Abstract
Purpose
Neuropsychiatric manifestation of lupus (NPSLE) is related with vitamin D (vit-D) deficiency which is possibly amenable to supplementation. This study was done to explore link of serum vit-D level and clinical mini-mental state examination (MMSE) with brain perfusion SPECT (BS) in patients with NPSLE.
Methods
Patients who underwent BS with the diagnosis of NPSLE and had serum levels of vit-D and MMSE within a span of 1 month were retrospectively included. The BS DICOM data were used to generate 3D surface images of brain for visual identification of regional hypoperfusion, and the z-scores from eZIS software and then to perform voxel-based regression analysis in order to explore association between serum vit-D level and cerebral perfusion deficit using SPM8. Distribution of serum vit-D level was checked across MMSE and BS z-score using R.
Results
A total 19 patients with means ± SD age of 28.4 ± 9.2 years, having mean levels of serum vit-D of 18.7 ± 9.8 ng/ml and mean MMSE scores 24.2 ± 1.6, had undergone BS. The eZIS-derived z-score fall in the category of normal in six (31.6%), mild perfusion deficit (PD) in 10 (52.6%) and moderate PD in three (15.8%) with the means ± SD of z-score being 0.52 ± 0.2, 1.72 ± 0.2, and 2.33 ± 0.2. Voxel-based analysis revealed significant positive correlation between vit-D level and hypoperfusion in brain regions related to cognitive function (p<0.05). Serum vit-D levels were significantly lower in NPSLE patients with lower MMSE scores as well as in higher eZIS z-score (p < 0.01).
Conclusions
Our results may support the utility of vit-D supplementation in NPSLE and applicability of BS as a clinical adjunct for monitoring response to vit-D supplementation.
Keywords: Neuropsychiatric systemic lupus erythematosus, Brain, SPECT, Voxel-based analysis, Statistical parametric mapping, Vitamin D deficiency, Cognitive function
Introduction
Over the past 20 years, neuropsychiatric lupus has been responsible for more than 15% of mortality in patients with systemic lupus erythematosus (SLE) in Asia [1]. Deficiency of vitamin D (vit-D) coexists with systemic lupus erythematosus (SLE) [2] with one causing exacerbation of the other [3]. Vit-D deficiency independently predicted cognitive impairment in SLE patients [4]. The postulated role of vit-D in human learning and memory is supported by the observation of production of vit-D in cerebellum and cerebral cortex together with the presence of vit-D receptors in cortical, limbic system, and basal ganglia structures [5, 6]. Clinical evidences indicate an immunomodulatory role of vit-D supplementation [7] while the preclinical data tends to link vit-D supplementation with suppression of neuroinflammation with a concomitant upregulation of anti-inflammatory mediators and neurotrophins [6]. Although the vit-D deficiency has links with both the progression of SLE [3, 7] as well as with the risk of SLE-unrelated cognitive declines [8, 9], there is a perceived lack of clinical evidence to demonstrate the relation of vit-D status or effect of its supplementation in patients with neuropsychiatric systemic lupus erythematosus (NPSLE).
Early research works demonstrated the roles of brain perfusion SPECT (BS) in SLE patients to find perfusion deficit despite a normal EEG [10], to find reversible lesions or subclinical CNS involvement [11] and to correlate the severity of neuropsychiatric symptoms with different combinations of cortical involvement [12]. Qualitative approaches with BS in patients with structural lesions remained unsuccessful to demonstrate an association of hypoperfusion with cognitive deficit [13, 14]. Further reports of BS in patients with NPSLE have widened the understanding of patterns and incidences of hypoperfusion [15–18] even in some cases with normal brain MRI [19], with correlation to disease duration, symptom [20, 21], and severity of cognitive dysfunction [22] as well as serum markers of cumulative tissue damage[23]. Moreover, changes of perfusion in response to immunomodulatory therapy [24–27] and discrimination between potential responders and non-responders [28] with identification of patients at risk for progression of structural change in the future [29] were also demonstrated using BS. Additional improvement of quantification methods based on SPECT [30, 31] and voxel-based morphometry [32] allowed distinction between NPSLE and non-CNS lupus. However, there have been no reports of interpretation of BS data from patients with NPSLE using the easy z-score imaging system (eZIS) which has been applied for quantitative evaluation of BS data for more than a decade [33] and being increasingly proven robust for assessment of patients with major dementias and various other neurological disorders [34–37].
This study was done on a small group of patients with rheumatologically diagnosed NPSLE undergoing radionuclide BS, to explore if their clinical severities of cognitive impairment, their serum vit-D levels, and the BS imaging features were mutually linked so that an inference can be drawn about the possible utility of vit-D supplementation for their disease improvement where BS will have an extended role for the assessment of response.
Patients and Method
Study Population
All consenting patients who underwent brain perfusion SPECT at the workplace of the first author, with the clinical diagnosis of NPSLE between January 2020 and February 2021, were retrospectively included in this study. The clinical diagnoses in these patients were confirmed in the preceding 1 to 7 years from the workplace of the fifth author with adherence to the American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes [38]. This study was approved by the Medical Research Ethics Committee (MREC) from the workplace of the first author.
Serum vitamin D assay
The serum level of vit-D was determined in each patient within one month of undergoing BS. The quantitative chemiluminescent immunoassay (CLIA) method was used for non-specific detection of both the free forms of vit-D in the serum as well as the bound forms after they got dissociated from the binding protein during the incubation. According to the laboratory reference, a level of 30–100 ng/ml was categorized as sufficient, less than 30 up to 20 ng/ml as insufficient, and less than 20 ng/ml as deficient.
Assessment of Ancillary Biomarkers
Nutritional status was assessed by anthropometric measures such as body mass index (BMI) and waist-hip ratio (WHR) as well as by blood hemoglobin concentration (Hb%). The serological tests included anti-ds DNA antibody and anti-phospholipid antibody for rheumatological confirmation of SLE and creatinine for exclusion of renal functional impairment which often occurs as a consequence of SLE and thyrotropin (TSH) for exclusion of hypothyroidism which can also cause a cognitive decline.
Assessment of Cognitive Function by the Clinician
All patients also had undergone an assessment of cognitive function within a month before undergoing the BS with the mini-mental state examination (MMSE), a clinician-administered 30-point questionnaire for the assessment of cognitive impairment (CI). A score of 30 to 25 was clinically categorized as No CI, 24 to 20 as mild CI, 19 to 10 as moderate CI, and lower than 10 as severe CI. The neuropsychiatric symptoms or a diagnosis in each patient were available from the rheumatologists’ note which was done by some other physicians belonging to the specialty of neurology or psychiatry. The operational criteria for neuropsychiatric evaluation were unknown to the authors.
SPECT Data Acquisition and Reconstruction
A pre-imaging consultation session was attended by each patient along with his or her caregiver. The purpose was an assessment of the patient’s ability to comply and cooperate during the BS imaging procedure, verbal communication regarding patient preparation and imaging procedure, documentation of clinical data, and obtaining the patient’s consent. The requirement to refrain from caffeine and alcohol on the day of the test was explained and consumption of other drugs with known effects on cerebral blood flow was checked. On the day of the test, the patient was instructed to void, and shortly thereafter they had an intravenous line in place about 10 to 20 min before the administration of radiotracer. Each patient got an intravenous injection of 600 MBq of Tc-99m-ethyl cysteinate dimer (ECD) while reclining or sitting comfortably in a dimly lit, quiet, well-ventilated space with indoor air temperature around 25°C. The patients received specific instructions to keep their eyes and ears open and refrain from speaking or reading. The resting phase or uptake phase lasted for 30 min from the injection of the radiotracer. Thereafter, BS was performed with dual-headed gamma camera (Symbia Evo Excel, Siemens, USA) equipped with low-energy and high-resolution parallel-hole collimators. For each camera, projection data were obtained in a 128 × 128 matrix through 360° rotations at steps of 2.8° for 20 s per view. Filtered back-projection and Butterworth and Ramp filter were used for SPECT image reconstruction.
Derivation of z-Score from BS DICOM Data
The easy z-score imaging system (eZIS) [36], free software released under the Asia Oceania Research Initiative Network (AORIN) Brain, was used to generate 3D surface images of the brain and z-score from BS DICOM data of each patient. The 3D surface images were visually interpreted to identify the areas of hypoperfusion. This SPM-based software is capable of voxel-based comparison of BS data with an age-matched normal database comprising of Tc-99m-ECD BS data. A z-score of more than 2 and a cluster size of more than 300 voxels were considered to indicate a severe decline in brain perfusion.
Voxel-Based Analysis
Voxel-based analysis of BS DICOM data was done using SPM 8 which included anatomical normalization into a Montreal Neurological Institute (MNI) space using the SPM8, followed by smoothing with a 12-mm FWHM Gaussian kernel. Regression analysis was then performed between brain perfusion SPECT and serum vit-D level with age as a nuisance covariate. Statistically, a height threshold of p<0.05 without multiple comparisons and an extent threshold of 300 voxels were considered significant.
Relationship Between Variables
Correlation among brain regional hypoperfusion from eZIS-generated three-dimensional (3D) surface images of brain and clinical manifestations and hierarchical clustering of those features were done using the “heatmaply” function on R. Correlation of BS z-scores and serum levels of vit-D were plotted using the “ggplot2” and the distribution of serum levels of vit-D was checked across the MMSE categories as well as across the BS z-score categories using the “ggstatsplot” [39] functions on R.
Result
Nineteen patients (16 females) with means age of 28.4 ± 9.2 years (range 18 to 45 years) having mean serum levels of vit-D of 18.7 ± 9.8 ng/ml (range 6.5 to 39.0 ng/ml), with a mean MMSE score of 24.2 ± 1.6 (range 22 to 27), mean BMI of 19.1 ± 1.9 (range 16 to 25), and waist-hip ratio 0.8 ± 0.1 (range 0.6 to 1.0), had undergone BS within the period of inclusion. The clinical manifestations or complaints among these patients included depression, forgetfulness, anxiety, headache, confusion, impaired quality of life, movement disorders, irritability, mood disorders, and speech disorders, as noted during the first consultation. All patients were serologically positive for anti-ds DNS antibody and all except one was positive for anti-phospholipid antibody. Table 1 shows the characteristics of the study patients.
Table 1.
Characteristics of study patients (n=19)
| Traits | Frequency (percentage) | mean ± SD |
|---|---|---|
| Gender and mean ± SD age in year | ||
| Female | 16 (84%) | 27.1 ± 8.7 |
| Male | 3 (16%) | 35.3 ± 10.7 |
| Symptoms* | ||
| Depression | 15 | |
| Forgetfulness | 14 | |
| Anxiety | 11 | |
| Headache | 11 | |
| Confusion | 5 | |
| Impaired quality of life | 5 | |
| Movement disorder | 4 | |
| Irritability | 1 | |
| Mood disorder | 1 | |
| Speech disorder | 1 | |
| Vitamin D status categories and mean ± SD serum levels in ng/ml | ||
| Normal | 4 (21%) | 34.3 ± 3.4 |
| Insufficient | 3 (15.8%) | 21.3 ± 0.6 |
| Deficient | 12 (63.2%) | 12.8 ± 4.9 |
| Cognitive function categories and mean ± SD of MMSE score | ||
| No CI | 6 (31.6%) | 26.2 ± 0.4 |
| Mild CI | 13 (68.4%) | 23.2 ± 0.9 |
| Areas of hypoperfusion on Brain SPECT (eZIS 3D surface view)* | ||
| No-area involved | 4 | |
| Frontal lobe | 15 | |
| Parietal lobe | 9 | |
| Precuneus | 8 | |
| Cingulate gyri | 4 | |
| Basal ganglia | 2 | |
| Temporal lobe | 1 | |
| Cerebellum | 1 | |
| eZIS Z-score from brain SPECT and mean ± SD of Z score | ||
| Normal | 6 (31.6%) | 0.52 ± 0.2 |
| Mild deficit | 10 (52.6%) | 1.72 ± 0.2 |
| Moderate deficit | 3 (15.8%) | 2.33 ± 0.2 |
*One patient may have more than one symptoms or area involved
The most common findings on eZIS-generated 3D surface images of brain were frontal hypoperfusion with or without hypoperfusion in other regions. Although four patients (two males and two female) had normal 3D surface images with their z-score belonging to the category of normal, another two patients (one male and one female) with both having MMSE scores belong to the category of “No CI” and frontal hypoperfusions on 3D surface images but normal z-scores owing to absence of hypoperfusion in their parietal lobes and precunei.
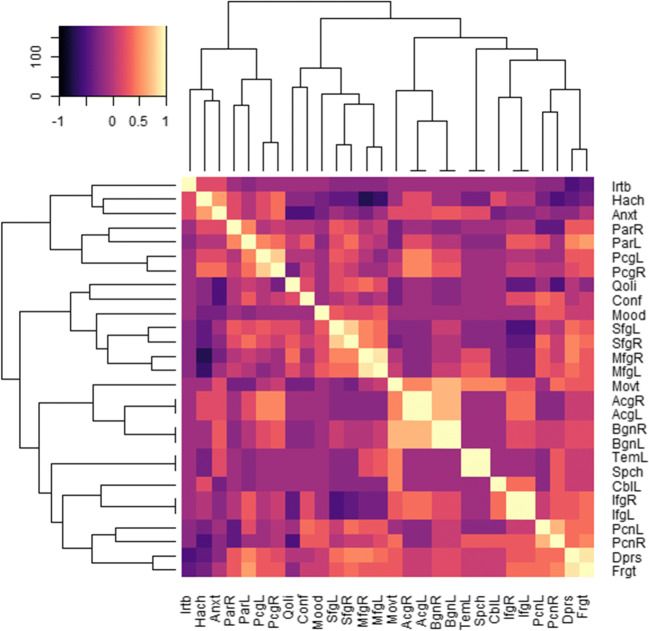
Two distinct clustered combinations of presenting features with the regional cerebral hypoperfusion were noted among the study patients (Fig. 1). Irritability, anxiety, headache, and movement disorder features were in a cluster with hypoperfusion in the regions of posterior cingulate, parietal, anterior cingulate, basal ganglia, cerebellum, and inferior frontal gyri. Movement-related features had correlations with the hypoperfusion in the bilateral basal ganglia, bilateral anterior cingulate gyri, cerebellum, and bilateral inferior frontal gyrus. Whereas, speech disorder, impaired quality of life, depression, forgetfulness, confusion, and mood-related features were in a cluster with hypoperfusion in the regions of temporal, middle frontal, superior frontal, and precuneus gyri. Temporal hypoperfusion was strongly correlated with speech-related features. Hypoperfusion in the bilateral superior frontal gyrus and bilateral middle frontal gyrus was correlated to impaired quality of life. Depression and forgetfulness along with confusion and mood-related features were correlated with hypoperfusion of bilateral precuneus. Depression and forgetfulness were further correlated with bilateral superior and middle frontal gyrus. In addition, correlation of parietal hypoperfusion with depression and forgetfulness, as well as a correlation of movement-related features with hypoperfusion in the right sided precuneus, was noted.
Fig. 1.
Correlation matrix of 19 brain regions of hypoperfusion with 10 clinical features. The rows and columns of the matrix have been ordered using a hierarchical clustering algorithms. The dendrograms are shown on the top and left side. The right cerebellum and the right temporal lobe were excluded in this plot because no patients showed hypoperfusion in those two regions. The regional hypoperfusions were identified by visual interpretation of eZIS-generated 3D surface view images of the brain and the clinical features were assigned by some other physicians belonging to the specialty of neurology or psychiatry available through the rheumatologists’ note. The regions were as follows: AcgR and AcgL, anterior cingulate gyrus right and left; BgnR and BgnL, basal ganglia right and left; CblR and CblL, cerebellum right (excluded) and left; IfgR and IfgL, inferior frontal gyrus right and left; MfgR and MfgL middle frontal gyrus right and left; PcgR and PcgL, posterior cingulate gyrus right and left; PcnR and PcnL, precuneus right and left; ParR and ParL, parietal gyrus right and left; SfgR and SfgL, superior frontal gyrus right and left; TemR and TemL, temporal lobe right (excluded) and left. The clinical features were as follows: Anxt, anxiety; Conf, confusion; Dprs, depression; Frgt, forgetfulness; Hach, headache; Irtb, irritability; Mood, mood disorder; Movt, movement disorder; Qoli, impaired quality of life; Spch, speech disorder
The mean duration of rheumatological diagnosis in all patients with NPSLE was 4.3 ± 2.1 years (range 1 to 7 years). The duration of disease was significantly shorter in vit-D insufficient category in comparison to the other two vit-D level categories while the patients with normal serum vit-D levels tended to be significantly older (Table 2). The duration of disease was also significantly shorter with the patients being younger in the frontal hypoperfusion category in comparison to the no-area involved category. Patients with parietal hypoperfusion tended to have a lower waist-hip ratio in this series with all patients being female. The higher was the z-score category, the lower the BMI tended to be.
Table 2.
Distribution of pre-test parameters across the categories of vitamin D level, MMSE score, and z-score
| Duration (year) | Age (year) | Hb% (gm/dL) | Creatinine (mg/dL) | TSH (mIU/L) |
BMI (kg/m2) |
WHR (cm/cm) | |
|---|---|---|---|---|---|---|---|
| Vitamin D level | |||||||
| Normal | 6±0.8 | 42.3±1.9* | 11.9±0.8 | 0.97±0.05 | 3.2±0.64 | 21.6±2.4 | 0.87±0.1 |
| Insufficient | 1.7±0.6* | 20.3±2.5 | 10.7±0.3 | 0.99±0.01 | 2.3±1.0 | 17.8±2.0 | 0.77±0.2 |
| Deficient | 4.3±2.0 | 25.8±6.5 | 11.1±1.2 | 0.98±0.05 | 2.3±0.9 | 18.5±1.0 | 0.74±0.1 |
| MMSE categories | |||||||
| No CI | 4.5±2.4 | 35.0±11.4 | 11.5±0.8 | 0.98±0.04 | 2.7±0.9 | 20.4±2.9 | 0.86±0.1 |
| Mild CI | 4.2±2.0 | 25.4±6.5 | 11.1±1.1 | 0.98±0.05 | 2.4±0.9 | 18.4±1.0 | 0.73±0.1 |
| Areas of hypoperfusion† | |||||||
| No-area involved | 6.0±0.8 | 42.3±1.9 | 11.9±0.8 | 0.98±0.05 | 3.2±0.6 | 21.6±2.4 | 0.87±0.1 |
| Frontal | 3.8±2.1‡ | 24.7±6.3‡ | 11.0±1.1 | 0.98±0.05 | 2.3±0.8 | 18.4±1.2 | 0.75±0.1 |
| Parietal | 3.8±2.2 | 24.3±7.5 | 11.2±0.9 | 0.98±0.06 | 2.4±0.9 | 18.3±1.2 | 0.72±0.1‡ |
| Precuneus | 4.5±1.9 | 25.5±4.5 | 10.9±1.4 | 0.99±0.06 | 2.5±0.9 | 18.4±0.7 | 0.75±0.1 |
| Z-score categories | |||||||
| Normal | 4.5±2.4 | 35±11.4 | 11.5±0.8 | 0.97±0.04 | 2.7±0.9 | 20.4±2.9* | 0.86±0.1 |
| Mild deficit | 4.2±1.9 | 26.2±6.6 | 10.9±1.2 | 0.99±0.05 | 2.3±0.9 | 18.7±1.0* | 0.73±0.1 |
| Moderate deficit | 4.0±2.6 | 22.7±6.4 | 11.7±0.3 | 0.97±0.05 | 2.7±0.9 | 17.7±2.9* | 0.76±0.1 |
*Significantly different than other two with t-test p-value < 0.05
†Temporal lobe and basal ganglia statistics were not generated due to single sample
‡Significantly different from patients with dissimilar perfusion trait including normal and deficit perfusions with t-test p value < 0.05
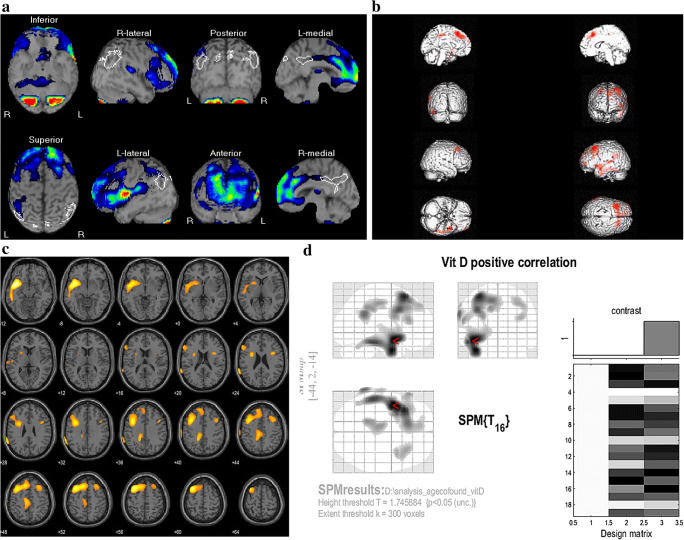
The 3D surface-rendered images of brain generated by eZIS from BS DICOM data (Fig. 2a) showed significant hypoperfusion in 15 (78.9%) out of 19 SPECT scans. Hypoperfused areas were observed in frontal lobes of 15, parietal lobes of nine, precuneus of eight, cingulate gyri of four, basal ganglia of two, temporal lobe of one, and cerebellum of one patient. Among the 15 having frontal hypoperfusion, the regions involved were the superior, middle, and inferior frontal gyri in nine, eight, and five patients. Among those, the eZIS-derived z-score fell in the category of normal in six (31.6%), a mild deficit in 10 (52.6%), and moderate deficit in three (15.8%) with the means ± SD of z-score being 0.52 ± 0.2, 1.72 ± 0.2, and 2.33 ± 0.2.
Fig. 2.
Brain perfusion SPECT images. a Representative 3D surface images of the brain generated by eZIS showing perfusion deficit in bilateral superior and middle frontal gyri (this patient had a Z-score of 1.25); b SPM brain surface rendering; c SPM axial slice views; and d SPM glass brain with design matrix in patients with NPSLE (n=19) showing the regional cerebral hypoperfusion in bilateral upper frontal lobes, left temporal lobe, and cingulate gyrus that correlated with serum level of vit-D (P<0.05)
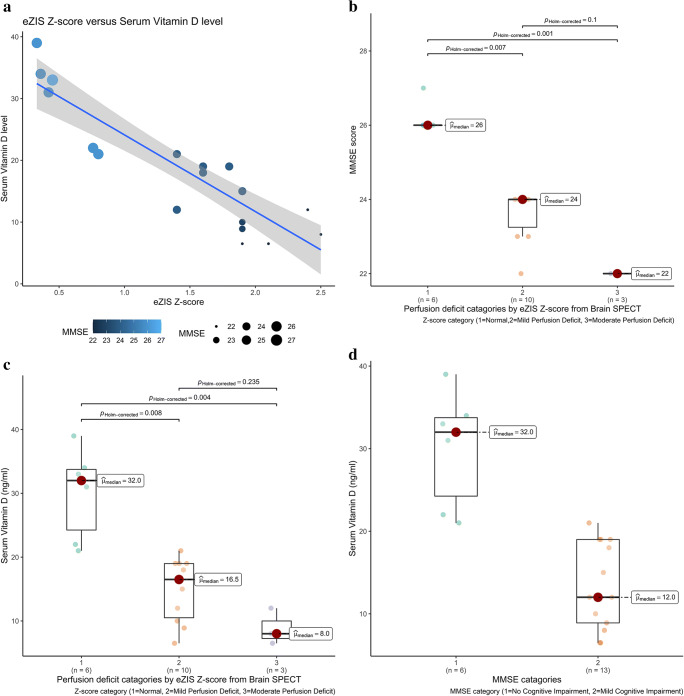
Voxel-based analysis (Fig. 2b, c, d) revealed a significant positive correlation between vit-D level and hypoperfusion in brain regions related to cognitive function (p<0.05) mainly in the bilateral upper frontal lobes, left temporal lobe, and cingulate gyrus. Table 3 details the additional information from the SPM analysis. In addition, there was a negative correlation (Fig. 3a) between serum level of vit-D- and eZIS-derived z-score from BS (Pearson r = −0.896, p < 0.001); a negative correlation between MMSE- and eZIS-derived z-score from BS (Pearson r = −0.943, p < 0.001); and a positive correlation between vit-D and MMSE (Pearson r = 0.893, p < 0.001). The distribution of MMSE scores across the z-score category is shown in (Fig 3b). The median MMSE scores for eZIS PD category normal, mild, and moderate are 26, 24 and 22 respectively. There is a significant difference between normal and mild PD (Kruskal-Wallis p-value = 0.005) and between normal and moderate PD (Kruskal-Wallis p-value = 0.001). Although the median MMSE score in moderate PD was lower than that for mild PD, the difference did not reach a statistical significance. The distribution of serum level of vit-D across the z-score category is shown in (Fig 3c). The median levels of serum vit-D for eZIS perfusion deficit category normal, mild, and moderate are 32, 16.5, and 8 ng/ml respectively. There is a significant difference in serum vit-D between normal and mild (Kruskal-Wallis p-value = 0.006) and between normal and moderate (Kruskal-Wallis p-value = 0.004). The difference of median vit-D levels in moderate and mild PD did not reach statistical significance. The serum vit-D level is significantly lower in mild CI in comparison to No CI, Mann-Whitney p-value = 0.001 (Fig. 3d) with median serum vit-D being 12 and 32 ng/ml.
Table 3.
Voxel-based analysis on SPM8 showing significant positive correlation between serum vitamin D level and regional brain hypoperfusion in patients with NPSLE (n=19)
| T-value(peak voxel) | MNI coordinate | Location of peak voxels | ||
|---|---|---|---|---|
| X | Y | Z | ||
| 3.21 | −44 | 2 | -14 | Left posterior insula |
| 3.09 | −40 | 0 | -30 | Left temporal pole |
| 2.95 | −34 | 20 | 58 | Left middle frontal gyrus |
| 2.49 | −68 | −48 | 28 | Left supramarginal gyrus |
| 2.21 | −14 | −44 | 40 | Left precuneus/posterior cingulate gyrus |
| 2.11 | 34 | 20 | 46 | Right middle frontal gyrus |
Fig. 3.
Relationship among variables; a scatterplot showing correlation between eZIS-generated BS z-score (x-axis) and serum vit-D level (y-axis) with color and size of marker corresponding to the MMSE score (lighter color and larger size indicates higher MMSE); b box plots along with jittered data points showing distribution of MMSE score across eZIS-generated z-score categories with the median MMSE scores shown on right side of each box and the p-values (Kruskal-Wallis) for pairwise comparison mentioned on top of bars between each pair of boxes; c box plots showing distribution of serum level of vit-D across eZIS-generated z-score categories with the median serum vit-D level shown on right side of each box and the p-values (Kruskal-Wallis) for pairwise comparison mentioned on top of bars between each pair of boxes; d box plots showing distribution of serum level of vit-D across MMSE score categories with the median serum vit-D level shown on right side of each box and the p-value (Mann-Whitney) for pairwise comparison is 0.001 (not displayed)
Discussion
The patients in this study were diagnosed cases of NPSLE sampled from a tertiary hospital comprising of 16% males and an overall incidence of mild CI in 68.4%. Recent data from hospital-based cohorts in Bangladesh shows a 50% incidence of CI among females with NPSLE [40]. While the visual interpretation of 3D surface images of brain was normal for 21% of patients in this study, application of eZIS-generated z-score-based interpretation resulted in a further increase of the normalcy rate to 31.6% due to normal z-score in two patients owing to the absence of perfusion deficit in their parietal cortices and precunei. The overall incidence of hypoperfusion by visual interpretation of 3D surface images of brain in this study was 79% with the incidence of hypoperfusion in males and females being 33.3 and 87.5% which is larger than the previously reported data, based on clinical examination showing organic brain syndrome in 6–43% in patients with NPSLE from Bangladesh [40, 41]. Although the parietal hypoperfusion was seen solely in female patients in this series, the inadequate proportion of male patients did not allow postulation of sex-based difference in regional hypoperfusion which has been a problem since always [15, 19, 25–27].
The clustering and correlation of symptoms with regional hypoperfusion were in concordance with the reported facts that the dorsal anterior cingulate cortex is a modulator of the supplementary motor area [42] or engagement of precuneus during regulation of emotion [43]. The current data also demonstrated a distinct association of anxiety with inferior frontal and cingulate hypoperfusion as well as an association of depression with middle and superior frontal hypoperfusion which is concordant with the report that anxiety and depression in NPSLE are related to limbic and frontostriatal hypoperfusion [44].
The duration of disease was found to be shorter in patients with frontal hypoperfusion. This though contradicts with a previous report [20] which might be due to differences in size and other traits of samples. Although the higher BMI in normal z-score categories was contributed by the male patients, the females with moderate perfusion deficit indeed had a significantly lower BMI than the females with mild perfusion deficit. It could be due to their younger age if the difference could reach a statistical significance. However, a similar inverse association of BMI with cognitive impairment was observed in chronic schizophrenia [45], Alzheimer’s, and other dementias [46–49].
The most frequent regions of hypoperfusion in the patients with NPSLE in this series were the frontal cortices followed by the parietal cortices and then the precuneus. The frontal hypoperfusion in NPSLE was reported as the most frequent by the majority [10, 15, 20] with one observation of the parietal cortex as the most frequently involved region [12]. However, the frontal, temporal [24], and parietal cortices [25, 26] showed a change of perfusion in response to immunomodulatory therapy, and both the frontal and parietal lobes were spared in non-CNS SLE [27]. Hypoperfusion of precuneus was found in 42% of patients in this series, which has been reported as a characteristic feature of impairment of memory [17] and major psychiatric symptoms [18] associated with NPSLE. The current study also finds an ascending order of means of z-score with the additional involvement of parietal cortex and precuneus with the frontal hypoperfusion, a fact that complements the previously reported decline of cognitive function in correlation with the degree of hypoperfusion in the frontal lobes [22], precuneus [17], and cerebellum [16]. Moreover, the z-score approach is capable of overcoming the limitation of previously used quantification approaches like asymmetry index [10] or formula-based brain perfusion index [30] and does not require arterial blood sampling [31].
This study used voxel-based morphometry with controlling of the age to demonstrate the correlation of hypoperfusion with vit-D deficiency which not only suggests the utility of vit-D supplementation in NPSLE but also expands the current view of correlated hypoperfusion with cognitive function and serum markers of cumulative tissue damage in SLE [23]. The suggestion of utility of vit-D supplementation is further supported by the statistical demonstration of mutual positive correlation between the vit-D and the MMSE while both of those being negatively correlated with eZIS-derived z-score: both the median MMSE and median vit-D levels being significantly higher in the patients having normal z-score than in those who belong to the category of mild or moderate perfusion deficit according to corresponding z-scores; and a significantly lower median vit-D level in patients belonging to the category of mild CI according to corresponding MMSE score, in comparison to those having no CI.
Although this data shows an indication of vit-D supplementation in 78.9% of patients with NPSLE by their serum levels of vit-D, the 3D surface view generated by eZIS showed hypoperfusion in all of those patients and an abnormal z-score in 68% of them except in the two patients (one male and one female) who had insufficient levels of vit-D with MMSE scores belonging to the category of “No CI” with frontal hypoperfusions but normal z-scores. Thus it may be permitted to assume that the appearance of hypovitaminosis precedes the progression of hypoperfusion in NPLSE where vit-D may have prophylactic implications. Reports suggest that NPSLE is rather associated with a reduced expression of vit-D binding protein in serum [50] than with genetic aberration of vit-D receptors [51]. Thus, the incidence of vit-D deficiency in SLE [2, 3] can therefore be partially attributed to lowered vit-D binding protein resulting in a lowered bound form of serum vit-D level [52]. On the contrary, serum level of vit-D itself is known to be unrelated to the expression of vit-D binding protein [52] and exert its effects related to neurotransmission, neuroprotection, and immunomodulation by directly binding with vit-D receptors in the cortical and subcortical structures [5, 6]. However, supplementation of vit-D with a specific intention to alleviate NPSLE or prevent its progression is absent in the current management recommendation for NPSLE which although recognizes the need for supplementation of vit-D due to its deficiency that may occur due to SLE-related compromise of renal function or due to therapeutic exposure of glucocorticoid or cautionary deprivation from sunlight [53].
There were several limitations to this study. The small sample size precluded formation of age, sex, disease severity, or symptom-matched subgroups of patients belonging to normal and subnormal categories of vit-D level or MMSE scores and then compare the eZIS score among those subgroups. A further sub-classification of patients according to their symptoms in to groups like psychiatric SLE and neurological SLE or the management of the patients’ neuropsychiatric symptoms was not taken into account during the analysis. Identification of morphological abnormalities in brain structures or assessment of local brain volumes and cortical thicknesses by structural imaging techniques and their correlation with regional hypoperfusion or were not performed in this study.
Conclusions
The patients in the current series are ideal candidates in whom the indication of vit-D supplementation is proven by their serum vit-D level and the utility of supplementation can be proven through a subsequent study by comparing their BS-demonstrated perfusion deficits and z-score with corresponding post-vit-D supplementation BS. The diagnostic, immediate therapeutic, and short- and long-term prognostic implication of this seminal work on brain perfusion in patients with NPSLE remains under our longitudinal surveillance which is also expected to facilitate its incorporation into the conventional standard of care in the institute. The dependency of clinical workup of NPSLE on the American College of Rheumatology nomenclature and case definitions [38] lacks specificity [54] and requires modification to support translational research and clinical trials in NPSLE [55]. BS in recent days with improved hardware [56] and robust methods for quantitative evaluation [34] should be considered as an ideal stepping stone towards such modification of care.
Acknowledgements
We acknowledge the Department of Rheumatology of BSMMU for the cooperation.
Authors Contribution
The first and corresponding author, as a principal investigator, was responsible for study design, overall management of the study, inter institute communication, clinical interpretation of data, and manuscript drafting. The co-first author was responsible for statistical analysis and manuscript drafting. The second author was responsible for clinical interpretation of data and statistical analysis. The third author was responsible for data documentation and analysis. The fourth author was responsible for patient management, study design, and clinical interpretation. The fifth and sixth authors were responsible for study design.
Funding
This study was partially funded by a grant from IAEA (research contract no 20402).
Data Availability
The data and material can be made be available upon communication with the corresponding author.
Declarations
Competing Interests
Nasreen Sultana, Azmal Kabir Sarker, Hiroshi Matsuda, Md. Amimul Ihsan, Syed Atiqul Haq, Md. Saidul Arefin, and Sheikh Nazrul Islam declare no conflict of interest.
Ethics Approval and Consent to Participate
The questionnaire and methodology of this study were approved by the Medical Research Ethics Committee of the National Institute of Nuclear Medicine and Allied Sciences (NINMAS), Bangladesh Atomic Energy Commission. All participants made signatures on an official consent for brain perfusion SPECT after they were verbally informed about the radionuclide scan procedure and anonymized use of patient data for research purpose under the surveillance of the institute.
Consent for publication
The participants signed consent regarding publishing their data and photographs.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nasreen Sultana, Email: nasreeninm@yahoo.com.
Azmal Kabir Sarkar, Email: azmal.sarker@gmail.com.
Hiroshi Matsuda, Email: mathiroshinucl@gmail.com.
Md Amimul Ihsan, Email: amimulihsan@iut-dhaka.edu.
Syed Atiqul Haq, Email: haqsyedatiqul@gmail.com.
Md Saidul Arefin, Email: masaidularefindmc@gmail.com.
Sheikh Nazrul Islam, Email: sheikhnazrul09@gmail.com.
References
- 1.Barber MRW, Drenkard C, Falasinnu T, Hoi A, Mak A, Kow NY, et al. Global epidemiology of systemic lupus erythematosus. Nat Rev Rheumatol. 2021;17:515–532. doi: 10.1038/s41584-021-00668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Islam MA, Khandker SS, Alam SS, Kotyla P, Hassan R. Vitamin D status in patients with systemic lupus erythematosus (SLE): a systematic review and meta-analysis. Autoimmun Rev. 2019;18:102392. doi: 10.1016/j.autrev.2019.102392. [DOI] [PubMed] [Google Scholar]
- 3.Hassanalilou T, Khalili L, Ghavamzadeh S, Shokri A, Payahoo L, Bishak YK. Role of vitamin D deficiency in systemic lupus erythematosus incidence and aggravation. Auto Immun Highlights. 2017;9:1. doi: 10.1007/s13317-017-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tay SH, Ho CS, Ho RC, Mak A. 25-Hydroxyvitamin D3 deficiency independently predicts cognitive impairment in patients with systemic lupus erythematosus. PLoS One. 2015;10:e0144149. doi: 10.1371/journal.pone.0144149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui X, Gooch H, Petty A, McGrath JJ, Eyles D. Vitamin D and the brain: genomic and non-genomic actions. Mol Cell Endocrinol. 2017;453:131–143. doi: 10.1016/j.mce.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 6.Lang F, Ma K, Leibrock CB. 1,25(OH)2D3 in brain function and neuropsychiatric disease. Neurosignals. 2019;27:40–49. doi: 10.33594/000000182. [DOI] [PubMed] [Google Scholar]
- 7.Islam MA, Khandker SS, Kotyla PJ, Hassan R. Immunomodulatory effects of diet and nutrients in systemic lupus erythematosus (SLE): a systematic review. Front Immunol. 2020;11:1477. doi: 10.3389/fimmu.2020.01477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maretzke F, Bechthold A, Egert S, Ernst JB, Melo van Lent D, Pilz S, et al. Role of vitamin D in preventing and treating selected extraskeletal diseases-an umbrella review. Nutrients. 2020;12:969. doi: 10.3390/nu12040969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Oliva R, Geribaldi-Doldan N, Dominguez-Garcia S, Carrascal L, Verastegui C, Nunez-Abades P, et al. Vitamin D deficiency as a potential risk factor for accelerated aging, impaired hippocampal neurogenesis and cognitive decline: a role for Wnt/beta-catenin signaling. Aging (Albany NY) 2020;12:13824–13844. doi: 10.18632/aging.103510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colamussi P, Giganti M, Cittanti C, Dovigo L, Trotta F, Tola MR, et al. Brain single-photon emission tomography with 99mTc-HMPAO in neuropsychiatric systemic lupus erythematosus: relations with EEG and MRI findings and clinical manifestations. Eur J Nucl Med. 1995;22:17–24. doi: 10.1007/BF00997243. [DOI] [PubMed] [Google Scholar]
- 11.Falcini F, De Cristofaro MT, Ermini M, Guarnieri M, Massai G, Olmastroni M, et al. Regional cerebral blood flow in juvenile systemic lupus erythematosus: a prospective SPECT study. Single photon emission computed tomography. J Rheumatol. 1998;25:583–588. [PubMed] [Google Scholar]
- 12.Lin WY, Wang SJ, Yen TC, Lan JL. Technetium-99m-HMPAO brain SPECT in systemic lupus erythematosus with CNS involvement. J Nucl Med. 1997;38:1112–1115. [PubMed] [Google Scholar]
- 13.Waterloo K, Omdal R, Sjoholm H, Koldingsnes W, Jacobsen EA, Sundsfjord JA, et al. Neuropsychological dysfunction in systemic lupus erythematosus is not associated with changes in cerebral blood flow. J Neurol. 2001;248:595–602. doi: 10.1007/s004150170138. [DOI] [PubMed] [Google Scholar]
- 14.Olazaran J, Lopez-Longo J, Cruz I, Bittini A, Carreno L. Cognitive dysfunction in systemic lupus erythematosus: prevalence and correlates. Eur Neurol. 2009;62:49–55. doi: 10.1159/000215879. [DOI] [PubMed] [Google Scholar]
- 15.Castellino G, Padovan M, Bortoluzzi A, Borrelli M, Feggi L, Caniatti ML, et al. Single photon emission computed tomography and magnetic resonance imaging evaluation in SLE patients with and without neuropsychiatric involvement. Rheumatology (Oxford) 2008;47:319–323. doi: 10.1093/rheumatology/kem354. [DOI] [PubMed] [Google Scholar]
- 16.Huang WS, Chiu PY, Tsai CH, Kao A, Lee CC. Objective evidence of abnormal regional cerebral blood flow in patients with systemic lupus erythematosus on Tc-99m ECD brain SPECT. Rheumatol Int. 2002;22:178–181. doi: 10.1007/s00296-002-0224-9. [DOI] [PubMed] [Google Scholar]
- 17.Oh DH, Kim SH, Jung S, Sung YK, Bang SY, Bae SC, et al. Precuneus hypoperfusion plays an important role in memory impairment of patients with systemic lupus erythematosus. Lupus. 2011;20:855–860. doi: 10.1177/0961203310394895. [DOI] [PubMed] [Google Scholar]
- 18.Oda K, Matsushima E, Okubo Y, Ohta K, Murata Y, Koike R, et al. Abnormal regional cerebral blood flow in systemic lupus erythematosus patients with psychiatric symptoms. J Clin Psychiatry. 2005;66:907–913. doi: 10.4088/JCP.v66n0714. [DOI] [PubMed] [Google Scholar]
- 19.Chen JJ, Shiau YC, Wang JJ, Ho ST, Kao A. Abnormal regional cerebral blood flow in primary antiphospholipid antibody syndrome patients with normal magnetic resonance imaging findings. A preliminary report. Scand J Rheumatol. 2002;31:89–93. doi: 10.1080/03009740252937603. [DOI] [PubMed] [Google Scholar]
- 20.Giovacchini G, Mosca M, Manca G, Della Porta M, Neri C, Bombardieri S, et al. Cerebral blood flow in depressed patients with systemic lupus erythematosus. J Rheumatol. 2010;37:1844–1851. doi: 10.3899/jrheum.100121. [DOI] [PubMed] [Google Scholar]
- 21.Nobili F, Mignone A, Rossi E, Morbelli S, Piccardo A, Puppo F, et al. Migraine during systemic lupus erythematosus: findings from brain single photon emission computed tomography. J Rheumatol. 2006;33:2184–2191. [PubMed] [Google Scholar]
- 22.Driver CB, Wallace DJ, Lee JC, Forbess CJ, Pourrabbani S, Minoshima S, et al. Clinical validation of the watershed sign as a marker for neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2008;59:332–337. doi: 10.1002/art.23308. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Longo FJ, Carol N, Almoguera MI, Olazaran J, Alonso-Farto JC, Ortega A, et al. Cerebral hypoperfusion detected by SPECT in patients with systemic lupus erythematosus is related to clinical activity and cumulative tissue damage. Lupus. 2003;12:813–819. doi: 10.1191/0961203303lu470oa. [DOI] [PubMed] [Google Scholar]
- 24.Frantellizzi V, Morreale M, Pontico M, Francia A, Drudi FM, Farcomeni A, et al. (99m)Tc-HMPAO brain SPECT in the monitoring of cerebral vasculitis therapy. Rev Esp Med Nucl Imagen Mol (Engl Ed) 2018;37:211–217. doi: 10.1016/j.remn.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Sun SS, Huang WS, Chen JJ, Chang CP, Kao CH, Wang JJ. Evaluation of the effects of methylprednisolone pulse therapy in patients with systemic lupus erythematosus with brain involvement by Tc-99m HMPAO brain SPECT. Eur Radiol. 2004;14:1311–1315. doi: 10.1007/s00330-003-2166-3. [DOI] [PubMed] [Google Scholar]
- 26.Liu FY, Huang WS, Kao CH, Yen RF, Wang JJ, Ho ST. Usefulness of Tc-99m ECD brain SPECT to evaluate the effects of methylprednisolone pulse therapy in lupus erythematosus with brain involvement: a preliminary report. Rheumatol Int. 2003;23:182–185. doi: 10.1007/s00296-002-0282-z. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Zhu Z, Zhang F, Shu H, Li F, Dong Y. Diagnostic value of single-photon-emission computed tomography in severe central nervous system involvement of systemic lupus erythematosus: a case-control study. Arthritis Rheum. 2005;53:845–849. doi: 10.1002/art.21591. [DOI] [PubMed] [Google Scholar]
- 28.Mauro L, Manuela M, Valentina M, Sara C, Chondrogiannis S, Maria DF, et al. Role of brain perfusion SPECT with 99mTc HMPAO in the assessment of response to drug therapy in patients with autoimmune vasculitis: a prospective study. N Am J Med Sci. 2015;7:135–142. doi: 10.4103/1947-2714.156008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castellino G, Govoni M, Padovan M, Colamussi P, Borrelli M, Trotta F. Proton magnetic resonance spectroscopy may predict future brain lesions in SLE patients: a functional multi-imaging approach and follow up. Ann Rheum Dis. 2005;64:1022–1027. doi: 10.1136/ard.2004.026773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tatsukawa H, Ishii K, Haranaka M, Kumagi M, Hino I, Yoshimatsu H. Evaluation of average amount of cerebral blood flow measured by brain perfusion index in patients with neuropsychiatric systemic lupus erythematosus. Lupus. 2005;14:445–449. doi: 10.1191/0961203305lu2127oa. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida A, Shishido F, Kato K, Watanabe H, Seino O. Evaluation of cerebral perfusion in patients with neuropsychiatric systemic lupus erythematosus using 123I-IMP SPECT. Ann Nucl Med. 2007;21:151–158. doi: 10.1007/s12149-006-0006-7. [DOI] [PubMed] [Google Scholar]
- 32.Appenzeller S, Amorim BJ, Ramos CD, Rio PA, de CEEC CEE, et al. Voxel-based morphometry of brain SPECT can detect the presence of active central nervous system involvement in systemic lupus erythematosus. Rheumatology (Oxford) 2007;46:467–472. doi: 10.1093/rheumatology/kel255. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda H. Neurological diseases and SPECT--analysis using easy Z-score imaging system (eZIS) Brain Nerve. 2007;59:487–493. [PubMed] [Google Scholar]
- 34.Tokumitsu K, Yasui-Furukori N, Takeuchi J, Yachimori K, Sugawara N, Terayama Y, et al. The combination of MMSE with VSRAD and eZIS has greater accuracy for discriminating mild cognitive impairment from early Alzheimer’s disease than MMSE alone. PLoS One. 2021;16:e0247427. doi: 10.1371/journal.pone.0247427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasaki M, Nakagawa E, Sugai K, Shimizu Y, Hattori A, Nonoda Y, et al. Brain perfusion SPECT and EEG findings in children with autism spectrum disorders and medically intractable epilepsy. Brain Dev. 2010;32:776–782. doi: 10.1016/j.braindev.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Matsuda H, Mizumura S, Nagao T, Ota T, Iizuka T, Nemoto K, et al. Automated discrimination between very early Alzheimer disease and controls using an easy Z-score imaging system for multicenter brain perfusion single-photon emission tomography. AJNR Am J Neuroradiol. 2007;28:731–736. [PMC free article] [PubMed] [Google Scholar]
- 37.Ishibashi M, Kimura N, Sumi K, Aso Y, Matsubara E. Comparison of brain perfusion patterns in dementia with Lewy bodies patients with or without cingulate island sign. Geriatr Gerontol Int. 2019;19:197–202. doi: 10.1111/ggi.13586. [DOI] [PubMed] [Google Scholar]
- 38.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599–608. [DOI] [PubMed]
- 39.Patil I. Visualization with statistical details: The ‘ggstatsplot’ approach. Journal of Open Source Software. 2021;61:3167. doi: 10.21105/joss.03167. [DOI] [Google Scholar]
- 40.Hossain F, Hawlader MDH, Mitra DK, Nabi MH, Rahman MM. Pattern and prevalence of neuropsychiatric lupus: a retrospective study from a tertiary level hospital in Bangladesh. Egypt J Neurol Psychiatry Neurosurg. 2021;57:77. doi: 10.1186/s41983-021-00334-z. [DOI] [Google Scholar]
- 41.Miah T, Haque MA, Mahmood T, Tarafder BK. Clinical profile, management and outcome of lupus. Mymensingh Med J. 2008;17:S6–11. [PubMed] [Google Scholar]
- 42.Asemi A, Ramaseshan K, Burgess A, Diwadkar VA, Bressler SL. Dorsal anterior cingulate cortex modulates supplementary motor area in coordinated unimanual motor behavior. Front Hum Neurosci. 2015;9:309. doi: 10.3389/fnhum.2015.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loeffler LAK, Radke S, Habel U, Ciric R, Satterthwaite TD, Schneider F, et al. The regulation of positive and negative emotions through instructed causal attributions in lifetime depression - a functional magnetic resonance imaging study. Neuroimage Clin. 2018;20:1233–1245. doi: 10.1016/j.nicl.2018.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papadaki E, Kavroulakis E, Bertsias G, Fanouriakis A, Karageorgou D, Sidiropoulos P, et al. Regional cerebral perfusion correlates with anxiety in neuropsychiatric SLE: evidence for a mechanism distinct from depression. Lupus. 2019;28:1678–1689. doi: 10.1177/0961203319887793. [DOI] [PubMed] [Google Scholar]
- 45.Wei CW, Chen YQ, Ma M, Xiu MH, Zhang XY. Sex differences in the association of body mass index with symptoms and cognitive deficits in Chinese patients with chronic schizophrenia. Transl Psychiatry. 2020;10:18. doi: 10.1038/s41398-020-0717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michaud TL, Siahpush M, Farazi PA, Kim J, Yu F, Su D, et al. The association between body mass index, and cognitive, functional, and behavioral declines for incident dementia. J Alzheimers Dis. 2018;66:1507–1517. doi: 10.3233/JAD-180278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suemoto CK, Gilsanz P, Mayeda ER, Glymour MM. Body mass index and cognitive function: the potential for reverse causation. Int J Obes (Lond). 2015;39:1383–1389. doi: 10.1038/ijo.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim S, Kim Y, Park SM. body mass index and decline of cognitive function. PLoS One. 2016;11:e0148908. doi: 10.1371/journal.pone.0148908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan Y, Li J, Zhang N, Fu P, Jing Z, Yu C, et al. Body mass index and mild cognitive impairment among rural older adults in China: the moderating roles of gender and age. BMC Psychiatry. 2021;21:54. doi: 10.1186/s12888-021-03059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen C, Geng L, Xu X, Kong W, Hou Y, Yao G, et al. Comparative proteomics analysis of plasma protein in patients with neuropsychiatric systemic lupus erythematosus. Ann Transl Med. 2020;8:579. doi: 10.21037/atm.2020.04.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho RC, Ong H, Thiaghu C, Lu Y, Ho CS, Zhang MW. Genetic variants that are associated with neuropsychiatric systemic lupus erythematosus. J Rheumatol. 2016;43:541–551. doi: 10.3899/jrheum.150884. [DOI] [PubMed] [Google Scholar]
- 52.Bikle DD, Schwartz J. Vitamin D binding protein, total and free vitamin d levels in different physiological and pathophysiological conditions. Front Endocrinol (Lausanne) 2019;10:317. doi: 10.3389/fendo.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gordon C, Amissah-Arthur MB, Gayed M, Brown S, Bruce IN, D’Cruz D, et al. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults: Executive Summary. Rheumatology (Oxford). 2018;57:14–18. doi: 10.1093/rheumatology/kex291. [DOI] [PubMed] [Google Scholar]
- 54.Ainiala H, Hietaharju A, Loukkola J, Peltola J, Korpela M, Metsanoja R, et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum. 2001;45:419–423. doi: 10.1002/1529-0131(200110)45:5<419::AID-ART360>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 55.Vivaldo JF, de Amorim JC, Julio PR, de Oliveira RJ, Appenzeller S. Definition of NPSLE: does the ACR nomenclature still hold? Front Med (Lausanne) 2018;5:138. doi: 10.3389/fmed.2018.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bordonne M, Chawki MB, Marie PY, Zaragori T, Roch V, Grignon R, et al. High-quality brain perfusion SPECT images may be achieved with a high-speed recording using 360 degrees CZT camera. EJNMMI Phys. 2020;7:65. doi: 10.1186/s40658-020-00334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and material can be made be available upon communication with the corresponding author.