Abstract
Introduction
Approval of sunitinib and everolimus for the treatment of progressive, unresectable or metastatic well-differentiated pancreatic neuroendocrine tumors (pNETs) was obtained in France in 2011 and 2012, respectively. OPALINE was set up as an observational study to evaluate the efficacy of sunitinib and everolimus compared to usual pNET treatments of chemotherapies and somatostatin analogues that had been previously recommended by the health authorities.
Methods
The OPALINE study assessed the efficacy of everolimus and sunitinib in terms of survival, disease progression and tolerance. Patients (N = 144) were enrolled from May 2015 to September 2017, and their disease characteristics were analyzed from diagnosis to 2 years post-enrollment.
Results
At inclusion most patients had comorbidities, and about 95% presented metastases. Patients received on average 3.2 lines of treatment from diagnosis to inclusion and two lines throughout the 2-year follow-up. Seventy-nine patients (59.0%) received at least one targeted therapy (TT) during their care path. For these patients, the overall survival (OS) was approximatively 176.5 months (95% CI: 97.2-not evaluable), with a 2-year survival rate estimated at 93.6% (SD 2.6%). Similar survival rates were observed whether the TTs were prescribed sooner or later in the treatment path. The main reasons for discontinuation of TTs were disease progression (54 patients) and adverse events (26 patients). Most patients receiving TTs did not change their dose during the follow-up reflecting the good treatment tolerability over time. No new safety alert was reported for everolimus and sunitinib during this study.
Conclusion
Given their good tolerance and positive impact on estimated OS, the two TTs have an important role to play in the care path of patients with pNETs.
ClinicalTrials.gov National Clinical Trial Number
Keywords: Ambispective study, Everolimus, Pancreatic neuroendocrine tumor, pNET, Sunitinib, 2-Year morbi-mortality
Key Summary Points
| Why carry out this study? |
| Two targeted therapies (TTs), everolimus and sunitinib, demonstrated efficacy in the treatment of patients with unresectable pancreatic neuroendocrine tumors (pNETs) in randomized placebo-controlled phase III clinical trials based on progression-free survival. |
| In its opinions of 2011 and 2012 concerning extension of the indication respectively for sunitinib (Sutent®, Pfizer) and everolimus (Afinitor®, Novartis Pharma), the French health authority (Haute Autorité de Santé) requested data assessing the impact compared with other treatments of sunitinib and everolimus from a pNET registry. |
| OPALINE study was conducted to assess in a real-world setting the use of everolimus and sunitinib in the treatment of progressive, unresectable or metastatic well-differentiated pNETs in terms of survival, disease progression and tolerance during 2-year follow-up period. |
| What was learned from the study? |
| OPALINE study provided a real-world picture of the care of patients with pNETs in France between 2015 and 2019 and described the place of TTs in their care path. |
| Given their good tolerance and positive impact on the estimated overall patient survival, everolimus and sunitinib have an important and specific role to play in the care path of patients with pNETs. |
Introduction
Pancreatic neuroendocrine tumors (pNETs) are a group of uncommon tumors arising from hormone-producing cells in the pancreas. When adjusted to the world population, pNETs only represent a small proportion (2.8%) of pancreatic cancers [1–5], but the prevalence is higher, being estimated at 10% of pancreatic cancers because of a more prolonged patient survival than for pancreatic adenocarcinoma. Patient management varies according to the degree of differentiation based on the World Health Organization (WHO) 2019. Neuroendocrine tumors (NETs) are classified as well-differentiated tumors (grade 1–3) vs. poorly differentiated carcinomas (grade 3) [6]. The survival rates for well-differentiated pNET (85%) have been reported to range between 38 and 43% at 5 years [2, 7, 8]. The decision criteria for patient management are tumor extension, tumor activity and symptoms. In France, all patient cases are discussed in expert multidisciplinary meetings of the RENATEN network, and inclusion in therapeutic trials is always considered. Depending on tumor aggressiveness the recommended French guidelines for first-line treatments of patients with progressive or metastatic well-differentiated pNETs are somatostatin analogues and chemotherapy. The recommended second-line treatments include somatostatin analogues, chemotherapy, everolimus, sunitinib and locoregional therapies. [177Lu-DOTA(0),Tyr3] Octreotate somatostatin receptor-targeted radionuclide therapy (Lu-DOTATATE) is approved for the treatment of gastroenteropancreatic neuroendocrine tumors including pNETS in Europe and the US, but was considered not to have sufficient clinical interest by the French health authorities [Haute Autorité de Santé (HAS)] to justify health insurance reimbursement in non-intestinal neuroendocrine tumors. Targeted therapies (TTs) are optional first-line treatments when chemotherapy is contraindicated [9].
In the years preceding the OPALINE study two TTs, sunitinib and everolimus, demonstrated efficacy in the treatment of patients with unresectable pNETs in phase III randomized clinical studies vs. placebo, based on the progression-free survival (PFS) criterion [10, 11]. In its opinions of 2011 and 2012, concerning extension of the indication respectively for sunitinib (Sutent®, Pfizer) and everolimus (Afinitor®, Novartis Pharma), HAS requested data assessing the impact compared with other treatments of sunitinib and everolimus from a pNET registry. Here, we describe the use of the TTs in a real-world setting, with regard to other therapies, in adult patients treated for a progressive unresectable or metastatic well-differentiated pNET in terms of efficacy, morbidity and mortality 2 years post-treatment initiation. The characteristics of the patients and disease at inclusion, PFS and 2-year overall survival (OS) rates were assessed as well as their tolerance to the study treatments (TTs and other treatments).
Methods
OPALINE study (NCT02264665) was a national, observational, descriptive, ambispective, multicenter study conducted in France.
Study Objectives
The primary objective of this observational study was to describe, in a real-world setting, the evolution of adult patients treated (TTs and other treatments) for a progressive unresectable or well-differentiated metastatic pNET in terms of morbi-mortality at 2 years. PFS and 2-year OS rates as well as the tolerance of the study treatments (TTs and other treatments) were assessed.
The secondary objective of this study was to describe the characteristics of the population of patients treated for progressive unresectable or well-differentiated metastatic pNETs.
Study Population
Physicians specialized in oncology, gastroenterology and endocrinology were contacted to participate in the study and consecutively enrolled patients according to the following inclusion criteria: adult patients (> 18 years), treated for histologically confirmed progressive unresectable or metastatic well-differentiated pNETs according to the judgment of the investigator with a TT (everolimus or sunitinib) or another treatment (chemotherapy, analogues of somatostatin, metabolic radiotherapy or interferon-alpha). Patients treated beyond the fourth line, those who had already received the TT in a previous line of treatment (rechallenged patient) and those with a poorly differentiated neuroendocrine carcinoma were excluded from the study. Patients were recruited over a period of approximately 28 months (from May 12, 2015, to September 18, 2017) and were followed for 24 months starting from their inclusion in the study. The therapeutic management of each patient was not modified by their participation in the study and followed the recommendations made during a Multidisciplinary Consultation Meeting in accordance with the recommendations of good practice. The follow-up visits were therefore carried out during the patient's usual consultations for tumor assessments. The reference population included patients who met the eligibility criteria and received at least one documented dose of TT or other treatment during the study prospective follow-up. The tolerance population included all the patients who received at least one documented dose of TT or other treatment during the prospective phase of the study.
Data Collection
Data were extracted from patient records collected during the usual patient consultations in the centers, i.e., at inclusion and during tumor assessment visits carried out every 2–3 months. The patient electronic records were collected in an electronic case report form (eCRF) and uploaded directly to the study database. In this study, both TTs, sunitinib and everolimus, were considered as one entity. Indeed, sunitinib is an oral multi-targeted kinase inhibitor and everolimus is an oral mammalian target of rapamycin (mTOR) inhibitor, and they have been evaluated in similar phases III trials [10, 11] that led to market authorization. Patient data were analyzed prospectively for 24 months, and retrospective history of treatment was collected. The retrospective analysis included the initial diagnosis of tumor and prior anti-cancer treatments and tolerance. The prospective analysis included tumor changes, treatment modifications after the inclusion in the study and tolerance. The characteristics of patients and pathology at the treatment initiation as well as data related to the treatment were also recorded and analyzed.
Overall and Progression-Free Survival Rates
The follow-up of patients was carried out according to investigators’ standard of care. The evaluations were carried out using computed tomography (CT) scan and/or magnetic resonance imaging (MRI). Radiological and clinical responses based on examinations to assess tumor development were collected to define the PFS and OS rate at 2 years. OS was defined by the time between the date of diagnosis and the death from any cause. PFS was defined by the time between treatment initiation (of the ongoing line at the time of inclusion in the study) and the date of the first evidence of progression of the disease or death from any cause during the main treatment received at inclusion.
Safety and Tolerance
Treatment tolerance (TTs and other treatments) was evaluated during the study period prospectively or retrospectively from the date of initiation of treatment. The evaluation included the treatment discontinuations and their reason, adverse events (AEs, grading according to Common Terminology Criteria for Adverse Events version 4.0 of May 2009) as well as their possible complications observed during the study. An AE starting after the last treatment intake could be attributed to treatment if it occurred up to 28 days after stopping the drugs or the last assessment. If the investigator considered an AE as related to a given treatment, this AE was included in this treatment group.
Statistical Analysis
Quantitative variables were described using counts, mean and median, standard deviation (SD), interquartile range [Q1–Q3], minimum and maximum as well as the number of missing data. Estimates of OS and PFS were measured by the Kaplan-Meier method. Due to its nature, the study exhibited truncations and left censors. A Kaplan-Meier estimator was used to estimate the distribution of a left-truncated and right-censored estimator [12]. The median survival was estimated and presented with its 95% confidence interval (CI). Statistical analyses were performed with the SAS software version 9.4.
Ethics Statement
This study was conducted according to the guidelines of the Declaration of Helsinki of 1964 and its later amendments. All participants provided informed consent to participate in the study. This study does not fall within the scope of the program law no. 2006-450 of April 18, 2006, for research or in law no. 2004-806 of August 9, 2004, article 88 chapter II article L1121-1; the project was therefore not submitted to the National Agency for the Safety of Medicines and Health Products (Agence National de Sécurité du Médicament, ANSM) or to a French ethics committee (Comité de Protection des Personnes, CPP). However, the investigators were paid to participate in this study. This study was thus submitted to the French National Medical Council (Conseil National de l’Ordre des Médecins, CNOM). This study required the collection and processing of personal data for the purpose of health research, therefore falling under Chapter IX of the Data Protection Act of January 6, 1978, as amended. An opinion from the advisory committee on the processing of information in the field of health research (Comité Consultatif sur le Traitement de l’Information en matière de Recherche dans le domaine de la Santé, CCTIRS) was therefore requested as well as an authorization from the Commission Nationale Informatique et Libertés (CNIL).
Results
Physician and Patient Disposition
The study lasted 54 months. Among the 72 centers contacted, 35 physicians in 35 centers agreed to participate in the study. The profiles of the active (those who have included at least one patient) and non-active doctors were relatively similar, in terms of specialty, type of exercise (public, private, mixed), structure (hospital, city doctor/clinic, anti-cancer center) and the estimated number of patients with pNET. Their profiles are summarized in Fig. 1. The mean number of patients seen for pNET at the time of inclusion was estimated at 16 patients per active center.
Fig. 1.
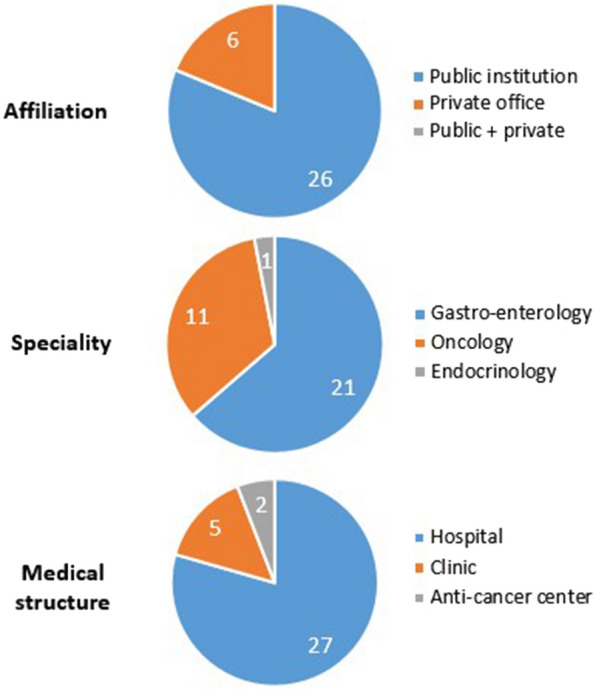
Physician population. Profile of the active physicians, per specialty, affiliation and medical structure. Physicians actively participating in the study were mainly gastroenterologists or oncologists, mainly affiliated to public institutions and more particularly practicing in hospital structures
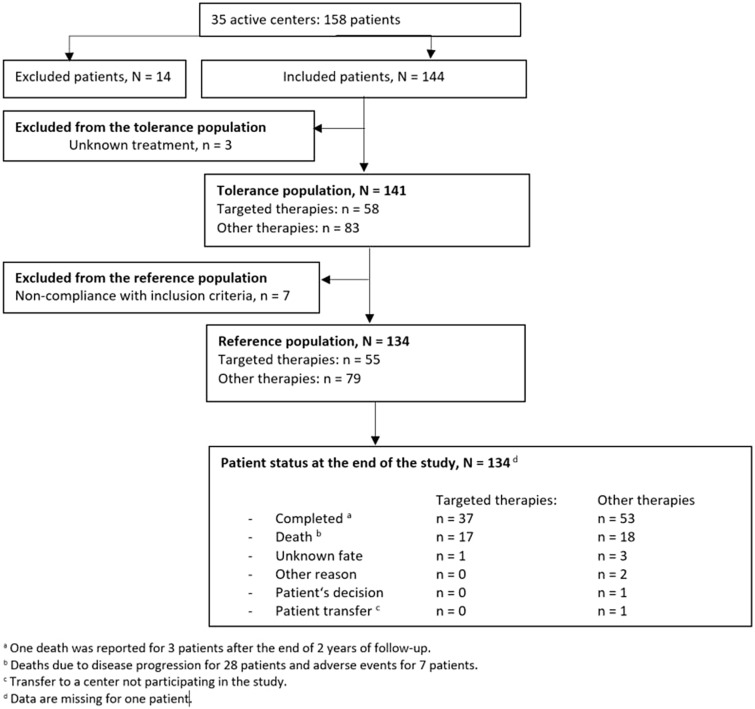
A total of 144 patients were included in the study. A summary of the patient dispositions is shown in Fig. 2. The patients were divided into two groups, patients who received at least one line with a TT during their care path (TT group) and those who did not receive any line with a TT (OT group). OT patients received chemotherapy, somatostatin analogues, metabolic radiotherapy or interferon alpha during their treatment path.
Fig. 2.
Patient population
Demographics and Characteristics of the Disease
The patients included in the reference population were aged between 34 and 93 [mean age: 63.4 (SD 12.6)] years, and the sex ratio was 1.5 (Table 1). Overall, the patients had Eastern Cooperative Oncology Group (ECOG) performance index ≤ 1 (90.5% of patients). Ninety-three (69.4%) patients presented at least one comorbidity or history, and most patients had between one and three comorbidities (data not shown) such as lung disease, heart failure, stroke, arterial hypertension, diabetes and hypercholesterolemia. The most common comorbidities were generally treated (> 70% of patients) (Table 2).
Table 1.
Patient characteristics at inclusion (reference population)
| Number of patients | |
|---|---|
| Age at inclusion (years) | |
| Mean (SD) | 63.4 (12.6) |
| Median [Q1; Q3] | 65.3 [54.6; 71.7] |
| Gender | |
| Men, n (%) | 81 (60.4%) |
| Women, n (%) | 53 (39.6%) |
| At least one comorbidity or history, n | 134 |
| Present, n (%) | 93 (69.4%) |
| Arterial hypertension, n | 134 |
| Present, n (%) | 48 (35.8%) |
| Treated, n (%) | 45 (93.8%) |
| Diabetes, N | 134 |
| Present, n (%) | 48 (35.8%) |
| Untreated, n (%) | 45 (93.8%) |
| Hypercholesterolemia, N | 134 |
| Present, n (%) | 14 (10.4%) |
| Treated, n (%) | 10 (71.4%) |
| Renal function (clearance), N | 99 |
| < 30 ml/min | 0 (0.0%) |
| Between 30 and 60 ml/min | 11 (11.1%) |
| > 60 ml/min | 88 (88.9%) |
| Other comorbidities or histories of interesta, N | 134 |
| Present, n (%) | 76 (56.7%) |
Patients’ age, gender, comorbidities and history were analyzed at the time of their inclusion in the study. Most patients were men, aged of 63 years on average. Most had comorbidities at diagnosis, mainly hypertension, diabetes and high cholesterol
Q1, first quartile; Q3, third quartile; SD, standard deviation
aIncluding stroke, left ventricular ejection fraction (when available), pulmonary disease, heart failure and coronary insufficiency
Table 2.
Disease characteristics at diagnosis and at inclusion (reference population)
| Number of patients | |
|---|---|
| Type of tumor at diagnosis, N | 134 |
| Localized, n (%) | 40 (29.9%) |
| Metastatic, n (%) | 94 (70.1%) |
| Pathology characteristics at inclusion, N | 134 |
| Presence of metastases, n (%) | 127 (94.8%) |
| Localizationa | |
| Liver | 120 (89.6%) |
| Hepatic invasion | |
| ≤ 50% | 102 (76.1%) |
| > 50% | 18 (13.4%) |
| Peritoneum | 19 (14.2%) |
| Bone | 18 (13.4%) |
| Adenopathy | 18 (13.4%) |
| Lung | 5 (3.7%) |
| Functional symptomatology | |
| Present, n (%) | 13 (9.7%) |
| Gastrinoma, n (%) | 2 (1.5%) |
| Insulinoma, n (%) | 4 (3.0%) |
| Glucagonoma, n (%) | 2 (1.5%) |
| VIPoma, n (%) | 1 (0.7%) |
| Others, n (%) | 4 (3.0%) |
Metastases were frequent in the study participants, and they were mainly located in the liver at the time of inclusion in the study. Functional symptoms, mainly insulinoma, could be found in a few patients at the time of inclusion in the study
The percentages were calculated based on the total number of patients
aAt the initiation of the current treatment line or initiated at inclusion
Metastatic tumors were common at diagnosis (70.1% patients) and at inclusion (about 95% of patients). The tumor assessment at inclusion showed that metastases were generally located in one or two sites, liver being the most common site [120 (89.6%) patients] (Table 2). Less than 10% of patients had functional symptoms, insulinoma being the most frequent.
Most of the study participants received locoregional cancer treatments, mainly primary surgery [60 (44.8%) patients], liver metastasis surgery [26 (19.4%) patients] and (chemo)embolization [25 (18.7%) patients], regardless of the group considered (TT or OT) (Table 3). At the time of inclusion, the Ki-67 proliferation index was ≤ 20% for 96/134 patients. A lower number of patients had a determined mitotic index (53/134 patients at inclusion) and had always < 20 mitoses per field. Some patients benefited from a review by the TenPath network (42 patients, information not provided for 32 patients), and overall, the examinations had been performed approximatively 18 months and less than a year preceding the patient inclusion in the study for Octreoscan® (67 patients) and fluoro-deoxy-glucose positron emission tomography (PET) scan (35 patients), respectively (Table 3).
Table 3.
Previous locoregional treatments and tests (reference population)
| Number of patients | |
|---|---|
| Previous locoregional treatments at inclusion | |
| N | 134 |
| Received, n (%) a | 75 (56.0%) |
| Primary surgery | 60 (44.8%) |
| Surgery for liver metastases | 26 (19.4%) |
| Chemoembolization/embolization | 25 (18.7%) |
| Radiofrequency ablation | 11 (8.2%) |
| Other treatments | 4 (3.0%) |
| Recent tests at inclusion | |
| Octreoscan®, N | 134 |
| Recently performed, n (%) | 67 (50.0%) |
| Not reported, n (%) | 7 (5.2%) |
| Seniority (months)a, N | 67 |
| Mean (SD) | 18.5 (20.3) |
| Median [Q1; Q3] | 10.4 [4.3; 25.1] |
| Fixation, n | 58 |
| PET scan, N | 134 |
| Recently performed, n (%) | 35 (26.1%) |
| Not reported, n (%) | 7 (5.2%) |
| Seniority (months) a, N | 35 |
| Mean (SD) | 9.52 (13.3) |
| Median [Q1; Q3] | 4.5 [1.1; 11.9] |
| Fixation, n | 29 |
| Ki-67 value, N | 134 |
| Recently obtained, n (%) | 108 (80.6%) |
| < 3%, n (%) | 22 (16.4%) |
| [3–20%], n (%) | 74 (55.2%) |
| > 20%, n (%) | 12 (9.0%) |
| ≤ 10%, n (%) | 78 (58.2%) |
| > 10%, n (%) | 30 (22.4%) |
| Mitotic index, N | 134 |
| Recently obtained, n (%) | 53 (39.6%) |
| < 2 mitoses per field, n (%) | 17 (12.7%) |
| [2–20] mitoses per field, n (%) | 36 (26.9%) |
| Reviewed by TenPath, N | 134 |
| Recently obtained, n (%) | 42 (31.3%) |
| Not reported, n (%) | 32 (23.9%) |
Most of the study patients had received locoregional cancer treatments [mainly primary surgery, liver metastasis surgery, and (chemo)embolization]. The Ki-67 proliferation index was graded ≤ 20% for most of the patients who had a recent Ki-67 index analysis at the time of inclusion. A lower number of patients had a determined mitotic index which was always < 20 mitoses per field. Some patients benefited from a review TenPath network, an Octreoscan® and/or a PET scan in the 2 years preceding their inclusion in the study
aPercentage calculated on the number of patients who received previous locoregional treatment
Q1, first quartile; Q3, third quartile; PET, positron emission tomography; SD, standard deviation
Treatments Prior to Inclusion
At the time of their enrollment in the study, most patients (65.7%) had received at least one previous treatment line. The study patients had received on average 3.2 (SD 1.7) lines before their inclusion in the study. Only 46 (34.3%) patients initiated their anti-cancer treatment at inclusion in the study (Table 4). These previous lines of treatment included mainly chemotherapy (67.0% patients), followed by omatostatin analogues (46.6%) and TT (30.7%). The reason for discontinuation of these previous treatments was mainly disease progression (about 67% of patients for TT, 56% for chemotherapy and up to 90% of patients for somatostatin analogue), followed by scheduled discontinuation and AE. Discontinuation due to AE was mostly reported for previous lines with TTs [9 (33.3%) patients]. In the patients who had received multiple lines of treatment before inclusion in the study, the first line of treatment had been mainly chemotherapy (53/88 patients) followed by somatostatin analogues (27 patients); only 8 patients had a TT as first line of treatment before their inclusion in the study. Among patients who received a second line of treatment before their inclusion in the study, chemotherapy (15/40 patients) and TT (15 patients) were the mostly used for this second line. For patients who had a third line of treatment before their inclusion in the study, an equivalent proportion of patients received a TT or chemotherapy (9 patients and 8 patients, respectively, out of 24 patients). Disease progression was the main reason for discontinuing the different treatments regardless of the treatment line.
Table 4.
Previous treatment lines (reference population)
| Number of treatment lines per patient | |
|---|---|
| N | 134 |
| 0, n (%) | 46 (34.3%) |
| 1, n (%) | 48 (35.8%) |
| 2, n (%) | 16 (11.9%) |
| 3, n (%) | 24 (17.9%) |
| Patients who received at least one line of treatment | |
|---|---|
| Per rank, N | 134 |
| No previous line, n (%) | 46 (34.3%) |
| 1st line, n (%) | 88 (65.7%) |
| 2nd line, n (%) | 40 (29.9%) |
| 3rd line, n (%) | 24 (17.9%) |
The treatment lines received before inclusion in the study were analyzed retrospectively. The majority of patients had already received a previous treatment line
Treatments at Inclusion
Treatments at inclusion were everolimus (32 [58.2%] patients) and sunitinib (23 [41.8%] patients) in patients initiating TT. The other treatments at inclusion were mainly chemotherapy [temozolomide 23 (29.1%) patients, capecitabine 21 (26.6%) patients, 5-fluorouracil 15 (19.0%) patients, streptozocin 10 (12.7%) patients] and analogues of somatostatin [lanreotide 23 (29.1%) patients and octreotide 5 (6.3%) patients]. TTs were more commonly prescribed as monotherapy (in > 70% of patients) at a dose in line with the summary of product characteristics. At initiation, sunitinib dose was 37.5 mg/day in accordance with recommendations (for 87% of patients), whereas a reduced dose of 25 mg/day was given to 13% of patients. The initiation dose of everolimus was 10 mg (for 84.4%) and reduced to 5 mg (15.6%). When prescribed in combination, it was with analogues of somatostatin.
Treatments During the Entire Care Path
The treatment was modified or stopped during the 2 years of prospective follow-up for 112 patients (83.6% of the reference population). Patients received two lines of treatment during the study follow-up period. Therefore, up to 79 (59.0%) patients received at least one TT during their care path, which was mainly administered in the second (42 patients), third (26 patients), or fourth line (24 patients) (Table 5). For those patients, the median duration of the TT was 18 months. The main reasons for discontinuation were the same regardless of the period considered (disease progression and AE). The median duration of the TT decreased with the rank of treatment line, where the concerned TT was administered [from 24 (7; 48) months for the first line (N = 15) to 5 (2; 16) months for the fourth line (N = 24)]. The main reasons for discontinuation of TTs were disease progression (54 patients) and AEs (26 patients), regardless of the treatment line (Table 5).
Table 5.
Use and reasons for discontinuation of the main treatments (targeted therapies and other treatments) over the entire care path (according to the main treatment lines) (reference population)
| Number of patients who received at least one line of targeted therapy | ||||
| N | 134 | |||
| Targeted therapy | 79 (59.0%) | |||
| None | 55 (41.0%) | |||
| Main treatments | 1st line | 2nd line | 3rd line | 4th line |
| N | 134 | 110 | 79 | 54 |
| Targeted therapies | 16 | 42 | 26 | 24 |
| Chemotherapy | 74 | 40 | 37 | 20 |
| SSA | 44 | 19 | 13 | 6 |
| Metabolic radiotherapy | 0 | 3 | 1 | 4 |
| Others | 0 | 6 | 2 | 0 |
| Duration of the targeted therapy (months) | ||||
| N | 15 | 41 | 26 | 24 |
| Mean (SD) | 28.8 (24.8) | 20.4 (17.4) | 11.2 (10.5) | 8.8 (9.7) |
| Median (Q1; Q3) | 24 [7; 48] | 18 [8; 26] | 10 [3; 14] | 5 [2; 16] |
| Overall reasons for discontinuation, regardless of the therapy | ||||
| N | 123 | 94 | 68 | 44 |
| Disease progression | 70 (56.9%) | 61 (64.9%) | 33 (48.5%) | 22 (50.0%) |
| AE | 9 (7.3%) | 8 (8.5%) | 18 (26.5%) | 8 (18.2%) |
| Death | 5 (4.1%) | 1 (1.1%) | 0 (0.0%) | 3 (6.8%) |
| Scheduled discontinuation | 17 (13.8%) | 7 (7.5%) | 2 (2.9%) | 0 (0.0%) |
| Physician choice | 15 (12.2%) | 14 (14.9%) | 12 (17.7%) | 11 (25.0%) |
| Patient choice | 2 (1.6%) | 3 (3.2%) | 3 (4.4%) | 0 (0.0%) |
| Others | 5 (4.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Main reasons for discontinuation of the targeted therapiesa | ||||
| N | 16 | 42 | 26 | 24 |
| Disease progression | 9 | 24 | 9 | 12 |
| AE | 2 (16.7%) | 5 (11.9%) | 12 (46.2%) | 7 (29.2%) |
Use and reasons for discontinuation of the main treatments over the entire care path. Throughout the 2 years of prospective follow-up study period, a large proportion of patients received the targeted therapies, which were mainly administered in the second or third line. Patients rather received chemotherapy or a somatostatin analogue as first-line treatment. During the study follow-up period, the main reasons for discontinuation were disease progression, scheduled discontinuation or physician’s decision, regardless of the therapy or the treatment line. For the targeted treatments, the main reasons for discontinuation were disease progression and AE
aThe percentages are calculated based on the number of patients receiving the targeted therapy
AE, adverse event; Q1, first quartile; Q3, third quartile; SSA, somatostatin analogue
All treatments combined, patients largely received chemotherapy (74 patients) or somatostatin analogues (44 patients) compared to TTs in first line. Overall, the reasons for treatment discontinuation over the entire care path were primarily disease progression (up to 70 patients), scheduled discontinuation (up to 17 patients) or physician decision (up to 15 patients), regardless of the therapy or the treatment line, for treatments received in first to fourth line (Table 5).
Follow-Up, Progression-Free Survival and Overall Survival Rates
The follow-up of patients was carried out according to investigators’ standard of care. Most of the patients (113 patients) received 3–5 examinations per year. The reported evaluations were mainly carried out using CT scan and/or MRI (> 50% of patients). Survival analyses were carried out using the initiation date of the treatment line (in progress or initiated at the inclusion visit) as the starting date.
In the reference population, the median OS, using the date of diagnosis as starting date, was estimated at 46.3 months [95% CI 32.8–not evaluable (NE)], and the 2-year survival rate was estimated at 68.7% (SD 4.9%; 95% CI 58.0–77.1%). For the 79 patients receiving at least one TT during their care path, the OS was 176.5 months (95% CI 97.2–NE). The median OS was 128.4 months (95% CI 46.7–NE) in patients who did not receive a TT (non-significant difference, test score p = 0.1946). In TT patients who received a TT in first or second line (52 patients), the median OS was estimated at 190.8 months (95% CI 97.2–NE); when the TT was prescribed in the third line or later (27 patients), the median OS was estimated at 103.3 months (95% CI 78.0–NE) (non-significant difference, test score p = 0.2258). In this TT population, the 2-year survival rate was estimated at 93.6% (SD 2.6%) and was maintained across treatment lines: the 2-year survival rate was estimated at 95.7% (95% CI 83.9–98.9%) and 95.1% (70.1–99.3%) when the TT was prescribed in first or second line and in third line or later, respectively.
In patients who received at least one TT, the median PFS duration was estimated at 9.1 months (95% CI 6.6–15.5) (Table 6).
Table 6.
Analysis of the progression-free survival in patients who received at least one line with a targeted therapy throughout the entire care path (reference population)
| Progression-free survival in patients who received at least one targeted therapy | |
| N | 79 |
| Data not available | 0 |
| Number of events | 44 |
| Progression | 43 |
| Death | 1 |
| Censored data | 35 |
| Unknown fate | |
| Other reasons | 35 |
| Median (months) (95% CI) | 9.1 (6.6–15.5) |
| Minimum survival (months) | 0.9 |
| Maximum survival (months) | 87.0 |
The PFS was defined by the time between treatment initiation and the date of the first evidence of progression of the disease or death from any cause during the main treatment received at inclusion. Patients receiving targeted therapies were generally advanced in their care path regarding the number of previous treatment lines
CI, confidence interval
Safety
Out of the 141 patients included in the tolerance population, 119 (84.4%) reported a total of 816 AEs. Nine AEs reported for eight patients (7 during the study period and 1 after the 2-year follow-up period) resulted in death. When AEs were analyzed according to the treatment taken at least once during the study, the most common AEs suspected to be related to the treatment were known treatment side effects. For everolimus, the most common AEs were inflammation of the mucosa (8 patients) and stomatitis (5 patients). For sunitinib the common AEs included diarrhea (9 patients), neutropenia (8 patients), upper abdominal pain (4 patients) and dysgeusia (4 patients). In patients receiving chemotherapy, the most common AEs were nausea (11 patients), peripheral neuropathy (9 patients), thrombopenia (9 patients) and diarrhea (8 patients). During treatment with somatostatin analogues, the most common AE was diarrhea (8 patients) (Table 7).
Table 7.
Adverse events suspected of being related to the study treatment, by received treatment (at least once during the study), SOC and PT for the most frequent treatments (everolimus, sunitinib, chemotherapy and somatostatin analogue) (reported by > 5% patients for at least one treatment) (tolerance population)
| SOC PT n (%) |
Everolimus N = 52 |
Sunitinib N = 36 |
CT N = 82 |
SSA N = 65 |
|---|---|---|---|---|
| At least one related AE | 31 (59.6%) | 24 (66.7%) | 48 (57.8%) | 13 (20.0%) |
| General disorders and anomalies at the administration site | 16 (30.8%) | 8 (22.2%) | 25 (30.1%) | 2 (3.1%) |
| General deterioration of the state of health | 0 (0.0%) | 0 (0.0%) | 2 (2.4%) | 0 (0.0%) |
| Inflammation of the mucosa | 8 (15.4%) | 2 (5.6%) | 2 (2.4%) | 0 (0.0%) |
| Peripheral edema | 5 (9.6%) | 1 (2.8%) | 2 (2.4%) | 0 (0.0%) |
| Gastrointestinal disorders | 9 (17.3%) | 12 (33.3%) | 20 (24.1%) | 10 (15.4%) |
| Diarrhea | 1 (1.9%) | 5 (13.9%) | 8 (9.6%) | 8 (12.3%) |
| Nausea | 0 (0.0%) | 0 (0.0%) | 11 (13.3%) | 2 (3.1%) |
| Vomiting | 2 (3.8%) | 2 (5.6%) | 6 (7.2%) | 1 (1.5%) |
| Upper abdominal pain | 2 (3.8%) | 4 (11.1%) | 1 (1.2%) | 0 (0.0%) |
| Stomatitis | 5 (9.6%) | 1 (2.8%) | 0 (0%) | 0 (0.0%) |
| Gastroesophageal reflux | 0 (0.0%) | 2 (5.6%) | 2 (2.4%) | 0 (0.0%) |
| Blood and lymphatic system disorders | 8 (15.4%) | 10 (27.8%) | 13 (15.7%) | 1 (1.5%) |
| Neutropenia | 1 (1.9%) | 8 (22.2%) | 6 (7.2%) | 0 (0.0%) |
| Thrombopenia | 3 (5.8%) | 3 (8.3%) | 9 (10.8%) | 1 (1.5%) |
| Anemia | 3 (5.8%) | 2 (5.6%) | 3 (3.6%) | 0 (0.0%) |
| Nervous system disorders | 5 (9.6%) | 4 (11.1%) | 20 (24.1%) | 3 (4.6%) |
| Dysgeusia | 2 (3.8%) | 4 (11.1%) | 3 (3.6%) | 2 (3.1%) |
| Peripheral neuropathy | 0 (0.0%) | 0 (0.0%) | 9 (10.8%) | 0 (0.0%) |
| Paresthesia | 0 (0.0%) | 0 (0.0%) | 6 (7.2%) | 0 (0%) |
| Infections and infestations | 5 (9.6%) | 0 (0.0%) | 5 (6.0%) | 0 (0.0%) |
| Skin and subcutaneous tissue disorders | 8 (15.4%) | 11 (30.6%) | 5 (6.0%) | 0 (0.0%) |
| Hand-foot syndrome | 0 (0.0%) | 4 (11.1%) | 1 (1.2%) | 0 (0.0%) |
| Change in hair color | 0 (0.0%) | 3 (8.3%) | 0 (0.0%) | 0 (0.0%) |
| Investigations | 4 (7.7%) | 4 (11.1%) | 4 (4.8%) | 2 (3.1%) |
| Weight loss | 3 (5.8%) | 2 (5.6%) | 1 (1.2%) | 1 (1.5%) |
| Metabolism and nutrition disorders | 8 (15.4%) | 2 (5.6%) | 7 (8.4%) | 0 (0.0%) |
| Respiratory, thoracic and mediastinal disorders | 6 (11.5%) | 3 (8.3%) | 0 (0.0%) | 0 (0.0%) |
| Epistaxis | 1 (1.9%) | 2 (5.6%) | 0 (0.0%) | 0 (0.0%) |
| Hemoptysis | 0 (0.0%) | 2 (5.6%) | 0 (0.0%) | 0 (0.0%) |
| Kidney and urinary tract disorders | 3 (5.8%) | 0 (0.0%) | 4 (4.8%) | 0 (0.0%) |
AE, adverse event; CT, chemotherapy; PT, preferred term; SOC, system organ class; SSA, somatostatin analogue
A total of 155 AEs reported for 66 patients were SAEs. Sixty-four AEs reported for 38 patients led to permanent treatment discontinuation; 38 AEs and 12 SAEs suspected of being treatment-related, and reported for 22 and 8 patients, respectively, led to permanent treatment discontinuation. Grade 3 or ≥ 4 SAEs suspected of being related to the treatment have been reported for the four treatments of interest (everolimus, sunitinib, chemotherapy, analogues of somatostatin). For everolimus treatment, four patients had grade 3 AEs (algoneurodystrophy, pneumonitis and fever), one patient had one grade ≥ 4 AE (venous thrombosis), and one patient had four events of grade 3 (mucositis, erythema, urticaria and edema). All these events led to permanent discontinuation of the treatment except for venous thrombosis (unknown) and fever (dose reduction). For sunitinib treatment a grade 3 hepatic cytolysis led to permanent discontinuation of the treatment. For chemotherapy treatment, grade 3 and grade ≥ 4 AEs were reported for two and four patients, respectively. The grade 3 AE was physical health deterioration; the grade ≥ 4 AEs were grade 4 heart failure (one patient), grade 4 infectious endocarditis (one patient), grade 4 water inflation and grade 5 heart failure reported in the same patient. All of these events led to discontinuation of the treatment except for infectious endocarditis and water inflation (without consequence on treatment). For analogues of somatostatin, grade 3 vomiting, without consequence for the ongoing treatment, was reported in one patient.
A total of 38 patients died during the 2-year study follow-up period. The vast majority of deaths (31 patients, 81.6% of deaths) was due to disease progression and was not considered as related to the treatment. The remaining deaths (7 patients, 18.4% of deaths) occurred following an AE that occurred during the study period and were considered as unrelated to the treatment, except one event, a multifactorial acute renal failure, which was suspected to be related to the chemotherapy received by the patient as first-line treatment. No fatal AEs occurred in patients treated with TT.
Discussion
The OPALINE study provides a picture of the care path and describes the place of TTs in a real-world setting of patients with pNETs in France between 2015 and 2019. Thus, chemotherapies and analogues of somatostatin remained the main treatments used as first line (55% and 40%, respectively), unlike the TTs (12% of patients) that were more frequently used in subsequent therapeutic lines [13]. Despite their relatively recent introduction in France at the time of the study initiation, the uncommon use of TTs first at baseline suggests that there is no “fashion effect” behind the use of these treatments and that their first-line use is reasonable in the context of pNETs.
The use of TTs was then in line with the recommendations of expert societies (Thesaurus National de Cancerologie Digestive, TNCD, European Neuroendocrine Tumor Society, ENETS) [9, 15], which restrict their use initially after chemotherapy or somatostatin analogues. However, their use in first line is better documented now, and more patients may receive TT with a favorable profile (low burden disease, Ki-67 < 10%, low progression curve) [16].
This study does not allow comparison between the two TTs or clearly defining their place regarding chemotherapy. Indeed, this study is a reflection of real life, where decisions are made on a case-by-case basis in a multidisciplinary consultation meeting. The only randomized sequential trial in pNETs that is currently underway is the European Neuroendocrine Tumors Society/Spanish Task Force Group for Neuroendocrine Tumors (GETNE) trial called SEQTOR (NCT02246127). The SEQTOR trial seeks to compare the efficacy and safety of everolimus followed by chemotherapy with streptozocin-fluorouracil (STZ-5FU) upon progression or the reverse sequence in advanced progressive pNETs and will allow better assessment of the impact of the first therapeutic sequence.
For the 79 (59.0%) patients who used at least one TT during their care path, the median OS time was estimated at approximatively 176.5 months. This is in line with the survival duration reported in the literature for pNET patients. In our study we show that the results obtained in terms of PFS (9.1 months in patients who took at least one TT) are worse when patients are treated in later lines. Indeed, the use of successive treatment lines leads to selection of the most resistant tumor clones [17]. In the OPALINE study, some patients received TT beyond the third line, with a markedly reduced efficacy in terms of PFS: the median duration of TT decreased significantly beyond the fourth line of treatment, from 24 to 4 months on average, underlying the better efficacy observed for treatment-naïve patients [16].
Treatment decision for advanced pNET depends on multiple factors including the extent of the disease and tumor characteristics (grade, Ki-67 status, morphology), tumor functionality, tumor biomarkers, tumor burden (large liver tumor load and presence of extrahepatic metastases), individual factors (comorbidities), prior treatment regimens and responses to them including side effects, progression rate and other symptoms [18]. Ki-67 is considered a crucial element of the decision in the OPALINE study, even if the cut-off of 10% separated the survival curves (data not shown) it was not possible to evaluate it according to the treatment because of low numbers per subgroup. A multidisciplinary approach is therefore crucial for the management of patients with rare diseases like pNET. Moreover, as treatment options increase, the potential to change the order of each treatment grows and the concept of individualized care becomes more relevant. The challenge is to be able to determine the right sequence strategy for each individual patient, with the difficulty of selecting not only the first line but also the subsequent lines of therapy. Although some ongoing clinical trials (e.g., SEQTOR) may answer some questions regarding the two therapies (chemotherapy vs. TT in the SEQTOR study), others in progress in GastroEnteropancreatic Neuroendocrine Tumors (GEP-NETs) are questioning the place of peptide receptor radionuclide therapy (PRRT) vs. other therapies (chemotherapy or everolimus) in the COMPOSE trial (NCT04919226), PRRT and everolimus in the COMPETE trial (NCT03049189), and PRRT and sunitinib in OCLURANDOM trial (NCT02230176). PRRT seems to present an interesting efficiency, but could not be evaluated in our study because of its unavailability in France for this indication [19]. The different clinical trials will not answer all the questions regarding other potential sequence combinations if all available therapies are taken into account. Additional research therefore is needed to evaluate possible correlations between different therapeutic sequences and survival and/or tolerance. For example, patients participating in the OPALINE study received on average 3.2 lines of treatment during their care path, and having a larger therapeutic arsenal available would have brought real added value.
As recently reported by another real-world study, management is generally personalized and dependent on the patient’s profile [14]. The best treatment sequence should be investigated to allow patients to benefit from a maximum of therapeutic lines. The care decision-making processes are then personalized, and it becomes complex and inappropriate to compare the effectiveness of different treatments. In this context, TTs (everolimus and sunitinib) really appear to be additional therapeutic options in the care of pNET patients. In addition, TTs are oral treatments that can be managed on an outpatient basis and as such offer a degree of comfort compared with treatments administered parenterally.
Although the selection criteria were not restrictive, the population analyzed in the OPALINE study remained comparable to clinical registry studies regarding age of the patients, their ECOG performance status and the incidence of metastases. In both cases (randomized clinical studies and OPALINE study), the proportion of patients with metastases was ≥ 90%. However, functional symptoms seem less frequent in the OPALINE study compared to clinical studies (< 10% vs. 24% for everolimus [11] and around 50% for sunitinib [10]). The treatment duration seemed longer in the OPALINE study compared with the clinical registry studies (median treatment duration of 18 months, with 57% of patients on TTs for at least 12 months in the OPALINE study vs. 9–10 months for everolimus and 4–5 months for sunitinib in clinical registry studies). In randomized clinical studies, the criteria for treatment discontinuation might be more strictly defined (objective evaluation criteria such as response evaluation criteria in solid tumors, RECIST) than in real-world settings where the clinical benefit of the patient is potentially more determinant. In addition, the median PFS estimated in OPALINE is similar to that reported in clinical registration studies. These results indicate a good tolerance of the TTs in real-world settings and a real benefit from their real-world use in a non-selected population. Indeed, as highlighted in the OPALINE study, the main reason for stopping treatment, in particular for the first lines of treatment, is still disease progression, which seems different from other recent series [20].
No particular safety signal was reported during this study. AEs reported for the TTs were expected events already documented in the respective summary of product characteristics for everolimus and sunitinib. During the follow-up period, final discontinuations for toxicity were relatively rare compared to those linked to disease progression. In this unselected population, the safety profile was comparable to that established during the registry studies using a selected population. The use of TTs did not compromise the establishment of new treatment lines after their discontinuation. The median duration of treatment with TT was longer (18 months) compared to that in the registry studies. These two observations denote a good tolerance of TTs and a real benefit from their use in current practice in an unselected population. Relatively few severe or serious events were reported that required permanent discontinuation of treatment. No death was suspected to be related to the TTs. The real-world safety profile of the TTs is therefore not very different from that observed in clinical registry studies.
The analysis of the characteristics of patients on inclusion showed that the enrolled population was relatively representative of the general population of patients with pNET: sex ratio of 1.5 [7], mainly non-functional (approximately 90% of patients) and presenting a high rate of metastases (approximately 95%, generally hepatic), with comorbidities frequently encountered in these patients (diabetes and hypertension). The results of this study can therefore be extrapolated to some extent to all patients with pNETs.
Nonetheless, the OPALINE study has several limitations due to its observational nature and the difficulty of recruiting a sufficient number of patients with a rare condition. This disease affects a limited number of people (portal for rare diseases and orphan drugs, ORPHA.net, ORPHA reference: 97,253, prevalence 1–5/10,000). This is reflected by the average number of patients seen in consultation by the recruiting centers (16 patients per center) and the average number of patients included in the study by each active center (4 patients per center). This study also included both patients initiating therapy at the time of inclusion and patients undergoing treatment at inclusion, regardless of the line of treatment. Therefore, the analyses of OS used the date of diagnosis as the starting date to eliminate any bias linked to treatment lines that the patients received before inclusion in the study. Finally, this study was carried out in reference centers for the management of pNETs leading to an inevitable selection bias.
Conclusion
OPALINE evaluated the use of TTs (everolimus and sunitinib) in a real-world setting in patients with progressive unresectable or metastatic well-differentiated pNETs. In this pNET population, the use of the TTs subsequent to treatment with chemotherapy or analogues of somatostatin was in line with French health recommendations. The safety profile of TTs over a longer follow-up period confirmed the one established during registration studies. Finally, the use of either one or the other of these treatments was compatible with the introduction of new treatment lines after their discontinuation. In conclusion, the TTs everolimus and sunitinib are key therapeutic agents in the context of “chronic” pathology that requires a personalized and progressive management.
Acknowledgements
We thank the study participants for their involvement in the study. Participating physicians: M. Baconnier, D. Smith, S. Dominguez, R. Coriat, O. Dubreuil, E. Terrebonne, B. Goichot, I. Cumin, T. Lecomte, C. Lombard-Bohas, K. Bouhier-Leporrier, G. Cadiot, B. Roques, G. Geslin, L. Venat Bouvet, P. L. Etienne, C. Poisson Ligeza, C. Petorin, C. Lepage, K. Bideau, F. Khemissa Akouz, J. F. Seitz, M. Mabro, T. Walter, J. Forestier, V. Hautefeuille, X. Caroli Bosc, E. Baudin, H. Brixi, J. Bertherat, P. Deguiral, J. M. Phelip, J. L. Legoux, F. Portales and E. Samalin-Scalzi. We thank every team member of the participating physicians. Scientific committee: C. Lombard-Bohas, D. Smith, C. Lepage and E. Vicaut.
Funding
This study, including the journal’s Rapid Service and Open Access Fees, was funded equally by Novartis and Pfizer.
Medical Writing, Editorial and Other Assistance
Editorial assistance in the preparation of this article was provided by Dr. Ange-Clarisse Dusabineza and Dr. Anna Tury of Keyrus Life Science. Support for this assistance was funded by Novartis and Pfizer.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility of the work as a whole and have given their approval for this version to be published.
Author Contributions
Denis Smith, Catherine Lombard-Bohas and Côme Lepage were OPALINE study investigators and members of the scientific committee. Sophie Dominguez, Romain Coriat, Olivier Dubreuil, Thierry Lecomte, Eric Baudin, Laurence Venat Bouvet, Emmanuelle Samalin and Bernard Goichot were OPALINE study investigators. Eric Vicaut was the OPALINE study statistician and member of the scientific committee. All the named authors read, reviewed and approved the final manuscript.
Prior Presentation
Intermediary results of the OPALINE study have previously been presented at the following congresses: 14th and 15th Annual ENETS Conferences (Barcelona, March 2017 and March 2018, respectively), JFHOD Congress 2017 and 2018 (France, March 2017 and March 2018, respectively) and Colloque Données de Santé en vie réelle 2017 et 2018 (France, June 2017 and June 2018, respectively).
Disclosures
Denis Smith is a member of the Advisory Board and consultant for Ipsen and Pfizer. Côme Lepage is a member of the Advisory Board for Bayer, Amgen, Novartis and Advanced Accelerator Applications (AAA). Eric Vicaut reports fees from Abbott, Amgen, Bristol Myers Squibb, Celgene, LFB, Pfizer and Sanofi. Romain Coriat reports fees for consulting from Bayer and Amgen; honoraria from AAA, Amgen, Novartis, Keocyt, Ipsen, Bayer, Sanofi and Merck; and grants from IPSEN. Olivier Dubreuil reports honoraria from Ipsen, Novartis and Pfizer. Thierry Lecomte reports personal fees from Amgen, Merck Serono, Servier and Sanofi Genzyme and non-financial support from Amgen and Servier. Eric Baudin reports personal financial interests, including serving on expert boards for Ipsen, Novartis, Advanced Accelerator Applications, Pfizer and Hutchinson Pharma and drug supply for Pfizer and AAA; institutional financial interests, including research grants from Novartis and HRA and serving as a principal investigator for Ipsen; non-financial interests, including serving as a past president of the French Group of Endocrine Tumors (GTE), a coordinator of the Neuroendocrine and French Adrenal Cancer Networks and on the advisory board of ENSAT and ENETS networks. Emmanuelle Samalin reports honoraria for consulting from Amgen, BMS, Pierre Fabre Oncology, Servier, Roche, Novartis, Pfizer and Sanofi. Alexandre Santos and Ségolène Bisot-Locard are Novartis employees. Odile Borie is a Pfizer employee. Bernard Goichot reports fees for consulting and non-financial support from Novartis, AAA, Ipsen and Pfizer outside the submitted work. Catherine Lombard-Bohas reports honoraria from Novartis. Sophie Dominguez and Laurence Venat Bouvet participated in the OPALINE study as investigators.
Compliance with Ethics Guidelines
This study was conducted according to the guidelines of the Declaration of Helsinki of 1964 and its later amendments. All participants provided informed consent to participate in the study. This study does not fall within the scope of the program law no. 2006–450 of April 18, 2006, for research or in law no. 2004–806 of August 9, 2004, article 88 chapter II article L1121-1; the project was therefore not submitted to the National Agency for the Safety of Medicines and Health Products (Agence National de Sécurité du Médicament, ANSM) or to a French ethics committee (Comité de Protection des Personnes, CPP). However, the investigators were paid to participate in this study. This study was thus submitted to the French National Medical Council (Conseil National de l’Ordre des Médecins, CNOM). This study required the collection and processing of personal data for the purpose of health research, therefore falling under Chapter IX of the Data Protection Act of January 6, 19786 as amended. An opinion from the advisory committee on the processing of information in the field of health research (Comité Consultatif sur le Traitement de l’Information en matière de Recherche dans le domaine de la Santé, CCTIRS) was therefore requested as well as an authorization from the Commission Nationale Informatique et Libertés (CNIL).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Denis Smith, Email: denis.smith@chu-bordeaux.fr.
Alexandre Santos, Email: alexandre.santos@novartis.com.
References
- 1.Delaunoit T, Neczyporenko F, Rubin J, Erlichman C, Hobday TJ. Medical management of pancreatic neuroendocrine tumors. Am J Gastroenterol. 2008;103:475–483. doi: 10.1111/j.1572-0241.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, Phan AT, Jehl V, Shah G, Meric-Bernstam F. Everolimus in advanced pancreatic neuroendocrine tumors: the clinical experience. Cancer Res. 2013;73:1449–1453. doi: 10.1158/0008-5472.CAN-12-3923. [DOI] [PubMed] [Google Scholar]
- 4.David M, Lepage C, Jouve JL, et al. Management and prognosis of pancreatic cancer over a 30-year period. Br J Cancer. 2009;101:215–218. doi: 10.1038/sj.bjc.6605150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727–1733. doi: 10.1093/annonc/mdn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bierley J, Gospodarowicz MK, Wittekind C. International union against cancer. TNM classification of malignant tumours. 8. Oxford: Wiley-Blackwell; 2017. [Google Scholar]
- 7.Lepage C, Bouvier AM, Phelip JM, Hatem C, Vernet C, Faivre J. Incidence and management of malignant digestive endocrine tumours in a well defined French population. Gut. 2004;53:549–553. doi: 10.1136/gut.2003.026401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepage C, Rachet B, Coleman MP. Survival from malignant digestive endocrine tumors in England and Wales: a population-based study. Gastroenterology. 2007;132:899–904. doi: 10.1053/j.gastro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 9.de Mestier L, Lepage C, Baudin E, et al. Digestive Neuroendocrine Neoplasms (NEN): French Intergroup clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, GTE, RENATEN, TENPATH, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR) Dig Liver Dis. 2020;52:473–492. doi: 10.1016/j.dld.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 11.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data, Springer: statistics for biology and health; 1997.
- 13.Espinosa-Olarte P, La Salvia A, Riesco-Martinez M, et al. Chemotherapy in NEN: still has a role? Rev Endocr Metab Disord. 2021;22:595–614. doi: 10.1007/s11154-021-09638-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berdelou A, Boige V, Arfi-Rouche J, et al. Not all patients with a pancreatic neuroendocrine tumour will benefit from all approved or recommended therapeutic options: a real-life retrospective study. Neuroendocrinology. 2017;105:26–34. doi: 10.1159/000446988. [DOI] [PubMed] [Google Scholar]
- 15.Pavel, M, Öberg K, Falconi M, et al. ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020;31:844–860. [DOI] [PubMed]
- 16.Raymond E, Kulke MH, Qin S, et al. Efficacy and safety of sunitinib in patients with well-differentiated pancreatic neuroendocrine tumours. Neuroendocrinology. 2018;107:237–245. doi: 10.1159/000491999. [DOI] [PubMed] [Google Scholar]
- 17.Landau DA, Tausch E, Taylor-Weiner AN, et al. Mutations driving CLL and their evolution in progression and relapse. Nature. 2015;526:525–530. doi: 10.1038/nature15395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Megdanova-Chipeva VG, Lamarca A, Backen A, et al. Systemic treatment selection for patients with advanced pancreatic neuroendocrine tumours (PanNETs) Cancers (Basel) 2020;12:1988. doi: 10.3390/cancers12071988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan S, Stamp E, Sammon C, Brabander T, de Herder W, Pavel M. Matching-adjusted indirect treatment comparison of [177Lu]Lu-DOTA-TATE, everolimus and sunitinib in advanced, unresectable gastroenteropancreatic neuroendocrine tumours: relative effectiveness of [177Lu]Lu-DOTA-TATE in gastroenteropancreatic neuroendocrine tumours. Eur J Cancer. 2021;16:5–13. doi: 10.1016/j.ejcsup.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panzuto F, Rinzivillo M, Fazio N, et al. Real world study of Everolimus in advanced progressive neuroendocrine tumors. Oncologist. 2014;19:966–974. doi: 10.1634/theoncologist.2014-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.