Abstract
Introduction
Dysmenorrhea is a physical and mental burden for women, negatively affecting health-related quality of life (HRQL) and work productivity. However, data on HRQL and work productivity of Japanese women are scarce.
Methods
In this prospective observational study, 397 Japanese women received low-dose estrogen/progestin (LEP) or non-LEP treatment (non-steroidal anti-inflammatory drugs or Chinese herbal medicines) for primary/secondary dysmenorrhea and completed survey questionnaires online regarding menstrual symptoms, HRQL, and work productivity. Regression analysis was performed to compare the groups and evaluate outcomes over time using the paired t test. Subgroup analysis was performed using stratification by patient background, and correlations between improvement in menstrual symptoms/HRQL and work productivity were investigated using Spearman’s rank correlation coefficient.
Results
Significant reductions in the modified Menstrual Distress Questionnaire (mMDQ) total score were shown in the LEP group (n = 251) (P < 0.01), but not the non-LEP group (n = 146). Significant improvements in HRQL, measured by the 36-Item Short-Form Health Survey v2.0 (SF-36v2.0), were shown in the LEP group, but not the non-LEP group. Improvements were seen in mental component summary and 7/8 domains (role physical, bodily pain, general health, role emotional, mental health, vitality, and social functioning) in the LEP group, but not the non-LEP group. There were no differences in the physical component summary and role functioning in either group. Improvements in work productivity, measured by the modified Work Productivity and Activity Impairment Questionnaire (mWPAI), were greater in the LEP group vs. non-LEP group. Regression analysis showed differences in improvements between the groups in the mMDQ total score, SF-36v2.0, and mWPAI. A correlation between mMDQ or HRQL and work productivity was seen.
Conclusion
In Japanese women, dysmenorrhea is associated with reduced HRQL and work productivity. In real-world clinical practice, improvements in physical and mental menstrual symptoms, HRQL, and work productivity were observed with LEP treatment.
Trial Registration
NCT04607382 (ClinicalTrials.gov).
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02118-0.
Keywords: Dysmenorrhea, Health-Related Quality of Life, LEP, Menstrual Symptoms, PRO/QOL: Trial Based, Women’s Health, Work Productivity
Key Summary Points
| Why carry out this study? |
| Dysmenorrhea affects up to 90% of women, negatively affecting health-related quality of life (HRQL) and work productivity. |
| Studies of low-dose estrogen/progestin (LEP) as treatment for dysmenorrhea investigating the impact of extended LEP regimens on HRQL or examining the relationship between HRQL and work productivity in Japanese women are lacking. |
| Patient-reported outcomes from patients receiving LEP or non-LEP treatment for dysmenorrhea in real-world clinical practice may provide insight into the HRQL and work productivity in Japanese women by LEP treatment. |
| What is learned from this study? |
| In real-world clinical practice, physical and mental menstrual symptoms, HRQL, and work productivity improved significantly with LEP treatment in Japanese women with dysmenorrhea. |
| Patients with secondary dysmenorrhea taking LEP tended to have a lower improvement in HRQL than those with primary dysmenorrhea, while absenteeism of patients with secondary dysmenorrhea showed a significant improvement with LEP (not significant for patients with primary dysmenorrhea). |
Introduction
Dysmenorrhea is the most common gynecological condition worldwide, affecting up to 90% of women during their reproductive years [1]. Symptoms vary, but commonly include pain, headache, nausea, insomnia, fatigue, anxiety, depression, weakness, and diarrhea [2]. On the basis of pathophysiology, dysmenorrhea can be classified as primary dysmenorrhea, where no organic disease is present, or secondary dysmenorrhea, which is due to an underlying pelvic abnormality [3]. Primary dysmenorrhea is characterized by spasmodic, cramping menstrual pain and discomfort in the absence of pelvic pathology [4], while secondary dysmenorrhea is associated with a specific pelvic pathology, such as endometriosis, adenomyosis, or uterine fibroids [3, 5].
According to a survey conducted in 2011 in 254 Japanese women aged 15–49 years, almost 75% experienced menstrual symptoms, half of which were attributed to dysmenorrhea [6]. In another survey, one-third of Japanese women characterized their dysmenorrhea as severe, and generally required analgesics for pain relief [7]. Annually, approximately 900,000 women with dysmenorrhea seek medical attention in Japan [8]; however, because many women may not seek treatment, the actual number affected is undoubtedly much higher. Indeed, fewer Japanese women visit a gynecologist and access appropriate treatments than women in the USA and European countries, and Japanese women may be reluctant to discuss sex and reproductive health topics [6].
The impact of dysmenorrhea on daily life may be substantial. Data indicate that more than one-quarter of women reduced their working hours or were absent from work for at least 1 day per 6 months as a result of menstrual pain [9]. The annual economic burden due to menstrual symptoms in Japanese women was estimated at approximately 683 billion Japanese yen, and over 70% may be attributed to a loss in work productivity [6]. Thus, dysmenorrhea is not just a women’s health disease but also a societal issue. Additionally, many national and international studies have shown that adult or adolescent female individuals with dysmenorrhea have a significantly lower health-related quality of life (HRQL) [10–12] and have reported that dysmenorrhea has a negative impact on friendships, family relationships, work/school performance, absenteeism, and recreational activities compared with those without [13–15]. Thus, gynecologists understand the importance of pain management in dysmenorrhea and the need to improve HRQL.
Current Japanese guidelines recommend analgesics (e.g., non-steroidal anti-inflammatory drugs [NSAIDs]), low-dose estrogen/progestin (LEP) combination, and/or levonorgestrel-releasing intrauterine systems as first-line treatments for primary dysmenorrhea [16]. For pain management of secondary dysmenorrhea, the guidelines also recommend NSAIDs as the first-line treatment and LEP as the second-line treatment [16].
Although numerous studies have investigated the impact of dysmenorrhea on HRQL, only one publication investigated the benefits of taking LEP in Japan [17]. A patient-reported outcome study of Japanese women with dysmenorrhea treated with ethinylestradiol/drospirenone (EE/DRSP) showed HRQL improvements, as measured by the 36-Item Short-Form Health Survey version 2.0 (SF-36v2.0) [17]. However, studies investigating the impact of cyclic or extended LEP regimens on HRQL or examining the relationship between HRQL and work productivity in Japanese women are lacking.
The primary objectives of this study were to compare LEP and non-LEP groups in terms of menstrual symptoms, HRQL, and work productivity and to evaluate their changes over time. Secondary objectives were to investigate the relationship between improvements in HRQL and work productivity at day 120, and conduct a subgroup analysis by characterization of patients’ background and medical history.
Methods
Study Setting and Population
This was a prospective, non-interventional, observational study (ClinicalTrials.gov, NCT04607382) of Japanese women who received LEP or non-LEP treatment for primary or secondary dysmenorrhea in real-world clinical practice. The study was approved by the Takahashi Clinic Institutional Review Board, Hyogo, Japan, and was conducted in accordance with Good Post-Marketing Study Practice [18].
To acquire study participants from throughout Japan, we divided Japan into nine regions and selected at least one facility from each. Additionally, patients were recruited from clinics or hospitals in suburban as well as urban areas. Prospective participants were provided with a written explanation of the study and asked to submit an online informed consent form. Following agreement to participate in the study, gynecologists provided their patients with QR codes or a URL, enabling online access to answer questionnaires. All treatment decisions, including choice of LEP or non-LEP treatment and LEP dosage, were determined by shared decision-making between the patients and gynecologists, and treatment was administered in the context of routine clinical practice.
The patient registration period was between September 2020 and January 2021. The study observation period was 120 days, and data were collected at enrollment (day 1), day 60, and day 120, although this applied only if patients were seen during the relevant time periods as part of routine clinical follow-up. Eligible patients were aged 16–39 years; diagnosed with primary or secondary dysmenorrhea by their gynecologist; were able to visit the clinic or hospital every 6 months for consultations regarding dysmenorrhea treatment; planned to be treated with drugs for dysmenorrhea after enrollment in this study; and could access the website to complete questionnaires. Patients receiving non-LEP therapies (such as NSAIDs or Chinese herbal medicines, available without a prescription) were eligible for the non-LEP cohort. Exclusion criteria were use of LEP for dysmenorrhea in the 2 months prior to enrollment; presence of psychiatric disorders, severe infectious diseases, or malignant tumors; or contraindications for LEP.
Study Outcomes
The primary outcome was the modified Menstrual Distress Questionnaire (mMDQ [19]; reduction indicates improvement), the SF-36v2.0 (increase indicates improvement), and the modified Work Productivity and Activity Impairment Questionnaire General Health (mWPAI [20]; reduction indicates improvement). For the full details of each measure, please see the appendix in the Supplementary Material.
Secondary outcomes were the degree of correlation between improvement in HRQL and improvement in work productivity (LEP group), and the subgroup analysis based on patient baseline characteristics, including medical history.
Statistical Analysis
The sample size was calculated using the results of an interim analysis of a post-marketing study of EE/DRSP (Yaz Flex®) [21]. Change in mean mMDQ score and standard deviation (SD) were assumed to be − 8.0 and 25.0, respectively. To confirm that the difference in scores from baseline would be less than 0, the number of required patients for the analyses was calculated to be 129 with α = 0.05 (2-sided) and 1 − β = 0.95. If one assumed a discontinuation rate of 25%, the required number of patients was approximately 175. We planned to assess two cohorts, and the final sample size was 350.
Patients’ baseline characteristics and medical history were descriptively summarized. Changes in mMDQ, SF-36v2.0, and mWPAI scores from day 1 to day 60 and day 120 were expressed as summary statistics. In an exploratory analysis, a mixed-effect model with repeated measures (MMRM) was used. Regression analyses were performed to confirm differences in improvements between the groups, adjusting for age, body mass index, marital status, pregnancy history, type of dysmenorrhea, time of diagnosis, concomitant drugs, and severity. For the mMDQ, the total and six domain scores were summarized. For the SF-36v2.0, eight domain scores and the physical and mental component scores (PCS and MCS) were summarized. For the mWPAI, the following four items were summarized as percentages: work time missed because of health (absenteeism), impairment while working because of health (presenteeism), overall work impairment because of health, and activity impairment because of health. The paired t test was used to assess if there was a significant change in mMDQ, SF-36v2.0, and mWAPI over time (day 1 vs. day 60 and day 1 vs. day 120). A P value of less than 0.05 was considered statistically significant. Wherever applicable, tests were adjusted for multiplicity using Bonferroni correction.
For the secondary outcome, Spearman’s rank correlation coefficient was used to investigate the correlation between improvement in menstrual symptoms or HRQL and work productivity at day 120. As the LEP regimen (extended/cyclic), concomitant drug (yes/no), and severity (none/mild/moderate/severe) may cause bias in the results, outcomes were stratified to check bias. Considering that the type of dysmenorrhea may largely affect the results, we divided the patients into LEP and non-LEP groups, and then stratified them by type of dysmenorrhea to check for bias.
There were no missing values in any of the questionnaires because the system did not allow transmission until all responses were completed. In the current data analysis, all statistical analyses were performed using SAS software, version 9.4 for Windows (SAS Institute Inc., Cary, NC, USA).
Results
Of 495 women with dysmenorrhea who received LEP or non-LEP treatment and were enrolled in this study, 98 (71 from the LEP group and 27 from the non-LEP group) were excluded because they did not complete a follow-up questionnaire at day 60 and day 120 and/or did not have menstruation during the observation period. Thus, 397 patients had data available for analysis (LEP n = 251; non-LEP n = 146).
Patient Characteristics
In the LEP group, the mean (SD) age was 26.3 (5.5) years, and in the non-LEP group, the mean age was 28.7 (6.0) years (Table 1). Many patients in the LEP group (73.3%) and the non-LEP group (65.1%) had primary dysmenorrhea. Of the women with secondary dysmenorrhea, the most common gynecological organic disease in both groups was endometriosis (LEP 19.1%; non-LEP 24.7%), followed by uterine fibrosis (6.8% and 13.0%, respectively) and adenomyosis (4.4% and 3.4%, respectively). Additionally, almost 50% of patients in both groups had premenstrual syndrome. Among patients in the LEP group, 13.1% reported severe dysmenorrhea (defined as bedridden for at least 1 day and unable to carry out work, including schoolwork and housework), 47.4% reported moderate symptoms, 33.1% reported mild symptoms, and 6.4% reported no symptoms; a similar trend was observed in the non-LEP group: 11.0%, 44.5%, 34.9%, and 9.6%, respectively (Table 1).
Table 1.
Baseline patient demographic and clinical characteristics
| LEP (n = 251) |
Non-LEP (n = 146) |
|
|---|---|---|
| Age (years), mean ± SD | 26.3 ± 5.5 | 28.7 ± 6.0 |
| BMI (kg/m2), mean ± SD | 20.4 ± 2.4 | 21.5 ± 4.2 |
| Marital status (married), n (%) | 50 (19.9) | 58 (39.7) |
| Education, n (%) | ||
| Junior high school | 4 (1.6) | 7 (4.8) |
| High school | 98 (39.0) | 62 (42.5) |
| Undergraduate | 141 (56.2) | 68 (46.6) |
| Postgraduate | 8 (3.2) | 9 (6.2) |
| Smokers, n (%) | 26 (10.4) | 17 (11.7) |
| History of pregnancy, n (%) | 59 (23.5) | 43 (29.5) |
| Type of dysmenorrhea, n (%) | ||
| Primary | 184 (73.3) | 95 (65.1) |
| Secondarya | 67 (26.7) | 51 (34.9) |
| Endometriosis | 48 (19.1) | 36 (24.7) |
| Uterine fibrosis | 17 (6.8) | 19 (13.0) |
| Adenomyosis | 11 (4.4) | 5 (3.4) |
| Concomitant medication for dysmenorrhea,b n (%) | ||
| No | 112 (44.6) | 21 (14.4) |
| Yes | ||
| NSAIDs | 106 (42.2) | 107 (73.3) |
| Chinese herbal medicine | 34 (13.5) | 64 (43.8) |
| Other | 3 (1.2) | 4 (2.7) |
| Severity,c n (%) | ||
| None | 16 (6.4) | 14 (9.6) |
| Mild | 83 (33.1) | 51 (34.9) |
| Moderate | 119 (47.4) | 65 (44.5) |
| Severe | 33 (13.1) | 16 (11.0) |
| Type of LEP,a n (%) | ||
| EE/DRSP (Yaz®) | 50 (19.9) | 0 (0.0) |
| EE/DRSP (Yaz® Flex) | 72 (28.7) | 0 (0.0) |
| EE/LNG (Jemina®) | 10 (4.0) | 0 (0.0) |
| EE/NET (Lunabell®) | 53 (21.1) | 0 (0.0) |
| EE/NET (Frewell®) | 71 (28.3) | 0 (0.0) |
| Type of LEP regimena | ||
| Cyclic (Yaz®, Lunabell®, Frewell®) | 174 (69.3) | 0 (0.0) |
| Extended (Yaz® Flex, Jemina®) | 82 (32.7) | 0 (0.0) |
BMI body mass index, DRSP drospirenone, EE ethinylestradiol, LD low dose, LEP low-dose estrogen/progestin (cyclic or extended), LNG levonorgestrel, NET norethindrone, NSAIDS non-steroidal anti-inflammatory drugs, SD standard deviation, ULD ultra-low dose
aIncludes duplicate cases
bDrugs that had been prescribed by hospitals for the treatment of dysmenorrhea (menstrual symptoms) and were still being taken
cDegree of disruption to overall daily life due to dysmenorrhea. None, no impairment; mild, slight impairment of work, including schoolwork and housework; moderate, impairs work, including schoolwork and housework to the degree one wants to lie down and take a rest; severe, bedridden for at least 1 day and unable to carry out work, including schoolwork and housework
Approximately half of the LEP group used extended LEP, and the remainder used a cyclic regimen (Table 1). In the LEP group, 106 and 34 patients were taking NSAIDs or Chinese herbal medicine on day 1 (baseline); however, the respective numbers decreased to 28 (11.4%) and 12 (4.9%) by day 60, and 18 (7.9%) and 8 (3.5%) by day 120.
Between-Group Regression Analysis
Regression analyses were performed to identify a difference in improvements between the LEP and non-LEP groups (Table 2). The results revealed differences between the groups in the total score and all mMDQ domains. As for SF-36v2.0, there were no differences in PCS and role functioning (RF); however, differences were observed on day 60 and/or day 120 for domains other than PCS and RF. Furthermore, as for mWPAI, differences were observed on day 60 and day 120 in all items except for absenteeism.
Table 2.
Comparison of the change in mMDQ, SF-36v2.0, and mWPAI scores between the LEP and non-LEP groups using regression analysis
| Differencea (95% CI) | P value | |||
|---|---|---|---|---|
| Day 60 | Day 120 | Day 60 | Day 120 | |
| mMDQ | ||||
| Before menstruation | ||||
| Total score | − 12.7 (− 21.3, − 4.2) | − 23.8 (− 32.3, − 15.2) | P < 0.01 | P < 0.01 |
| Pain | − 2.1 (− 3.9, − 0.4) | − 4.0 (− 5.8, − 2.3) | P < 0.01 | P < 0.01 |
| Concentration | − 2.5 (− 4.5, − 0.5) | − 5.3 (− 7.3, − 3.2) | P < 0.01 | P < 0.01 |
| Behavioral effect | − 2.0 (− 3.6, − 0.5) | − 4.1 (− 5.7, − 2.5) | P < 0.01 | P < 0.01 |
| Autonomic response | − 0.9 (− 1.8, 0.1) | − 1.5 (− 2.4, − 0.5) | NS | P < 0.01 |
| Water retention | − 0.9 (− 2.1, 0.2) | − 2.1 (− 3.2, − 0.9) | NS | P < 0.01 |
| Negative affect | − 4.3 (− 6.8, − 1.9) | − 6.9 (− 9.3, − 4.4) | P < 0.01 | P < 0.01 |
| During the menstrual period | ||||
| Total score | − 13.0 (− 20.7, − 5.3) | − 20.7 (− 28.4, − 13.0) | P < 0.01 | P < 0.01 |
| Pain | − 2.7 (− 4.4, − 1.0) | − 4.2 (− 5.9, − 2.5) | P < 0.01 | P < 0.01 |
| Concentration | − 2.8 (− 4.8, − 0.9) | − 4.5 (− 6.5, − 2.6) | P < 0.01 | P < 0.01 |
| Behavioral effect | − 2.0 (− 3.5, − 0.5) | − 3.5 (− 5.0, − 2.0) | P < 0.01 | P < 0.01 |
| Autonomic response | − 1.1 (− 2.1, − 0.1) | − 1.6 (− 2.6, − 0.6) | P < 0.05 | P < 0.01 |
| Water retention | − 1.1 (− 2.0, − 0.1) | − 1.9 (− 2.8, − 0.9) | P < 0.05 | P < 0.01 |
| Negative affect | − 3.7 (− 5.8, − 1.5) | − 5.3 (− 7.4, − 3.1) | P < 0.01 | P < 0.01 |
| SF-36v2.0 | ||||
| During menstruation | ||||
| Physical component summary | 0.7 (− 1.4, 2.8) | 1.0 (− 1.2, 3.1) | NS | NS |
| Mental component summary | 3.4 (1.1, 5.6) | 2.6 (0.3, 4.9) | P < 0.01 | P < 0.05 |
| Physical functioning | − 0.7 (− 2.5, 1.2) | 0.2 (− 1.6, 2.1) | NS | NS |
| Role physical | 1.8 (− 0.7, 4.4) | 3.8 (1.3, 6.4) | NS | P < 0.01 |
| Bodily pain | 5.5 (2.6, 8.4) | 5.8 (2.9, 8.7) | P < 0.01 | P < 0.01 |
| General health | 1.6 (− 0.3, 3.6) | 2.0 (0.1, 4.0) | NS | P < 0.05 |
| Vitality | 3.0 (0.6, 5.4) | 2.1 (− 0.2, 4.5) | P < 0.01 | NS |
| Social functioning | 1.7 (− 1.1, 4.4) | 3.3 (0.6, 6.0) | NS | P < 0.01 |
| Role emotional | 2.4 (− 0.4, 5.2) | 4.6 (1.8, 7.3) | NS | P < 0.01 |
| Mental health | 2.8 (0.4, 5.2) | 3.7 (1.3, 6.1) | P < 0.05 | P < 0.01 |
| mWPAIb | ||||
| Absenteeismc | − 0.4 (− 4.9, 4.2) | − 0.4 (− 4.9, 4.2) | NS | NS |
| Presenteeismd | − 10.9 (− 19.4, − 2.5) | − 11.8 (− 20.2, − 3.4) | P < 0.01 | P < 0.01 |
| Overall work impairment | − 10.7 (− 19.2, − 2.1) | − 11.8 (− 20.3, − 3.3) | P < 0.01 | P < 0.01 |
| Activity impairment | − 14.5 (− 23.0, − 6.1) | − 9.2 (− 17.7, − 0.6) | P < 0.01 | P < 0.05 |
CI confidence interval, LEP low-dose estrogen/progestin (cyclic or extended), mMDQ modified Menstrual Distress Questionnaire, NS not significant, SF-36v2.0 36-Item Short-Form Health Survey v2.0, mWPAI modified Work Productivity and Activity Impairment Questionnaire
aDifference from non-LEP group based on the change in the LEP group
bWorking conditions for the past 7 days at each point
cPercentage work time missed due to health
dPercentage impairment while working due to health
Changes in Menstrual Symptoms Over Time
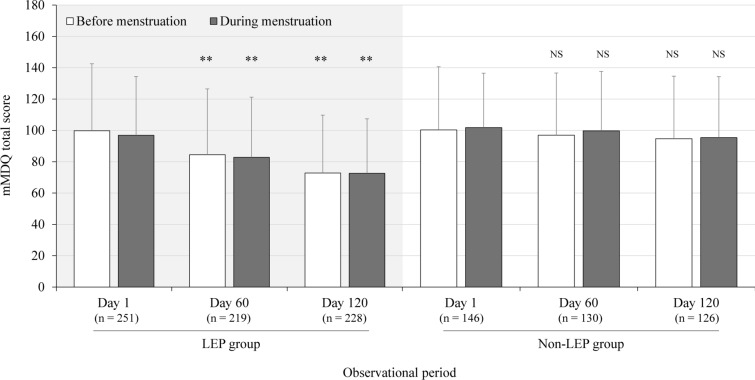
Changes in the mMDQ total scores before and during menstrual periods are shown in Fig. 1. Day 1 scores before and during menstruation in the LEP (99.8 and 96.9, respectively) and non-LEP (100.33 and 101.8, respectively) groups were consistent. Scores were significantly reduced at days 60 and 120 in the LEP, but not in the non-LEP group: in the premenstrual period, mean total mMDQ scores in the LEP group decreased to 84.5 by day 60 (P < 0.01) and to 72.8 by day 120 (P < 0.01); respective scores in the non-LEP group were 96.9 and 94.7 at day 60 and day 120 (not significant). A similar trend was observed during the menstrual period: mean total mMDQ scores in the LEP group decreased to 82.8 at day 60 (P < 0.01) and to 72.6 at day 120 (P < 0.01); respective scores in the non-LEP group were 99.7 at day 60 and 95.4 at day 120 (not significant).
Fig. 1.
Changes in mMDQ total score in the LEP and non-LEP groups before and during menstruation. LEP low-dose estrogen/progestin (cyclic or extended), mMDQ modified Menstrual Distress Questionnaire, NS not significant. **P < 0.01 (vs. day 1)
During the premenstrual and menstrual periods, significant reductions from baseline were observed at day 60 and day 120 in all six domains of the mMDQ in the LEP group (P < 0.01 for all) (Supplementary Table S1). By contrast, in the non-LEP group, numerical decreases occurred in each domain, and only water retention (before menstruation) and pain and behavioral change (during menstruation) showed significant changes at day 120.
Change in HRQL Over Time
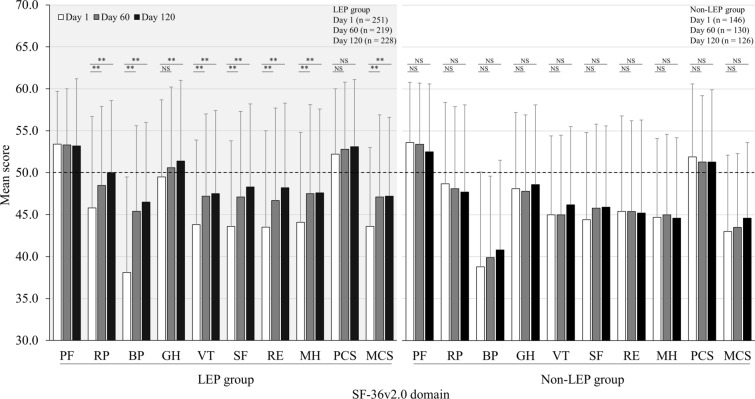
The SF-36v2.0 PCS at day 1 was 52.2 in the LEP group and increased to 52.8 at day 60 and 53.1 at day 120; in the non-LEP group, PCS was 51.9 at day 1 with negligible changes during the study period. In the LEP group, an improvement in the MCS was observed over time, with values on days 1, 60, and 120 of 43.6, 47.1, and 47.2, respectively. In the non-LEP group, MCS increased mildly throughout the study period.
Changes in HRQL according to each domain of the SF-36v2.0 are shown in Fig. 2. Notably, bodily pain (BP) in the LEP group was significantly improved. The value at day 1 was 38.1, which was much lower (indicating worse pain) than the Japanese standard norm of 50; however, the domain value increased to 45.4 at day 60 and 46.5 at day 120. In the non-LEP group, BP also improved mildly from the day 1 value of 38.8 to 39.9 and 40.8 at days 60 and 120, respectively. In the LEP group, the role emotional (RE) and mental health (MH) domains showed improvement. The mean RE value at day 1 (43.5) increased to 46.7 and 48.2 at days 60 and 120, respectively, while the mean MH value at day 1 (44.1) increased to 47.5 and 47.6 at days 60 and 120, respectively. By contrast, in the non-LEP group, RE and MH values did not change appreciably from baseline throughout the study period. Similar trends were observed for vitality (VT) and social functioning, with significant improvements over time observed in the LEP group and only mild changes in the non-LEP group.
Fig. 2.
Change in SF-36v2.0 total score in the LEP and the non-LEP groups during menstrual periods. The dotted line indicates the standardized mean Japanese norm score. BP bodily pain, GH general health, LEP low-dose estrogen/progestin (cyclic or extended), MCS mental component score, MH mental health, NS not significant, PCS physical component score, PF physical functioning, RE role emotional, RP role physical, SF social functioning, SF-36v2.0 36-Item Short-Form Health Survey version 2.0, VT vitality. **P < 0.01 (vs. day 1)
Change in Work Productivity Over Time
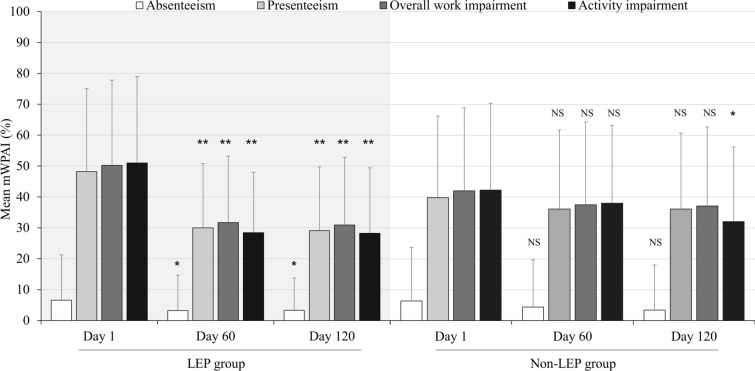
mWPAI data demonstrated that although rates of absenteeism at day 1 were consistent between the LEP (6.6%) and non-LEP (6.4%) groups (Fig. 3), absenteeism gradually decreased over time in both groups, with rates at day 120 of 3.3% and 3.4%, respectively.
Fig. 3.
Change in mWPAI in the LEP and the non-LEP groups during menstrual periods. LEP low-dose estrogen/progestin (cyclic or extended), NS not significant, mWPAI modified Work Productivity and Activity Impairment Questionnaire. *P < 0.05 (vs. day 1), **P < 0.01 (vs. day 1)
Baseline presenteeism was 48.2% and 39.8% in the LEP and the non-LEP groups, respectively, with the LEP group showing greater health-related impairments while working at day 1. There was a substantial improvement in presenteeism in the LEP group, with a 19.1% decrease by day 120, while in the non-LEP group, there was a 3.7% decrease in presenteeism throughout the 120-day study period.
Similar trends were observed in overall work impairment due to health and activity impairment. Mean overall work impairment due to health substantially improved from 50.2% to 31.7% (day 60; P < 0.01). Mean activity impairment also improved from 51.0% to 28.4% by day 60. Both factors continued to improve, albeit mildly, until day 120 (Fig. 3). By contrast, in the non-LEP group, there were only mild decreases in overall work impairment and activity impairment: 37.5 and 38.0 at day 60, and 37.1 and 32.1 at day 120, respectively (Fig. 3).
Subgroup Analysis
Subgroup analyses were performed by stratifying the following four categories: extended/cyclic LEP regimen, type of dysmenorrhea, presence of concomitant drugs, and severity of dysmenorrhea. After stratification by regimen and concomitant drugs, changes in mMDQ, SF-36v2.0, and mWPAI scores remained largely unaffected (Supplementary Tables S2, S3, S4, and S5). However, in terms of severity of dysmenorrhea, changes in mMDQ, SF-36v2.0, and mWPAI scores were affected (Supplementary Table S2); thus, severity may be a confounder in this analysis. After the patients were divided into LEP or non-LEP groups and stratified by dysmenorrhea type, those with secondary dysmenorrhea showed a slower improvement trend than those with primary dysmenorrhea on several subscales of SF-36v2.0 (Supplementary Table S3). Conversely, absenteeism in patients with primary dysmenorrhea was not improved by LEP on day 60 and day 120, while patients with secondary dysmenorrhea showed improvement on both day 60 and day 120 (P < 0.05, P < 0.01, respectively) (Supplementary Table S3).
Although the subanalysis by regimen and concomitant drug did not affect the change in mMDQ total score, SF-36v2.0, or mWPAI scores, in some domains of SF-36v2.0, P values tended to be smaller with an extended regimen than with a cyclic regimen, especially at day 60 (Supplementary Table S5). In addition, there was a difference in absenteeism throughout the observational period. Change in absenteeism from baseline in patients who received an extended regimen was − 4.4% at day 60 (P < 0.01) compared with − 1.4% (not significant) for cyclic LEP. By day 120, change in absenteeism from baseline in patients who received an extended regimen was − 3.4% (P < 0.05); it was − 3.0% with a cyclic regimen (not significant) (Supplementary Table S5).
Correlation Between mMDQ/SF36 and mWPAI at Day 120
The correlation coefficients between mMDQ and presenteeism, overall work impairment, and activity impairment were greater than 0.4 (Supplementary Table S6). For SF-36v2.0, PCS and MCS did not show a correlation (0.3); however, a correlation was observed for role physical (RP), BP, VT, RE, and MH (0.3 or higher).
Discussion
This non-interventional, prospective, observational study of patients with dysmenorrhea treated with LEP or non-LEP demonstrated substantial improvements in menstrual symptoms, HRQL, and work productivity before and during menstruation with LEP treatment. The data in the LEP group remained consistent throughout the MMRM, strongly suggesting that LEP may contribute to improvements in dysmenorrhea symptoms. The results indicated that the LEP group showed greater improvements for LEP vs. non-LEP in all domains of the mMDQ, in most subscales of the SF-36v2.0, and mWPAI, although these results were exploratory and may need to be interpreted with caution.
Notably, some of the most burdensome symptoms of dysmenorrhea appear to be ameliorated by LEP treatment, based on the statistically significant improvements in all mMDQ domain scores both before and during menstruation. From the perspective of a distribution-based method of minimal clinically important difference, there was a change in mMDQ total score of more than 0.5 SDs in the premenstrual and menstrual periods, which may be considered as a clinically meaningful change [22, 23]. Although there was a significant decrease in negative affect scores in the LEP group, the scores changed only marginally in the non-LEP group. Thus, we can hypothesize that LEP treatment may positively impact MH. By contrast, the result suggests that non-LEP treatment ameliorated pelvic pain during menstruation; however, it did not contribute to improvement in pain over a short period; furthermore, it did not appear to contribute to relief of other symptoms.
In the SF-36v2.0 domain values, there were no statistically significant changes in PCS or PF values throughout the study period. This was inconsistent with results from a previous post-marketing surveillance study of EE/DRSP (Yaz®) [6], in which PCS, MCS, and all SF-36v2.0 domain values showed statistically significant improvements from baseline following treatment. Notably, there were more patients with mild symptoms and fewer patients with severe symptoms in our study (none, mild, moderate, severe: 6.4%, 33.1%, 47.2%, 13.1%, respectively) compared with the previous study (2.7%, 23.7%, 53.2%, 20.4%, respectively) [6]. Baseline PCS was already 52, which was the same PCS as that after taking LEP in the previous study. This may have affected the results.
The greatest change in SF-36v2.0 domains was observed in BP, which was consistent with the mMDQ results. The minimal important difference (MID) of SF-36 is a change of at least 5, which corresponds to a change of 0.5 SDs; in this analysis, BP and RE showed a change of greater than 5, indicating a clinically meaningful significant improvement. Moreover, the statistically significant changes in the MCS, RE, and MH scores were consistent with the improvement in the negatively affected mMDQ domain score. Taken together, these data suggest that LEP treatment is beneficial for reducing pelvic pain due to dysmenorrhea and may also relieve negative emotions, such as depression and anxiety, before and during menstruation.
It is well known that dysmenorrhea is a societal issue because it can negatively affect relationships with friends, family, and colleagues [13–15]. We found that the SF-36v2.0 SF values substantially improved with LEP treatment; thus, using LEP could benefit both patients and society. Conversely, no statistically significant differences occurred in domain values related to MH in the non-LEP group. Therefore, LEP may be recommended over non-LEP for patients who are prone to experiencing depressive feelings or anxiety and pain before and during menstruation.
Regarding work productivity in the LEP group, all domains of the mWPAI improved significantly. These results suggest that LEP treatment in women with dysmenorrhea can contribute to improved work productivity. Notably, there was also a substantial decrease from pre- to post-treatment in presenteeism (P < 0.01). It has been reported that financial loss related to work productivity is largely related to presenteeism rather than absenteeism [24]. Thus, improvement in work productivity is another measure from which we can conclude that LEP treatment could benefit both patients and society. Subgroup analysis showed that no improvement in absenteeism was found in patients with primary dysmenorrhea, but improvement was shown in those with secondary dysmenorrhea. In general, patients with secondary dysmenorrhea tend to have more severe symptoms including pelvic pain, and LEP may improve absenteeism in these patients. The non-LEP group also showed a decrease in absenteeism from 6.4% to 3.4%, similar to that seen in the LEP group; however, it was not statistically significant. The reason for this may be that the sample size of the non-LEP group was smaller than that of the LEP group and that the degree of data variation was greater in the non-LEP group than in the LEP group. Although only activity impairment at day 120 showed a statistically significant improvement from baseline, these results could have been different with a larger sample size. Nevertheless, on the basis of the aforementioned results and the results of group comparisons, LEP may alleviate various symptoms and benefit patients.
In the 2020 update to the Japanese treatment guidelines for dysmenorrhea [25], an extended rather than cyclic LEP regimen is recommended. Our subgroup analysis showed that there was a tendency for patients receiving an extended regimen to have a greater reduction in mMDQ scores by day 60. Moreover, in terms of SF-36v2.0 scores, an extended regimen elicited statistically significant improvements in some subscales (RP, RE, SF, and MH) at day 60, whereas a cyclic regimen did not. However, although improvement may have occurred more rapidly with the extended regimen, the scores for each regimen were similar at day 120, indicating that a cyclic regimen can ultimately produce the same outcome for patients.
In the LEP group, concomitant use of NSAIDs and/or Chinese herbal medicine was allowed as these medications can be purchased at drugstores and pharmacies specializing in Chinese herbal medicine without a prescription in Japan, making it difficult to control the patients’ use of NSAIDs or Chinese herbal medicine in this observational study. As patients begin to take LEP, the proportion of concomitant drugs decreases, but we stratified the results to see if there was an impact on each score, as concomitant drug use could potentially bias the findings. The results showed that stratification did not significantly change the scores for mMDQ and SF-36v2.0; however, for absenteeism in mWPAI, the group without concomitant medication (LEP only) showed a statistically significant difference but not for the group with concomitant medication. This may be because the group with the combination drug included more patients with high severity and did not show enough improvement with treatment to show a significant difference in absenteeism. Our subgroup analysis showed that a significant reduction in absenteeism was seen with the extended regimen, thus indicating fewer absences from work or school. Our study showed that the cyclic LEP regimen relieved pelvic pain and burdensome symptoms due to dysmenorrhea. However, cyclic regimens allow hormone withdrawal bleeding to occur at regular intervals, and it is known that pain due to dysmenorrhea generally peaks around the time of menstruation [26]. An extended LEP regimen available in Japan makes it possible to take EE/DRSP or levonorgestrel/EE for up to 120 and 77 days, respectively, resulting in a reduced menstrual frequency, which may reduce the number of painful episodes for patients with dysmenorrhea and may limit situations in which patients miss work or school.
The correlation coefficients between mWPAI subscales other than absenteeism and mMDQ were 0.4 or higher, indicating a correlation; thus, some improvement in mMDQ will be related to improved work productivity. Of the SF-36v2.0 domains, RP, BP, VT, RE, and MH were correlated with all subscales other than absenteeism. Thus, LEP can positively impact physical and mental improvements for women with dysmenorrhea; in turn, these improvements may translate into improved work productivity.
Study limitations included the possibility of drug selection bias and patient selection bias due to the non-interventional observational study design; the disparate number of patients in each group (there were fewer in the non-LEP group), which may have caused selection bias and reduced the power to observe statistical significance; the lack of a washout period to offset the influence of previous treatments; and that the timing of treatment initiation may have differed between the LEP and non-LEP groups, potentially confounding the data. Patients visiting gynecologists often have relatively severe dysmenorrhea symptoms, resulting in possible participant bias. Each LEP formulation has a different amount of EE and type of progestin. Because the response of patients with secondary dysmenorrhea to LEP depends on these factors, the possibility of bias cannot be ruled out. Additionally, severely affected patients may have taken other non-prescription medications before visiting a gynecologist; hence, the true day 1 scores or values may be masked. Finally, the follow-up period for this study was 120 days, which may be too short to accurately evaluate outcomes. In the future, it would be interesting to conduct a study with a more extended follow-up period, which may reveal changes in items for which no significant differences were found in the current study.
Conclusions
This study showed that physical and mental symptoms, HRQL, and work productivity improved significantly after LEP treatment in Japanese patients with dysmenorrhea. In addition, improvements in menstrual symptoms appeared to correlate with work productivity for patients with dysmenorrhea. LEP may be an effective treatment option for women with significant benefits to improve the physical, emotional, and societal aspects of dysmenorrhea.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients, their families, and all investigators involved in the study.
Funding
This study, including the journal’s Rapid Service fees, was supported by Bayer Yakuhin, Ltd., Japan. The sponsor reviewed and provided comments on the study concept and protocol. The sponsor was also involved in the review and approval process for this manuscript.
Medical Writing Assistance
Medical writing assistance was provided by Nila Bhana, MSc (Hons), of Edanz, Japan, in accordance with Good Publication Practice guidelines (https://www.ismpp.org/gpp3) and was funded by Bayer Yakuhin, Ltd. Japan.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Osamu Yoshino, Noriko Takahashi; Acquisition of data: Noriko Takahashi; Analysis and interpretation of data: Osamu Yoshino, Noriko Takahashi, Yoshimi Suzukamo; Writing—original draft preparation: Osamu Yoshino, Noriko Takahashi, Yoshimi Suzukamo; Writing—review and editing: Osamu Yoshino, Noriko Takahashi, Yoshimi Suzukamo; Statistical analysis: Osamu Yoshino, Noriko Takahashi, Yoshimi Suzukamo; Provision of study materials or patients: Osamu Yoshino, Noriko Takahashi; Funding acquisition: Noriko Takahashi; Administrative, technical, or logistic support: Noriko Takahashi; Supervision: Osamu Yoshino, Yoshimi Suzukamo.
Prior Presentation
The main results of this study have been published at the 36th Annual Meeting of the Japanese Society for Menopause and Women's Health (Osaka, Japan) on 16–19 November 2021.
Disclosures
Noriko Takahashi is an employee of Bayer Yakuhin, Ltd. Osamu Yoshino and Yoshimi Suzukamo have no conflicts of interest to declare. The sponsor (Bayer Yakuhin, Ltd.) funded the research and publication costs including Rapid Service Fees.
Compliance with Ethics Guidelines
The study was approved by the Takahashi Clinic Institutional Review Board, Hyogo, Japan (approval no. 202009-01), and was conducted in accordance with the Declaration of Helsinki, Ethical Guidelines for Medical and Health Research Involving Human Subjects, and Japanese law and regulation. All patients provided informed consent via an online consent form in order to participate in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Ju H, Jones M, Mishra G. The prevalence and risk factors of dysmenorrhea. Epidemiol Rev. 2014;36:104–113. doi: 10.1093/epirev/mxt009. [DOI] [PubMed] [Google Scholar]
- 2.Chen CX, Draucker CB, Carpenter JS. What women say about their dysmenorrhea: a qualitative thematic analysis. BMC Womens Health. 2018;18:47. doi: 10.1186/s12905-018-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proctor M, Farquhar C. Diagnosis and management of dysmenorrhoea. BMJ. 2006;332:1134–1138. doi: 10.1136/bmj.332.7550.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update. 2015;21:762–768. doi: 10.1093/humupd/dmv039. [DOI] [PubMed] [Google Scholar]
- 5.Nagy H, Khan MAB. Dysmenorrhea. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK560834/. 2021. Accessed Aug 31, 2021.
- 6.Tanaka E, Momoeda M, Osuga Y, et al. Burden of menstrual symptoms in Japanese women: results from a survey-based study. J Med Econ. 2013;16:1255–1266. doi: 10.3111/13696998.2013.830974. [DOI] [PubMed] [Google Scholar]
- 7.Osuga Y, Hayashi K, Kobayashi Y, et al. Dysmenorrhea in Japanese women. Int J Gynaecol Obstet. 2005;88:82–83. doi: 10.1016/j.ijgo.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Japan Society of Obstetrics and Gynecology, and Japan Association of Obstetricians and Gynecologists. [Guideline for gynecological practice in Japan, 2017]. https://jglobal.jst.go.jp/detail?JGLOBAL_ID=200902266877023542. Accessed Nov 17, 2021. (In Japanese).
- 9.Ministry of Health, Labour and Welfare. [Health Sciences Research: Children’s Household Comprehensive Research Project; a questionnaire survey of 10,000 general women]. https://research-er.jp/projects/view/129384. Accessed Sep 4, 2021. (In Japanese).
- 10.Unsal A, Ayranci U, Tozun M, Arslan G, Calik E. Prevalence of dysmenorrhea and its effect on quality of life among a group of female university students. Ups J Med Sci. 2010;115:138–145. doi: 10.3109/03009730903457218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong CL. Health-related quality of life among Chinese adolescent girls with dysmenorrhoea. Reprod Health. 2018;15:80. doi: 10.1186/s12978-018-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabuchi Y, Yoshidome A, Ban N, Kumagai Y, Kusama T. A survey of the relationship between HRQoL and menstrual symptoms in Japanese patients with endometriosis. Health Care. 2016;58:567–572. [Google Scholar]
- 13.Eryilmaz G, Ozdemir F, Pasinlioglu T. Dysmenorrhea prevalence among adolescents in eastern Turkey: its effects on school performance and relationships with family and friends. J Pediatr Adolesc Gynecol. 2010;23:267–272. doi: 10.1016/j.jpag.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Wong LP, Khoo EM. Dysmenorrhea in a multiethnic population of adolescent Asian girls. Int J Gynaecol Obstet. 2010;108:139–142. doi: 10.1016/j.ijgo.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz MI, Rangel-Flores E, Carrillo-Alarcon LC, Veras-Godoy HA. Prevalence and impact of primary dysmenorrhea among Mexican high school students. Int J Gynaecol Obstet. 2009;107:240–243. doi: 10.1016/j.ijgo.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Kawaguchi R, Matsumoto K, Akira S, et al. Guidelines for office gynecology in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2017 edition. J Obstet Gynaecol Res. 2019;45:766–786. doi: 10.1111/jog.13831. [DOI] [PubMed] [Google Scholar]
- 17.Momoeda M, Akiyama S, Tanaka K, Suzukamo Y. Quality of life in Japanese patients with dysmenorrhea treated with ethinylestradiol 20 μg/drospirenone 3 mg in a real-world setting: an observational study. Int J Womens Health. 2020;12:327–338. doi: 10.2147/IJWH.S238460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumano S. GPSP: good post-marketing study practice. Nihon Yakurigaku Zasshi. 2012;140(2):81–84. doi: 10.1254/fpj.140.81. [DOI] [PubMed] [Google Scholar]
- 19.Moos RH. The development of a menstrual distress questionnaire. Psychosom Med. 1968;30:853–867. doi: 10.1097/00006842-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto S, Ishimori S, Sunaya T, Watanabe A, Sato S. The evaluation of safety and efficacy of drospirenone- and ethinylestradiol-containing tablet in patients with pain associated with endometriosis and patients with dysmenorrhea—report of interim analysis of use-result survey. Therapeutic Res. 2020;41:381–396. [Google Scholar]
- 22.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;4:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 23.King MT. A point of minimal important difference (MID): a critique of terminology and methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11:171–184. doi: 10.1586/erp.11.9. [DOI] [PubMed] [Google Scholar]
- 24.Tsuno Y. Health and productivity of medical professionals in medical institutions: from the viewpoint of health management. Soc Secur Res. 2019;3:492–504. [Google Scholar]
- 25.The Japan Society of Obstetrics and Gynecology/The Japan Society for Menopause and Women’s Health. [Guidelines concerning the use of OC/low-dose estrogen–progestin 2020]. 2021. https://www.jsog.or.jp/modules/journal/index.php?content_id=2. Accessed Sep 4, 2021. (In Japanese).
- 26.Momoeda M, Akiyama S, Yamamoto S, Kondo M, Fukai T. Burden of menstrual pain measured by heatmap visualization of daily patient-reported data in Japanese patients treated with ethinylestradiol/drospirenone: a randomized controlled study. Int J Womens Health. 2020;12:175–185. doi: 10.2147/IJWH.S242864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.