Abstract
Background
Genomic aberrations in the cell cycle and PI3K/Akt/mTOR pathways have been reported in diffuse intrinsic pontine glioma (DIPG) and high-grade glioma (HGG). Dual inhibition of CDK4/6 and mTOR has biologic rationale and minimal overlapping toxicities. This study determined the recommended phase 2 dose (RP2D) of ribociclib and everolimus following radiotherapy in children with DIPG and HGG.
Methods
Patients were enrolled according to a Rolling-6 design and received ribociclib and everolimus once daily for 21 and 28 days, respectively. All patients with HGG and biopsied DIPG were screened for retinoblastoma protein presence by immunohistochemistry. Pharmacokinetics were analyzed.
Results
Nineteen patients enrolled (median age: 8 years [range: 2-18]). Three patients enrolled at each dose level 1 and 2 without dose-limiting toxicities (DLT). Thirteen patients were enrolled at dose level 3, with one patient experiencing a DLT (grade 3 infection). One patient came off therapy before cycle 9 due to cardiac toxicity. The most common grade 3/4 toxicities were neutropenia (33%), leucopenia (17%), and lymphopenia (11%). Steady-state everolimus exposures in combination were 1.9 ± 0.9-fold higher than single-agent administration. Median overall survival for 15 patients with DIPG was 13.9 months; median event-free survival for four patients with HGG was 10.5 months. Two longer survivors had tumor molecular profiling identifying CDKN2A/B deletion and CDK4 overexpression.
Conclusion
The combination of ribociclib and everolimus following radiotherapy in children with newly diagnosed DIPG and HGG was well tolerated, with a RP2D of ribociclib 170 mg/m2 and everolimus 1.5 mg/m2. Results will inform a molecularly guided phase II study underway to evaluate efficacy.
Keywords: diffuse intrinsic pontine glioma (DIPG), high-grade glioma (HGG), pediatric, phase I, ribociclib and everolimus
Key Points.
This study defined the RP2D of ribociclib and everolimus in children with DIPG/HGG.
Therapy was well tolerated, with the potential impact of ribociclib on everolimus pharmacokinetics.
Results will inform a phase II study, under development, to evaluate efficacy.
Importance of the Study.
Joint inhibition of CDK4/6 and mTOR has the potential to benefit children and adolescents with diffuse intrinsic pontine glioma (DIPG) and high-grade glioma (HGG). This study defines the recommended phase 2 dose (RP2D) of ribociclib and everolimus among pediatric patients with newly diagnosed DIPG and HGG following radiotherapy. The combination was well tolerated, and pharmacokinetics and drug-drug interactions corroborate adult data. Results will inform a phase II study, currently under development, evaluating efficacy in patients with DIPG/HGG harboring specific genetic alterations of cell cycle and PI3K/mTOR pathways.
Despite aggressive multimodal therapy, diffuse intrinsic pontine glioma (DIPG) and high-grade glioma (HGG) remain the leading cause of cancer-related death in children and young adults.1–4 Focal gains of CDK4/6 and Cyclin D1-3 and/or homozygous loss of CDKN2A have been identified across cancer types, implicating cell cycle disruption in tumorigenesis.5 The PI3K/Akt/mTOR pathway is also commonly activated in human cancer.6–8 Up to 60% of patients with DIPG and HGG exhibit aberrations of checkpoint cell cycle regulators (eg, CDK4/6, CDK2NA/B/C, CCND2), and 26%-79% have activation of the PI3K/Akt/mTOR pathway (eg, PIK3CA, PIK3R1, PTEN),9–12 supporting inhibition of CDK4/6 and mTOR as combination therapy for these intractable brain tumors. Furthermore, genome-wide analyses have identified enrichment of specific pathway alterations within molecularly distinct subtypes of HGG and DIPG, with amplifications of CCND2 and deletions of CDKN2C predominating in pontine tumors, CDKN2A/B deletions or CDK6 amplifications presenting more commonly in hemispheric HGGs, and alterations of the PI3K/mTOR pathway occurring in H3.1-mutant tumors.9 Additionally, epigenetic repression of CDKN2A (p16) may be an important and targetable component of H3.3K27M-driven gliomagenesis in DIPG.13 Given the prevalence of cell cycle and PI3K/mTOR pathway aberrations in DIPG and HGG, as well as emerging preclinical evidence of synergy between CDK4/6 and mTOR inhibition across cancer types, including HGGs.14–16 we investigated the combination of ribociclib and everolimus.
Everolimus (RAD001; Novartis Pharmaceuticals) inhibits mTOR by targeting mTOR-raptor signal transduction complex 1 (mTORC1) and reducing tumor cell proliferation, glycolysis, and angiogenesis. Everolimus is FDA-approved for advanced breast and renal cancers17–20 as well as tuberous sclerosis-associated subependymal giant cell astrocytoma (SEGA) and partial-onset seizures,21 with a pediatric recommended phase 2 dose (RP2D) of 5 mg/m2 daily.22 Ribociclib (LEE011; Novartis Pharmaceuticals), an orally bioavailable CDK4/6 inhibitor that induces G1 cell cycle arrest by hypo-phosphorylating RB,23,24 is FDA-approved for the treatment of advanced breast cancer, and has been studied in combination with everolimus and exemestane,18,20,25 with the RP2D of 300 mg/day ribociclib (3 weeks on/1 week off), 2.5 mg/day everolimus (continuous), and 25 mg/day exemestane (continuous) [NCT01857193].20 In another dose-escalation study in adults, everolimus exposure increased 2- to 4-fold in the presence of ribociclib,26,27 suggesting that lower doses of both drugs may be used in the pediatric population.
We recently published a phase I study of single-agent ribociclib in children with newly diagnosed DIPG and HGG following radiotherapy. The RP2D was 350 mg/m2, with 12-month and median overall survival (OS) among 10 patients enrolled in the study of 89% and 16.1 months, respectively.28 The Pediatric Brain Tumor Consortium also completed a phase I and surgical study of ribociclib and everolimus in pediatric patients with refractory central nervous system (CNS) tumors. The RP2D of ribociclib and everolimus in this recurrent/refractory population was 120 and 1.2 mg/m2 for 21 and 28 days, respectively, and ribociclib concentrations were achieved in cerebrospinal fluid and tumor tissue, although variability was observed.29
Herein, we report the results of a phase I trial of ribociclib and everolimus in children with newly diagnosed DIPG and HGG post-radiation, to assess safety, evaluate tolerability, and establish the RP2D of this combination therapy in the upfront setting.
Materials and Methods
Objectives
The primary objectives of this phase I study (NCT02607124) were to (1) determine the maximally tolerated dose (MTD) and/or RP2D of ribociclib administered in combination with everolimus following radiotherapy, (2) describe toxicities, and (3) characterize ribociclib and everolimus pharmacokinetics and the potential for drug-drug interactions. Secondary objectives were to (1) estimate OS (in patients with DIPG) and event-free survival (EFS; in patients with HGG) in the context of a phase I study, (2) determine the proportion of patients experiencing pseudoprogression, (3) explore tumor molecular profiling when available, and (4) assess dexamethasone-based mouthwash for mucositis prevention.
Patient Eligibility
Patients were newly diagnosed with either imaging-confirmed DIPG (aged 1-30 years) or histologically confirmed HGG (1-21 years). DIPG tumors with typical neuroimaging features (pontine epicenter and diffuse intrinsic [>50%] pontine involvement)30 did not require histological confirmation; if these patients underwent pontine biopsy, histology needed to confirm infiltrating WHO grade II-IV glioma to enroll. Patients with pontine tumors with atypical imaging for DIPG were eligible only if tumor was histologically confirmed infiltrating WHO grade II-IV glioma. HGG and other brainstem tumors had to be histologically confirmed WHO grade III/IV. All biopsied/resected tumor tissue was evaluated for the presence of the retinoblastoma (RB) protein by immunohistochemistry in a CLIA-certified laboratory, with the requirement of RB positivity (>20% nuclear staining in tumor tissue) for eligibility. Patients with primary spinal cord tumors were eligible.
Patients must have initiated radiotherapy within 30 days of radiographic diagnosis or definitive surgery (whichever was later). Study enrollment occurred following the completion of radiotherapy, with the initiation of study treatment mandated within 2-4 weeks of radiotherapy completion. Patients must have received within 10% of standard dose of radiotherapy (DIPG: 54 Gy; non-brainstem HGG: 59.4 Gy) administered in 1.8 Gy daily fractions over 6 weeks to the planning target volume. Other eligibility criteria included: Lansky (≤16 years) or Karnofsky (>16 years) performance scores ≥50%; no prior therapy other than surgery, radiation, and/or steroids; adequate laboratory data, including (a) bone marrow function (hemoglobin ≥9 g/dL, absolute neutrophil count ≥1000 mm3, platelets ≥100 000/mm3 [transfusion-independent, defined as no platelet transfusion within 7 days before enrollment]), (b) renal function (age-adjusted normal serum creatinine or glomerular filtration >70 mL/minutes/1.73 m2), (c) liver function (total bilirubin, alanine transaminase [ALT], and aspartate aminotransferase [AST] ≤3× institutional upper limit of normal, albumin ≥2 g/dL); and recovered from acute radiation-related toxicities (≤grade 2). Patients with controlled seizures on non-enzyme-inducing anticonvulsants were eligible. Patients of childbearing or child-fathering potential must have agreed to use medically acceptable birth control while on the study and for 8 weeks’ post-treatment.
Patients were excluded if they (a) were pregnant, (b) had disseminated disease, (c) received a radiosensitizer, investigational agent, or additional adjuvant therapy during radiotherapy, (d) were on potent CYP3A4 inducers/inhibitors, (e) had significant cardiac disease, hypertension, cardiac dysfunction, or cardiomyopathy (left ventricular ejection fraction <50% or QTc >450 ms), (f) were on warfarin or other warfarin-derived anticoagulants, and/or (g) had major surgery within 14 days of first therapy doses.
Written informed consent and assent were obtained according to institutional guidelines. The protocol was approved by the institutional review boards (IRBs) of each participating site. Cincinnati Children’s Hospital Medical Center (CCHMC) was the IRB of reliance for participating institutions and maintained protocol approval throughout the study.
Drug Administration and Dose Escalation
Ribociclib (50 and 200 mg capsules or 30 mg/mL liquid formulation administered orally or via g-tube or nasogastric tube) and everolimus (2 mg oral dissolving tablets) were supplied by Novartis Pharmaceuticals. Administration followed a 28-day cycle, with ribociclib and everolimus taken daily for 21 and 28 days, respectively. Patients were encouraged to take both drugs at the same time.
Body surface area (BSA) restrictions were required for each dose level to accommodate the smallest capsule size of 50 mg for ribociclib and avoid overlaps between dose levels. The starting dosage for dose level 1 was ribociclib 120 mg/m2 and everolimus 1.2 mg/m2, requiring a BSA ≥0.75 m2. Dosage escalation was governed by the Rolling-6 statistical design.31 No intra-patient dose escalation was permitted. Only dose-limiting toxicities (DLTs) observed during the dose-finding period (cycle 1) of therapy were used to guide dose escalation/de-escalation. In the absence of disease progression or unacceptable toxicity, patients could receive up to 2 years (26 cycles) of treatment and continue beyond 2 years if evidence of continued response and approved by Novartis.
To prevent or minimize the severity of mucositis, dexamethasone mouthwash was administered for at least the first two cycles of therapy. Starting on cycle 1, day 3, patients were instructed to swish and spit the alcohol-free dexamethasone mouthwash (5-10 mL, 0.5 mg/5 mL) 3 times daily, and to remain NPO (nothing by mouth) for at least 1 hour afterwards (with exception of nystatin or other topical antifungals). Subsequent cycles did not mandate mouthwash, but patients could continue as tolerated. If grade 1 mucositis was noted, saltwater mouth rinse was administered (10 mL, 0.9%; swish and spit) 3 times daily, 10-15 minutes prior to the dexamethasone mouthwash. Swab sponges were used for patients unable to swish and spit. The number and grade of mucositis events were descriptively noted.
Toxicity Monitoring
Toxicity monitoring included the following during cycle 1 and prior to each subsequent cycle: (a) physical examinations (days 1 and 15), (b) EKG (baseline and day 15), (c) laboratory evaluations (complete blood counts [weekly], serum chemistries and renal and hepatic functions [day 15], lipid profiles [every other cycle]); (d) echo evaluation (baseline, pre-cycle 3, and as clinically indicated thereafter); and (e) disease evaluations with brain and/or spine MRI (baseline, every 8 weeks after cycles 2, 4, and 6, and then every 12 weeks thereafter through treatment or progression).
Definition of DLTs and MTD/RP2D Estimation
Patients were evaluable for estimating MTD/RP2D if they (1) completed all toxicity monitoring requirements, (2) received at least one dose of ribociclib and everolimus, and (3) either were taken off treatment for toxicity during cycle 1 (dose-finding period) or in the absence of toxicity, received ~85% of prescribed therapy during cycle 1 (≥18/21 doses of ribociclib and ≥22/26 doses of everolimus).
DLTs attributable to ribociclib and/or everolimus and requiring dose reduction were graded according to the NCI Common Terminology Criteria for Adverse Events (v5), unless otherwise specified. Hematological DLTs were defined as thrombocytopenia (≥grade 3) or neutropenia (grade 4) lasting >7 consecutive days or myelosuppression causing a ≥14-day delay between treatment cycles. Non-hematologic DLTs were defined as (a) any grade 3 non-hematologic toxicity (except grade 3 nausea or vomiting, grade 3 fever or infection lasting <5 days, grade 3 hypophosphatemia, hypokalemia, hypocalcemia, or hypomagnesemia responsive to oral supplementation, or grade 3 hyperlipidemia and anorexia); (b) ≥grade 2 QTc prolongation, mucositis, or pneumonitis; and (c) any grade 2 non-hematologic toxicity persisting for ≥7 days and considered medically significant or sufficiently intolerable.
The MTD was defined, based on the Rolling-6 design,31 as the highest dose at which six patients were treated with no more than one patient experiencing a DLT, and the next higher dose level was determined to be intolerable. Once the MTD and/or RP2D was estimated, six additional patients were then treated at this dose to ensure it was not too toxic.
Survival Outcomes and Radiographic Response Criteria
All patients who received at least one dose of study therapy were evaluable for assessing survival outcomes (OS in DIPG and EFS in HGG). OS was defined as time from diagnosis (radiographic diagnosis or definitive surgery) to death (to enable comparison with historical registry survival data2). EFS was defined as time from diagnosis (radiographic diagnosis or definitive surgery) to the earlier of death or progression.
Patients were evaluable for radiographic response assessment if they (1) had measurable disease at initiation of treatment, (2) received at least one dose of each of the study drugs, and (3) had at least one subsequent imaging evaluation. Tumor response was determined by changes on MRI (FLAIR and T2 or post-contrast T1-weighted images) according to Response Assessment in Neuro-Oncology (RANO) criteria.32 Complete response (CR), partial response (PR), and stable disease (SD) were all defined by a stable or decreasing dose of corticosteroids and a stable or improving neurologic examination for 8 weeks or longer, plus the following MRI responses: a CR required the disappearance of all evaluable tumor and mass effect; PR was ≥50% reduction in tumor size (vs baseline), measured as the product of the longest tumor dimension and its perpendicular; and SD patients had imaging that did not meet criteria for PR or progressive disease (PD). PD included one or more of the following: progressive neurologic abnormalities, worsening neurologic status not explained by causes unrelated to tumor progression, ≥25% increase in tumor size (vs smallest tumor measurement recorded), appearance of a new lesion, and/or increasing doses of corticosteroids to maintain stable neurological status, unless in the context of recent wean or transient neurologic change due to treatment effect.
Pharmacokinetics
Pharmacokinetic studies were conducted in all patients to characterize the disposition of ribociclib and everolimus administered alone and in combination. For each drug, 1 mL of blood was collected per sample. For cycle 1, day 1, ribociclib single-dose blood samples were collected pre-dose and post-dose at 1, 2, 4, 8 (±1), 24 (±4), 32 (±4), and 48 (±4) hours; on day 2, the ribociclib dose was held; and on day 17 (±2 days), ribociclib and everolimus steady-state blood samples were collected pre-dose and post-dose at 1, 2, 4, 8 (±1), and 24 (±4) hours. For cycle 2, day 1, everolimus blood samples were collected pre-dose and post-dose at 0.5, 1, 1.5, 2, 4, 6, 8 (±1), and 24 (±4) hours. Ribociclib and everolimus blood samples were collected in K2-EDTA vacutainer tubes. Ribociclib samples were immediately centrifuged (10 000 rpm, 2 minutes), and plasma was stored at −80°C.
Ribociclib and everolimus concentrations were determined using validated LC-MS/MS methods.22,33 The lower limit of quantification (LLOQ) for ribociclib in plasma was 0.058 µM and then reduced to 0.023 µM. The LLOQ for everolimus in whole blood was 1.04 nM. All plasma ribociclib and blood everolimus concentration-time data were analyzed using a non-compartmental pharmacokinetic approach with Phoenix WinNonlin software (v8.2). For each drug, peak concentration (Cmax) and time to Cmax (Tmax) were determined from the concentration-time profiles. Data below LLOQ before Tmax were set to zero and after Tmax were replaced by LLOQ/2.34 Log-linear terminal slope (β) and terminal half-life (t1/2 = ln(2)/β) were defined by the last 3 measurable data points in the serial sampling window for both single-dose and steady-state data. Areas under the concentration-time curves were calculated as follows: (1) from time zero to the last measurable sampling time-point (AUC0-Tlast), using the linear-up log-down trapezoidal rule and (2) from time zero to time infinity (AUC0-∞) by extrapolating AUC0-Tlast from the last measurable time-point (Clast) using the terminal log-linear slope: (AUC0-Tlast + Clast/β). Apparent oral single and steady-state clearances (CL/F) were calculated as the BSA-normalized dosage divided by AUC0-∞.
RB Immunohistochemistry and Molecular Profiling
Diagnostic tumor samples were collected from all patients prior to enrollment (except for those with DIPG who had not undergone biopsy). Biopsied tissue was formalin-fixed, paraffin-embedded, and sectioned. Slides (unstained and corresponding H&E-stained) were sent to a CLIA-certified laboratory—at either CCHMC or locally for RB staining, as previously described.35 Unstained sections were cut to 5 µm in thickness from a section containing ≥50% tumor cells. Tumor was denoted RB+ if ≥20% of tumor cells had positive nuclear staining in ≥3 20× fields evaluated per case. RB+ endothelial cells served as an internal positive control. All slides were reviewed by the neuropathologist (C.F.). Tumor molecular profiling was not formally conducted as part of the study, but if performed for clinical purposes by local institutions from biopsy and/or autopsy tissue and shared with the study team, results were obtained and summarized descriptively below.
Statistical Analysis
Continuous and categorical variables are described by median (range) and frequency (percentage), respectively. The Kaplan-Meier method was used to calculate median OS and 12-, 24-, and 36-month OS percentage (with corresponding 95% confidence intervals [CI]) in patients with DIPG. EFS data in patients with HGG were descriptively analyzed, given a much smaller sample.
Results
Patient Cohort
Between November 2017 and March 2020, 35 patients were screened, and 19 (53%) enrolled, ranging in age from 2 to 15 years (median: 6.5 years; Table 1). Reasons for not enrolling included parental decision, delay in starting radiation therapy, patient passed away, >4 weeks between radiation and start of study treatment, and labs or baseline conditions outside eligibility requirements (including 2 with negative RB status). Of the 19 enrolled patients, 15 were diagnosed with DIPG and four with HGG. Five patients with DIPG had biopsies and confirmed histology (Table 1). Specimens from the nine patients with tumor tissue available (5 DIPG, 4 HGG) were all RB+. Five of these nine patients had H3K27M-mutant tumors.
Table 1.
Patient Demographics and Tumor Diagnoses (n = 19)
| Age—median years (range) | |
| 6.5 (2-15) | |
| Gender | |
| Female | 14 (74%) |
| Male | 5 (26%) |
| Race/ethnicity | |
| White | 12 (63%) |
| Hispanic or Latino | 2 (10.5%) |
| Black | 2 (10.5%) |
| Unknown | 2 (10.5%) |
| Asian | 1 (5%) |
| Diagnoses | |
| DIPG (radiographic) | 10 (53%) |
| DIPG (biopsy) | 5 (26%) |
| Diffuse midline glioma, H3K27M-mutant, grade IV | 4 |
| High-grade glioma, not otherwise specified, H3K27-wildtypea | 1 |
| HGG | 4 (21%) |
| Grade III | 2 |
| Glioblastoma; grade IV, small-cell features | 1 |
| Diffuse midline glioma, H3K27M-mutant, grade IVb | 1 |
Abbreviations: DIPG, diffuse intrinsic pontine glioma; HGG, high-grade glioma.
Grades are all WHO-based.
aBrainstem, thalamus, cerebellum (Note: This patient was 6 years of age at diagnosis).
bPrimarily right thalamus.
Study Treatment and Toxicities
Three patients were enrolled on dose level 1 (ribociclib 120 mg/m2 days 1-21; everolimus 1.2 mg/m2 days 1-28), three patients were enrolled on dose level 2 (ribociclib 170 mg/m2 days 1-21; everolimus 1.2 mg/m2 days 1-28), and 12 patients were enrolled on dose level 3 (ribociclib 170 mg/m2 days 1-21; everolimus 1.5 mg/m2 days 1-28). One of the 19 enrolled patients received one dose of ribociclib and then withdrew consent to pursue another study (no toxicity observed); this patient was not evaluable for MTD/RP2D estimation and was replaced. Of the 18 patients who completed cycle 1, one elected to come off study after cycle 1 to pursue another trial. Of the 18 patients, 11 received six or more cycles (median: 6 cycles [range: 1-40 cycles]). At the time of data cutoff (July 2021), two patients remained on active treatment (both having received ≥37 cycles of therapy) and 16 patients had discontinued treatment for the following reasons: disease progression (n = 11), adverse events (AEs) (n = 2), non-compliance (n = 2), and decision to pursue another trial (n = 1).
Eighteen patients were evaluable for MTD/RP2D estimation (Table 2). None of the three patients at each dose level 1 and 2 experienced a DLT. Of the six patients enrolled on dose level 3, one experienced a DLT (grade 3 lung infection). An additional six patients were enrolled on dose level 3 as an expansion cohort; none experienced a DLT. Thus, the RP2D was 170 mg/m2 days 1-21 for ribociclib and 1.5 mg/m2 days 1-28 for everolimus; the MTD was not reached.
Table 2.
DLT Summary (n = 19)
| Dose Levels (mg/m2/dose)a | Treated Patients | Evaluable Patients | Patients With DLTs | ||
|---|---|---|---|---|---|
| Ribociclib | Everolimus | ||||
| Level 1 | 120 | 1.2 | 3 | 3 | 0 |
| Level 2 | 170 | 1.2 | 3 | 3 | 0 |
| Level 3 | 170 | 1.5 | 7 | 6 | 1b |
| Level 3, expansion cohort | 170 | 1.5 | 6 | 6 | 0 |
Abbreviation: DLT, dose-limiting toxicities.
aRibociclib administered on days 1-21; everolimus on days 1-28, cycle 1.
bGrade 3 lung infection.
The majority of AEs observed in the 18 evaluable patients were at least possibly related to both study drugs unless noted in Table 3 as ribociclib- or everolimus-only. The most common toxicities were grade 2 or lower, and included decreased white blood cell count (94%), lymphopenia (83%), neutropenia (78%), fatigue (72%), anemia (67%), hypercholesterolemia (72%), mucositis (67%), hypertriglyceridemia (50%), and increased AST (50%). The most common grade 3/4 toxicities were neutropenia (33%), leucopenia (17%), and lymphopenia (11%). Three patients (17%) required dose reduction or came off therapy due to toxicity. Dose reductions were required for two patients: one in the DLT period due to grade 3 lung infection and one in cycle 2 for grade 3 ALT elevation and grade 4 hypokalemia. A third patient came off study prior to cycle 9 due to grade 4 cardiac toxicity (conduction disorder with prolonged QTc, ventricular tachycardia, and arrhythmia; no history of previously diagnosed cardiac medical conditions); this patient recovered upon discontinuation of study medications. No patients died due to toxicity.
Table 3.
All Attributable Adverse Events (n = 18 Evaluable Patients)
| Adverse Events | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total Patients (%) | |
|---|---|---|---|---|---|---|
| Blood and lymphatic system disorders | Anemia | 9 (1a) | 3 | 0 | 0 | 12 (67%) |
| Cardiac disordersb | Atrioventricular block (first degree) | 1 | 0 | 0 | 0 | 1 (6%) |
| Conduction disorder | 0 | 0 | 0 | 1c | 1 (6%) | |
| Supraventricular tachycardia | 0 | 1 | 0 | 0 | 1 (6%) | |
| Ventricular arrhythmia | 0 | 0 | 0 | 1c | 1 (6%) | |
| Ventricular tachycardia | 0 | 0 | 0 | 1c | 1 (6%) | |
| Gastrointestinal disorders | Abdominal pain | 4 | 2 | 0 | 0 | 6 (33%) |
| Anal mucositis | 1 | 0 | 0 | 0 | 1 (6%) | |
| Constipation | 2 | 0 | 0 | 0 | 2 (11%) | |
| Diarrhea | 3 | 1 | 0 | 0 | 4 (22%) | |
| Dysphagia | 0 | 1 | 0 | 0 | 1 (6%) | |
| Oral mucositis | 10 (4a) | 1a | 1a | 0 | 12 (67%) | |
| Oral dysesthesia | 1 | 0 | 0 | 0 | 1 (6%) | |
| Oral pain | 2a | 0 | 0 | 0 | 2 (11%) | |
| Nausea | 4 | 1 | 0 | 0 | 5 (28%) | |
| Stomach pain | 0 | 1 | 0 | 0 | 1 (6%) | |
| Vomiting | 4 (1b) | 3 | 0 | 0 | 7 (39%) | |
| General disorders | Fatigue | 6 (1b) | 7 (2b) | 0 | 0 | 13 (72%) |
| Feverb | 1 | 0 | 0 | 0 | 1 (6%) | |
| Infections and infestations | Lung infectiond | 0 | 0 | 1a,e | 0 | 1 (6%) |
| Paronychiaa | 0 | 1 | 0 | 0 | 1 (6%) | |
| Skin infection | 1 | 1 | 0 | 0 | 2 (11%) | |
| Urinary tract infection | 0 | 1 | 0 | 0 | 1 (6%) | |
| Investigations | Alanine aminotransferase increased | 4 | 2 | 2 | 0 | 8 (44%) |
| Aspartate aminotransferase increased | 7 | 2 | 0 | 0 | 9 (50%) | |
| Cholesterol high | 11 (10a) | 0 | 2a | 0 | 13 (72%) | |
| Creatinine increased | 3 (1b) | 1 | 0 | 0 | 4 (22%) | |
| Ejection fraction decreased | 1 | 0 | 0 | 0 | 1 (6%) | |
| Electrocardiogram QT corrected interval prolongedb | 2 | 0 | 0 | 0 | 2 (11%) | |
| Electrocardiogram T wave abnormalb,d | 0 | 1 | 0 | 0 | 1 (6%) | |
| Lymphocyte count decreased | 6 | 7 | 2 | 0 | 15 (83%) | |
| Neutrophil count decreased | 0 | 8 | 3 | 3 | 14 (78%) | |
| Platelet count decreased | 4 | 0 | 0 | 0 | 4 (22%) | |
| Weight loss | 1b | 2 | 0 | 0 | 3 (17%) | |
| White blood cell count decreased | 7 | 7 | 3 | 0 | 17 (94%) | |
| Metabolism and nutrition disorders | Anorexia | 3 (1b) | 1b | 0 | 0 | 4 (22%) |
| Dehydration | 1 | 0 | 0 | 0 | 1 (6%) | |
| Hyperglycemia | 5 (4a) | 0 | 0 | 0 | 5 (28%) | |
| Hypertriglyceridemiaa | 7 | 1 | 1 | 0 | 9 (50%) | |
| Hypokalemia | 1b | 0 | 0 | 1c | 2 (11%) | |
| Hypophosphatemia | 6 | 2 (1b) | 0 | 0 | 8 (44%) | |
| Musculoskeletal and connective tissue disorders | Arthralgiaa | 1a | 0 | 0 | 0 | 1 (6%) |
| Back paina | 0 | 1a | 0 | 0 | 1 (6%) | |
| Myalgiaa | 1a | 0 | 0 | 0 | 1 (6%) | |
| Neck pain | 1 | 0 | 0 | 0 | 1 (6%) | |
| Pain in extremity | 2 (1a;1b) | 1a | 0 | 0 | 3 (17%) | |
| Nervous system disorders | Headache | 3 | 3 | 0 | 0 | 6 (33%) |
| Intracranial hemorrhage | 1a | 0 | 0 | 0 | 1 (6%) | |
| Psychiatric disorders | Insomnia | 1 | 1a | 0 | 0 | 2 (11%) |
| Renal and urinary disorders | Glucosuriaa | 1 | 0 | 0 | 0 | 1 (6%) |
| Hematuriaa | 2 | 0 | 0 | 0 | 2 (11%) | |
| Proteinuriaa | 2 | 0 | 0 | 0 | 2 (11%) | |
| Urinary tract paina | 1 | 0 | 0 | 0 | 1 (6%) | |
| Urinary urgencya | 1 | 0 | 0 | 0 | 1 (6%) | |
| Urinary frequencya | 0 | 1 | 0 | 0 | 1 (6%) | |
| Reproductive system and breast disorders | Vaginal inflammationa | 0 | 1 | 0 | 0 | 1 (6%) |
| Respiratory, thoracic, and mediastinal disorders | Cougha | 1 | 1 | 2 (11%) | ||
| Dyspepsiab | 0 | 1 | 0 | 0 | 1 (6%) | |
| Dyspnea | 1 | 0 | 0 | 0 | 1 (6%) | |
| Epistaxis | 1 | 0 | 0 | 0 | 1 (6%) | |
| Skin and subcutaneous tissue disorders | Alopeciab | 2 | 0 | 0 | 0 | 2 (11%) |
| Dry skina | 0 | 2 | 0 | 0 | 2 (11%) | |
| Eczema | 1 | 0 | 0 | 0 | 1 (6%) | |
| Hyperhidrosisa | 1 | 0 | 0 | 0 | 1 (6%) | |
| Pruritus | 1 | 0 | 0 | 0 | 1 (6%) | |
| Rash maculo-papularb | 1 | 0 | 0 | 0 | 1 (6%) | |
| Vascular disorders | Hypertension | 0 | 5a | 1b | 0 | 6 (33%) |
Abbreviations: AEs, adverse events; DLT, dose-limiting toxicities
aEverolimus attribution;
bRibociclib attribution;
cDMT, DMT Dose Modifying Toxicity;
dSerious;
eDLT.
All AEs deemed attributable to study agents; reporting only the highest grade reported per participant during study treatment.
Survival Outcomes and Treatment Response
All 19 enrolled patients were evaluable for assessing survival. As of July 2021, three patients had completed ≥36 months of active therapy (all with DIPG) and seven patients were still alive (1 HGG, 6 DIPG), with median follow-up (defined as time from treatment initiation to July 2021 data capture) of 36 months (range: 14-39 months). Median OS for the 15 patients with DIPG was 13.9 months, with 12-, 24-, and 36-month OS of 53.3% (95% CI: 33%-86%), 38.9% (20%-74%), and 38.9% (20%-74%), respectively. When excluding two patients diagnosed with DIPG before 3 years of age, median OS of remaining DIPG cohort was 10.8 months, with 12-, 24-, and 36-month OS of 46.2% (26%-83%), 28.8% (12%-70%), and 28.8% (12%-70%). Three of the 15 patients with DIPG underwent re-irradiation after radiographic and/or clinical progression on ribociclib and everolimus (OS: 13, 14, and >21 months [still alive]); none of the patients with DIPG alive beyond 24 months had undergone re-irradiation. Note that among 15 patients with DIPG, all had typical diagnostic imaging features by local reports, except for one patient (6 years of age at diagnosis, OS >18 months) with imaging demonstrating a T2-hyperintense expansile, infiltrative pontine mass with extension into the cerebellum and surrounding brainstem, yet with areas of leptomeningeal enhancement; given the latter findings, this patient underwent biopsy, consistent with HGG, not otherwise specified. Among four patients with HGG, EFS outcomes were 6, 9, 12, and >18 months [still alive], respectively (median: 10.5 months). Among 18 patients evaluable for assessing radiographic response, no objective responses (CR or PR) were observed. Six patients had apparent progression prior to cycle 5; four of the six were deemed pseudoprogression upon follow-up MRI 4 weeks later and remained on study.
Long-term DIPG survivors.
—As of the July 2021 data capture, three patients with DIPG were alive >24 months (and remain alive), with OS of 36, 36, and 39 months and each have completed ≥36 cycles of active therapy, as noted above. This includes the two aforementioned patients diagnosed at 2 years of age, as well as one patient diagnosed at 3 years of age. One of these patients (with typical diagnostic imaging features, as shown in Supplementary Figure S1) underwent biopsy at initial diagnosis, with pathology consistent with diffuse midline glioma, H3K27M-mutant (a H3F3A [H3.3] mutation was identified on targeted sequencing; no cell cycle or PI3K/mTOR pathway alterations were identified as discussed further below). The other two patients (also with typical imaging features of DIPG at diagnosis with T2-hyperintense expansile, infiltrative pontine masses comprising the majority of the pons and lacking significant contrast enhancement or diffusion restriction) have not undergone biopsy. All three patients had presenting symptoms (ataxia and/or cranial nerve palsies) of <6 months duration preceding their diagnosis. None received re-irradiation.
Pharmacokinetics
Ribociclib pharmacokinetic studies were performed for a total of 18 patients. Steady-state profiles obtained for two patients were excluded from the analysis: one profile was not complete for clinical reasons, and the 24-hour time-point of the second profile was inadvertently collected after a second dose administration, preventing characterization of drug clearance.
Everolimus cycle 1, day 17 pharmacokinetic data were available for 18 patients; however, one profile was not complete for logistical reasons and thus, was excluded from the analysis. Everolimus cycle 2, day 1 pharmacokinetic data were only available for 16 patients as two patients came off the study before cycle 2. Two profiles were unevaluable as the 24-hour time-points were inadvertently collected after a second dose administration. There was no drug interruption between cycles 1 and 2 for eight patients, in whom pharmacokinetic data were all considered at steady state. However, for the remaining six patients, the start of cycle 2 was delayed, mainly due to toxicities. Thus, the associated everolimus concentrations were not at steady state.
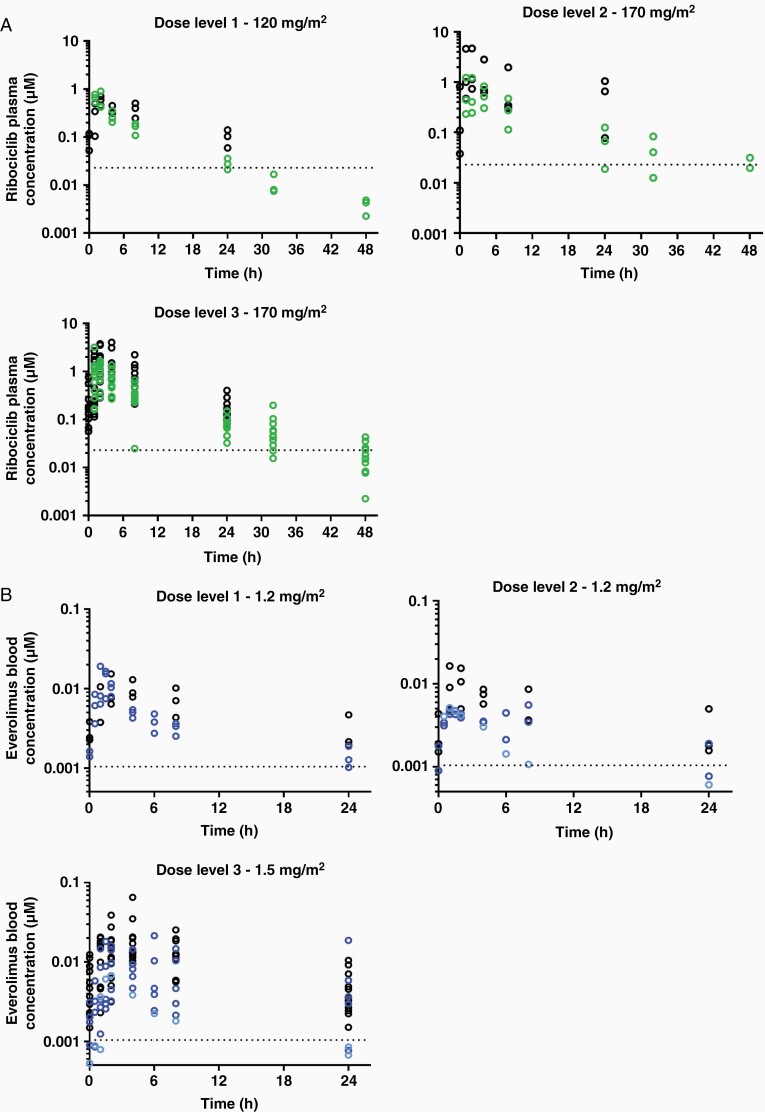
Large inter-individual variabilities for both drugs were observed, as shown in a summary of the pharmacokinetic parameters for plasma ribociclib and blood everolimus after single dose and at steady state (Table 4) as well as in concentration-time profiles stratified by dosage level (Figure 1). After a single dose of 120 or 170 mg/m2 ribociclib, the total AUC0-∞ ranged from 3.99 to 5.66 vs 4.08 to 15.3 h·µM, respectively. The mean ± standard deviation (SD) accumulation of ribociclib from single dose alone to steady state in combination, assessed by AUC0-24 h ratios, was 2.2 ± 1.1-fold across dosage levels. For everolimus, the slope of the terminal elimination phase was not ideally characterized, as the extrapolated AUC0-24 h represented >25% of total AUC0-∞ for nine patients. The mean ± SD accumulation of everolimus from single dose alone (cycle 2, day 1 delayed) to steady state in combination was 3.2 ± 0.4-fold across dosage levels. Steady-state everolimus AUC0-24 h values when given in combination were 1.9 ± 0.9-fold higher than steady-state everolimus AUC0-24 h administered alone (cycle 2, day 1 not delayed).
Table 4.
Ribociclib and Everolimus Plasma Pharmacokinetic Parameters
| Median (Range) | |||||||
|---|---|---|---|---|---|---|---|
| Dose (mg/m2) | Cycle/day | N | T max (h) | C max (µM) | AUC0-24 h (h·µM) | Half-life (h) | CL/F (L/h/m2) |
| Ribociclib Plasma Pharmacokinetic Parameters | |||||||
| Level 1 120 |
C1/D1 SD | 3 | 1 (1-2) | 0.78 (0.53-0.90) | 3.59 (3.04-4.71) | 11.7 (11.6-11.9) | 49.7 (41.1-69.6) |
| C1/D17 SS | 3 | 2 (2-8) | 0.60 (0.51-0.71) | 6.86 (5.11-7.42) | 10.2 (8.0-11.5) | 34.0 (30.4-54.4) | |
| Level 2 170 |
C1/D1 SD | 3 | 1 (1-4) | 0.52 (0.45-1.23) | 5.03 (3.13-10.6) | 11.6 (11.2-11.8) | 61.5 (35.7-92.6) |
| C1/D17 SS | 2a | 2-2 | 1.15-4.70 | 7.79-43.4 | 6.6-9.7 | 48.0-10.6 | |
| Level 3 170 |
C1/D1 SD | 12 | 2 (1-8) | 1.32 (0.30-3.17) | 7.42 (2.08-13.1) | 9.5 (8.4-14.6) | 46.7 (24.9-159) |
| C1/D17 SS | 11b | 2 (1-8) | 1.39 (0.34-4.01) | 13.6 (5.18-41.9) | 8.09 (5.9-11.2) | 26.4 (10.6-78.4) | |
| Everolimus Plasma Pharmacokinetic Parameters | |||||||
| Level 1 1.2 |
C1/D17 SS | 3 | 4 (2-4) | 8.8 (7.8-15.3) | 120.6 (95.4-207) | 10.8 (9.7-13.8) | 10.6 (5.8-12.9) |
| C2/D1 SS | 3 | 1 (0.5-1.5) | 16.5 (8.4-19.1) | 70.9 (68.8-98.6) | 16.2 (5.8-17.5) | 17.5 (12.9-18.7) | |
| Level 2 1.2 |
C1/D17 SS | 3 | 2 (1-4) | 15.4 (7.5-16.4) | 96.9 (8.1-183) | 13.1 (12.9-21.9) | 13.1 (6.8-15.0) |
| C2/D1 SS | 2 | 1.5-8 | 4.7-5.6 | 50.4-88.0 | 7.6-18.0 | 14.0-25.5 | |
| C2/D1 SD | 1 | 1 | 5.1 | 34.0 | 13.4 | 28.0 | |
| Level 3 1.5 |
C1/D17 SS | 11c | 4 (1-8) | 20.0 (9.5-35) | 204.6 (108-409) | 10.4 (6.2-20.9) | 7.7 (3.6-14.6) |
| C2/D1 SS | 3d | 4 (2-6) | 16.0 (14.4-21.5) | 179.5 (57.7-212) | 8.0 (7.4-9.6) | 8.6 (7.2-28.6) | |
| C2/D1 SD | 5 | 4 (1.5-8) | 9.2 (3.9-10) | 66.5 (43.3-128) | 18.1 (7.6-45.3) | 14.1 (3.95-30.3) |
Abbreviations: SD, single dose; SS, steady state.
On cycle 1 day 1 (C1D1), ribociclib was administered alone. On cycle 1 day 17 (C1D17), ribociclib was given in combination with everolimus.
aOne profile considered unevaluable as the 24-h time-point was suspected to be collected after a second dose of ribociclib.
bOne profile considered unevaluable as for patient logistic reasons, the 8- and 24-h time-points were not collected.
Everolimus: SD, single dose (cycle 2/day 1 delayed, mainly due to toxicities); SS, steady state (cycle 2/day 1 not delayed).
On course 2 day 1 (C2D1), everolimus was administered alone. On course 1 day 17 (C1D17), everolimus was given in combination with ribociclib.
cOne profile was considered unevaluable as for patient logistic reasons, the 8- and 24-h time-points were not collected.
dTwo profiles were considered unevaluable as the 24-h time-point was suspected to be collected after a second dose of everolimus.
Figure 1.
Plasma ribociclib and blood everolimus concentration-time profiles. (A) Ribociclib plasma concentration-time data after single dose on cycle 1, day 1 (green) and at steady state on cycle 1, day 17 (black). Dotted lines represent lower limits of quantification (0.023 µM). (B) Everolimus blood concentration-time data at steady-state on cycle 1, day 17 (black) and on cycle 2, day 1 after drug interruption (light blue) or at steady state (dark blue). Dotted lines represent lower limits of quantification (1.04 nM). In both, each circle represents individual concentrations.
Tumor Molecular Profiling (Descriptive Analysis With Focus on Cell Cycle and PI3K/mTOR Pathway Alterations)
Although not mandated or conducted by the study at the time of publication, tumor molecular profiling was performed for clinical purposes by respective treating institutions in a subset of patients. Herein, for a preliminary, descriptive analysis, we report on available results allowing evaluation of alterations relevant to the cell cycle or PI3K/mTOR pathway from this small cohort (Table 5), though emphasize cautious interpretation given the heterogeneity of testing assays and lack of comprehensive genomic profiling for most patients. Eight patients (DIPG [n = 5], HGG [n = 3]) had molecular testing performed on tumor tissue from biopsy/pre-treatment (n = 6) or autopsy (n = 2). Two patients were found to have cell cycle aberrations, with CDK4 amplification in one patient with HGG who remains alive at >37 months, and CDKN2A/B deletion in one patient with HGG with OS of 20 months (who had undergone re-irradiation). Alterations of the PI3K/mTOR pathway were identified in two patients, including a PTEN deletion (HGG) and PIK3R1 mutation (DIPG), with OS of 11 and 10 months, respectively. Four additional patients had tumor molecular profiling performed without evidence of cell cycle or PIK3/mTOR pathway aberrations. One patient (with DIPG, OS: 9 months) had detailed sequencing (whole-exome and RNA sequencing), which was unrevealing. In the remaining three patients (OS range: 13 to >39 months), targeted sequencing was performed, which may not have captured all relevant genes or aberration types.
Table 5.
Individual Patient Tumor Molecular Profiling Results, and Clinical Features, for Patients Who Had Testing Performed at Biopsy or Autopsy Allowing Evaluation of Alterations Relevant to the Cell Cycle or PI3K/mTOR Pathways
| Patient Age at Diagnosis (years) | Diagnosis | H3K27 Mutation Status | Tissue Source of Molecular Profiling (Biopsy and/or Autopsy) | Molecular Testing Performed | Molecular Profiling Results, Relevant to Cell Cycle, and PI3K/mTOR Pathways | Additional Alterations | Overall Survival (months) | Event-free Survival (months) for Patients With HGG | Number of Cycles | Post-study Re-irradiation | Post-study additional systemic therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HGG | |||||||||||
| 10 | HGG | Wildtype | Biopsy | Targeted sequencing | CDKN2A/B deletion | TP53 mutation, wildtype for IDH and BRAFV600 | 20 | 12 | 9 | Yes | Yes, details not known |
| 11 | HGG | H3K27M-mutant | Biopsy | Targeted sequencing | PTEN deletion (heterozygous) | Wildtype for IDH and BRAFV600 | 11 | 9 | 6 | No | Not known |
| 12 | HGG | Wildtype | Biopsy | Targeted sequencing | CDK4 amplification | TP53 mutation, wildtype for IDH and BRAFV600 | >37a | >18 | 17 | No | Yes, ribociclib and everolimus off-trial (came off study treatment due to non-compliance); temozolomide |
| DIPG | |||||||||||
| 2 | DIPG | H3K27M-mutant (H3.3 [H3F3A]] |
Biopsy | Targeted sequencing | No relevant alterations identifiedc | N/A | >39a | >40b | N/A | N/A | |
| 5 | DIPG | H3K27M-mutant | Biopsy | Targeted sequencing | No relevant alterations identifiedc | MLL2 R185H mutation | 13 | 6 | Yes | No | |
| 7 | DIPG | H3K27M-mutant (H3.3 [H3F3A]] |
Autopsy | Whole exome and RNA sequencing | No relevant alterations identifiedc | TP53 mutation | 9 | 4 | No | Yes, bevacizumab | |
| 10 | DIPG | H3K27M-mutant (H3.3 [H3F3A]] |
Autopsy | Whole exome and RNA sequencing | PIK3R1 mutation | TP53 mutation | 10 | 5 | No | Yes, vorinostat | |
| 15 | DIPG | H3K27M-mutant | Biopsy | Targeted sequencing | No relevant alterations identifiedc | TP53 mutation, PDGFRA/KIT amplification | >21a | 1 | Yes | Yes, intraventricular radioimmunotherapy with omburtamab, avastin, etoposide, and “anti-neoplastins” |
aPatient is alive at the time of data capture for manuscript submission.
bPatient remains on active therapy at the time of data capture for manuscript submission.
cNo genetic alterations involving cell cycle (CDK4/6, CDKN2A/B) or PI3K/mTOR (PIK3R1, PIK3CA, PTEN) pathways were identified, based on available results from molecular testing performed. Note that molecular profiling assays were variable across patients and often focused targeted sequencing panels, most commonly by Foundation Medicine.
Discussion
This is the first study to evaluate the safety, tolerability, and pharmacokinetics of the combination of ribociclib and everolimus following radiotherapy in children with newly diagnosed DIPG and HGG. Eighteen patients were evaluable for MTD/RP2D determination; a MTD was not reached. The RP2D is ribociclib 170 mg/m2 daily for 21 days and everolimus 1.5 mg/m2 daily for 28 days, which is equivalent to the adult RP2D,18 confirming safety and feasibility following radiotherapy.
Ribociclib and everolimus combination therapy was well tolerated with a safety profile similar to adults.20 As expected, the most common grade 3/4 toxicities were neutropenia (33%), leucopenia (17%), and lymphopenia (11%), similar to the recent phase I trial of this combination in children with recurrent CNS tumors.29 One patient came off study prior to cycle 9 due to grade 4 cardiac toxicity (conduction disorder with prolonged QTc, ventricular tachycardia, and arrhythmia), which recovered upon discontinuation of therapy. The majority of oral mucositis was grade 1 or 2, with no grade 4 toxicity identified, supporting the efficacy of dexamethasone mouthwash for mucositis prevention (compared with >30% historical incidence of everolimus-related grade 3/4 mucositis36). Grade 3/4 thrombocytopenia was not observed in our chemotherapy-naïve patient cohort, suggesting this combination can be safely used in high-grade CNS tumors at risk of hemorrhage.
Large interpatient pharmacokinetic variability for both ribociclib and everolimus was observed in this pediatric population. Ribociclib exhibited similar dose-dependent pharmacokinetic behavior compared to previous reports in children with rhabdoid tumors, neuroblastoma, and other solid tumors receiving ribociclib alone (280-470 mg/m2/d).37 The observed accumulation of ribociclib from day 1 alone to steady state in combination with everolimus (~2.2 ± 1.1-fold) agreed with prior studies in children and adults receiving ribociclib alone, which both reported a 2- to 3-fold ribociclib accumulation at steady state.37,38 Overall, there was no evidence of an impact of everolimus on ribociclib pharmacokinetics. When administered alone, everolimus displayed comparable pharmacokinetic profiles with those reported in both children and adults.22,39,40 Steady-state exposures of everolimus in combination with ribociclib were about 2-fold higher than that of everolimus administered alone, which may suggest an impact of ribociclib on everolimus pharmacokinetics. However, considering the large interpatient variabilities, results should be interpreted with caution. This potential drug-drug interaction was previously observed in women with ER+/HER2-metastatic breast cancer, where everolimus exposure (when administered at 2.5 mg daily) increased 2- to 4-fold in the presence of 300 mg ribociclib.20 A population pharmacokinetic analysis will be performed to further characterize the interaction between ribociclib and everolimus, and to determine the influence of potential patient covariates.
Given the above potential dose-dependent impact of ribociclib on everolimus pharmacokinetics as well as evidence of synergy of dual CDK4/6 and mTOR inhibition across preclinical tumors models, including glioblastoma,14–16 it is not surprising that the RP2D of ribociclib and everolimus in combination (170 mg/m2 days 1-21 and 1.5 mg/m2 days 1-28) is less than respective single-agent administration (350 mg/mg2 days 1-21 and 5 mg/m2 days 1-28). Furthermore, the RP2D of this combination in pediatric patients newly diagnosed with HGG and DIPG following radiotherapy is greater than the RP2D in the recurrent/refractory pediatric CNS tumor population (120 mg/m2 days 1-21 and 1.2 mg/m2 days 1-28),29 suggesting higher tolerability in the upfront setting prior to additional systemic therapy. Based on results from the latter phase I and surgical cohort study, ribociclib dosing of 120 mg/m2 was suspected to achieve ribociclib concentrations above the IC50 value in tumor tissue,29 supporting adequate CNS penetration of the RP2D of the present trial, though continued research will be needed.
The median OS for patients with DIPG in our cohort was 13.9 months, with 12-month OS of 53.3% and both 24- and 36-month OS of 38.9% by Kaplan-Meier methodology. Among 15 evaluable patients with DIPG, three patients remained on active therapy beyond 36 cycles. Serving as a historical control, the International and European Society for Pediatric Oncology DIPG registries reported a median OS of 11 months among 1008 patients with radiographically confirmed DIPG, with less than 10% surviving at least 2 years,2 although comparison of survival of this study to registry data is limited by stringent eligibility criteria in the former as well as heterogeneity in patient clinical features and treatment received in the latter. Additionally, of note, two patients in our study with OS >36 months (including one with biopsy-confirmed diffuse midline glioma, H3K27M-mutant) were <3 years at diagnosis, corroborating prior reports that long-term DIPG survivors are more commonly diagnosed at the age extremes2 and possibly confounding outcomes, given that median OS of the DIPG cohort after excluding these patients decreased to 10.8 months and no partial or objective responses were observed. However, potentially improved percentage survival at 24 and 36 months (both 38.9%), including when excluding the two above infant patients (both 28.8%), despite lack of re-irradiation among the long-term survivors, suggest that the combination of ribociclib and everolimus may contribute to prolonged disease stabilization in a subset of patients who deserve further characterization; these results should be interpreted with caution given the small sample size, including those with sufficiently long follow-up, as well as by lack of biopsy tissue and real-time central neuroimaging review of diagnostic MRIs for patients with DIPG, such that further assessment must be formally performed in the context of a phase II study with careful imaging and/or tissue requirements. Among the four evaluable patients with HGG, median EFS was 10.5 months; the small numbers limit conclusions in this specific disease population, also warranting further study.
Molecular profiling of tumor tissue at biopsy or autopsy was performed in a subset of patients, providing preliminary insight about targeting relevant activated pathways in DIPG and HGG. Cell cycle upregulation was identified in two of the patients with longer OS, with CDK4 amplification in one patient (still alive at >37 months) and CDKN2A/B deletion in the other (OS of 20 months, had undergone re-irradiation). PI3K/mTOR pathway activation was observed in two patients whose tumors harbored a PIK3R1 mutation and PTEN deletion, respectively, though without improved outcomes. Several long-term survivors did not have tissue available for sequencing or had focused gene panels performed, which may not have captured all pertinent actionable aberrations. Given the small number of cases, variation in molecular assays conducted clinically, and lack of comprehensive profiling for most tumors, results must be interpreted cautiously. However, based on the known relevance of cell cycle and PI3K/mTOR pathway upregulation in DIPG and HGGs9–12 as well as the presence of activating somatic cell cycle alterations in at least some of the longer survivors treated in our cohort, the continued investigation will be essential. An improved understanding of how DIPG and HGG tumor molecular landscapes—evaluated prospectively with consistent, detailed sequencing—correlate with survival and response to targeted agents is critical and will be incorporated in future research efforts planned by our team.
This study defined the RP2D, toxicity profiles, and pharmacokinetics of ribociclib and everolimus in pediatric patients with newly diagnosed DIPG and HGG following radiotherapy. This combination was well tolerated, and results will inform future research evaluating efficacy in this patient population, with a corresponding phase II study with integrated molecular profiling currently in development.
Supplementary Material
Acknowledgments
We thank the patients and their families for their support and commitment to this study. We gratefully acknowledge Amy Jones, Michelle Deutsch, and Mythili Murala for operational support through CONNECT. We gratefully acknowledge Dr. Renee Doughman for regulatory support as well as Jan Englehart and Maria Mathews for data management support.
Contributor Information
Mariko DeWire, Cancer and Blood Diseases Institute, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA.
Margot Lazow, Pediatric Neuro-Oncology Program, Nationwide Children’s Hospital, Columbus, Ohio, USA; The Ohio State University College of Medicine, Columbus, Ohio, USA.
Olivia Campagne, Department of Pharmaceutical Sciences, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
James Leach, Division of Radiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA.
Christine Fuller, Division of Pathology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA; Department of Pathology, Upstate Medical University, Syracuse, New York, USA.
Shiva Senthil Kumar, The Ohio State University College of Medicine, Columbus, Ohio, USA.
Joseph Stanek, Pediatric Neuro-Oncology Program, Nationwide Children’s Hospital, Columbus, Ohio, USA; The Ohio State University College of Medicine, Columbus, Ohio, USA.
Peter de Blank, Cancer and Blood Diseases Institute, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA.
Trent R Hummel, Cancer and Blood Diseases Institute, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA.
Natasha Pillay-Smiley, Cancer and Blood Diseases Institute, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA.
Ralph Salloum, Pediatric Neuro-Oncology Program, Nationwide Children’s Hospital, Columbus, Ohio, USA; The Ohio State University College of Medicine, Columbus, Ohio, USA.
Charles B Stevenson, Division of Neurosurgery, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA.
Patricia Baxter, Texas Children’s Cancer Center, Baylor College of Medicine, Houston, Texas, USA.
David Gass, Cancer and Blood Disorders Department, Atrium Health Levine Children’s Hospital, Charlotte, North Carolina, USA.
Stewart Goldman, Phoenix Children’s Hospital, University of Arizona College of Medicine-Phoenix, Phoenix, Arizona, USA.
Sarah E S Leary, Cancer and Blood Disorders Center, Seattle Children’s Hospital, Seattle, Washington, USA.
Adam Carle, Anderson Center Health Systems Excellence, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA.
Leonie Mikael, Pediatric Neuro-Oncology Program, Nationwide Children’s Hospital, Columbus, Ohio, USA.
Dorothy Crabtree, Pediatric Neuro-Oncology Program, Nationwide Children’s Hospital, Columbus, Ohio, USA.
Brooklyn Chaney, Pediatric Neuro-Oncology Program, Nationwide Children’s Hospital, Columbus, Ohio, USA.
Adam Lane, Division of Biostatistics, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA.
Rachid Drissi, The Ohio State University College of Medicine, Columbus, Ohio, USA; Center for Childhood Cancer & Blood Disorders, Nationwide Children’s Hospital, Columbus, Ohio, USA.
Clinton F Stewart, Department of Pharmaceutical Sciences, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Maryam Fouladi, Pediatric Neuro-Oncology Program, Nationwide Children’s Hospital, Columbus, Ohio, USA; The Ohio State University College of Medicine, Columbus, Ohio, USA.
Funding
This work was financially supported by Novartis Pharmaceuticals CLEE011XUS17T, The Cure Starts Now Foundation, Curing Kids Cancer, Hope for Caroline Foundation, Julian Boivin Courage for Cures Foundation, Abbie’s Army, Michael Mosier Defeat DIPG Foundation, Reflections of Grace Foundation, The Cure Starts Now Australia, Brooke Healey Foundation, Soar With Grace Foundation, Jeffrey Thomas Hayden Foundation, Cure Brain Cancer Foundation, The Jones Family Foundation, Musella Foundation, Pray, Hope Believe Foundation, Smiles for Sophie Foundation, Benny’s World, Love Chloe Foundation, Aiden’s Avengers, A Cure from Caleb Society, the Operation Grace White Foundation, Ryan’s Hope, Wayland Villars DIPG Foundation, American Childhood Cancer Organization, Juliana Rose Donnelly Trust, Sheila Jones & Friends, the Ellie Kavalieros DIPG Research Fund, Voices Against Brain Cancer, and the DIPG Collaborative. Novartis provided ribociclib, everolimus, and financial support for trial conduct, patient research costs, operations costs, and correlative studies. Novartis also reviewed the manuscript but did not have a direct role in trial design, patient recruitment, data collection, analyses, or manuscript preparation.
Conflict of interest statement. None.
Authorship statement. Conception and design: M.D. and M.F. Data analyses/interpretation: M.D., A.L., S.E.S.L., and M.L. Pathologic analyses: C.F. Pharmacokinetic studies: C.B.S. and O.C. Imaging design/review: J.L. Manuscript writing and editing: M.D. and M.L. with critical feedback from all authors. Final approval: all authors.
References
- 1. Cohen KJ, Jabado N, Grill J. Diffuse intrinsic pontine gliomas-current management and new biologic insights. Is there a glimmer of hope? Neuro Oncol. 2017;19(8):1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, et al. . Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): a collaborative report from the International and European Society for Pediatric Oncology DIPG Registries. J Clin Oncol. 2018;36(19):1963–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7(3):241–248. [DOI] [PubMed] [Google Scholar]
- 4. Ostrom QT, Cioffi G, Gittleman H, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21(Suppl 5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24(11):1770–1783. [DOI] [PubMed] [Google Scholar]
- 6. Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8(8):627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sartelet H, Oligny LL, Vassal G. AKT pathway in neuroblastoma and its therapeutic implication. Expert Rev Anticancer Ther. 2008;8(5):757–769. [DOI] [PubMed] [Google Scholar]
- 8. Kawauchi K, Ogasawara T, Yasuyama M, Otsuka K, Yamada O. Regulation and importance of the PI3K/Akt/mTOR signaling pathway in hematologic malignancies. Anticancer Agents Med Chem. 2009;9(9):1024–1038. [DOI] [PubMed] [Google Scholar]
- 9. Mackay A, Burford A, Carvalho D, et al. . Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017;32(4):520–537.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu G, Diaz AK, Paugh BS, et al. . The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46(5):444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buczkowicz P, Hoeman C, Rakopoulos P, et al. . Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet. 2014;46(5):451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paugh BS, Broniscer A, Qu C, et al. . Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J Clin Oncol. 2011;29(30):3999–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cordero FJ, Huang Z, Grenier C, et al. . Histone H3.3K27M represses p16 to accelerate gliomagenesis in a murine model of DIPG. Mol Cancer Res. 2017;15(9):1243–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olmez I, Brenneman B, Xiao A, et al. . Combined CDK4/6 and mTOR inhibition is synergistic against glioblastoma via multiple mechanisms. Clin Cancer Res. 2017;23(22):6958–6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bardia A, Hurvitz SA, DeMichele A, et al. . Phase I/II trial of exemestane, ribociclib, and everolimus in women with HR+/HER2− advanced breast cancer after progression on CDK4/6 inhibitors (TRINITI-1). Clin Cancer Res. 2021;27(15):4177–4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weinberg BA, Wang H, Witkiewicz AK, et al. . A phase I study of ribociclib plus everolimus in patients with metastatic pancreatic adenocarcinoma refractory to chemotherapy. J Pancreat Cancer. 2020;6(1):45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Motzer RJ, Escudier B, Oudard S, et al. . Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. [DOI] [PubMed] [Google Scholar]
- 18. Bardia A, Modi S, Gregor MC-M, et al. . Phase Ib/II study of LEE011, everolimus, and exemestane in postmenopausal women with ER+/HER2-metastatic breast cancer. J Clin Oncol. 2014;32(15_suppl):535–535.24419119 [Google Scholar]
- 19. Beaver JA, Park BH. The BOLERO-2 trial: the addition of everolimus to exemestane in the treatment of postmenopausal hormone receptor-positive advanced breast cancer. Future Oncol. 2012;8(6):651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bardia A, Modi S, Oliveira M, et al. . Phase Ib dose-escalation/expansion trial of ribociclib in combination with everolimus and exemestane in postmenopausal women with HR+, HER2− advanced breast cancer. Clin Cancer Res. 2020;26(24):6417–6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franz DN, Belousova E, Sparagana S, et al. . Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381(9861):125–132. [DOI] [PubMed] [Google Scholar]
- 22. Fouladi M, Laningham F, Wu J, et al. . Phase I study of everolimus in pediatric patients with refractory solid tumors. J Clin Oncol. 2007;25(30):4806–4812. [DOI] [PubMed] [Google Scholar]
- 23. Lukas J, Parry D, Aagaard L, et al. . Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995;375(6531):503–506. [DOI] [PubMed] [Google Scholar]
- 24. Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov. 2016;6(4):353–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shah A, Bloomquist E, Tang S, et al. . FDA approval: ribociclib for the treatment of postmenopausal women with hormone receptor-positive, HER2-negative advanced or metastatic breast cancer. Clin Cancer Res. 2018;24(13):2999–3004. [DOI] [PubMed] [Google Scholar]
- 26. Kovarik JM, Hartmann S, Figueiredo J, et al. . Effect of food on everolimus absorption: quantification in healthy subjects and a confirmatory screening in patients with renal transplants. Pharmacotherapy. 2002;22(2):154–159. [DOI] [PubMed] [Google Scholar]
- 27. Urva S, Bouillaud E, Delaney R, Jappe A, Cheung W. A phase I study evaluating the effect of everolimus on the pharmacokinetics of midazolam in healthy subjects. J Clin Pharmacol. 2013;53(4):444–450. [DOI] [PubMed] [Google Scholar]
- 28. DeWire M, Fuller C, Hummel TR, et al. . A phase I/II study of ribociclib following radiation therapy in children with newly diagnosed diffuse intrinsic pontine glioma (DIPG). J Neurooncol. 2020;149(3):511–522. [DOI] [PubMed] [Google Scholar]
- 29. DeWire MD, Fuller C, Campagne O, et al. . A phase I and surgical study of ribociclib and everolimus in children with recurrent or refractory malignant brain tumors: a pediatric brain tumor consortium study. Clin Cancer Res. 2021;27(9):2442–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barkovich AJ, Krischer J, Kun LE, et al. . Brain stem gliomas: a classification system based on magnetic resonance imaging. Pediatr Neurosurg. 1990;16(2):73–83. [DOI] [PubMed] [Google Scholar]
- 31. Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. 2008;26(2):190–195. [DOI] [PubMed] [Google Scholar]
- 32. Wen PY, Macdonald DR, Reardon DA, et al. . Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 33. Kala A, Patel YT, Davis A, Stewart CF. Development and validation of LC-MS/MS methods for the measurement of ribociclib, a CDK4/6 inhibitor, in mouse plasma and Ringer’s solution and its application to a cerebral microdialysis study. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1057:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keizer RJ, Jansen RS, Rosing H, et al. . Incorporation of concentration data below the limit of quantification in population pharmacokinetic analyses. Pharmacol Res Perspect. 2015;3(2):e00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goldhoff P, Clarke J, Smirnov I, et al. . Clinical stratification of glioblastoma based on alterations in retinoblastoma tumor suppressor protein (RB1) and association with the proneural subtype. J Neuropathol Exp Neurol. 2012;71(1):83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martins F, de Oliveira MA, Wang Q, et al. . A review of oral toxicity associated with mTOR inhibitor therapy in cancer patients. Oral Oncol. 2013;49(4):293–298. [DOI] [PubMed] [Google Scholar]
- 37. Geoerger B, Bourdeaut F, DuBois SG, et al. . A phase I study of the cdk4/6 inhibitor ribociclib (LEE011) in pediatric patients with malignant rhabdoid tumors, neuroblastoma, and other solid tumors. Clin Cancer Res. 2017;23(10):2433–2441. [DOI] [PubMed] [Google Scholar]
- 38. Infante JR, Cassier PA, Gerecitano JF, et al. . A phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin Cancer Res. 2016;22(23):5696–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Santana VM, Sahr N, Tatevossian RG, et al. . A phase 1 trial of everolimus and bevacizumab in children with recurrent solid tumors. Cancer. 2020;126(8):1749–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kirchner GI, Meier-Wiedenbach I, Manns MP. Clinical pharmacokinetics of everolimus. Clin Pharmacokinet. 2004;43(2):83–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.