postoperative Delirium Rating Scale-98 score. CSF and plasma biomarkers all increased postoperatively (all p < .05, n = 13 paired preoperative–postoperative samples). Delirium severity was associated with peak changes in CSF tTau (p = .007, r = .710) and pTau (p = .01, r = .667) but not NfL (p = .09, ρ = .491) or Ab42/40-ratio (p = .18, ρ = .394). Sensitivity analysis with exclusion of participants with putative spinal cord ischemia shifted the NfL result to significance (p < .001, ρ = .847). Our data show that changes in tau and biomarkers of neurodegeneration in the CSF are associated with delirium severity. These data should be considered hypothesis-generating and future studies should identify if these changes are robust to confounding.
Keywords: Biology of aging, Biomarkers, Dementia
Epidemiological data suggest that delirium and dementia are associated (1–3), but evidence for a causal association remains weak. Establishing robust links to pathophysiological mechanisms through which delirium may contribute to the accumulation of dementia pathologies and/or cognitive decline would greatly enhance (i) the plausibility of relationships between delirium and dementia and (ii) the possibility of identifying interventions to either treat delirium or prevent subsequent dementia. Herein, we leverage the “Amyloid-Tau-Neurodegeneration” (ATN) framework that has been proposed for considering Alzheimer’s disease and related dementias (4). Using this structure, we tested whether changes in amyloid disease (using the ratio of amyloid-β 42/40 [Ab42/40-ratio]), tau pathology (using total tau [tTau] or phosphorylated tau [pTau]), or neurodegeneration (using neurofilament light [NfL], an axonal structural protein) correlated with changes in delirium symptom severity induced by elective vascular surgery.
We recently reported that delirium is associated with increases in plasma NfL (5) and tTau (6), thus providing a plausible link between delirium and the pathologies of dementia. Other studies have found that preoperative cerebrospinal fluid (CSF) levels of Ab42 (7) or the ratio of Ab42/tau (8) (evidence of Alzheimer’s pathology) may predispose to delirium. Cross-sectional data suggest that CSF NfL is associated with delirium (9), but it may be confounded by predelirium differences in neurodegeneration; data linking changes in CSF with delirium are lacking. Herein, we sought to extend our prior findings, which were based on plasma samples (5,6), with data collected from CSF, to determine whether similar changes occur centrally in the perioperative period. Paired CSF and blood samples were collected preoperatively and postoperatively in patients with spinal drains placed for clinical reasons prior to surgery. The plasma samples were sent for analysis separately to our previous reports and have not been reported elsewhere. We hypothesized that changes in ATN biomarkers would correlate with delirium severity based on the concept that a biological gradient for changes enhances plausibility for a causal relationship. In addition, we investigated whether ATN biomarkers varied based on a binary “delirium ever” incidence.
Method
The data are derived primarily from an ongoing prospective perioperative cohort study registered with ClinicalTrials.gov (NCT02926417) and approved by the University of Wisconsin-Madison Institutional Review Board (2015-096). The larger plasma cohort was collected under a separate ongoing prospective perioperative cohort study also registered with ClinicalTrials.gov (NCT03124303) and approved by the University of Wisconsin-Madison Institutional Review Board (2015-0374). Thirty-two adult patients who were scheduled for major elective nonintracranial surgery with at least a 2-day hospital stay (described in our recent publication on NfL (5)) were recruited. One patient died intraoperatively and did not have any postoperative data to contribute to the following analyses. All patients received general anesthesia during surgery. A Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) diagram is available in Supplementary Figure 1, and the inclusion and exclusion criteria for the study are presented in Supplementary Table 1. In brief, patients were eligible if they were undergoing thoracoabdominal aneurysm repair with anticipated placement of a spinal drain to allow CSF drainage for potential, postoperative reductions in CSF pressure. This decision was a clinical standard of care and was not influenced by the study. All demographic information was obtained via interview and validated in participants’ electronic medical records.
Delirium Assessments
Participants consented to postoperative study visits for 4 days or until their delirium resolved. Preoperatively, and twice daily postoperatively, participants underwent delirium assessments with the Confusion Assessment Method (CAM)/3D-CAM (10) or the Confusion Assessment Method for the intensive care unit (11) if the patient was intubated. Participants were defined as delirious if they had a positive 3D-CAM or CAM-ICU at any point postoperatively. All 32 participants were nondelirious before surgery. Delirium symptom severity was assessed postoperatively with the validated Delirium Rating Scale—revised-98 (DRS) (12). The peak DRS was defined as the highest total delirium severity score recorded in the perioperative period.
CSF Collection
CSF was collected at placement of a spinal drain for thoracoabdominal aneurysm repair (open = 23, endovascular = 8), and is referred to as a preoperative sample as the operation had not yet begun. Postoperative samples were collected dependent on whether the drain was still in use clinically. If possible, CSF samples were taken twice daily at the time of the morning delirium assessment. At a minimum, we aimed to collect samples on postoperative day 1 (POD1) whenever possible. CSF was collected, centrifuged at 3000 revolutions per minute (rpm) for 10 minutes, and stored at −80 °C.
Blood Sample Collection
Blood samples were collected in ethylenediaminetetraacetic acid-containing tubes preoperatively, in the morning (05:00–10:00) and in the afternoon (16:00–20:00) of each POD as close as possible to the delirium assessment interviews. Blood samples were centrifuged for 10 minutes at 3,000 rpm and stored at −80 °C.
ATN Fluid Biomarker Analyses
Samples were sent to the University of Gothenburg for analysis. Plasma NfL, tTau, pTau, Ab42, and Ab40 concentrations were measured using Simoa methods (Quanterix, Billerica, MA), as previously described in detail (5,13). CSF NfL concentration was measured using an in-house enzyme-linked immunosorbent assay, as previously described (14). CSF tTau, pTau, Ab42, and Ab40 concentrations were measured using Lumipulse assays (Fujirebio, Ghent, Belgium). All measurements were performed in one round of experiments using one batch of reagents by board-certified laboratory technicians who were blind to clinical data. Intra-assay coefficients of variation were below 10%. Because they showed a strong skew, all biomarker data were log transformed. In total, 26 participants had preoperative CSF samples analyzable for ATN biomarkers; 13 participants had sufficient peak postoperative CSF and 11 had POD1 CSF samples for ATN analysis.
Statistical Analysis
A power analysis was not conducted for these analyses. Samples were sent for analysis after recruitment was paused in 2020 for change of principal investigator and the coronavirus pandemic. The Ab42/Ab40-ratio was calculated by dividing observed concentrations of Ab42 by Ab40 for each participant at each timepoint. Demographic variables were assessed for normality with the Shapiro–Wilk test and all but body mass index showed a non-normal distribution. Median, interquartile range (IQR), sample size, and percentage of sample size were provided when applicable. Difference estimates and corresponding p values were calculated with Wilcoxon rank sum test for continuous variables. Odds ratios (ORs) and corresponding p values were calculated with Fisher’s exact test for dichotomous variables.
Biomarker levels were log transformed to accommodate the strong skew in the raw data. For each biomarker, the change was calculated by subtracting the log-transformed peak, or POD1 value, from the log-transformed preoperative value for each participant. The distributions of change values were assessed for normality with Shapiro–Wilk tests. When Shapiro–Wilk p values were less than .05, Spearman correlations and Mann–Whitney–Wilcoxon tests were performed. Inversely, when p values were higher than .05, Pearson correlations and Welch 2-sample t tests were performed.
The primary outcome was the peak change in biomarkers compared with peak postoperative DRS score. The peak change in biomarkers was an a priori planned outcome for the association of biomarkers with delirium as (i) we could not be sure when we could use the spinal drain (as it depended on its use clinically) and (ii) we felt that peak changes in the central nervous system would be both specific to central changes in dementia pathology and peak change levels would be most likely to have clinical significance. However, we also analyzed the POD1 change in biomarkers for consistency with our prior data (5,6). Peak DRS indicates the greatest severity of delirium symptoms at any assessment time point, which we consider a useful clinical outcome. Our overarching premise is that for a biomarker to be worth further investigation, a change in the biomarker that is proportional to delirium severity should be observed.
Secondary outcomes included biomarker associations with surgery itself, delirium incidence (yes/no), and ATN biomarker changes in plasma and the associations of delirium severity and incidence. Due to the small sample sizes, we did not attempt multivariable analyses.
Results
Demographics of the CSF and total plasma cohorts are shown in Table 1. Delirious participants incurred more blood loss (Mann–Whitney, p = .006), had longer operations (Mann–Whitney, p = .008), and were significantly more likely to have open type surgery (Fisher’s exact OR = 55.2, 95% CI: 4.4–3352.3; p < .001). A STROBE diagram is available in Supplementary Figure 1. Preoperative cognitive data beyond the 3D-CAM were not collected in the Interventions for Postoperative Delirium: Biomarker-2 (IPOD-B2) study, and this is one of several reasons that prompted the development of the IPOD-B3 study. No patient in either study had dementia according to their electronic medical records. Montreal Cognitive Assessment scores for the 19 dual-enrolled B3 participants, not including the participant who died intraoperatively, ranged from 18 to 27. A total of 54 CSF samples were analyzed for tTau, pTau, NfL, Ab42, and Ab40. In addition, 118 plasma samples were analyzed for tTau, NfL, Ab42, and Ab40. Only 53 plasma samples were analyzed for pTau as this analysis was conducted last and only plasma samples exactly paired to CSF were sent for analysis. Plasma was collected twice daily for typically 4 days after surgery for each participant, though the exact number of samples per participant depended on their recovery time and whether delirium persisted during hospitalization; some participants had fewer than 4 days recovery while some had longer recoveries and thus longer plasma sampling periods. CSF sampling was only possible until the CSF catheter was removed. A time course of the changes in the 4 CSF biomarkers is plotted in Figure 1 for descriptive purposes. Supplementary Figure 2 shows the equivalent plasma changes.
Table 1.
Cohort Demographics for All Participants With Postoperative Delirium Assessments (n = 31)
| Delirious (n = 22) | Nondelirious (n = 9) | OR (95% CI) | Difference (95% CI) | p Value | |||
|---|---|---|---|---|---|---|---|
| Median (IQR) | n (%) | Median (IQR) | n (%) | ||||
| Age | 68.5 (61.5–73) | 72 (67–77) | −4.0 (−11.0 to 2.0) | .32 | |||
| Sex (female) | 10 (45%) | 3 (33%) | 1.6 (0.3–12.8) | .70 | |||
| BMI | 27.50 (24.11–31.36) | 29.58 (27.85–29.86) | −2.07 (−6.64 to 2.92) | .53 | |||
| ASA | 3 (3–3) | 3 (3–4) | −6.64e-05 (−1.0e-01 to 2.79e-05) | .64 | |||
| NSQIP-D | 3.25 (1.83–6.18) | 1 (0.60–5.80) | 1.51 (−0.80 to 4.0) | .19 | |||
| Diabetes | 5 (23%) | 1 (11%) | 2.3 (0.2–124.9) | .64 | |||
| CHF | 1 (5%) | 0 | Inf (0.01–Inf) | >.99 | |||
| Smoker ever | 19 (86%) | 6 (67%) | 3.0 (0.3–29.4) | .32 | |||
| COPD | 12 (55%) | 3 (33%) | 2.3 (0.4–18.2) | .43 | |||
| OSA | 3 (14%) | 1 (11%) | 1.3 (0.1–74.5) | >.99 | |||
| Hypertension | 21 (95%) | 9 (100%) | 0 (0–95.2) | >.99 | |||
| Stroke/TIA | 3 (14%) | 0 | Inf (0.2–Inf) | .54 | |||
| PVD | 9 (41%) | 4 (44%) | 0.9 (0.1–5.7) | >.99 | |||
| Renal failure | 1 (5%) | 1 (11%) | 0.4 (0.005–33.6) | .50 | |||
| CAD | 7 (32%) | 2 (22%) | 1.6 (0.2–19.8) | .69 | |||
| Surgery type (open) | 21 (95%) | 2 (22%) | 55.2 (4.4–3352.3) | <.001 | |||
| Blood loss (mL) | 6300 (4025–7875) | 200 (150–400) | 5200 (3000–7150) | .006 | |||
| Operative time (min) | 565 (449.25–715.25) | 374 (272–469) | 188 (19–333) | .03 | |||
| AUC 10% | 86543.42 (31640.21–250588.04) | 103444.93 (50449.57–186602.55) | −11291.34 (−107694.6 to 102985.3) | .75 |
Notes: ASA = American Society of Anesthesiologists score; AUC 10% = area under the curve 10% below relative; BMI = body mass index; CAD = coronary artery disease; CHF = congestive heart failure; CI = confidence interval; COPD = chronic obstructive pulmonary disease; IQR = interquartile range; NSQIP-D = National Surgical Quality Improvement Program for risk of death; OR = odds ratio; OSA = obstructive sleep apnea; PVD = peripheral vascular disease; TIA = transient ischemic attack. One participant not included because the participant died intraoperatively and did not have any postoperative delirium assessments to contribute. The OR and upper bound of the CIs for CHF and stroke/TIA were undefined due to zero nondelirious patients having these diagnoses before surgery; regardless, the p values show no significant differences by delirium status.
Figure 1.
Descriptive plots for the time course of cerebrospinal fluid changes for each biomarker by participant, (A) pTau, (B) tTau, (C) NfL, and (D) Ab42/40-ratio. Ab42/40-ratio = amyloid-β 42/40 ratio; NfL = neurofilament light; pTau = phosphorylated tau; tTau = total tau.
Prior to surgery, 4–8 participants (of 31) were positive for the biomarkers based on internally validated laboratory cutoffs (Supplementary Table 2). Peak postoperative CSF samples tended to occur on POD1 or POD2 (median = 2, IQR = 1, mode = 1). Peak postoperative plasma samples tended to occur on POD4 (median = 4, IQR = 3.65, mode = 4). Six out of the 13 participants in the peak difference analysis were intubated during their peak postoperative CSF time points. Four out of 11 participants in the POD1 analysis were intubated on POD1. Correlations with peak DRS are unaffected by this, as peak DRS values were derived from patients who were not intubated.
Correlation of CSF and Plasma Biomarkers
We correlated the change in POD1 CSF biomarkers with the change in POD1 plasma biomarkers. Our aim was to elucidate whether the biomarkers positively correlated at this fixed point in time after surgery; this was to investigate whether the biomarkers rose or fell in a similar manner in both mediums; however, a key assumption is that the time courses of these 2 factors covary with the same time constants. This analysis showed that the POD1 changes in CSF tTau (Spearman correlation, p = .024, ρ = .857), NfL (Pearson correlation, p = .033, r = .848), Ab42 (Pearson correlation, p = .033, r = .793), and Ab40 (Spearman correlation, p = .007, ρ = .929) significantly correlated with the POD1 changes in plasma biomarkers. POD1 changes in CSF and plasma pTau were not significantly correlated (Spearman correlation, p = .752, ρ = −.143), but both increased after surgery.
Surgery Is Associated With Increases in ATN CSF Changes
Peak postoperative levels of all the CSF biomarkers increased significantly from preoperative levels: CSF tTau (Welch t test, p = .001), pTau (Welch t test, p = .003), NfL (Mann–Whitney, p = .044), and Ab42/40-ratio (Mann–Whitney, p < .001; Supplementary Figure 3). Peak postoperative levels of plasma tTau (Mann–Whitney, p < .001), pTau (t test, p < .001), NfL (Welch t test, p < .001), and Ab42/40-ratio (Mann–Whitney, p = .021) also increased significantly from preoperative levels (Supplementary Figure 4).
Primary Outcome: Associations of ATN Peak CSF Changes and Delirium Severity
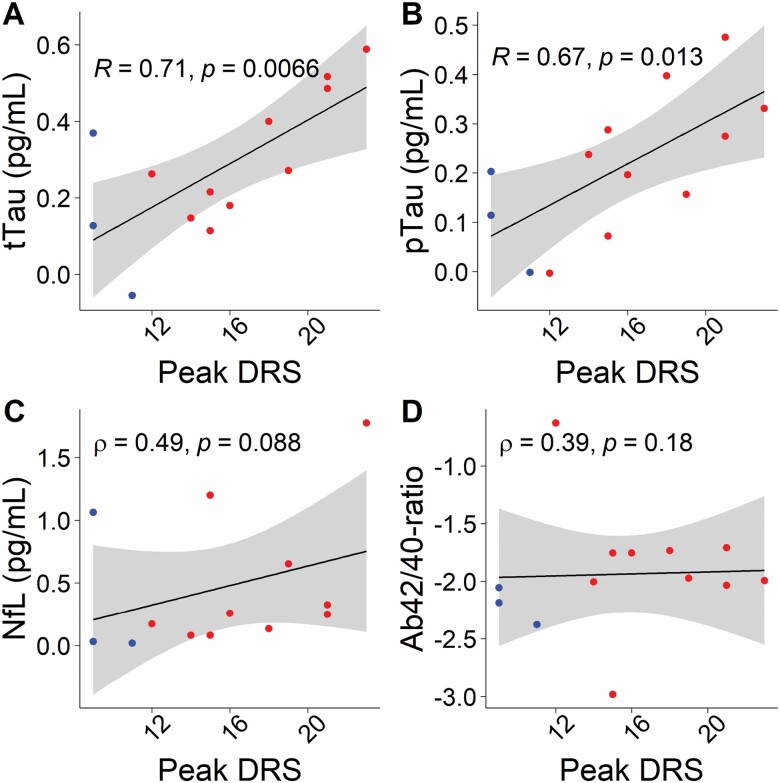
Delirium severity or incidence was not associated with preoperative levels of CSF or plasma ATN biomarkers (all p > .05; data not shown). Peak changes in CSF tTau (Pearson correlation, p = .007, r = .710) and pTau (Pearson correlation, p = .01, r = .667) were significantly associated with delirium severity (Figure 2A and B), but peak changes in CSF NfL (Spearman correlation, p = .09, ρ = .491) or CSF Ab42/40-ratio (Spearman correlation, p = .18, ρ = .394) were not (Figure 2C and D).
Figure 2.
Peak delirium severity correlates with peak postoperative changes in cerebrospinal fluid tTau (A) and pTau (B) but not NfL (C) or Ab42/40-ratio (D). The y-axis is on a log(10) scale. Ab42/40-ratio = amyloid-β 42/40 ratio; DRS = Delirium Rating Scale; NfL = neurofilament light; pTau = phosphorylated tau; tTau = total tau.
Secondary Outcome: Associations of ATN Peak Plasma Changes and Delirium Severity
From plasma samples, peak change in pTau suggested a possible association with delirium severity (Pearson correlation, p = .053, r = .570) (Figure 3B). No association with delirium severity was observed in tTau (Pearson correlation, p = .160, ρ = .310), NfL (Pearson correlation, p = .068, r = .371), or Ab42/40-ratio (Pearson correlation, p = .357, ρ = .206) (Figure 3A, C, and D).
Figure 3.
Peak delirium severity did not correlate with changes in plasma tTau (A), pTau (B), NfL (C), or Ab42/40-ratio (D). The y-axis is on a log(10) scale. Ab42/40-ratio = amyloid-β 42/40 ratio; DRS = Delirium Rating Scale; NfL = neurofilament light; pTau = phosphorylated tau; tTau = total tau.
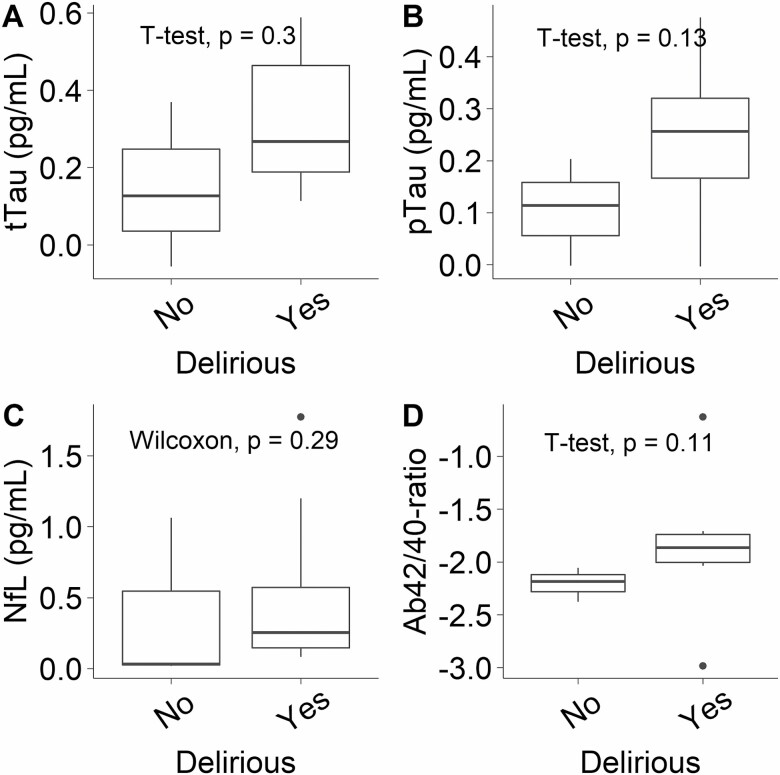
Secondary Outcome: Associations of ATN Peak CSF and Plasma Changes and Delirium Incidence
Peak changes in CSF tTau (Welch t test, p = .297), pTau (Welch t test, p = .129), NfL (Mann–Whitney, p = .287), and Ab42/40-ratio (Welch t test, p = .114) were not significantly associated with delirium incidence (Figure 4). Peak postoperative changes in plasma pTau (Welch t test, p < .001) and NfL (Welch t test, p = .026) were significantly higher in delirious participants (Supplementary Figure 5B and C). Peak postoperative changes in plasma tTau (Mann–Whitney, p = .155) and Ab42/40-ratio (Mann–Whitney, p = .971) were not significantly higher in delirious participants (Supplementary Figure 5A and D).
Figure 4.
Peak changes in cerebrospinal fluid biomarkers, tTau (A), pTau (B), NfL (C), or Ab42/40-ratio (D), were not associated with delirium incidence. The y-axis is on a log(10) scale. Ab42/40-ratio = amyloid-β 42/40 ratio; NfL = neurofilament light; pTau = phosphorylated tau; tTau = total tau.
Secondary Outcome: Associations of ATN POD1 CSF and Plasma Changes
Only POD1 changes in CSF tTau significantly correlated with peak delirium severity (Pearson correlation, p = .044, r = .615; Supplementary Figure 6A). POD1 changes in plasma pTau (Pearson correlation, p = .039, r = .731) and NfL (Pearson correlation, p = .013, r = .543) were significantly correlated with delirium severity (Supplementary Figure 7B and C), while plasma tTau (Spearman correlation, p = .102, ρ = .367) and Ab42/40-ratio (Spearman correlation, p = .646, r = .135) were not (Supplementary Figure 7A and D).
POD1 changes in CSF tTau (Welch t test, p = .057), pTau (Welch t test, p = .132), NfL (Welch t test, p = .271), and Ab42/40-ratio (Welch t test, p = .420) were not higher in delirious participants (Supplementary Figure 8). POD1 changes in plasma tTau (Mann–Whitney, p = .014) and NfL were significantly higher (Welch t test, p = .003) in delirious participants (Supplementary Figure 9A and C). POD1 changes in plasma Ab42/40-ratio (Welch t test, p = .164) were not significantly higher in delirious participants (Supplementary Figure 9D). There was insufficient plasma pTau samples at POD1 to include in this analysis.
Sensitivity Analysis: Exclusion of Patients With Putative Spinal Cord Ischemia
Two patients were thought to have sustained neuronal injury to the spinal cord, as noted by their attending physician in the discharge summary following their surgery. As the drains were placed to help manage this situation, we conducted a sensitivity analysis by removing participants with this putative injury. One patient was delirious and the other was not delirious during their hospital stays. Sensitivity analyses with removal of these patients’ data showed that peak changes in CSF tTau (Pearson correlation, p < .001, r = .865) and pTau (Pearson correlation, p = .007, r = .757) were still associated with delirium severity; peak change in CSF NfL became significantly associated (Spearman correlation, p < .001, ρ = .847) with delirium severity only after these patients were removed (Supplementary Figure 10). POD1 change in CSF tTau (Pearson correlation, p = .067, r = .601) was no longer statistically significant, but POD1 change in CSF NfL (Pearson correlation, p = .023, r = .704) became significantly associated with delirium severity.
Discussion
We have previously shown that changes in plasma tTau and NfL correlate with delirium severity. A major limitation with these analyses is that plasma biomarkers, especially tTau (15), may measure a peripheral release of these biomarkers to the bloodstream. Herein, we address this limitation though a proof-of-principle study of paired CSF samples to assay central nervous system changes after surgery. CSF changes in tTau, pTau, and NfL correlated with delirium severity, though for NfL this was only apparent in the sensitivity analysis after removing the participants with putative spinal cord ischemia. This seems plausible, as spinal cord ischemia would involve neuronal injury but would not be related to delirium. Future studies should address the relationship of these biomarkers to delirium in situations where spinal cord injury is less likely. Overall, our data support and extend our recent finding that delirium severity correlates with increases in plasma NfL and tTau (5,6). Interestingly, we have uncovered novel associations with pTau in CSF and plasma that appear similar to tTau. Given multiple recent papers emphasizing the prognostic value of different plasma and CSF pTau markers for dementia (16–20), these data suggest a tantalizing link between delirium and dementia. Finally, we did not find effects on Ab42/40-ratio that correlated with delirium severity or incidence, though surgery itself was associated with increases in Ab42/40-ratio. Increases in this ratio, however, seems to run counter to lower Ab42/40 ratios being associated with amyloid deposition on positron emission tomography and dementia (21). One possible explanation is that perioperative inflammation leads to decreases in cerebral amyloid load through microglial activation (22) and this leads to increases in the Ab42/40-ratio and plasma. Our data do not appear to be consistent with the concept that anesthesia leads to accumulation of amyloid-β (23). Perhaps of most importance for the field, our results indicate that plasma samples may be considered largely as a surrogate of CSF changes, though the changes in pTau on POD1 were not correlated (despite CSF and plasma levels both rising). This may be due to changes in the sensitivity of different biomarkers to blood–brain barrier integrity. Tau proteins directly interact with the blood–brain barrier, determining its integrity (24), and this may alter the acute dynamics of CSF:plasma ratios of biomarkers. We plan to further explore this in future research.
Our data emphasize that changes in tau (total and phosphorylated-181 forms) and NfL may occur in delirium, providing links to neuronal injury in the brain and dementia pathologies (4). Of course, the duration of the rise in biomarkers remains unclear. It is also unclear whether whether acute (and possibly transient) changes in the perioperative period can contribute to long-term changes in the burden of pathology. In particular, the associations with tau disease are intriguing. Given that tau may spread by seeding across the brain (25), these data suggest that tau disease may change following an episode of surgery, especially with delirium. In turn, it is possible this contributes to subtle effects on long-term cognition following surgery (26).
Overall, the CSF and plasma data appear concordant, except that pTau and NfL levels in plasma could differentiate delirious and nondelirious participants, whereas CSF levels could not. Of note, we observed previously that plasma tTau changes could differentiate delirious and nondelirious participants, so it is possible that the lack of significance for plasma tTau in the present study relates to the small sample size. On the other hand, and intriguingly, it is possible that plasma levels may predict delirium incidence better than CSF levels. We speculate that this is due to a breakdown in the blood–brain barrier in delirium (27) exaggerating the difference between nondelirious and delirious participants, potentially implying a greater neuropathological change than would be detected centrally in delirium. In contrast, plasma samples may underestimate central changes in nondelirious participants (where the blood–brain barrier is likely largely intact). Future studies are required to help clarify the respective central and plasma effects of these biomarkers. While associations with blood–brain barrier integrity are the next step in our field of enquiry, this work has important implications for the development of neuropathological biomarkers and interpretation of studies in which blood–brain barrier breakdown may be expected (5,6) or not (28).
Our study does have some important limitations. Firstly, the sample size was small, meaning we could not adjust for confounders. However, it is important to stress that analysis of paired samples removes much of the confounding from preoperative factors, through analysis of the “within-subject” change. Furthermore, while this study is small, it did allow us to track the dynamic changes that occur with delirium, which is difficult or impossible with cross-sectional case–control analyses because of the significant confounding from the premorbid state of neuropathology. Nonetheless data were lacking on many potential risk factors for delirium, and while it is presently unclear whether these factors would modify associations of the biomarker with delirium, future studies should investigate this possibility. Overall, our data should be considered as hypothesis-generating rather than definitive. Secondly, we did not seek to adjust p values for multiple comparisons across the 4 biomarkers tested. Using the conservative Bonferroni correction would yield a p value threshold of <.0125. At this threshold of our primary outcome, we would still show significant effects for tTau with a borderline effect for pTau in the CSF. Thirdly, the sample was potentially biased by the clinical indication for the drain and our approach of requiring a drain to be present for us to collect a sample. These restrictions were required for approval by our ethics committee. Nonetheless, our sensitivity analysis of removing participants with putative spinal cord ischemia showed stronger effects, suggesting that the indication for the drain would not explain the results. Hence, while this study is coherent with our recent analyses of plasma data, and emphasize the potential utility of ATN plasma biomarkers, we consider these experiments hypothesis-generating, and suggest larger cohorts enrolling from many different types of surgeries will be important to increase generalizability of the findings. Fourthly, while we detected a change, presently it is unclear what a clinically meaningful acute change in these biomarkers is. Future work must clarify if these short-term changes translate to long-term effects or not.
Conclusions
Our data show that delirium severity is associated with proportional central changes in tau disease and neuronal injury (the latter after removing participants with spinal cord ischemia). This adds to the weight of evidence that delirium is associated with dementia-related neuropathological changes. The next steps are to track these changes against changes in the blood–brain barrier and also test whether these biomarker changes are associated with changes in long-term cognition.
Supplementary Material
Funding
R.D.S., R.A.P., M.P., M.W., C.C., D.K., A.B., and R.L. are supported by R01AG063849. H.Z. is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the European Research Council (#681712), Swedish State Support for Clinical Research (#ALFGBG-720931), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C, and #ADSF-21-831377-C), the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2019-0228), the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 860197 (MIRIADE), and the UK Dementia Research Institute at UCL. K.B. is supported by the Swedish Research Council (#2017-00915), the ADDF, USA (#RDAPB-201809-2016615), the Swedish Alzheimer Foundation (#AF-742881), Hjärnfonden, Sweden (#FO2017-0243), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF agreement (#ALFGBG-715986), and European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236).
Conflict of Interest
H.Z. has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, and CogRx; has given lectures in symposia sponsored by Fujirebio, Alzecure, and Biogen; and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). K.B. has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu, Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers, and is a cofounder of BBS, which is a part of the GU Ventures Incubator Program.
References
- 1. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. J Am Med Assoc. 2010;304:443–451. doi:10.1001/jama.2010.1013 [DOI] [PubMed] [Google Scholar]
- 2. Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72:1570–1575. doi:10.1212/WNL.0b013e3181a4129a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanders RD, Pandharipande PP, Davidson AJ, Ma D, Maze M. Anticipating and managing postoperative delirium and cognitive decline in adults. Br Med J. 2011;343:d4331. doi:10.1136/bmj.d4331 [DOI] [PubMed] [Google Scholar]
- 4. Jack CR Jr., Bennett DA, Blennow K, et al. ; Contributors . NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi:10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casey CP, Lindroth H, Mohanty R, et al. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2020;143:47–54. doi:10.1093/brain/awz354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ballweg T, White M, Parker M, et al. Association between plasma tau and postoperative delirium incidence and severity: a prospective observational study. Br J Anaesth. 2021;126:458–466. doi:10.1016/j.bja.2020.08.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cunningham EL, McGuinness B, McAuley DF, et al. CSF beta-amyloid 1-42 concentration predicts delirium following elective arthroplasty surgery in an observational cohort study. Ann Surg. 2018. doi:10.1097/SLA.0000000000002684 [DOI] [PubMed] [Google Scholar]
- 8. Xie Z, Swain CA, Ward SA, et al. Preoperative cerebrospinal fluid β-amyloid/tau ratio and postoperative delirium. Ann Clin Transl Neurol. 2014;1:319–328. doi:10.1002/acn3.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Halaas NB, Blennow K, Idland AV, et al. Neurofilament light in serum and cerebrospinal fluid of hip fracture patients with delirium. Dement Geriatr Cogn Disord. 2018;46:346–357. doi:10.1159/000494754 [DOI] [PubMed] [Google Scholar]
- 10. Marcantonio ER, Ngo LH, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161:554–561. doi:10.7326/M14-0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). J Am Med Assoc. 2001;286:2703–2710. doi:10.1001/jama.286.21.2703 [DOI] [PubMed] [Google Scholar]
- 12. Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale—revised-98: comparison with the Delirium Rating Scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229–242. doi:10.1176/jnp.13.2.229 [DOI] [PubMed] [Google Scholar]
- 13. Gisslén M, Price RW, Andreasson U, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine. 2016;3:135–140. doi:10.1016/j.ebiom.2015.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaetani L, Höglund K, Parnetti L, et al. A new enzyme-linked immunosorbent assay for neurofilament light in cerebrospinal fluid: analytical validation and clinical evaluation. Alzheimers Res Ther. 2018;10:8. doi:10.1186/s13195-018-0339-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mattsson N, Zetterberg H, Janelidze S, et al. ; ADNI Investigators . Plasma tau in Alzheimer disease. Neurology. 2016;87:1827–1835. doi:10.1212/WNL.0000000000003246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palmqvist S, Janelidze S, Stomrud E, et al. Performance of fully automated plasma assays as screening tests for Alzheimer disease-related β-amyloid status. JAMA Neurol. 2019;76:1060–1069. doi:10.1001/jamaneurol.2019.1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Janelidze S, Berron D, Smith R, et al. Associations of plasma phospho-Tau217 levels with tau positron emission tomography in early Alzheimer disease. JAMA Neurol. 2021;78:149–156. doi:10.1001/jamaneurol.2020.4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med. 2020;26:379–386. doi:10.1038/s41591-020-0755-1 [DOI] [PubMed] [Google Scholar]
- 19. Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19:422–433. doi:10.1016/S1474-4422(20)30071-5 [DOI] [PubMed] [Google Scholar]
- 20. Mattsson-Carlgren N, Janelidze S, Palmqvist S, et al. Longitudinal plasma p-tau217 is increased in early stages of Alzheimer’s disease. Brain. 2020;143:3234–3241. doi:10.1093/brain/awaa286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature. 2018;554:249–254. doi:10.1038/nature25456 [DOI] [PubMed] [Google Scholar]
- 22. Ghosh S, Wu MD, Shaftel SS, et al. Sustained interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer’s mouse model. J Neurosci. 2013;33:5053–5064. doi:10.1523/JNEUROSCI.4361-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xie Z, Xu Z. General anesthetics and β-amyloid protein. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:140–146. doi:10.1016/j.pnpbp.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blair LJ, Frauen HD, Zhang B, et al. Tau depletion prevents progressive blood–brain barrier damage in a mouse model of tauopathy. Acta Neuropathol Commun. 2015;3:8. doi:10.1186/s40478-015-0186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vogel JW, Iturria-Medina Y, Strandberg OT, et al. ; Alzheimer’s Disease Neuroimaging Initiative; Swedish BioFinder Study . Spread of pathological tau proteins through communicating neurons in human Alzheimer’s disease. Nat Commun. 2020;11:2612. doi:10.1038/s41467-020-15701-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krause BM, Sabia S, Manning HJ, Singh-Manoux A, Sanders RD. Association between major surgical admissions and the cognitive trajectory: 19 year follow-up of Whitehall II cohort study. Br Med J. 2019;366:l4466. doi:10.1136/bmj.l4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hughes CG, Pandharipande PP, Thompson JL, et al. Endothelial activation and blood–brain barrier injury as risk factors for delirium in critically ill patients. Crit Care Med. 2016;44:e809–e817. doi:10.1097/CCM.0000000000001739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deiner S, Baxter MG, Mincer JS, et al. Human plasma biomarker responses to inhalational general anaesthesia without surgery. Br J Anaesth. 2020;125:282–290. doi:10.1016/j.bja.2020.04.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.