Abstract
Introduction
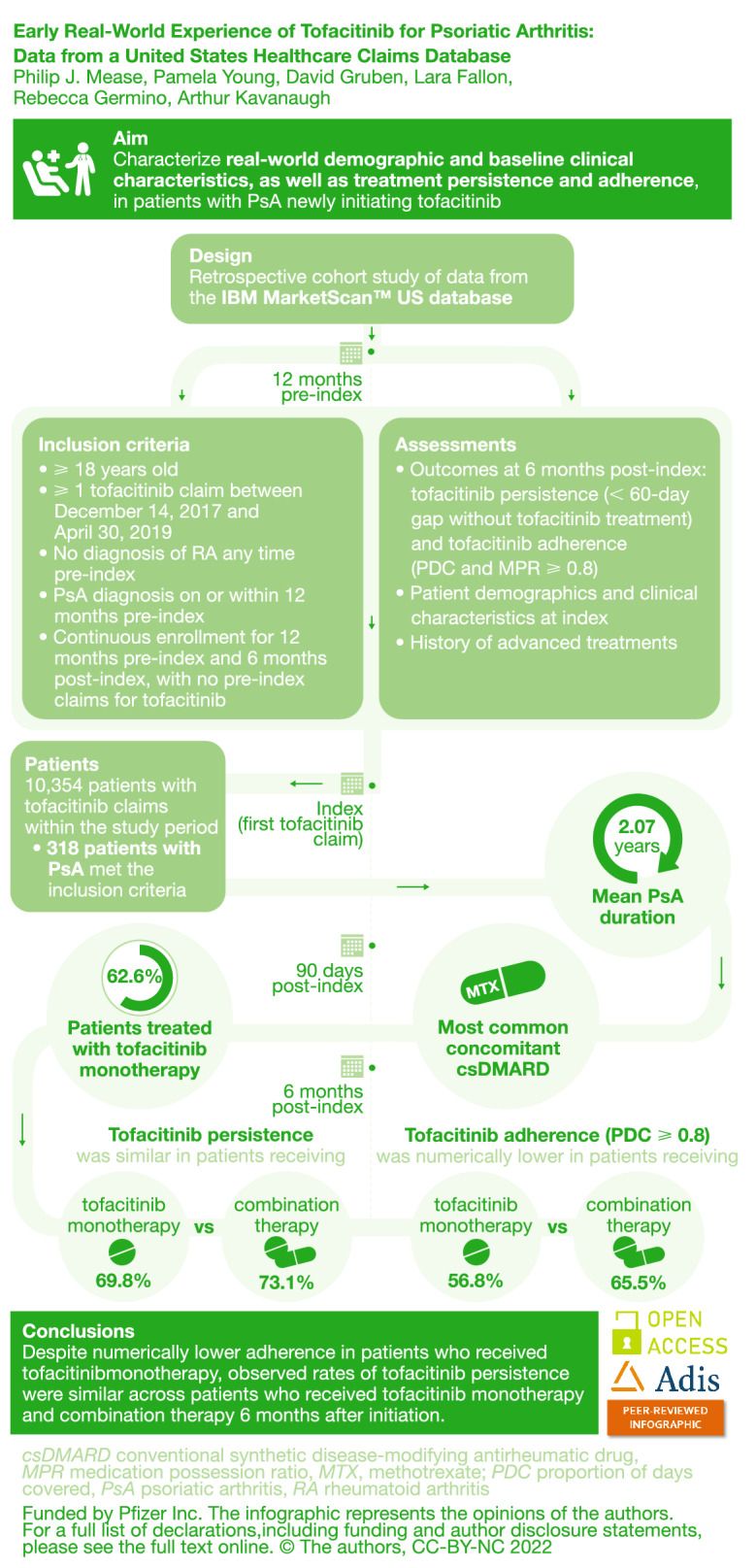
This study characterized real-world demographic and baseline clinical characteristics, as well as treatment persistence and adherence, in patients with psoriatic arthritis (PsA) who had newly initiated tofacitinib treatment.
Methods
This retrospective cohort study included patients aged 18 years or older in the IBM MarketScan™ US database with at least one tofacitinib claim (first = index) between December 14, 2017 and April 30, 2019; PsA diagnoses on/within 12 months pre-index; and no diagnoses of rheumatoid arthritis any time pre-index. Patients were continuously enrolled for 12 months pre-index and 6 months post-index, with no pre-index claims for tofacitinib. Patient demographic and clinical characteristics on the day of index, and history of advanced treatments (including tofacitinib monotherapy or combination therapy), were recorded. Outcomes at 6 months post-index included tofacitinib persistence (less than 60-day gap without tofacitinib treatment) and adherence (proportion of days covered [PDC] and medication possession ratio 80% or higher).
Results
Of the 10,354 patients with tofacitinib claims within the study period, 318 patients with PsA met the inclusion criteria. More than 60% of patients received tofacitinib monotherapy post-index, with a mean duration of PsA of 760.5 days at index. For patients who received tofacitinib combination therapy post-index, methotrexate was the most common concomitant conventional synthetic disease-modifying antirheumatic drug. At 6 months post-index, persistence was similar in patients receiving tofacitinib monotherapy (69.8%) versus combination therapy (73.1%); adherence (as measured by PDC ≥ 0.8) was numerically lower in patients receiving tofacitinib monotherapy (56.8%) versus combination therapy (65.5%).
Conclusions
This analysis of US-based claims data described patients who had newly initiated tofacitinib treatment an average of 2 years after PsA diagnosis, with approximately two-thirds of patients receiving tofacitinib monotherapy. Observed rates of tofacitinib persistence were similar across patients who received tofacitinib monotherapy and combination therapy 6 months after initiation; adherence rates were numerically lower in patients receiving monotherapy.
Graphical Abstract
Keywords: Claims database, JAK inhibitor, Psoriatic arthritis, Real-world evidence, Tofacitinib, Treatment adherence, Treatment persistence
Plain Language Summary
Tofacitinib is a drug approved to treat patients with psoriatic arthritis (PsA) that has been shown to improve PsA symptoms and quality of life in controlled clinical trials. However, there is not much information on everyday use of tofacitinib outside of clinical trials in the USA. This study is one of the first to describe the characteristics of patients with PsA in the USA who take tofacitinib, including their typical age, sex, where they live, how long they have had PsA, and how they use tofacitinib. Use of tofacitinib included how patients followed tofacitinib prescription timings and dose (adherence) and how long they took tofacitinib for after it was prescribed (persistence). We used data collected from a US health insurance claims database (IBM MarketScan™) for patients with PsA and at least one claim for tofacitinib. In total, 318 patients were included and over 60% of them received tofacitinib therapy only (monotherapy; no conventional synthetic disease-modifying antirheumatic drug [csDMARD] therapy). For patients treated with both tofacitinib and a csDMARD (combination therapy), methotrexate was the most common drug prescribed. Six months after their first prescription of tofacitinib, around 70% of patients were still taking tofacitinib (monotherapy or combination therapy). However, a slightly lower number of patients taking tofacitinib monotherapy were taking it as originally instructed (adherence 57%), compared with those taking tofacitinib combination therapy (adherence 66%). Our results provide valuable information on the use of tofacitinib in US real-life settings outside of clinical trials and could help to improve the quality of care for patients with PsA.
Key Summary Points
| Why carry out this study? |
| Tofacitinib is an oral Janus kinase inhibitor for the treatment of psoriatic arthritis (PsA), and there are currently only limited studies in the real-world setting that provide evidence and context for the efficacy of tofacitinib in patients with PsA, which was demonstrated in a clinical setting. |
| This real-world data analysis assessed demographic and baseline clinical characteristics, as well as treatment persistence/adherence from patients with PsA in the IBM MarketScan™ US Commercial Claims and Encounters database and the Medicare Supplemental database with at least one tofacitinib claim. |
| What was learned from the study? |
| The results from this US-based claims analysis showed that patients newly initiated tofacitinib treatment an average of 2 years after PsA diagnosis, with the majority of patients receiving tofacitinib monotherapy, and high levels of tofacitinib persistence and adherence observed 6 months after treatment initiation. |
| This is one of the first real-world studies specific to tofacitinib for the treatment of PsA in the USA and findings from this study could help provide additional context for associations between tofacitinib treatment patterns and response rates, efficacy, and safety in US clinical settings, potentially offering better quality of care for patients with PsA. |
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disease characterized by peripheral joint inflammation, enthesitis, dactylitis, axial disease, and cutaneous manifestations [1]. Patients with PsA experience impairments in sleep, physical functioning, emotional well-being, and overall health-related quality of life [2–4]. PsA has been estimated to affect approximately 0.06–0.25% [5] of the United States (US) general population, and develops in up to 30% of patients with psoriasis [6]. Additionally, it has been suggested that approximately 16% of patients with psoriasis globally have undiagnosed PsA [7].
Treatment guidelines for patients with PsA have been developed by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA; 2021) [8], the European Alliance of Associations for Rheumatology (EULAR; 2020) [9], and the American College of Rheumatology and National Psoriasis Foundation (ACR/NPF; 2019) [10]. The recommendations vary depending on several different factors including disease severity, responsiveness to prior treatment, patient disease characteristics, comorbidities, and published data at the time of development [11]. However, all current treatment guidelines include Janus kinase (JAK) inhibitors as a recommended treatment strategy for PsA [8–10] and, most recently, the 2021 GRAPPA guidelines also included JAK inhibitors as a recommended treatment for most PsA domains including axial disease [8].
Tofacitinib is an oral JAK inhibitor for the treatment of PsA. The efficacy and safety of tofacitinib 5 and 10 mg twice daily (BID) have been previously demonstrated in two phase 3 randomized controlled trials (NCT01877668 and NCT01882439) in patients with active PsA and an inadequate response to either conventional synthetic disease-modifying antirheumatic drugs [csDMARDs] or tumor necrosis factor inhibitors, and in an open-label, long-term extension study (NCT01976364) [12–14]. Tofacitinib 5 mg BID is approved in the USA for the treatment of adult patients with active PsA who have had an inadequate response or intolerance to methotrexate or other DMARDs [15], and it is included in the aforementioned ACR/NPF guidelines [10].
While randomized controlled trial data are important to assess and establish the efficacy and safety profiles of a drug, it should not be assumed that research outcomes of the sample patient population represent those of an entire patient population [16]. Studies in a real-world setting can provide evidence and context for a drug’s efficacy and safety, in a heterogeneous, uncontrolled patient population representative of real-life clinical practice [16]. Furthermore, they can also expand our understanding of patient adherence to treatment outside of a trial setting [17], which can impact treatment response and outcome.
The objective of this study was to use data from a real-world setting to assess demographic and baseline clinical characteristics, as well as treatment persistence and adherence, in patients with PsA who had newly initiated tofacitinib treatment in the USA.
Methods
Data Source and Patients
This retrospective population-based study was based on data from the IBM MarketScan™ US Commercial Claims and Encounters database and the Medicare Supplemental database. The Commercial Claims and Encounters database included a collection of health insurance claims for working adults and retirees with employer-sponsored health insurance. The Medicare Supplemental database included data from retirees with Medicare supplemental insurance paid for by employers. All reported health insurance claims were from December 14, 2017 through October 31, 2019 (the study period). The first prescription fill date of tofacitinib in the database was defined as the index date; only index dates up to April 30, 2019 were included in this study.
Patients were included in the study if they were aged 18 years or older; had at least one tofacitinib claim during the study period; had a PsA diagnosis (at least one inpatient or at least two outpatient [30–365 days apart]) on or within 12 months pre-index; had not had a rheumatoid arthritis diagnosis any time pre-index; and had continuously enrolled for 12 months pre-index and at least 6 months post-index, with no pre-index claims for tofacitinib. International Classification of Diseases, 10th Revision, Clinical Modification codes were used during data collection.
Assessments and Outcomes
Patient demographics and clinical characteristics were recorded, including age at index date, sex, location, payer type, duration of PsA (defined as the number of days between PsA diagnosis and index date, excluding the end date), and PsA-related and inflammatory comorbidities at 12 months pre-index. A pre-index Quan Charlson comorbidity score was also calculated for all patients.
The databases included records for patient history of other advanced therapy within 12 months pre-index, defined as at least one claim for any of the following: apremilast, abatacept, ixekizumab, secukinumab, ustekinumab, adalimumab, etanercept, certolizumab, golimumab, or infliximab biosimilars.
Tofacitinib treatment regimen was assessed on or within 90 days post-index as monotherapy or combination therapy (defined as at least one claim for the csDMARDs listed earlier or apremilast). Changes in all concomitant medications, including glucocorticoid, opioid, and non-steroidal anti-inflammatory drug (NSAID) therapy, were assessed 90 days pre- and post-index.
All outcomes were assessed at 6 months post-index and included tofacitinib persistence (proportions of patients with less than 60-day gap without tofacitinib treatment) and adherence (proportions of patients with at least 80% of days covered [PDC ≥ 0.8] and proportions of patients with at least 80% medication possession ratio [MPR ≥ 0.8]) [18]. Tofacitinib treatment patterns, including the proportions of patients who were persistent, discontinued, restarted, or switched to alternative treatments, were also assessed at 6 months post-index. Claims for alternative treatments included those for apremilast, abatacept, ixekizumab, secukinumab, ustekinumab, adalimumab, etanercept, certolizumab, golimumab, and infliximab.
Statistical Analyses
Descriptive statistics were used for all study assessments and outcomes. Categorical data were presented as counts and percentages of patients within each category. The mean and standard deviation (SD) were used to describe all continuous variables, with the exception of data for duration of PsA, which were presented as the median and interquartile range.
The data were statistically de-identified, compliant with the Health Insurance Portability and Accountability Act of 1996, and the research was deemed exempt from institutional review board approval.
Results
Patients
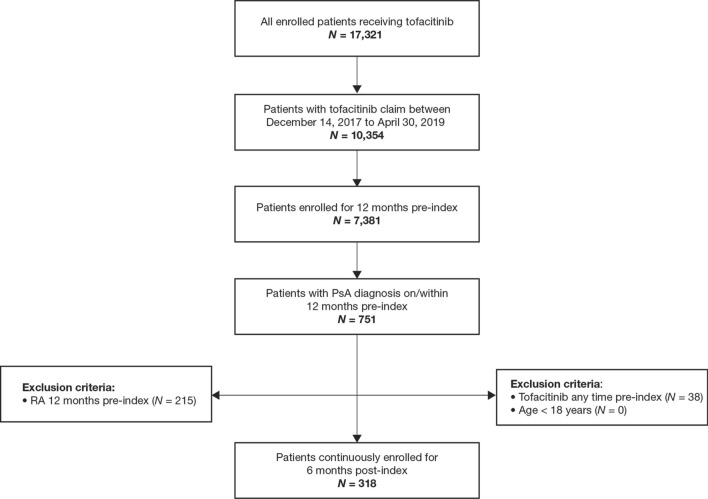
Of the 17,321 patients receiving tofacitinib, 10,354 patients with tofacitinib claims between December 14, 2017 and April 30, 2019 were initially identified. Following eligibility criteria, including the exclusion of 215 patients with a diagnosis of rheumatoid arthritis 12 months pre-index, 318 patients with a PsA diagnosis continuously enrolled for 6 months post-index were included in the analysis (Fig. 1).
Fig. 1.
Patient disposition. N number of patients, PsA psoriatic arthritis, RA rheumatoid arthritis
Patient Demographics and Clinical Characteristics at Index
Of the 318 patients included 6 months post-index, 199 (62.6%) had claims for tofacitinib monotherapy and 119 (37.4%) for combination therapy post-index (Table 1). In this cohort, the mean (SD) age at index date was 51.8 (11.4) years for patients receiving tofacitinib monotherapy and 52.5 (10.0) years for patients receiving combination therapy; most patients were female and enrolled in a commercial health plan. The mean (SD) duration of PsA at index date for patients receiving tofacitinib monotherapy was 760.5 (318.8) days (2.08 years) and that for tofacitinib combination therapy was 748.3 (319.7) days (2.05 years). Additional patient demographics and clinical characteristics at index are listed in Table 1. A total of 183 patients (57.6%) had concomitant psoriasis.
Table 1.
Demographics and clinical characteristics of patients with PsA initiating tofacitinib, stratified by post-index tofacitinib treatment regimen
| Patients with PsA initiating tofacitinib (N = 318) | ||
|---|---|---|
| Tofacitinib monotherapy (n = 199) | Tofacitinib combination therapy (n = 119) | |
| Age, yearsa | ||
| Mean (SD) | 51.8 (11.4) | 52.5 (10.0) |
| Median (IQR) | 52 (46–59) | 54 (47–59) |
| Sex, n (%) | ||
| Female | 138 (69.3) | 80 (67.2) |
| Male | 61 (30.7) | 39 (32.8) |
| US region, n (%)a | ||
| Midwest | 31 (15.6) | 18 (15.1) |
| Northeast | 51 (25.6) | 30 (25.2) |
| South | 65 (32.7) | 41 (34.5) |
| West | 25 (12.6) | 10 (8.4) |
| Unknown | 27 (13.6) | 20 (16.8) |
| Payer, n (%)b | ||
| Commercial | 175 (87.9) | 107 (89.9) |
| Medicare | 24 (12.1) | 12 (10.1) |
| PsA disease duration daysc | ||
| Mean (SD) | 760.5 (318.8) | 748.3 (319.7) |
| Median (IQR) | 818 (493.5–1036.5) | 827 (489.0–985.5) |
IQR interquartile range, N number of patients in each cohort, n number of patients in each category, PsA psoriatic arthritis, SD standard deviation, US United States
aRecorded on the index date
bFirst payer over the index date
cDays between PsA diagnosis and index date, excluding the end date
Comorbidities
In total, 90.6% of patients had one or more comorbidity, with neurological disorders (54.1%), respiratory diseases (53.8%), and hypertension (46.2%) being the most common (Table 2).
Table 2.
Comorbidities and comorbid inflammatory conditions in patients with PsA initiating tofacitinib
| Patients with PsA initiating tofacitinib (N = 318) | |
|---|---|
| Comorbidities, n (%) | |
| Overall | 288 (90.6) |
| Neurological disorder | 172 (54.1) |
| Respiratory diseases | 171 (53.8) |
| Hypertension | 147 (46.2) |
| Hyperlipidemia | 134 (42.1) |
| Cardiovascular disease | 79 (24.8) |
| Depression | 72 (22.6) |
| Obesity | 68 (21.4) |
| Anxiety | 66 (20.8) |
| Diabetes mellitus | 52 (16.4) |
| Fibromyalgia | 41 (12.9) |
| Liver disease | 24 (7.5) |
| Osteoporosis | 23 (7.2) |
| Uveitis | 7 (2.2) |
| Metabolic syndrome | 4 (1.3) |
| Comorbid inflammatory conditions, n (%) | |
| Ankylosing spondylitis | 17 (5.3) |
| Ulcerative colitis | 6 (1.9) |
| Crohn’s disease | 5 (1.6) |
| Rheumatoid arthritis | 0 (0) |
N number of patients in the cohort, n number of patients in each category, PsA psoriatic arthritis
At 12 months pre-index, mean (SD) Quan Charlson comorbidity scores were 0.59 (1.0) for the patient cohort. The proportion of patients with any Charlson comorbidity index code in the cohort was 33.7%.
Advanced Therapy
The majority (76.4%) of patients had claims for at least one other advanced therapy within 12 months pre-index; patients were most commonly treated with secukinumab (28.9%), adalimumab (18.6%), and apremilast (18.6%) (Table 3).
Table 3.
History of advanced therapy (within 12 months pre-index) in patients with PsA initiating tofacitinib
| Patients with PsA initiating tofacitinib (N = 318) |
|
|---|---|
| Patients exposed to advanced therapy, n (%)a | |
| Any advanced therapy | 243 (76.4) |
| Secukinumab | 92 (28.9) |
| Adalimumab | 59 (18.6) |
| Apremilast | 59 (18.6) |
| Etanercept | 47 (14.8) |
| Ustekinumab | 36 (11.3) |
| Certolizumab | 20 (6.3) |
| Ixekizumab | 15 (4.7) |
| Abatacept | 10 (3.1) |
| Golimumab | 8 (2.5) |
| Infliximab | 1 (0.3) |
| Number of unique advanced therapy prescriptions per patient | |
| Mean (SD) | 1.1 (0.8) |
| Median (IQR) | 1 (1–2) |
| Range | 0–4 |
| Patients with specified number of unique advanced therapies, n (%) | |
| 0 | 75 (23.6) |
| 1 | 155 (48.7) |
| 2 | 73 (23.0) |
| ≥ 3 | 15 (4.7) |
bDMARD biologic DMARD, DMARD disease-modifying antirheumatic drug, IQR interquartile range, N number of patients in each cohort, n number of patients in each category, PsA psoriatic arthritis, SD standard deviation
aPatients with at least one claim for bDMARDs or apremilast within 12 months pre-index
Tofacitinib Treatment Regimens
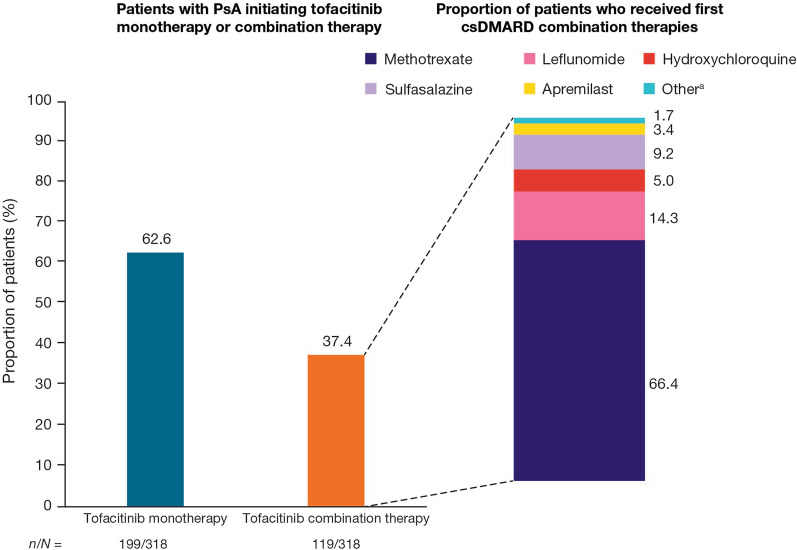
More than half (62.6%) of patients received tofacitinib monotherapy 90 days post-index (Fig. 2). Of the 37.4% of patients who received combination therapy post-index, concomitant methotrexate (66.4%) was the most common (Fig. 2).
Fig. 2.
Proportions of patients receiving tofacitinib monotherapy or combination therapy at 90 days post-index for patients with PsA initiating tofacitinib. csDMARD conventional synthetic disease-modifying antirheumatic drug, N number of patients in each cohort, n number of patients in each category, PsA psoriatic arthritis. aOther possible combination therapies included auranofin, aurothioglucose, azathioprine, chloroquine, cyclophosphamide, cyclosporine, gold sodium thiomalate, minocycline, penicillamine, tacrolimus, and thalidomide
Of interest, 114 (35.9%) with glucocorticoid prescriptions and 93 (29.3%) with opioid prescriptions of any kind were included in the study 90 days pre-index. Of these patients, 91 (79.8%) and 82 (88.2%), respectively, continued with glucocorticoid or opioid concomitant therapy 90 days post-index. Similarly, of the 77 (24.2%) patients with NSAID prescriptions, 76 (98.7%) patients continued with concomitant NSAID therapy 90 days post-index.
Tofacitinib Persistence and Adherence
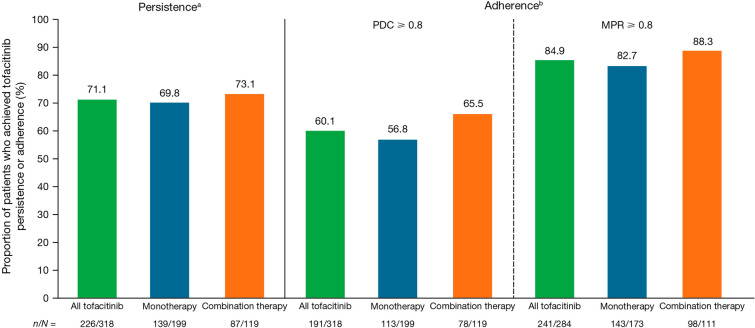
The proportions of patients with tofacitinib persistence at 6 months post-index were similar when patients initiated tofacitinib as monotherapy (69.8%) and combination therapy (73.1%) (all tofacitinib 71.1%) (Fig. 3). The proportions of patients with tofacitinib adherence per PDC ≥ 0.8 at 6 months post-index was 56.8% for patients receiving tofacitinib monotherapy, 65.5% for patients receiving combination therapy, and 60.1% for all patients with PsA initiating tofacitinib. Tofacitinib adherence per MPR ≥ 0.8 in both monotherapy and combination therapy groups was even higher with rates of 82.7% and 88.3%, respectively (all tofacitinib 84.9%) (Fig. 3). Mean (SD) MPR was 0.9 (0.12) for patients receiving tofacitinib monotherapy and 0.92 (0.12) for patients receiving combination therapy (all tofacitinib 0.91 [0.12]).
Fig. 3.
Proportion of patients who achieved tofacitinib persistence and adherence in patients with PsA initiating tofacitinib. MPR medication possession ratio, PDC proportion of days covered, PsA psoriatic arthritis. aPatients with less than 60-day gap without tofacitinib treatment. bAdherence was defined by patients with PDC ≥ 0.8 and MPR ≥ 0.8
Tofacitinib Treatment Patterns
While the majority (64.2%) of patients were persistent with tofacitinib therapy 6 months post-index, 12.9% of patients discontinued therapy and 2.2% subsequently restarted tofacitinib therapy within 6 months post-index. Furthermore, 18.6% of patients switched to alternative treatments within 60 days post-index (Fig. 4).
Fig. 4.
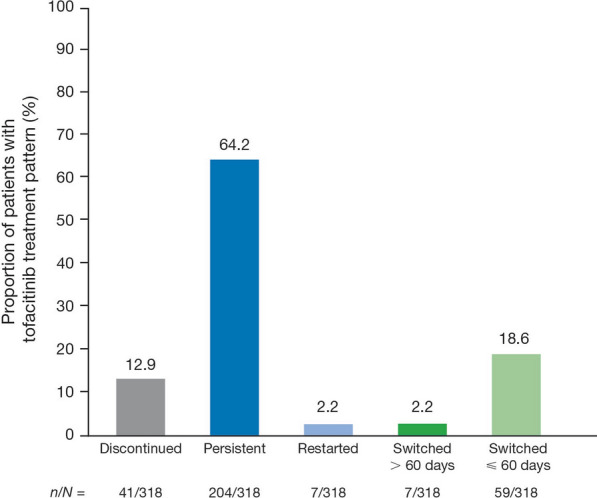
Tofacitinib treatment patterns in patients with PsA initiating tofacitinib within 6 months post-index. N number of patients assessed, n number of patients in each category, PsA psoriatic arthritis
Discussion
This analysis of US-based claims data from over 300 patients with PsA indicated that tofacitinib treatment was initiated an average of 2 years after PsA diagnosis. The majority of patients initiated tofacitinib as monotherapy (more than 60%) rather than combination therapy (more than 35%), with methotrexate the most common concomitant csDMARD therapy. Furthermore, most patients (more than 75%) had a claim for at least one other advanced therapy in the prior year. High levels of persistence (approx. 70%) and adherence (approx. 55–85%, depending on the measure) with tofacitinib were observed 6 months after treatment initiation, with similar levels of persistence observed for patients treated with monotherapy and combination therapy, and numerically higher levels of adherence in patients treated with tofacitinib combination therapy. Of the approximately 13% of patients who discontinued tofacitinib post-initiation, most switched to alternative treatments within 60 days (approx. 19%) rather than restarted tofacitinib (approx. 2%).
To our knowledge this is one of the first real-world studies specific to tofacitinib treatment for PsA in the USA. Previous studies in a real-world setting in patients with PsA have evaluated response rates, treatment patterns, persistence, and drug survival for other treatments, such as secukinumab, etanercept, and adalimumab [19–24]; there is also a study that evaluated demographic, treatment, and disease characteristics in patients with PsA in the USA alongside other European countries [25]. While randomized controlled trials are the gold standard for assessing efficacy and safety of advanced treatments—including biologic and targeted synthetic DMARDs—data from clinical practice, like the data presented here, provide valuable context and insights for approved treatments in heterogeneous patient populations with histories of various comorbidities and prior therapy. Adherence and persistence rates with biologic treatments are generally low and studies in a real-world setting could help identify if improvement of other factors, such as patient education and lower out-of-pocket costs, may support the care of patients with PsA [26].
High levels of tofacitinib adherence and persistence were observed in this study; however, it will be important to assess if these are maintained longer than the post 6-month period investigated in this study. Focusing on long-term follow-up of studies in a real-world setting could also provide additional insights into tofacitinib response rates, effectiveness, and safety profiles, all of which will provide clinicians with more context to improve shared treatment decisions with their patients with PsA.
The large proportion of patients having a claim for at least one other advanced therapy in the prior year in this study may be explained by the structure of the US healthcare system, and the fact that these are not necessarily newly diagnosed patients. Additionally, we believe that the results of our study reflect the use of opioids in inflammatory arthritis as a whole, with 88% of patients taking opioids at baseline continuing with this therapy after 90 days of tofacitinib treatment. It has been reported that a remarkably high number of patients with inflammatory arthritis, including PsA and rheumatoid arthritis, use opioids for pain management [27, 28], and it has previously been demonstrated that opioid use did not decline after patients started tumor necrosis factor inhibitor therapy for their PsA [29]. Furthermore, in a study of injectable biologic therapies for PsA, opioids were added as adjunctive medication in 17% of patients [30].
The large sampling size of the IBM MarketScan™ US Commercial Claims and Encounters database and the Medicare Supplemental database enabled the study to account for patient heterogeneity. Limitations of this study included its retrospective design, the inability to confirm that patients took the medication for which they filed a claim (resulting in potential bias of adherence rates), and the fact that claims data may potentially miscode the diagnosis of PsA, rheumatoid arthritis, and other autoimmune conditions. In addition, administrative claims mostly comprise commercial insurance and commercially managed Medicare/Medicaid programs and may not be generalizable beyond the commercially insured population. Furthermore, the impact of disease severity on tofacitinib initiation rates at index could have impacted persistence and adherence rates; however, this was not assessed as part of this study. The proportion of patients with concomitant psoriasis was surprisingly low (57.6%) and may have also been under-reported in the claims by investigators, possibly owing to partial clearance of skin disease at index date. Finally, reasons for treatment discontinuation were not captured, so no conclusions can be drawn as to why a patient was not persistent to a therapy, and all outcomes were assessed at 6 months post-index only.
Conclusions
This is one of the first reports of a real-world study of tofacitinib for the treatment of PsA in the USA and contributes to the limited number of studies in a real-world setting for targeted synthetic DMARD use in PsA. Claims data showed that the majority of patients with PsA receiving tofacitinib initiated tofacitinib monotherapy versus combination therapy, with high levels of persistence and adherence at 6 months for both treatment regimens. The findings from this study offer the potential to better understand tofacitinib treatment patterns in a US clinical practice setting, compared with a traditional randomized controlled trial. This study could also help inform future analyses to further assess associations between tofacitinib treatment patterns and clinical effectiveness and safety. Evaluation of tofacitinib treatment patterns in daily clinical practice can aid better understanding of patient outcomes and help healthcare providers to address challenges around treatment adherence and persistence, ultimately improving quality of care for patients with PsA.
Acknowledgements
Funding
This study, the Rapid Service Fee, and the Open Access fee were sponsored by Pfizer Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors were involved in study conception and design. PY, DG, LF, and RG were involved in acquisition of data. All authors were involved in analysis and interpretation of data, provided critical input and review of drafts, and approved the final version of the manuscript for submission.
Medical Writing and Editorial Assistance
Medical writing support, under the guidance of the authors, was provided by Tanya Guha, PhD, CMC Connect, McCann Health Medical Communications, and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–4).
Prior Presentation
This manuscript is based on previously presented work. Data from this analysis were previously presented as a poster at ACR Convergence 2020, November 5–9th (virtual meeting) and were published as an abstract only at EULAR 2021, June 2–5th (virtual meeting).
Disclosures
Philip J. Mease has received grant/research support from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer Inc, Sun, and UCB; has acted as a consultant for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, GSK, Janssen, Novartis, Pfizer Inc, Sun, and UCB; and has participated in speakers’ bureau for AbbVie, Amgen, Eli Lilly, Genentech, Janssen, Novartis, Pfizer Inc, and UCB. Pamela Young, David Gruben, Lara Fallon, and Rebecca Germino are employees and stockholders of Pfizer Inc. Arthur Kavanaugh has received grant/research support from Pfizer Inc.
Compliance with Ethics Guidelines
The data were statistically de-identified, compliant with the Health Insurance Portability and Accountability Act of 1996, and the research was deemed exempt from institutional review board approval.
Data Availability
The datasets generated during and/or analyzed during the current study are available from Pfizer, via VIVLI, on reasonable request. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
- 1.Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet. 2018;391:2273–2284. doi: 10.1016/S0140-6736(18)30830-4. [DOI] [PubMed] [Google Scholar]
- 2.Mease PJ, Menter MA. Quality-of-life issues in psoriasis and psoriatic arthritis: outcome measures and therapies from a dermatological perspective. J Am Acad Dermatol. 2006;54:685–704. doi: 10.1016/j.jaad.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Krajewska-Wlodarczyk M, Owczarczyk-Saczonek A, Placek W. Sleep disorders in patients with psoriatic arthritis and psoriasis. Reumatologia. 2018;56:301–306. doi: 10.5114/reum.2018.79501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee S, Mendelsohn A, Sarnes E. The burden of psoriatic arthritis: a literature review from a global health systems perspective. P T. 2010;35:680–689. [PMC free article] [PubMed] [Google Scholar]
- 5.Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41:545–568. doi: 10.1016/j.rdc.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376:957–970. doi: 10.1056/NEJMra1505557. [DOI] [PubMed] [Google Scholar]
- 7.Villani AP, Rouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242–248. doi: 10.1016/j.jaad.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Coates LC, Soriano E, Corp N, et al. The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) treatment recommendations 2021. Ann Rheum Dis. 2021;80(Suppl 1):139–140. doi: 10.1136/annrheumdis-2021-eular.4091. [DOI] [Google Scholar]
- 9.Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79:700–712. doi: 10.1136/annrheumdis-2020-217159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rhematol. 2019;71:5–32. doi: 10.1002/art.40726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogdie A, Coates LC, Gladman DD. Treatment guidelines in psoriatic arthritis. Rheumatology (Oxford) 2020;59:i37–i46. doi: 10.1093/rheumatology/kez383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377:1525–1536. doi: 10.1056/NEJMoa1615977. [DOI] [PubMed] [Google Scholar]
- 13.Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377:1537–1550. doi: 10.1056/NEJMoa1615975. [DOI] [PubMed] [Google Scholar]
- 14.Nash P, Coates LC, Kivitz AJ, et al. Safety and efficacy of tofacitinib in patients with active psoriatic arthritis: interim analysis of OPAL Balance, an open-label, long-term extension study. Rheumatol Ther. 2020;7:553–580. doi: 10.1007/s40744-020-00209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfizer Inc. Xeljanz® (tofacitinib): highlights of prescribing information. 2020. http://labeling.pfizer.com/ShowLabeling.aspx?id=959. Accessed 14 Oct 2021.
- 16.Kim HS, Lee S, Kim JH. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J Korean Med Sci. 2018;33:213. doi: 10.3346/jkms.2018.33.e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35:1763–1774. doi: 10.1007/s12325-018-0805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canfield SL, Zuckerman A, Anguiano RH, et al. Navigating the wild west of medication adherence reporting in specialty pharmacy. J Manag Care Spec Pharm. 2019;25:1073–1077. doi: 10.18553/jmcp.2019.25.10.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michelsen B, Georgiadis S, Di Giuseppe D, et al. Real-world 6 and 12-month drug retention, remission and response rates of secukinumab in 2,017 psoriatic arthritis patients in 13 European countries. Arthritis Care Res (Hoboken). 2021. 10.1002/acr.24560. [DOI] [PubMed]
- 20.Thomas ML, Shaddick G, Charlton R, et al. Tumor necrosis factor inhibitor monotherapy versus combination therapy for the treatment of psoriatic arthritis: combined analysis of European biologics databases. J Rheumatol. 2021;48:48–57. doi: 10.3899/jrheum.190815. [DOI] [PubMed] [Google Scholar]
- 21.Mease PJ, Accortt NA, Rebello S, et al. Persistence of tumor necrosis factor inhibitor or conventional synthetic disease-modifying antirheumatic drug monotherapy or combination therapy in psoriatic arthritis in a real-world setting. Rheumatol Int. 2019;39:1547–1558. doi: 10.1007/s00296-019-04345-1. [DOI] [PubMed] [Google Scholar]
- 22.Bonafede M, Johnson BH, Fox KM, Watson C, Gandra SR. Treatment patterns with etanercept and adalimumab for psoriatic diseases in a real-world setting. J Dermatolog Treat. 2013;24:369–373. doi: 10.3109/09546634.2012.755255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramonda R, Lorenzin M, Carriero A, et al. Effectiveness and safety of secukinumab in 608 patients with psoriatic arthritis in real life: a 24-month prospective, multicentre study. RMD Open. 2021;7:e001519. doi: 10.1136/rmdopen-2020-001519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klavdianou K, Lazarini A, Grivas A, et al. Real life efficacy and safety of secukinumab in biologic-experienced patients with psoriatic arthritis. Front Med (Lausanne) 2020;7:288. doi: 10.3389/fmed.2020.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogdie A, Schmerold L, Tillett W, Germino R, Cappelleri JC, Young P. Demographic, treatment and disease characteristics of patients with psoriatic arthritis receiving tofacitinib in the United States, France, Germany, Italy, Spain and the United Kingdom. Ann Rheum Dis. 2020;79(Suppl 1):1707–1708. doi: 10.1136/annrheumdis-2020-eular.982. [DOI] [Google Scholar]
- 26.Murage MJ, Tongbram V, Feldman SR, et al. Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: a systematic literature review. Patient Prefer Adherence. 2018;12:1483–1503. doi: 10.2147/PPA.S167508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stouten V, Pazmino S, Verschueren P, et al. Comorbidity burden in the first three years after diagnosis in patients with rheumatoid arthritis, psoriatic arthritis or spondyloarthritis: a general practice registry-based study. RMD Open. 2021;7:e001671. doi: 10.1136/rmdopen-2021-001671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YC, Kremer J, Guan H, Greenberg J, Solomon DH. Chronic opioid use in rheumatoid arthritis: prevalence and predictors. Arthritis Rheumatol. 2019;71:670–677. doi: 10.1002/art.40789. [DOI] [PubMed] [Google Scholar]
- 29.Palsson O, Love T, Wallman JK, Kapetanovic MC, Gunnarsson PS, Gudbjornsson B. Initiating TNF inhibitors in inflammatory arthritis does not decrease the average opioid analgesic consumption. Ann Rheum Dis. 2020;79(Suppl 1):58–59. doi: 10.1136/annrheumdis-2020-eular.2587. [DOI] [Google Scholar]
- 30.Walsh JA, Cai Q, Lin I, Fitzgerald T, Pericone CD, Chakravarty SD. Real-world 2-year treatment patterns among patients with psoriatic arthritis treated with injectable biologic therapies. Curr Med Res Opin. 2020;36:1245–1252. doi: 10.1080/03007995.2020.1754186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from Pfizer, via VIVLI, on reasonable request. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.