Abstract
Background:
Hospitalized children have high rates of tobacco smoke exposure; parents who smoke may be receptive to interventions during their child’s hospitalization.
Objective:
We tested the efficacy of a smoking cessation intervention for parents of hospitalized children.
Methods:
We conducted a randomized, single-blind clinical trial from 12/14–5/18 at the Children’s Hospital Colorado. Hospitalized children who had a parent who smoked tobacco were eligible. Intervention: Intervention participants received motivational interviewing sessions, 2 weeks of nicotine replacement therapy; both groups received referral to the Quitline Consenting parents completed a questionnaire; urine was collected from the child for measurement of cotinine. Our primary outcome was: 1) increase in reporting “no one is allowed to smoke anywhere” in the home (smoke-free home rule). Additional outcomes included: 2) change in child’s cotinine from baseline to 1 year, and 3) parental quitting at 1 year. Data were analyzed using Chi-square and t-tests for bivariable data, and multivariable logistic and linear regression.
Results:
Of 1641 eligible families approached, 252 were randomized (15%); 149 families had follow-up data at 12 months (59%). In the adjusted analysis, there was no difference between the groups in smoke free home rules, or child cotinine level; in an intention-to-treat analysis, 15% in the intervention group vs. 8% of controls reported quit (p=0.07).
Conclusions:
A smoking cessation intervention can be delivered to parents of hospitalized children. While hospitalization provides an opportunity to help parents quit smoking, more efficient and effective engagement strategies are needed to optimize tobacco control success.
Keywords: Smoking cessation, Secondhand tobacco smoke exposure, Clinical trials
Introduction:
Child tobacco smoke exposure (TSE) causes or contributes to a variety of illnesses, from acute otitis media,1 asthma, bronchiolitis, and pneumonia, to sudden infant death syndrome.2,3–6,7,8.9 While cigarette smoking rates in the US have declined significantly,10 great disparities in smoking rates persist, with higher rates for those with lower incomes, and for young adults.10 In 2014, 37.9% of children ages 3–11 years had biological evidence of TSE,11 this was higher for those living in poverty (47.9%). Smoke free homes are an important, but not perfect, way to reduce children’s exposure and they are associated with increased cessation rates.12 In 2013 only 54% of smoking adults reported smoke free home rules, and this rate was much lower (36%) among 25–44 year olds, who are most likely to be parents.13
Approximately 40% of hospitalized children are exposed to tobacco smoke,9,14–16 and parents may be more receptive to counseling in this setting.17–20 The hospitalization of a child represents an important opportunity to connect parents who smoke with cessation and exposure reduction resources and information.17 Building cessation programs into pediatric hospitals represents an opportunity to expand access to services.
The 5As model of smoking cessation includes asking about tobacco use, assessing willingness to quit, advising the smoker to quit, assisting with cessation efforts, and arranging for follow-up.21 Studies in the outpatient setting have demonstrated success in helping parents quit using the 5As, or Ask, Advise, Refer models.22–26 Small studies in the inpatient setting have shown a consistent baseline cessation rate of 15–18%, but no significant impact from the intervention itself,18,27 though a large scale quality improvement initiative was able to increase the percent of parents receiving an intervention.28 Since there was limited evidence about the best way to intervene, we developed a 5As-based intervention designed specifically for parents of hospitalized children and completed a randomized, controlled trial to determine its efficacy. We hypothesized that our intervention group would have 1) increased report of a smoke free home rule (no one is allowed to smoke anywhere), and for our secondary hypotheses, 2) increased parent quit rates, 3) decreased reported child exposure, and 4) decreased child exposure as measured by cotinine, compared with our control group.
Methods:
Setting:
The study took place at Children’s Hospital, Colorado from 12/2014–5/2018. Prior to the study, providers were encouraged to refer parents to the Colorado Quitline.
Enrollment and eligibility:
All children were screened for smoke exposure with the question: “Does anyone who lives in your home or who cares for your child smoke”. Families of all children ≤17 years of age admitted to the hospital during the study period who screened positive for secondhand smoke exposure, who had at least one custodial parent who smoked and who spoke English or Spanish, and who were in the hospital for ≥24 hours were invited to participate in the study.
Ethics, consent and permissions:
We obtained written consent, and assent for children >6, for all study procedures. The Colorado Multiple Institutional Review Board approved the study. The study was overseen by a Data Safety Monitoring Committee, with no concerns raised.
Compensation:
We gave participating families $25 after completion of the baseline survey and child urine collection, and additional $25 upon completion of the 6 month follow-up survey, and $75 on completion of the 12 month follow-up survey and child and parent urine collections.
Families who resided more than 100 miles away were also provided reimbursement for mileage.
Baseline Procedures:
Baseline survey:
After consent, the participant completed the baseline survey. We assessed pediatric TSE and parental tobacco using the standardized questions developed by the American Academy of Pediatrics/Julius B. Richmond Center of Excellence.29 These questions include standard demographic information and information about other potential sources of exposure, and smoke-free home and car rules.30 We asked about the child’s health history, reason for admission, and primary care practice.
Baseline urine collection:
We collected up to 50 mLs of urine from patients at enrollment using a specimen cup, hat, cotton balls placed in the diaper, a catheter (if already in place for routine care), or a urine bag applied by a trained study team member. Urine was stored at −80˚C and shipped to the University of California San Francisco (UCSF) for analysis of cotinine by liquid chromatography-tandem mass spectrometry.31,32
Allocation:
After the survey was completed, the family was randomized using a block randomization scheme in REDCap to ensure balanced allocations. The research coordinator completing follow-up surveys was blind to the randomization group.
Provider Training:
As part of the study, the INSPIRE team provided educational sessions to providers and staff; all were asked to reinforce the benefits of reducing exposure and quitting smoking for the health of the children.33 To ensure that the control group received the standard of care, they received the Ask, Assess, and Advise components, and were also offered referral to their State’s Quitline on enrollment.
All participants:
Ask:
Any child with a positive screen was automatically provided a cessation coach consult order, and tobacco smoke exposure was added to the child’s medical problem list.
Assess:
A member of the research team performed an in-depth assessment of caregiver smoking behaviors, using our baseline survey tool.
Advise:
Cessation coaches gave brief advice about the importance of quitting smoking and/or reducing their child’s exposure.
Intervention arm only:
Assist:
Cessation coaches:
We identified a diverse cohort of personnel to deliver brief motivational interviewing (MI), including respiratory therapists and research staff. Cessation coaches attended a 3–4 hour online or in person workshop on MI at the University of Colorado, and a 1-hour tobacco-specific MI training. For intervention parents, the cessation coaches offered daily brief (15–30 minute) MI sessions; our goal was 3–5 sessions, and these were done by phone after discharge. The maximum number was 10. MI directs the provider to help clients explore and resolve ambivalence to change, and to create their own goals for success;34 MI and similar strategies have been demonstrated to decrease SHS among children.35,36 Parents received information about protecting children from smoking in the home, including other smokers, or visitors. For parents interested in quitting, we focused on resolving barriers, identifying triggers, promoting alternatives, and setting a quit date. The cessation coaches had ongoing practice sessions addressing different scenarios and assessing skills, as well as periodic in-person observation by study leadership. Intervention parents were also given a referral to their state’s Quitline at the conclusion of their MI sessions.
Nicotine replacement therapy (NRT):
We offered 14 days of free dual NRT (based on funding availability), with the patch, and lozenges or gum dosed according to number of cigarettes smoked per day. We provided standard guidance on NRT use from the package insert.
Arrange:
The cessation coaches completed a discharge summary at the end of the child’s hospital stay describing the interventions delivered to the parent.
6 Month follow-up:
Parents completed a survey by phone, web, or in person at 6 months post discharge. The questions included those asked at the baseline and follow up questions about their child’s health since discharge, and any additional visits for health care. We asked parents about their own quit attempts in the prior 6 months, and about their child’s current TSE.
12 Month follow-up visit:
At the final 12-month visit the parent completed another survey, with the same questions as at 6 months.
Follow-up urine collection:
We collected approximately 30 mLs of urine from both the parent and child at 12 months. The child’s urine was prepared and tested as at baseline at UCSF using liquid chromatography-tandem mass spectrometry. Parents provided a sample in a cup, which was tested for cotinine using chemiluminescent Immunoassay at the University of Colorado Laboratories.
Chart review:
We completed a chart abstraction using Epic, including reason for admission and any complications. We classified the primary diagnosis as “respiratory illness” or “not respiratory illness” using Clinical Classifications Software Refined (CCSR) for International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM).37
Outcome measures:
Our outcome measures were: 1) Parent report of having smoke-free homes 12 months after hospitalization as measured by questionnaire (primary outcome) with a response to the question “Where is smoking allowed in your home” with the answer “No one is allowed to smoke anywhere, 2) Decreases in child cotinine levels 12 months post-hospitalization as measured by child urine cotinine analysis; and 3) Parent quit rates 6 and 12 months after hospitalization as measured by questionnaire and confirmed by parent urine cotinine analysis at 12 months. The cutoff for cessation was set as < 10 ng/mL.31
Sample size calculation:
We a priori estimated sample size based on our primary specific aim. Overall, 30% of smoking parents in a British study had a home smoking ban in place.38 Prior studies found increases in smoke free homes of 33%−42%.38,39 Using a conservative estimate of a 15% increase in home smoking bans for the intervention group and 80% power (two-tailed alpha=0.05), we planned to recruit 150 families per arm, for a total of 300 patients. Ultimately, we enrolled 252 hospitalized children, with at least one parent who smoked. This would have allowed us to detect a ∼17% difference with 80% power (alpha=0.05) between intervention and control groups. 40
Statistical analysis:
Baseline characteristics were compared using Pearson’s χ2 (Fisher’s exact when appropriate) for categorical data and t tests or Wilcoxon rank sum tests for continuous variables. The number of motivational interviewing sessions was examined using descriptive statistics to assess intervention fidelity.
To compare changes in smoke free homes over time (primary outcome) between randomization groups, multivariable longitudinal logistic regression was used with an interaction term between time points (baseline and 12 months) and treatment group. Baseline demographics were chosen based on clinical importance and statistical association with outcome and added to the model as covariates (p<0.1).
To analyze child cotinine levels, single imputation was used for values below the limit of quantitation (LOQ), replacing those values <LOQ with LOQ/2. Children with differences in cotinine levels from baseline greater than 50 ng/ml were considered outliers. Regression analysis was performed with and without these outliers. Multivariable longitudinal linear regression with log transformed cotinine was performed with an interaction term for randomization group and time first, and again after adding covariates to the model. An analysis of only those with follow up for parent reported quitting (secondary outcome) was performed using multivariable longitudinal logistic regression. Confounders were chosen based on clinical importance and statistical association with outcome (p<0.1).
Three sensitivity analyses were performed. First, the potential effect of 5 outlying values was evaluated in the analysis of cotinine levels. Second, families who were unable to be reached at follow up were assumed to have not quit and were analyzed in the original group to which they were assigned for the primary analyses (intention to treat analysis, ITT). In the ITT analysis, cross-sectional multivariable logistic regression models were used assuming loss to follow up as not quit. Third, a sensitivity analysis of the intervention effect by confirmed quit based on cotinine level, parent report quit, and NRT/Ecig use was assessed by multivariable logistic regression. Since NRT or ECIG use could contribute to cotinine levels, these factors were used to further classify subjects who had high cotinine levels but self reported as quit. If NRT or ECIG was reported as used in these subjects, they were considered to be quit.
Other analyses included an agreement analysis of parent report with parent urinary cotinine levels, assessing kappa, Gwet’s AC1, and percent agreement. Parent cotinine values was dichotomized with <10 as quit and values >=10 considered not quit. Agreement was interpreted based on the guidelines of Landis & Koch:41 <0.2 poor, 0.21–0.4 fair, 0.41–0.6 moderate, 0.61–0.8 strong, >0.8 almost perfect. All statistical analyses were performed using SAS v9.4.42 All statistical tests were performed as two tailed tests with a level of significance of 0.05.
Results:
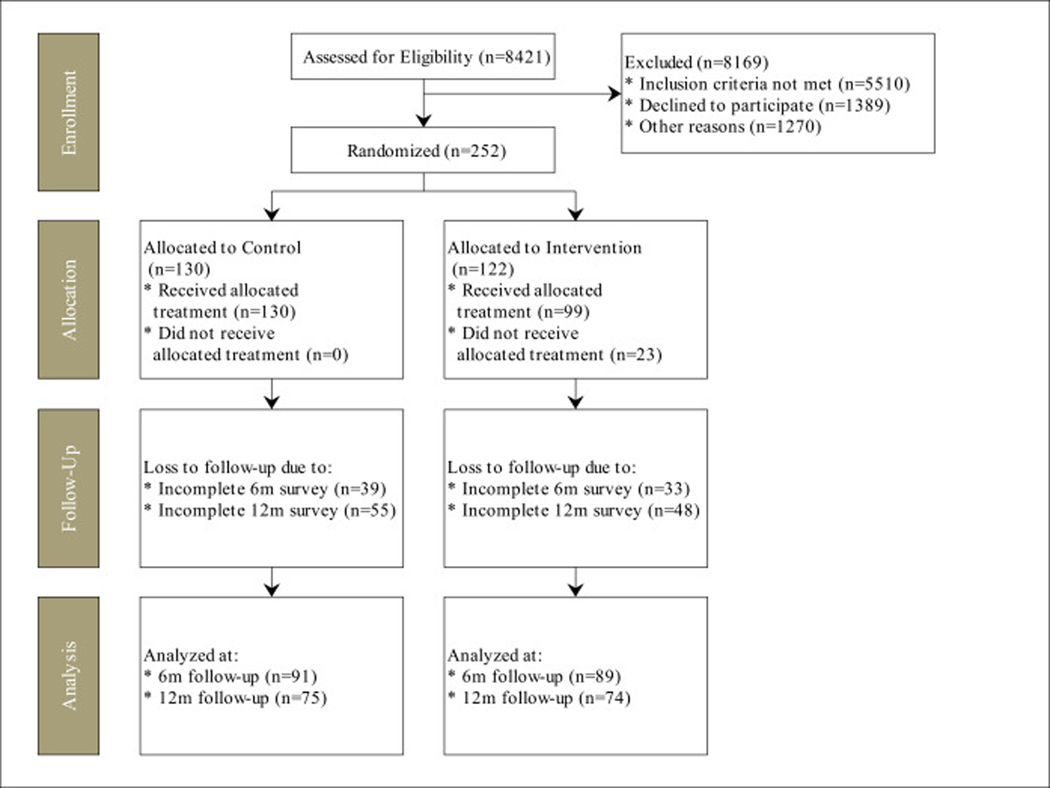
Of 1641 eligible families approached, 263 enrolled in the study (16%), 11 withdrew prior to randomization; our CONSORT diagram is in Figure 1. There were 122 families allocated to the intervention group, with 74 (61%) with 12 month follow up, and 130 families allocated to the control group, with 75 (58%) with 12 month follow up. Some children were unable to produce urine during the study visit; samples were obtained from 214 at baseline and 106 children at follow-up, and there were 115 parents with cotinine values at 12 months. At the baseline assessment (Table 1) 73% of the intervention vs. 64% of control parents reported smoke-free home rules (p=.13), and there was no difference in the child’s geometric mean cotinine level (1.0 ng/mL for the intervention group vs. 0.9 ng/mL for the control group; p=.70).
Figure 1.
CONSORT diagram for the INSPIRE Study
Table 1:
Baseline characteristics for control and intervention groups
| Variables | Total (n=252) | Control (n=130) | Intervention (n=122) | P-value |
|---|---|---|---|---|
| Child’s Gender | ||||
| Male | 145 (58) | 79 (61) | 66 (54) | 0.28 |
| Female | 107 (42) | 51 (39) | 56 (46) | |
| Age range of child | ||||
| Infants 0–1 yrs | 72 (29) | 34 (26) | 38 (31) | 0.72 |
| Toddlers 1–2 yrs | 54 (21) | 31 (24) | 23 (19) | |
| Preschool 3–4 yrs | 27 (11) | 15 (12) | 12 (10) | |
| Grade school 5–12 yrs | 76 (30) | 40 (31) | 36 (30) | |
| Teens 13+ | 23 (9) | 10 (8) | 13 (11) | |
| Race of child | ||||
| White | 136 (54) | 70 (54) | 66 (54) | 0.26 |
| Black or African American | 28 (11) | 10 (8) | 18 (15) | |
| Other | 23 (9) | 12 (9) | 11 (9) | |
| Multiracial | 65 (26) | 38 (29) | 27 (22) | |
| Ethnicity of Child | ||||
| Not Hispanic/Latino | 158 (63) | 78 (60) | 80 (66) | 0.35 |
| Hispanic/Latino | 92 (37) | 51 (40) | 41 (34) | |
| Subject’s Relationship to Child | ||||
| Mother | 170 (69) | 86 (68) | 84 (69) | 0.77 |
| Father | 78 (31) | 41 (32) | 37 (31) | |
| Household income | ||||
| Less than $20,000 | 78 (34) | 42 (36) | 36 (33) | 0.81 |
| $20,001–$50,000 | 96 (42) | 50 (42) | 46 (42) | |
| More than $50,000 | 54 (24) | 26 (22) | 28 (25) | |
| Parent education | ||||
| Some high school or less | 39 (16) | 17 (13) | 22 (18) | 0.45 |
| Grade 12 or GED (high school graduate) | 64 (26) | 37 (29) | 27 (23) | |
| College 1 year to 3 years (some college) | 119 (48) | 63 (49) | 56 (47) | |
| College 4 years or more (college graduate) | 25 (10) | 11 (9) | 14 (12) | |
| Relationship status | ||||
| Married or member of a couple | 154 (62) | 80 (62) | 74 (61) | 0.77 |
| Single (never been married) | 62 (25) | 30 (23) | 32 (26) | |
| Divorced, widowed, separated | 34 (14) | 19 (15) | 15 (12) | |
| Home ownership | ||||
| Own home | 49 (21) | 22 (19) | 27 (24) | 0.40 |
| Rent home | 180 (79) | 93 (81) | 87 (76) | |
| Housing type | ||||
| Stand-alone housing | 151 (61) | 76 (60) | 75 (63) | 0.67 |
| Attached housing | 96 (39) | 51 (40) | 45 (38) | |
| Government assistance for housing | ||||
| No | 208 (85) | 106 (83) | 102 (86) | 0.43 |
| Yes | 38 (15) | 22 (17) | 16 (14) | |
| Categorical hours child is out of the house | ||||
| 0–10 hours | 95 (45) | 48 (43) | 47 (47) | 0.49 |
| 11–40 hours | 76 (36) | 44 (39) | 32 (32) | |
| 40+ hours | 42 (20) | 20 (18) | 22 (22) | |
| Season of enrollment | ||||
| Spring(Mar-May) | 71 (28) | 37 (28) | 34 (28) | 0.92 |
| Summer(Jun-Aug) | 41 (16) | 23 (18) | 18 (15) | |
| Fall(Sep-Nov) | 59 (23) | 29 (22) | 30 (25) | |
| Winter(Dec-Feb) | 81 (32) | 41 (32) | 40 (33) | |
| Time to urine collection | ||||
| <=24 hours | 38 (17) | 18 (16) | 20 (19) | 0.54 |
| >24 hours | 180 (83) | 95 (84) | 85 (81) | |
| Parent’s Age | ||||
| MEAN (STD) | 32.0 (7.4) | 32.2 (7.6) | 31.8 (7.3) | 0.69 |
Intervention fidelity:
In the intervention group, 82% of the parents received motivational interviewing (the most common reason for not receiving was discharge prior to the cessation coach being able to engage). Parents receiving MI had on average 3.1 sessions (range 1 to 10), over an average of 5.5 weeks (range 1 to 23); the median total number of MI minutes was 452 (range 5 to 201). Most (59%) of the parents were given NRT, and 34% set a quit date at the first visit.
Outcomes:
In the unadjusted analyses, the primary outcome of smoke free home rules, at the 12 month follow up assessment, 75% of the intervention group and 73% of the control group reported smoke-free home rules (p=.74) (Table 2). For a secondary outcome of cotinine levels, geometric mean cotinine levels in both groups had increased, to 1.4 ng/mL in the intervention group and 1.6 ng/mL in the control group (p=.57). Of the parents who followed up at 12 months, 25% of those that received the intervention parents vs. 15% of those who received the control had quit smoking; p=0.13.
Table 2:
Bivariate comparison on outcomes measures for the control and intervention groups
| Time | Outcome | Total n(%) | Control n(%) | Intervention n(%) | P-value |
|---|---|---|---|---|---|
| Baseline | n =252 | n =130 | n =122 | ||
| Smoke free home rule (Primary) | |||||
| No one is allowed to smoke anywhere | 165 (68) | 79 (64) | 86 (73) | 0.13 | |
| Smoking is permitted in some places/any where | 77 (32) | 45 (36) | 32 (27) | ||
| Missing | 10 | 6 | 4 | ||
| Urinary Cotinine (n=214) | |||||
| Geometric Mean (95%CI) | 0.9 (0.8,1.1) |
1.0 (0.8,1.3) |
0.9 (0.7,1.2) | 0.70 | |
| 6 month | n =180 | n =91 | n =89 | ||
| Smoke free home rule (Primary) | |||||
| No one is allowed to smoke anywhere | 130 (73) | 66 (73) | 64 (73) | 0.98 (P) | |
| Smoking is permitted in some places/any where | 49 (27) | 25 (27) | 24 (27) | ||
| Missing | 1 | 0 | 1 | ||
| Parent report quit | |||||
| No | 134 (79) | 72 (83) | 62 (75) | 0.20 (P) | |
| Yes | 36 (21) | 15 (17) | 21 (25) | ||
| Missing | 10 | 4 | 6 | ||
| 12 month | n =149 | n =75 | n =74 | ||
| Smoke free home rule (Primary) | |||||
| No one is allowed to smoke anywhere | 109 (74) | 54 (73) | 55 (75) | 0.74 | |
| Smoking is permitted in some places/any where | 38 (26) | 20 (27) | 18 (25) | ||
| Missing | 2 | 1 | 1 | ||
| Urinary Cotinine (n=106) | |||||
| Geometric Mean (95%CI) Parent report quit No Yes Missing |
1.5 (1.1,2.1) 112 (80) 28 (20) 9 |
1.6 (1.0,2.7) 58 (85) 10 (15) 7 |
1.4 (0.8,2.3) 54 (75) 18 (25) 2 |
0.57 0.13 (P) |
In the regression with interaction analyses, the primary analysis of the trial (Table 3) showed no effect of the intervention in the outcome of smoking ban, with a difference in the proportion of homes with smoking bans of 75% (95% CI: 70, 80) vs 76% (95% CI: 72, 81) in the intervention versus the control group at 12 months (p=0.23). In the secondary outcomes there was no evidence of an intervention effect with differences of 1.85 (95% CI: 1.35,2.54) ng/mL vs +1.35 (95% CI: 1.06,1.72) ng/mL (p=0.24) in the geometric mean cotinine levels at 12 months, and 25% (95% CI: 21, 29) vs 17% (95% CI: 14,20) (p=0.26) in the proportion of report quit at 12 months. When analyzed using an intention-to-treat analysis of parent report quit, showed no significant difference between treatment and control groups, with a difference of 15% in the intervention group vs. 8% in the control group in the proportion of parent report quit (p=0.07) (Table 4).
Table 3.
Analysis of primary and secondary outcomes. Expected means of outcomes (95% confidence limits)^
| Outcome | Time (Months | Control | Intervention | Pvalue* |
|---|---|---|---|---|
| Smoke free home rule | 0 | 0.69 (0.65,0.73) | 0.70 (0.66,0.74) | 0.23 |
| Smoke free home rule | 6 | 0.77 (0.73,0.81) | 0.71 (0.67,0.76) | |
| Smoke free home rule | 12 | 0.76 (0.72,0.81) | 0.75 (0.70,0.80) | |
| Cotinine | 0 | 0.91 (0.75,1.10) | 0.93 (0.75,1.17) | 0.24 |
| Cotinine | 12 | 1.35 (1.06,1.72) | 1.85 (1.35,2.54) | |
| Parent report quit | 6 | 0.21 (0.18,0.24) | 0.21 (0.18,0.25) | 0.26 |
| Parent report quit | 12 | 0.17 (0.14,0.20) | 0.25 (0.21,0.29) |
Expected mean proportions are reported for smoke free home rule or parent report quit outcomes. Expected geometric means are reported for cotinine outcome.
pvalue for interaction between time and randomization group. The model contains a term for time and the interaction between time and randomization group.
Table 4:
Parent self-report of quit (intention to treat)
| Time | Variables | Control n(%) | Interventio n n(%) | P-value* | |
|---|---|---|---|---|---|
| 6 month | n =130 | n =122 | |||
| Do you consider yourself to now be quit? | |||||
| No | 115 (88) | 101 (83) | 0.20 (P) | ||
| Yes | 15 (12) | 21 (17) | |||
| 12 month | n =130 | n =122 | |||
| Do you consider yourself to now be quit? | |||||
| No | 120 (92) | 104 (85) | 0.07 (P) | ||
| Yes | 10 (8) | 18 (15) |
In the adjusted multivariable analyses with interaction all results were consistent with the primary analyses (Table 5). The adjusting covariates for each model are listed in Table 5. A sensitivity analysis performed without the 5 outlying child cotinine values was consistent with the results in Table 3 (results not shown).
Table 5:
Multivariable regression analysis on outcomes measures
| Primary Outcome | Secondary Outcomes | |||||
|---|---|---|---|---|---|---|
| home smoking bana,* | log cotinineb,** ^ | parent report quit at 12 monthsa,*** | ||||
| Predictor | Odds Ratio (95% CI) | Geometric Mean Ratio (95% CI) | Odds Ratio (95% CI) | |||
| Randomization: Itvn vs Control | 1.16 0(.60,2.23) | 0.69 (0.41,1.16) | 1.70 (0.73,3.95) | |||
| Time: 12 mo vs baseline | 1.35 (0.65,2.82) | 1.63 (1.04,2.54) | 0.847 (0.42,1.70)^^ | |||
| Time* randomization | 0.96 (0.33,2.82) | 1.55 (0.78,3.09) | 1.21 (0.485,3.02) | |||
Longitudinal Logistici regression
Longitudinal Linear regression
additional covariates in model include receiving government assistance for housing, car rules, allowing child to ride in car of smoker, and smoking in home in last 3 months
additional covariates in model include: time spent outside home, number of smokers in home, exposed in last 24 hours, receiving government assistance for housing, car rules, allowing child to ride in car of smoker, smoking in home in last 3 months, number of cigarettes smoked per day, home owner, and attached/detached housing
additional covariates in model include : car rules and parent education
Excluding 5 subjects with extreme differences from baseline
Reference category is 6 months
For the agreement analysis, four subjects were confirmed as quit by urinary cotinine with values <=10ng/ml, and 13 subjects reported as quit but were not confirmed as quit. Overall, there was substantial agreement between parent report quit and confirmed quit by urinary cotinine (Gwet’s AC1: 0.85 (0.74,0.96), percent agreement: 89%).
Discussion:
We fielded a randomized controlled trial of an evidence-based intervention to help parents of hospitalized children quit smoking and reduce their children’s exposure, demonstrating the feasibility of offering a comprehensive hospital-based program for parents. The differences observed were not statistically significant in report of smoke-free home rules, child cotinine levels, or parent-reported quit status; in fact, smoke free home rules increased more in the control group. However, our intervention group had a trend towards higher quit rates, with clinically meaningful differences.
While we had planned our primary outcome as smoke-free home rules based on a 2012 study showing a prevalence of 30% among smoking parents in a UK study,38 our baseline rates were much higher (68%). The prior study is also more consistent with the 36% smoke free home rule proportion in 24–45 year olds from the NYTS13. The high baseline SFH rates are a positive sign overall for children’s smoke exposure, and may have been impacted by higher rates of smoke free home rules overall in Colorado, than in the national population.43.
With the more conservative intention-to-treat estimate, we saw a 15% quit rate among intervention parents, compared to an 8% quit rate among controls (p=0.07, Table S2). This effect size is similar to the quit rate found in prior studies for inpatient interventions. However, the control group quit rate was much lower than the 20% found previously.18 While it is likely that there was some follow-up bias, the differences between the groups in the more- and less-conservative analyses are similar. We believe that the intervention was successful in helping some of our parents quit smoking, and that it provided added benefit over Quitline referral alone. It is important to recognize that Quitline referral was rarely delivered as an intervention for families prior to this study; while we weren’t structured to evaluate Quitline referral as a cessation tool, our study does suggest that it can be an effective, low-burden way to bring cessation services to inpatient settings.
We found that the cotinine levels were higher at follow up than at baseline, even when controlling for the number of smokers in the home, and time since last exposure. This finding highlights the challenge of reducing exposure even with cessation; further research is needed to understand the specific reasons behind this increase, such as parents who aren’t answering honestly, or the off-gassing from third hand smoke.
Providing the MI was a challenge. Our initial reliance on respiratory therapists as cessation coaches became untenable during the winter, and we trained research staff to provide the intervention. There was large variation in numbers of sessions delivered and in number of minutes per patient. While they performed well according to our training and observed performance, we may have had better outcomes with experienced tobacco cessation providers.
While we didn’t find a statistically significant impact from our intervention on smoking cessation, the hospitalization of a child remains a critical window of opportunity to address tobacco use and exposure. This study revealed some of the challenges with offering tobacco cessation to parents of hospitalized children, including finding personnel to do the counseling needed for success. Future research is needed on how to maximize the effectiveness of cessation interventions, and deliver them to more parents, at a cost that is manageable. Adding newer technologies such as automated referrals to Quitlines, smoke-free texting programs may help to refine the intervention for increased acceptability, lower cost, and improved effectiveness. Even in our study with NRT provided at no cost, we still had a significant proportion who did not receive it. While some insurance plans will cover NRT, not all do, and the cost can be significant. Offering NRT that is both free of charge and easy to obtain, especially during a child’s hospitalization, could help more parents quit as well.
Limitations:
Due to time constraints, we were unable to meet our enrollment target of 300 dyads; this and the high rate of loss to follow-up likely limited our power to detect a difference in our outcome measures. It is likely that our population represents parents with more motivation to quit smoking than the overall population of smokers. The high rate of parents who were lost to follow-up may have limited our power to detect a difference in smoking rates; distance to follow up may have impacted this as well. While we did get MI and NRT to most of our participants, there was significant variation in the number of MI settings and total MI minutes, which may have biased our results to the null. In addition, the control group received 3 of the 5 intervention elements, including enrollment in the Quitline that also provided free NRT to parents, which may have blunted the differences between groups. Finally, we were unable to completely assess all sources of tobacco smoke exposure for both the parent and the child; both could have been exposed in other settings, and likewise thirdhand smoke can remain a reservoir for nicotine exposure even after smoke-free home rules are in place.44
Financial Disclosure Statement:
The authors have no financial relationships relevant to this article to disclose.
Conclusion
Pediatric hospitals are an important site for engaging parents in tobacco cessation. A 5As based intervention can be delivered to parents of hospitalized children, and may help some parents quit smoking. However, more efficient and effective engagement strategies are needed to reach all of the parents who need help quitting.
What’s new:
We report the results of an RCT of a smoking cessation intervention for parents of hospitalized children; finding that delivering an intervention to parents in this setting is feasible, and we still need to improve cessation rates in this population.
Acknowledgements
We would like to acknowledge the assistance of Cordelia Elaiho, MPH, in the preparation of the manuscript.
This study was funded by the National Cancer Institute R01CA181207, Intervening with smoking parents of inpatients to reduce exposure (INSPIRE), as well as support from the Flight Attendant Medical Research Institute through a grant to the American Academy of Pediatrics’ Julius B. Richmond Center of Excellence, and the Children’s Hospital, Colorado Research Institute. None of these organizations had any role in the study design, collection, analysis, or decision to publish. We would like to recognize the assistance of Cordelia Elaiho, MPH, in the manuscript preparation.
Abbreviations:
- TSE
Tobacco smoke exposure
- NRT
Nicotine replacement therapy
- MI
Motivational interviewing
Footnotes
Conflicts of Interest Statement
The authors have no conflicts of interest related to this article to disclose.
Clinicaltrials.gov: NCT02281864
Clinical trial registration: NCT02281864
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Jones LL, Hassanien A, Cook DG, Britton J, Leonardi-Bee J. Parental smoking and the risk of middle ear disease in children: a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(1):18–27. [DOI] [PubMed] [Google Scholar]
- 2.Moritsugu KP. The 2006 Report of the Surgeon General: the health consequences of involuntary exposure to tobacco smoke. Am J Prev Med. 2007;32(6):542–543. [DOI] [PubMed] [Google Scholar]
- 3.Howrylak JA, Spanier AJ, Huang B, et al. Cotinine in children admitted for asthma and readmission. Pediatrics. 2014;133(2):e355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akinbami LJ, Kit BK, Simon AE. Impact of environmental tobacco smoke on children with asthma, United States, 2003–2010. Academic pediatrics. 2013;13(6):508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butz AM, Breysse P, Rand C, et al. Household smoking behavior: effects on indoor air quality and health of urban children with asthma. Maternal and child health journal. 2011;15(4):460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oberg M, Jaakkola MS, Woodward A, Peruga A, Pruss-Ustun A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377(9760):139–146. [DOI] [PubMed] [Google Scholar]
- 7.Merianos AL, Dixon CA, Mahabee-Gittens EM. Secondhand smoke exposure, illness severity, and resource utilization in pediatric emergency department patients with respiratory illnesses. J Asthma. 2017;54(8):798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiFranza JR, Masaquel A, Barrett AM, Colosia AD, Mahadevia PJ. Systematic literature review assessing tobacco smoke exposure as a risk factor for serious respiratory syncytial virus disease among infants and young children. BMC pediatrics. 2012;12:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn A, Edwards KM, Grijalva CG, et al. Secondhand Smoke Exposure and Illness Severity among Children Hospitalized with Pneumonia. J Pediatr. 2015;167(4):869–874.e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips E, Wang TW, Husten CG, et al. Tobacco Product Use Among Adults - United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(44):1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai J, Homa DM, Gentzke AS, et al. Exposure to Secondhand Smoke Among Nonsmokers - United States, 1988–2014. MMWR Morb Mortal Wkly Rep. 2018;67(48):1342–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Department of Health and Human Services. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2012. [Google Scholar]
- 13.Kruger J, Jana A, Homa DM, et al. Smoke-free home and vehicle rules by tobacco use status among US adults. Prev Med. 2015. Sep;789–13. [DOI] [PMC free article] [PubMed]
- 14.Wilson KM, Pier JC, Wesgate SC, Cohen JM, Blumkin AK. Second-Hand Tobacco Smoke Exposure and Severity of Influenza in Hospitalized Children. J Pediatr. 2012. [DOI] [PubMed]
- 15.Andrews AL, Shirley N, Ojukwu E, Robinson M, Torok M, Wilson KM. Is secondhand smoke exposure associated with increased exacerbation severity among children hospitalized for asthma? Hospital pediatrics. 2015;5(5):249–255. [DOI] [PubMed] [Google Scholar]
- 16.Wilson KM, Gates SC, Best D, Blumkin AK, J.D. K. Admission screening for secondhand tobacco smoke exposure. Hospital pediatrics. 2012;2(1):26–33. [DOI] [PubMed] [Google Scholar]
- 17.Winickoff JP, Hillis VJ, Palfrey JS, Perrin JM, Rigotti NA. A smoking cessation intervention for parents of children who are hospitalized for respiratory illness: the stop tobacco outreach program. Pediatrics. 2003;111(1):140–145. [DOI] [PubMed] [Google Scholar]
- 18.Ralston S, Grohman C, Word D, Williams J. A randomized trial of a brief intervention to promote smoking cessation for parents during child hospitalization. Pediatr Pulmonol. 2012:n/a-n/a. [DOI] [PubMed]
- 19.Farber HJ, Walley SC, Groner JA, Nelson KE. Clinical Practice Policy to Protect Children From Tobacco, Nicotine, and Tobacco Smoke. Pediatrics. 2015;136(5):1008–1017. [DOI] [PubMed] [Google Scholar]
- 20.Winickoff JP, Hibberd PL, Case B, Sinha P, Rigotti NA. Child hospitalization: an opportunity for parental smoking intervention. Am J Prev Med. 2001;21(3):218–220. [DOI] [PubMed] [Google Scholar]
- 21.A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. American journal of preventive medicine. 2008;35(2):158–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen LJ, Noach MB, Winickoff JP, Hovell MF. Parental smoking cessation to protect young children: a systematic review and meta-analysis. Pediatrics. 2012;129(1):141–152. [DOI] [PubMed] [Google Scholar]
- 23.Rosen LJ, Myers V, Hovell M, Zucker D, Ben Noach M. Meta-analysis of Parental Protection of Children From Tobacco Smoke Exposure. Pediatrics. 2014;133(4):698. [DOI] [PubMed] [Google Scholar]
- 24.Hovell MF, Zakarian JM, Matt GE, Hofstetter CR, Bernert JT, Pirkle J. Effect of counselling mothers on their children’s exposure to environmental tobacco smoke: randomised controlled trial. Bmj. 2000;321(7257):337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hovell MF, Meltzer SB, Zakarian JM, et al. Reduction of Environmental Tobacco Smoke Exposure Among Asthmatic Children: A Controlled Trial. Chest. 1994;106(2):440–446. [DOI] [PubMed] [Google Scholar]
- 26.Nabi-Burza E, Drehmer JE, Hipple Walters B, et al. Treating Parents for Tobacco Use in the Pediatric Setting: The Clinical Effort Against Secondhand Smoke Exposure Cluster Randomized Clinical Trial. JAMA pediatrics. 2019;173(10):931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ralston S, Roohi M. A randomized, controlled trial of smoking cessation counseling provided during child hospitalization for respiratory illness. Pediatr Pulmonol. 2008;43(6):561–566. [DOI] [PubMed] [Google Scholar]
- 28.Walley SC, Mussman GM, Lossius M, et al. Implementing Parental Tobacco Dependence Treatment Within Bronchiolitis QI Collaboratives. Pediatrics. 2018;141(6). [DOI] [PubMed] [Google Scholar]
- 29.Surveys, Questionnaires, and Assessment Tools. American Academy of Pediatrics Julius B. Richmond Center of Excellence. https://www.aap.org/en-us/advocacy-and-policy/aap-healthinitiatives/Richmond-Center/Pages/Measurement-Core.aspx. Accessed 02/22/2021.
- 30.McMillen RC, Winickoff JP, Klein JD, Weitzman M. US adult attitudes and practices regarding smoking restrictions and child exposure to environmental tobacco smoke: changes in the social climate from 2000–2001. Pediatrics. 2003;112(1 Pt 1):e55–60. [DOI] [PubMed] [Google Scholar]
- 31.Jacob P 3rd, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3’-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2011;879(3–4):267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goniewicz ML, Eisner MD, Lazcano-Ponce E, et al. Comparison of urine cotinine and the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their ratio to discriminate active from passive smoking. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2011;13(3):202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torok MR, Lowary M, Ziniel SI, et al. Perceptions of Parental Tobacco Dependence Treatment Among a Children’s Hospital Staff. Hospital pediatrics. 2018;8(11):724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundahl B, Moleni T, Burke BL, et al. Motivational interviewing in medical care settings: a systematic review and meta-analysis of randomized controlled trials. Patient Educ Couns. 2013;93(2):157–168. [DOI] [PubMed] [Google Scholar]
- 35.Hovell MF, Bellettiere J, Liles S, et al. Randomised controlled trial of real-time feedback and brief coaching to reduce indoor smoking. Tobacco control. 2020;29(2):183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blaakman SW, Borrelli B, Wiesenthal EN, et al. Secondhand Smoke Exposure Reduction After NICU Discharge: Results of a Randomized Trial. Academic pediatrics. 2015;15(6):605–612. [DOI] [PubMed] [Google Scholar]
- 37.Clinical Classification Software Refined (CCSR) for ICD-10-CM diagnoses [computer program]. Agency for Healthcare Research and Quality; 2019. [Google Scholar]
- 38.Evans KA, Sims M, Judge K, Gilmore A. Assessing the knowledge of the potential harm to others caused by second-hand smoke and its impact on protective behaviours at home. J Public Health (Oxf). 2012;34(2):183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alwan N, Siddiqi K, Thomson H, Lane J, Cameron I. Can a community-based ‘smoke-free homes’ intervention persuade families to apply smoking restrictions at homes? J Public Health (Oxf). 2011;33(1):48–54. [DOI] [PubMed] [Google Scholar]
- 40.PASS 2016: Power Analysis and Sample Size Software [Internet]. 20169. https://www.ncss.com/software/pass/. Accessed 02/11/2021.
- 41.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 42.SAS. In. Cary, NC: SAS Institute.
- 43.Young W, Karp S, Bialick P, et al. Health, Secondhand Smoke Exposure, and Smoking Behavior Impacts of No-Smoking Policies in Public Housing, CO, 2014–2015. Prev. Chron. Dis. 2016;13:160008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matt GE, Quintana PJ, Hoh E, et al. Remediating Thirdhand Smoke Pollution in Multiunit Housing: Temporary Reductions and the Challenges of Persistent Reserviors. Nicotine Tob Res. 2021. Jan 22;23(2):364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]