Abstract
Maize (Zea mays L.) is the leading cereal crop and staple food in many parts of the world. This study aims to develop nutrient-rich maize genotypes by incorporating crtRB1 and o2 genes associated with increased β-carotene, lysine, and tryptophan levels. UMI1200 and UMI1230, high quality maize inbreds, are well-adapted to tropical and semi-arid regions in India. However, they are deficient in β-carotene, lysine, and tryptophan. We used the concurrent stepwise transfer of genes by marker-assisted backcross breeding (MABB) scheme to introgress crtRB1 and o2 genes. In each generation (from F1, BC1F1–BC3F1, and ICF1–ICF3), foreground and background selections were carried out using gene-linked (crtRB1 3′TE and umc1066) and genome-wide simple sequence repeats (SSR) markers. Four independent BC3F1 lines of UMI1200 × CE477 (Cross-1), UMI1200 × VQL1 (Cross-2), UMI1230 × CE477 (Cross-3), and UMI1230 × VQL1 (Cross-4) having crtRB1 and o2 genes and 87.45–88.41% of recurrent parent genome recovery (RPGR) were intercrossed to generate the ICF1-ICF3 generations. Further, these gene pyramided lines were examined for agronomic performance and the β-carotene, lysine, and tryptophan contents. Six ICF3 lines (DBT-IC-β1σ4-4-8-8, DBT-IC-β1σ4-9-21-21, DBT-IC-β1σ4-10-1-1, DBT-IC-β2σ5-9-51-51, DBT-IC-β2σ5-9-52-52 and DBT-IC-β2σ5-9-53-53) possessing crtRB1 and o2 genes showed better agronomic performance (77.78–99.31% for DBT-IC-β1σ4 population and 85.71–99.51% for DBT-IC-β2σ5 population) like the recurrent parents and β-carotene (14.21–14.35 μg/g for DBT-IC-β1σ4 and 13.28–13.62 μg/g for DBT-IC-β2σ5), lysine (0.31–0.33% for DBT-IC-β1σ4 and 0.31–0.34% for DBT-IC-β2σ5), and tryptophan (0.079–0.082% for DBT-IC-β1σ4 and 0.078–0.083% for DBT-IC-β2σ5) levels on par with that of the donor parents. In the future, these improved lines could be developed as a cultivar for various agro-climatic zones and also as good genetic materials for maize nutritional breeding programs.
Subject terms: Biotechnology, Molecular biology, Plant sciences
Introduction
Maize, an important cereal, is life for millions in the global population, as a source of protein, vitamins, minerals, oils, and dietary fibre. The crop is cultivated widely in diverse agroecology across the globe and has the highest genetic yield potential among the cereals. It is grown in more than 160 countries with a total production of 1.05 million thousand tonnes and 28.90 million tonnes in India for the year 20191. Maize is a rich source of provitamin A and non-provitamin A carotenoids. The carotenoids are synthesized in the maize endosperm via the carotenoid biosynthesis pathway that originates from the isoprenoid precursor, geranyl pyrophosphate, supplied by the MEP pathway2. Through a series of enzyme-mediated reactions, phytoene, the first carotenoid compound, is synthesized and enzymatically converted to lycopene. This is the branch point of the pathway, and further conversion depends on the cyclization of the lycopene molecule. An asymmetric cyclization would produce an α-carotene molecule, and a symmetric cyclization would yield a β-carotene molecule, forming the provitamin A carotenoids in maize3.
Among the provitamin A carotenoids, β-carotene has the highest provitamin A potential due to the presence of two β-ionone rings. β-carotene is further hydroxylated to produce β-cryptoxanthin and further to zeaxanthin and ABA which are non-provitamin A carotenoids4. Hence, in normal maize, due to the conversion of β-carotene to non-provitamin A carotenoids, a micronutrient deficiency occurs, particularly the vitamin A deficiency (VAD). Maize is also a staple food in many of the sub-Saharan and Latin American countries, and hence, VAD would pose an important threat to the population, specifically the pregnant women and infants, resulting in complications such as blindness and growth retardation5,6. In 2018, a study conducted by UNICEF revealed that children aged between 6 and 59 months from East Asia and the Pacific regions received the highest two-dosage of vitamin A supplements with 75% from the African countries and 59% from the South Asian countries7. Therefore, there is a pressing need for alleviating this micronutrient complication, and since the carotenoid compounds are naturally accumulated in the edible part of the maize endosperm, it becomes an ideal crop for biofortification.
Several studies have identified various genes that are directly involved in the variation of the β-carotene pathway by directly or indirectly modifying the carotene biosynthesis pathway. The LcyE and the crtRB1 genes were shown to be directly involved in influencing the beta carotene levels in the maize endosperm8,9. The precise manipulation of the crtRB1 gene has shown to favorably increase the beta carotene concentration in previous studies10,11. Yan et al. identified the crtRB1 gene responsible for this conversion and also three polymorphisms that influence the variation in the carotenoid concentration. The polymorphism in the 3’TE region with the favorable allele (543 bp) increases the carotene concentration in maize9–11.
Maize also contains two protein fractions viz., zein and non-zein, where zein proteins are predominant. However, these zein proteins lack essential amino acids like lysine and tryptophan and hence induce Protein Energy Malnutrition (PEM). Several natural mutants (i.e., opaque 2 (o2)12, floury 2 (fl2)13, opaque 7 (o7)14, opaque 6 (o6)15, floury 3 (fl3)14) have shown to increase these essential amino acids in maize of which o2 has been widely studied. The o2 mutant is known to decrease the zein fraction and increase the non-zein fraction which is naturally high in essential amino acids16–18. The large genetic variation present in maize makes it an ideal crop for nutritional improvement specifically in regard to micronutrient deficiencies. Marker-assisted backcross breeding (MABB) has been shown to be a promising technique to introgress several nutritionally important genes in many crops including maize19. Nutritional traits viz., provitamin A, higher protein content, high Zn, Fe, and Se content have been improved in maize through the MABB technique8,20–23.
Several studies in India and other parts of the world have successfully introgressed either crtRB1 or o2 into popular elite lines and improved the β-carotene, lysine, and tryptophan contents24–30. The common determinant in all the previous studies is the introgression of a single factor into an established variety. By adopting the technique of gene pyramiding, varieties can be produced with broad sense capabilities and essentially more important genetic stocks. Especially by bringing improved versions of β-carotene, lysine, and tryptophan into a single genotype, the time required to improve the plants individually is reduced and would also provide a superior genotype with several favourable nutritional traits. This has now become possible due to the advances made in technology as well as the identification of new molecular markers and integrated techniques developed for efficient selection26,28,31–34. Considering these, this study is planned to develop an intercross population and pyramid the crtRB1 and o2 simultaneously in the background of elite genotypes.
Results
Transfer of crtRB1 and o2 genes into UMI1200
A total of 27 and 23 F1s were produced in cross-1 and cross-2, and their heterozygosity was confirmed via foreground markers associated with crtRB1 and o2 genes. The healthy F1s from both crosses were backcrossed with a recurrent parent to produce 106 and 232 BC1F1 lines, and again heterozygous conditions were confirmed in BC1F1 lines using foreground markers. All the heterozygous positives were subjected to background selection with 112 and 106 polymorphic markers. They showed 52.82–56.41% and 62.13–74.25% of RPGR with an average of 54.84% and 69.38% in cross-1 and cross-2. Further, one BC1F1 line from each cross with crtRB1 and o2 genes and maximum RPGR was selected and backcrossed with a recurrent parent to produce 136 and 218 BC2F1 lines. Following similar selection procedures, BC2F1 generation was advanced to BC3F1. A total of 85 and 109 BC3F1 lines were produced to the cross-1 and cross-2, and foreground selection revealed that the 24 and 31 BC3F1 lines had crtRB1 and o2 genes in the heterozygous condition. All these plants were subjected to background selection, and they exhibited 86.35–88.52% and 86.14–88.21% of RPGR with an average of 87.74 and 87.45% (Supplementary Tables S1, S2). Among them, two lines, DBT 1-1-1-17-5-14 from cross-1 and DBT 4-1-1-10-10-16 from cross-2 having maximum RPGR, were used to develop the intercross population (designated as DBT-IC-β1σ4).
Transfer of crtRB1 and o2 genes into UMI1230
With the support of foreground markers, crtRB1 and o2 genes heterozygous lines were confirmed in F1s from cross-3 and cross-4. The F1s were backcrossed with a recurrent parent to produce 121 and 160 BC1F1 lines. Among them, 42 and 68 BC1F1 lines possessing crtRB1 and o2 genes in their heterozygous condition were identified in cross-3 and cross-4 using foreground markers and were subjected to background selection with 114 and 90 polymorphic SSR markers. Background selection revealed 53.87–57.69% and 68.60–76.20% of RPGR with an average of 55.12% and 72.70%. One BC1F1 line from each cross possessing crtRB1 and o2 genes and maximum RPGR was selected and backcrossed with a recurrent parent to produce 146 and 153 BC2F1 lines. Applying the same strategy, 5 BC3F1 and 10 BC3F1 lines possessing crtRB1 and o2 genes and maximum RPGR were identified. The BC3F1 lines from cross-3 and cross-4 exhibited 86.75–88.84% and 87.56–89.42% of RPGR with an average of 87.84% and 88.41% (Supplementary Tables S1, S2). The two BC3F1 lines, (DBT 2-1-4-7-1-9) and (DBT 5-1-14-5-8-7) from cross-3 and 4 having maximum RPGR, were used to develop the intercross population (designated as DBT-IC-β2σ5).
Stacking of crtRB1 and o2 genes
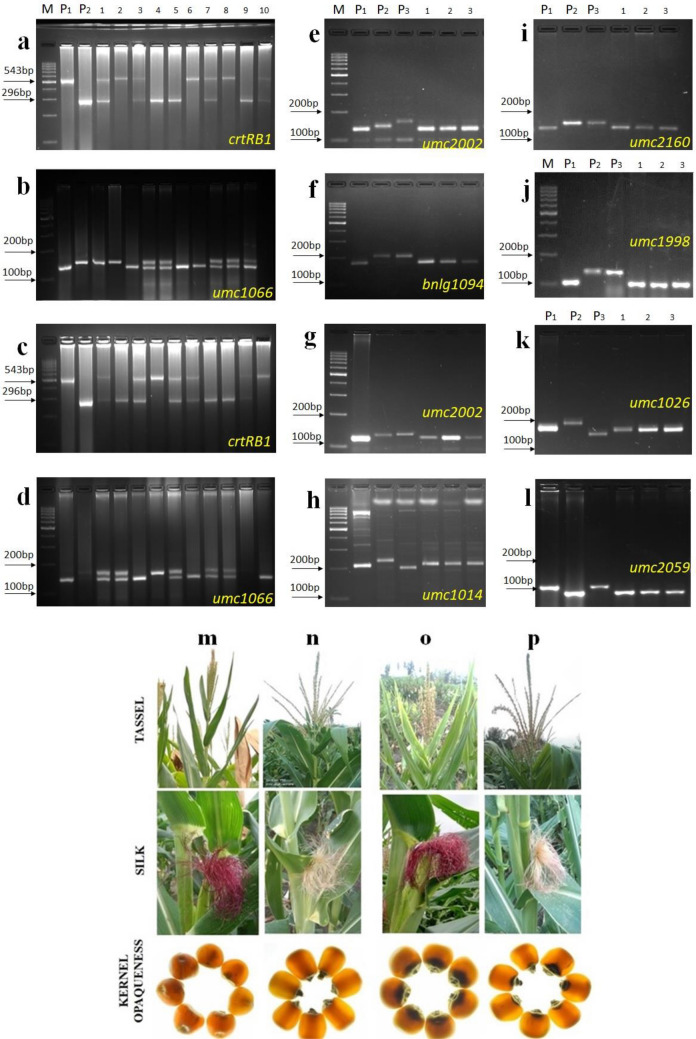
The line DBT 1-1-1-17-5-14 (derived from cross 1) was used as the female parent and DBT 4-1-1-10-10-16 (derived from cross 2) as the male parent in the development of intercross population (DBT-IC-β1σ4) to pyramid crtRB1 and o2 genes. Among the 128 ICF1 lines, 64 lines were confirmed to be heterozygous for two target genes. Of these, 64 ICF1 were selected and selfed to obtain 40 ICF2 lines. Foreground selection was conducted in ICF2 lines to trace the lines carrying a combination of two genes. Based on foreground selection and the phenotyping of kernels for opaqueness (25%), a total of 9 homozygous lines with crtRB1 and o2 genes were identified. Chi-square test on the 9 lines revealed that the population followed the expected Mendelian ratio of 1:2:1 (Table 1; Fig. 1). Background selection was done in those selected 9 lines with 148 polymorphic SSR markers and selfed to produce ICF3 generation (Supplementary Table S3). Eventually, ICF3 lines, (DBT-IC-β1σ4-4-8-8, DBT-IC-β1σ4-9-21-21, and DBT-IC-β1σ4-10-1-1 having 90.47%, 90.62%, and 89.54% of RPGR with 25% opaqueness, were developed. Likewise, to pyramid the crtRB1 and o2 genes, another intercross population (DBT-IC-β2σ5) was generated using the line DBT 2-1-4-7-1-9 (Cross 3) as the female parent and DBT 5-1-14-5-8-7 (Cross 4) as the male parent. Foreground markers confirmed the heterozygous form of crtRB1 and o2 genes in ICF1 lines. Then, 72 healthy ICF1 lines were selfed to produce 45 ICF2 lines. Foreground selection coupled with phenotyping of kernels for opaqueness resulted in 9 homozygous ICF2 lines with crtRB1 and o2 genes. All these lines were subjected to background selection and then selfed to produce ICF3 generation. Finally, 3 lines, DBT-IC-β2σ5-9-51-51, DBT-IC-β2σ5-9-52-52, and DBT-IC-β2σ5-9-53-53 having 91.71%, 89.05%, and 88.14% RPGR and opaqueness of 25% were generated (Supplementary Table S3; Fig. 1). Collectively, 6 ICF3 lines DBT-IC-β1σ4-4-8-8, DBT-IC-β1σ4-9-21-21, DBT-IC-β1σ4-10-1-1, DBT-IC-β2σ5-9-51-51, DBT-IC-β2σ5-9-52-52, and DBT-IC-β2σ5-9-53-53 containing crtRB1 and o2 genes were developed with 25% opaqueness.
Table 1.
Segregation pattern of o2 and crtRB1 allele in intercross (IC2) population of DBT-IC-β1σ4 and DBT-IC-β2σ5.
| o2 | crtRB1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ear | Total number of plants genotyped | Genotypic class | χ2 | P-value | Total number of plants genotyped | Genotypic class | χ2 | P value | ||||
| O2O2 | O2o2 | o2o2 | Allele3 | Allele3/Allele1 | Allele1 | |||||||
| DBT-IC-β1σ4 | ||||||||||||
| DBT-IC-β1σ4-4-1 | 110 | 27 | 58 | 25 | 0.400 ns | 0.819 | 110 | 28 | 58 | 24 | 0.618 ns | 0.734 |
| DBT-IC-β1σ4-4-3 | 95 | 25 | 49 | 21 | 0.432 ns | 0.806 | 95 | 25 | 50 | 20 | 0.789 ns | 0.674 |
| DBT-IC-β1σ4-4-6 | 116 | 30 | 57 | 29 | 0.052 ns | 0.974 | 116 | 31 | 60 | 25 | 0.759 ns | 0.684 |
| DBT-IC-β1σ4-4-8 | 115 | 28 | 60 | 27 | 0.235 ns | 0.889 | 115 | 31 | 58 | 26 | 0.443 ns | 0.801 |
| DBT-IC-β1σ4-9-19 | 92 | 23 | 50 | 19 | 1.043 ns | 0.593 | 92 | 25 | 50 | 19 | 1.217 ns | 0.544 |
| DBT-IC-β1σ4-9-21 | 121 | 30 | 62 | 29 | 0.091 ns | 0.956 | 121 | 32 | 63 | 26 | 0.802 ns | 0.670 |
| DBT-IC-β1σ4-9-23 | 105 | 28 | 55 | 22 | 0.924 ns | 0.630 | 105 | 28 | 55 | 22 | 0.924 ns | 0.630 |
| DBT-IC-β1σ4-10-1 | 102 | 27 | 53 | 22 | 0.647 ns | 0.724 | 102 | 27 | 51 | 24 | 0.176 ns | 0.916 |
| DBT-IC-β1σ4-10-4 | 94 | 26 | 47 | 21 | 0.532 ns | 0.766 | 94 | 24 | 50 | 20 | 0.723 ns | 0.696 |
| DBT-IC-β2σ5 | ||||||||||||
| DBT-IC-β2σ5-9-25 | 94 | 25 | 49 | 20 | 0.702 ns | 0.704 | 94 | 23 | 51 | 20 | 0.872 ns | 0.647 |
| DBT-IC-β2σ5-9-34 | 112 | 30 | 58 | 24 | 0.786 ns | 0.675 | 112 | 29 | 60 | 23 | 1.214 ns | 0.545 |
| DBT-IC-β2σ5-9-42 | 95 | 25 | 50 | 20 | 0.789 ns | 0.674 | 95 | 25 | 49 | 21 | 0.432 ns | 0.806 |
| DBT-IC-β2σ5-9-45 | 106 | 28 | 55 | 23 | 0.623 ns | 0.732 | 106 | 27 | 55 | 24 | 0.321 ns | 0.852 |
| DBT-IC-β2σ5-9-49 | 100 | 25 | 52 | 23 | 0.240 ns | 0.887 | 100 | 26 | 53 | 21 | 0.860 ns | 0.651 |
| DBT-IC-β2σ5-9-50 | 97 | 25 | 51 | 21 | 0.588 ns | 0.745 | 97 | 25 | 52 | 20 | 1.021 ns | 0.600 |
| DBT-IC-β2σ5-9-51 | 97 | 27 | 49 | 21 | 0.753 ns | 0.686 | 97 | 22 | 54 | 21 | 1.268 ns | 0.530 |
| DBT-IC-β2σ5-9-52 | 103 | 25 | 55 | 23 | 0.553 ns | 0.758 | 103 | 28 | 53 | 22 | 0.786 ns | 0.675 |
| DBT-IC-β2σ5-9-53 | 94 | 25 | 49 | 20 | 0.702 ns | 0.704 | 94 | 24 | 49 | 21 | 0.362 ns | 0.835 |
O2O2, Homozygous dominant; O2o2, Heterozygotes; o2o2, Homozygous recessive (Favourable); Allele 3 (Unfavourable); Allele 3/1 (Unfavourable); Allele 1 (Favourable); ns (Non significant).
Figure 1.
Foreground and background selection and morphological traits evaluation in ICF2 and ICF3 populations. (a) Foreground selection of ICF2 lines from DBT-IC-β1σ4 using crtRB1 gene specific marker crtRB1 3’TE, (M) Ladder (100 bp), (P1) CE477, (P2) UMI1200, (1–10) ICF2 plants; (b) Foreground selection of ICF2 lines from DBT-IC-β1σ4, using o2 gene linked marker umc1066, (M) Ladder (100 bp), (P1) UMI1200, (P2) VQL 1, (1–10) ICF2 plants; (c) Foreground selection of ICF2 lines from DBT-IC-β2σ5 using crtRB1 gene specific marker crtRB1 3’TE, (M) Ladder (100 bp), (P1) CE477, (P2) UMI1230, (1–10) ICF2 plants; (d), Foreground selection of ICF2 lines from DBT-IC-β2σ5 using o2 gene linked marker umc1066, (M) Ladder (100 bp), (P1) UMI1230, (P2) VQL 1, (1–10) ICF2 plants; (e, f, i and j), Background selection of ICF3 lines from DBT-IC-β1σ4, (M) Ladder (100 bp), (P1) UMI1200, (P2) CE477, (P3) VQL 1, (1–3) ICF3 plants; (g, h, k and l), Background selection of ICF3 lines from DBT-IC-β2σ5, (M) Ladder (100 bp), (P1) UMI1230, (P2) CE477, (P3) VQL 1, (1–3) ICF2 plants; (m–p), Evaluation of morphological traits in ICF3 lines. UMI 1200 (m), UMI1230 (n), DBT-ICβ1σ4-4-8-8 (o), DBT-IC-β2σ5-9-53-53 (p).
Evaluation of ICF3 generation for morphological traits
The newly developed 6 ICF3 line’s agronomical performance was evaluated (Fig. 1) by measuring 14 morphological traits and estimating the similarity percentage compared to the recurrent parent (Tables 2, 3). The three improved lines DBT-IC-β1σ4-4-8-8, DBT-IC-β1σ4-9-21-21, and DBT-IC-β1σ4-10-1-1 from the DBT-IC-β1σ4 population showed more than 90% similarity to the recurrent parent UMI1200 for most of the traits. The same was the case for the three improved lines from DBT-IC-β2σ5. In the DBT-IC-β1σ4 population, the similarity percentage ranged from 77.78% (NTB) to 99.31% (LB), and DBT-IC-β1σ4-4-8-8 showed the highest similarity percentage of 99.31% for LB, followed by DBT-IC-β1σ4-10-1-1 showing a similarity percentage of 99.28% for EW. In the DBT-IC-β2σ5 population, the similarity percentage ranged from 85.71% (NTB and NKRE) to 99.51% (LL), and DBT-IC-β2σ5-9-53-53 had the highest similarity percentage of 99.51% (LL), followed by DBT-IC-β2σ5-9-52-52 having a similarity percent of 98.86% for EL.
Table 2.
Comparison of the double positive lines in the ICF3 generation of DBT-IC-β1σ4 along with its recurrent parents for the recovery percentage of morphological traits.
| Morphological traits | Recurrent parent | Identified positive lines | Recovery percentage (%) | ||||
|---|---|---|---|---|---|---|---|
| DBT-IC-β1σ4 | UMI1200 | DBT-IC-β1σ4-4-8-8 | DBT-IC-β1σ4-9-21-21 | DBT-IC-β1σ4-10-1-1 | DBT-IC-β1σ4-4-8-8 | DBT-IC-β1σ4-9-21-21 | DBT-IC-β1σ4-10-1-1 |
| Days to tasseling (days) | 58.00 | 56.00 | 55.00 | 57.00 | 96.55 | 94.83 | 98.28 |
| Days to silking (days) | 60.00 | 59.00 | 58.00 | 60.00 | 98.33 | 96.67 | 98.33 |
| Plant height (cm) | 155.87 | 154.68 | 152.89 | 153.00 | 99.24 | 98.09 | 98.16 |
| Ear height (cm) | 76.84 | 73.85 | 67.75 | 72.02 | 96.11 | 88.17 | 93.73 |
| Tassel length (cm) | 21.34 | 20.42 | 21.08 | 19.68 | 95.69 | 98.78 | 92.22 |
| Number of tassel branches | 9.00 | 7.00 | 7.00 | 8.00 | 77.78 | 77.78 | 88.89 |
| Leaf length (cm) | 57.24 | 56.66 | 55.31 | 54.53 | 98.99 | 96.63 | 95.27 |
| Leaf breadth (cm) | 7.20 | 7.15 | 6.87 | 7.06 | 99.31 | 95.42 | 98.06 |
| Ear length (cm) | 15.60 | 14.40 | 14.80 | 14.50 | 92.31 | 94.87 | 92.95 |
| Number of kernel rows per ear | 12.00 | 10.00 | 10.00 | 10.00 | 83.33 | 83.33 | 83.33 |
| Number of kernels per row | 26.00 | 23.00 | 24.00 | 24.00 | 88.46 | 92.31 | 92.31 |
| Ear weight (g) | 121.47 | 120.20 | 119.90 | 120.60 | 98.95 | 98.71 | 99.28 |
| 100 kernel weight (g) | 28.76 | 26.50 | 27.20 | 25.80 | 92.14 | 94.58 | 89.71 |
| Single plant yield | 99.98 | 96.71 | 98.31 | 95.86 | 96.73 | 98.33 | 95.88 |
Table 3.
Comparison of the double positive lines in the ICF3 generation of DBT-IC-β2σ5 along with its recurrent parents for the recovery percentage of morphological traits.
| Morphological traits | Recurrent parent | Identified positive lines | Recovery percentage (%) | ||||
|---|---|---|---|---|---|---|---|
| DBT-IC-β2σ5 | UMI1230 | DBT-IC-β2σ5-9-51-51 | DBT-IC-β2σ5-9-52-52 | DBT-IC-β2σ5-9-53-53 | DBT-IC-β2σ5-9-51-51 | DBT-IC-β2σ5-9-52-52 | DBT-IC-β2σ5-9-53-53 |
| Days to tasseling (days) | 60.00 | 57.00 | 58.00 | 59.00 | 95.00 | 96.67 | 98.33 |
| Days to silking (days) | 62.00 | 59.00 | 60.00 | 61.00 | 95.16 | 96.77 | 98.39 |
| Plant height (cm) | 158.40 | 156.28 | 153.94 | 150.25 | 98.66 | 97.18 | 94.85 |
| Ear height (cm) | 81.00 | 79.06 | 77.75 | 76.57 | 97.60 | 95.99 | 94.53 |
| Tassel length (cm) | 31.30 | 29.92 | 30.70 | 29.18 | 95.59 | 98.08 | 93.23 |
| Number of tassel branches | 14.00 | 12.00 | 13.00 | 12.00 | 85.71 | 92.86 | 85.71 |
| Leaf length (cm) | 63.20 | 58.19 | 62.11 | 62.89 | 92.07 | 98.28 | 99.51 |
| Leaf breadth (cm) | 7.50 | 7.20 | 7.10 | 7.20 | 96.00 | 94.67 | 96.00 |
| Ear length (cm) | 17.50 | 17.20 | 17.30 | 17.00 | 98.29 | 98.86 | 97.14 |
| Number of kernel rows per ear | 14.00 | 12.00 | 12.00 | 12.00 | 85.71 | 85.71 | 85.71 |
| Number of kernels per row | 25.00 | 24.00 | 22.00 | 24.00 | 96.00 | 88.00 | 96.00 |
| Ear weight (g) | 106.90 | 104.56 | 103.70 | 102.20 | 97.81 | 97.01 | 95.60 |
| 100 kernel weight (g) | 25.20 | 22.12 | 23.21 | 24.11 | 87.78 | 92.10 | 95.67 |
| Single plant yield | 75.51 | 72.56 | 73.74 | 71.11 | 96.09 | 97.66 | 94.17 |
β-carotene, lysine, and tryptophan contents in ICF3 lines
The β-carotene content in the recurrent parents, UMI1200 and UMI1230, was found to be 0.60 μg/g and 1.20 μg/g respectively, whereas, for the donor parent, CE477, the β-carotene content was found to be 15.20 μg/g. In the DBT-IC-β1σ4 population, the highest β-carotene content was found in DBT-IC-β1σ4-9-21-21 (14.35 μg/g) followed by DBT-IC-β1σ4-10-1-1 (14.29 μg/g) and DBT-IC-β1σ4-4-8-8 (14.21 μg/g). For the DBT-IC-β2σ5 population, DBT-IC-β2σ5-9-53-53 was found to contain the highest β-carotene content (13.62 μg/g) followed by DBT-IC-β2σ5-9-51-51 (13.54 μg/g) and DBT-IC-β2σ5-9-52-52 (13.28 μg/g). The lysine and tryptophan levels of the recurrent parents, UMI1200 and UMI1230, were found to be 0.26%, 0.013% and 0.25%, 0.020% respectively. The lysine and tryptophan levels of the donor parent VQL1 were found to be 0.42% and 0.087%, respectively. In the DBT-IC-β1σ4 population, DBT-IC-β1σ4-4-8-8 recorded the highest level of lysine and tryptophan (0.33% and 0.082%), which is comparable with that of the donor parent. This was followed by DBT-IC-β1σ4-10-1-1 (0.32% and 0.081%) and DBT-IC-β1σ4-9-21-21 (0.31% and 0.079%). For the DBT-IC-β2σ5 population, DBT-IC-β2σ5-9-51-51 was found to contain the highest levels of lysine and tryptophan (0.34% and 0.083%). This was followed by DBT-IC-β2σ5-9-52-52 (0.32% and 0.081%) and DBT-IC-β2σ5-9-53-53 (0.31% and 0.078%). The average lysine and tryptophan levels for the improved lines were 0.32% and 0.080% (Table 4).
Table 4.
Lysine, tryptophan, and β carotene levels of the ICF3 improved double positive lines of DBT-IC-β1σ4 and DBT-IC-β2σ5.
| Trait | UMI1200 | UMI1230 | CE477 | VQL | DBT-IC-β1σ4 | DBT-IC-β2σ5 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| DBT-IC-β1σ4-4-8-8 | DBT-IC-β1σ4-9-21-21 | DBT-IC-β1σ4-10-1-1 | DBT-IC-β2σ5-9-51-51 | DBT-IC-β2σ5-9-52-52 | DBT-IC-β2σ5-9-53-53 | |||||
| β-Carotene | 0.60 | 1.20 | 15.20 | 0.7 | 14.21 | 14.35 | 14.29 | 13.54 | 13.28 | 13.62 |
| Lysine | 0.26 | 0.25 | 0.13 | 0.42 | 0.33 | 0.31 | 0.32 | 0.34 | 0.32 | 0.31 |
| Tryptophan | 0.013 | 0.020 | 0.021 | 0.087 | 0.082 | 0.079 | 0.081 | 0.083 | 0.081 | 0.078 |
Discussion
To improve maize lines with β-carotene, lysine, and tryptophan, in our study, we were able to pyramid two nutritionally important genes crtRB1 and o2 into a single genotype by way of intercrossing. In our breeding programme, four independent crosses (UMI1200 × CE477, UMI1200 × VQL1, UMI1230 × CE477, and UMI1230 × VQL1) were formed to incorporate the crtRB1 and o2 genes into two elite inbred lines UMI1200 and UMI1230. For the marker assisted backcrossing, selection using crtRB1 gene-specific and umc1066 markers was done to identify four BC3F1 lines from each cross having the desired genes and maximum RPG%. The lines DBT 1-1-1-17-5-14 from cross-1 and DBT 4-1-1-10-10-16 from cross-2 were intercrossed to produce the DBT-IC-β1σ4 population, which in hindsight improved UMI1200 for β-carotene, lysine, and tryptophan levels. Similarly, the lines DBT 2-1-4-7-1-9 from cross-3 and DBT 5-1-14-5-8-7 from cross-4 were intercrossed to produce the DBT-IC-β2σ5 population, which improved UMI1230 for β-carotene lysine and tryptophan levels.
In all the IC generations (ICF1–ICF3), the same markers were used to ensure that the final products were double homozygotes for both the crtRB1 and o2 genes. In the ICF2 generation, generated lines from both DBT-IC-β1σ4 and DBT-IC-β2σ5 populations were subjected to the chi-square test. The results revealed that the population segregated in the expected Mendelian ratio of 1:2:1 without any significant distortion for both the markers. Similar results were also obtained by Veldboom and Lee.35 and Lu et al.36. The selected double positive lines were then used to produce the ICF3 generation wherein the double homozygotes were ensured using the crtRB1 3’TE gene-specific and umc1066 markers. In this way, we were able to stack the nutritionally important genes and develop lines that were improved for β-carotene, lysine, and tryptophan levels. Similar studies were reported by other researchers26,28,31,33,34,37. However, in our study, we were able to achieve gene stacking by intercrossing homogenous lines that already had enhanced levels of β-carotene, lysine, and tryptophan thereby reducing the breeding cycle due to which we were able to produce a homogenous population that was highly similar to that of the recurrent parent in a short amount of time. Moreover, we were able to improve UMI1200 and UMI1230 which are the parents of a popular maize hybrid CO6 that is most suited to the climatic regions of South India.
Recovery of recurrent parent genome was also achieved in both ICF2 and ICF3 generation using a total of 148 polymorphic SSR markers. A high RPG% was obtained in the ICF2 generation itself due to the initial improved lines used to produce the intercross population having low levels of unwanted linkage drag. Once the ICF3 generation was developed we were able to identify three lines in both cross combinations that were double homozygotes and had a high recovery of recurrent parent genome. These results are in accordance with earlier reports19,26,32. The analysis of the opaqueness in the ICF2 generation showed that all the seeds showed 25% opaqueness for both the cross combinations. This was achieved because the lines that were used to produce the intercross population were already established for the 25% opaqueness using the lightbox screening method. Therefore, the progenies of the ICF3 generation also showed only 25% opaqueness. These results are in accordance with the previous findings24,29,33,38,39.
Morphological trait evaluation in the ICF3 generation for both DBT-IC-β1σ4 and DBT-IC-β2σ5 populations revealed that the improved lines were having more than 90% similarity with that of the recurrent parent without any major differences in important yield characters like SPY and EW. It showed that complete recovery of important phenotypic and yield characters of the recurrent parent was attained in the pyramided lines along with the desired genes. The lines DBT-IC-β1σ4-10-1-1 and DBT-IC-β1σ4-9-21-21 from the DBT-IC-β1σ4 population and the lines DBT-IC-β2σ5-9-51-51 and DBT-IC-β2σ5-9-52-52 from the DBT-IC-β2σ5 population were found to have the highest similarity to the respective recurrent parents as far as the yield characters were concerned. Similar results were also reported by former researchers28,34,40.
The evaluation of nutritional contents proved that the ICF3 lines had improved levels of β-carotene, lysine, and tryptophan levels in comparison with their normal recurrent parents. In the DBT-IC-β1σ4 population, DBT-IC-β1σ4-9-21-21 and DBT-IC-β1σ4-4-8-8 had the highest levels of β-carotene, lysine, and tryptophan respectively. Whereas, in the DBT-IC-β2σ5 population, DBT-IC-β2σ5-9-51-51 and DBT-IC-β2σ5-9-53-53 had the highest levels of β-carotene, lysine, and tryptophan respectively. Similar results were also obtained by earlier studies26,28,33,37. The improved lines in both cross combinations obtained from the ICF3 generation not only have the target donor genes with elevated nutrition levels but also has the high recovery of recurrent parent genome as well as highest phenotypic similarity to that of the recurrent parents rendering them crucial genetic materials for further hybrid synthesis and other genetic studies.
The present study has resulted in the development of improved lines possessing two genes (crtRB1 and o2) responsible for β-carotene, lysine, and tryptophan by marker-assisted gene pyramiding (MAGP) strategy. Thus, the pyramided inbred lines (UMI 1200 and UMI 1230) recorded a higher level of β-carotene, lysine, tryptophan, and better agronomic performance on par to donor parent and recurrent parents respectively. In the future, the promising improved lines could be developed as a cultivar for various agro-climatic zones and also as good genetic materials for maize nutritional breeding programs.
Materials and methods
Plant genetic materials
Maize inbreds, UMI1200, and UMI1230, well-adapted to tropical and semi-arid regions in India were selected as the recurrent parents. Because of their good combining ability, both were utilized to develop the CO6 hybrid. The inbreds seeds were obtained from the Department of Plant Genetic Resources, Centre for Plant Breeding and Genetics, Tamil Nadu Agricultural University, Coimbatore. VQL1 (Possessing o2 associated with high lysine and tryptophan contents) and CE477 (Possessing crtRB1 associated with high β-carotene content) were selected as donor parents. VQL1 was obtained from Vivekananda Parvatiya Krishi Anusandhan Sansthan (VPKAS), Almora, India, whereas CE477 was obtained from International Maize and Wheat Improvement Center, Mexico.
Foreground and background selection
Foreground selection was done using closely linked markers to crtRB1 and o2 genes. The crtRB1 gene located in chromosome 10 was selected using InDel marker crtRB1 3’TE9, whereas the o2 gene located in chromosome 7 was selected using the simple sequence repeat (SSR) marker umc106641. The background selection was done to examine the recurrent parent genome recovery (RPGR). It was performed using 248 SSR markers with known chromosomal positions distributing all ten maize chromosomes. All primer sequences were obtained from the maize genome database (www.maizegdb.org) and synthesized by Eurofins Ltd., Bangalore, India.
DNA extraction and PCR amplification
Genomic DNA was isolated from a two-week-old plant following the method by Murray and Thompson42. The PCR analysis for the crtRB1 gene-specific marker crtRB1 3′TE (65F: ACACCACATGGACAAGTTCG) and (62R: ACACTCTGGCCCATGAACAC, 66R: ACAGCAATACAGGGGACCAG) was carried out in a 10 μl reaction containing 2 μl of 20 ng template DNA, 2 mM of MgCl2, 1 mM of dNTPs, 2 μM of primer pair and 1.5U of Taq polymerase. The screening followed the ‘touch down’ technique of an initial denaturation for 5 min at 94 °C, followed by 19 cycles of denaturation for 45 s at 94 °C, annealing for 30 s at 62 °C with a reduction of 0.5 °C in every cycle down to 54 °C and extension for 1 min at 72 °C followed by another 20 cycles of denaturation at 94 °C for 45 s, annealing at 54 °C for 30 s, extension at 72 °C for 1 min and 20 s and a final extension at 72 °C for 10 min. The PCR analyses for the o2 gene-specific marker umc1066 (62R: ACACTCTGGCCCATGAACAC, 66R: ACAGCAATACAGGGGACCAG) and other background SSR markers were carried out in a 10 μl reaction containing 2 μl of 20 ng template DNA, 2 mM of MgCl2, 1 mM of dNTPs, 2 μM of primer pair, and 1.5U of Taq polymerase. The template DNA underwent an initial denaturation at 94 °C for 7 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s and extension at 72 °C for 45 s followed by a final extension at 72 °C for 7 min. The amplified PCR products were run using a 3% agarose gel for 3 h with the addition of 5 µl bromophenol blue, and the resolution was documented after 3 h.
Marker aided transfer of crtRB1 and o2 genes
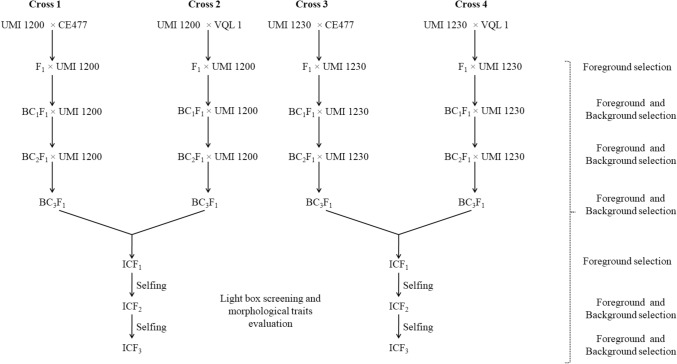
Four crossing programs, UMI1200 × CE477 (Cross-1), UMI1200 × VQL1 (Cross-2), UMI1230 × CE477 (Cross-3), and UMI1230 × VQL1 (Cross-4) were initiated to develop the nutrients rich lines using UMI1200 and UMI1230 (Recurrent) and CE477 and VQL1 (Donor) (Fig. 2). The F1s from all the four crosses were verified for the existence of crtRB1 and o2 genes in heterozygous form with foreground markers and then backcrossed with UMI1200 or UMI1230 to produce BC1F1. The BC1F1 lines having crtRB1 (Cross-1and 3) and o2 (Cross-2 and 4) in heterozygous form were selected with foreground markers. The foreground positives from BC1F1 were subjected to background selection to identify the plants with maximum recovery of recurrent parent genome using polymorphic SSR markers. Similarly, another two rounds of backcrossing followed by foreground and background selection generated BC3F1 lines having crtRB1 (Cross-1 and 3) and o2 (Cross-2 and 4) with maximum recovery of recurrent parent genome. The final lines were crossed to produce intercross F1s (ICF1) to combine the crtRB1 and o2 genes into a single plant. The heterozygous form in ICF1 was confirmed by foreground markers and then selfed to two generations to produce ICF3. The ICF2 and ICF3 generations were subjected to the foreground and background selection.
Figure 2.
Marker assisted backcrossing scheme (MABC) used to generate the intercross (IC) population. Cross 1 (UMI 1200 × CE477); Cross 2 (UMI1200 × VQL 1); Cross 3 (UMI1230 × CE477); Cross 4 (UMI1230 × VQL 1). Crossing between parents (Kharif season, June to September 2015), F1 (Rabi season, November to March 2015–2016), BC1F1 (Kharif season, June to September 2016), BC2F1 (Rabi season, November to March 2016–2017), BC3F1 (Kharif season, June to September 2017), ICF1 (Kharif season, June to September 2018), ICF2 (Rabi season, November to March 2018–2019), and ICF3 (Kharif season, June to September 2019).
Observation of kernel modification via lightbox screening
The o2o2 allele that is associated with the increased lysine and tryptophan content is also associated with an undesirable character of kernel softness that can be visualized as opaqueness in the kernels. Based on the opaqueness, the kernels can be categorized into five levels: 0%, 25%, 50%, 75%, and 100%. Usually, 25% and 50% kernels are selected since they are certain to contain the o2o2 gene in a homozygous recessive state. Whereas, the other categories contain the o2 gene in either heterozygous or homozygous dominant condition and are heavily susceptible to unfavourable irregularities. A lightbox apparatus is used to differentiate the level of kernel opaqueness as an indirect measure of the kernel softness. Hence, by the dual selection technique of lightbox screening and foreground selection, the o2o2 allele is guaranteed in the population. The ICF2 and ICF3 generation lines were subjected to the lightbox screening and the lines exhibiting 25% opaqueness are selected to fix the o2 allele in the homozygous recessive state.
Characterization of ICF3 lines for morphological traits
The newly developed intercross lines from both the cross combinations were planted along with the donor and recurrent parent. The plants were maintained with a distance of 20 cm, row spacing of 60 cm, and a row length of 3 m. Good agronomic practices were maintained during the growing period of the crop. Randomized Block Design (RBD) was performed with three replication. Randomly five plants were selected for the morphological trait evaluation. The recovery percentage of the recurrent parents was calculated according to the previous researchers29,33. The plants were examined for the agronomic performance by measuring 14 morphological characters viz., days to tasseling (DT, in days), days to silking (DS, in days), plant height (PH, cm), ear height (EH, cm), tassel length (TL, cm), number of tassel branches (NTB). leaf length (LL, cm), leaf breadth (LB, cm), ear length (EL, cm), number of kernels rows per ear (NKRE), number of kernels per row (NKR), ear weight (EW, g), 100 kernel weight (KW, g) and single plant yield (SPY, g). All the characterizations were done according to the descriptors suggested by the International Board for Plant Genetic Resources43.
Analysis of β carotene, lysine, and tryptophan
The kernels from the ICF3 generation were examined for β-carotene, lysine, and tryptophan. The extraction of β-carotene was done following the method given by Kurilich and Juvik44 and measured with the help of High-Performance Liquid Chromatography (HPLC). The final samples were eluted in a C30 column using a mobile phase consisting of acetonitrile: dichloromethane: methanol in the ratio of 75:20:5, and the flow rate was found to be 0.4 ml/min. The standard curve was constructed based on three different dilutions (1, 10, and 100 ppm) of standard beta carotene obtained from M/s Sigma Aldrich, USA. The lysine and tryptophan contents were measured following the colorimetric method45. The samples were measured using the spectrophotometer at a wavelength of 390 nm for lysine and 560 nm for tryptophan, and the levels were expressed in percent46.
Statement for the use of plant materials
The study complies with local and national regulations.
Supplementary Information
Acknowledgements
Financial support from the Department of Biotechnology (DBT), Government of India (GOI), through project entitled “Enrichment of nutritional quality in maize through molecular breeding” (BT/PR10922AGII/106/9442014 dt.25.3.2015) and Ministry of Science and Technology, Department of Biotechnology, Government of India DBT's Twinning programme for the NE for the project entitled “Marker assisted introgression of LyCE gene for enhanced ProA in maize” (BT/166/NE/TBP/2011 dt 12.12.2011) are acknowledged. The funders had no role in the work design, data collection and analysis, or decision and preparation of the manuscript.
Abbreviations
- EL
Ear length
- EW
Ear weight
- DS
Days to silking
- DT
Days to tasseling
- EH
Ear height
- HPLC
High performance liquid chromatography
- KW
Kernel weight
- LB
Leaf breadth
- LL
Leaf length
- MABB
Marker assisted backcross breeding
- MAGP
Marker assisted gene pyramiding
- NKR
Number of kernel rows
- NKRE
Number of kernel rows per ear
- NTB
Number of tassel branches
- PH
Plant height
- PCR
Polymerase chain reaction
- PME
Protein energy malnutrition
- RPGR
Recurrent parent genome recovery
- SPY
Single plant yield
- SSR
Simple sequence repeat
- TL
Tassel length
- UNICEF
United Nations International Childrens Emergency Fund
- USA
United States of America
- VAD
Vitamin A deficiency
Author contributions
Conceived and designed the experiments, S.N., HS.G., F.H.; Performed the experiments, N.C., N.R., B.P., S.C., D.M., A.K., S.V.; Analyzed the data, N.C., S.C., N.R., B.P.; Suggestions, G.K.N., M.S., R.R., V.M.; Writing—review & editing, N.C., A.K., S.C., S.N.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Neelima Chandrasekharan, Nagalakshmi Ramanathan, Bharathi Pukalenthy and Sarankumar Chandran.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-11585-y.
References
- 1.Knoema. "Maize Production Quantity". (New Knoema). https://knoema.com/atlas/topics/Agriculture/Crops-Production-Quantity-tonnes/Maize-production (2019).
- 2.Rodríguez-Concepción M. Supply of precursors for carotenoid biosynthesis in plants. Arch. Biochem. Biophys. 2010;504:118–122. doi: 10.1016/j.abb.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham FX, Gantt E. One ring or two? Determination of ring number in carotenoids by lycopene ɛ-cyclases. Proc. Natl. Acad. Sci. 2001;98:2905–2910. doi: 10.1073/pnas.051618398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.North HM, et al. The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J. 2007;50:810–824. doi: 10.1111/j.1365-313X.2007.03094.x. [DOI] [PubMed] [Google Scholar]
- 5.Sommer A, Davidson FR. Assessment and control of vitamin A deficiency: The Annecy Accords. J. Nutr. 2002;132:2845–2850. doi: 10.1093/jn/132.9.2845S. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Global database on vitamin A deficiency. Global prevalence of vitamin A deficiency in populations at risk. 1995–2005. http://whqlibdoc.who.int/publications/2009/9789241598019_eng.pdf (2009).
- 7.United Nations Children's Fund. Vitamin A deficiency. https://data.unicef.org/topic/nutrition/vitamin-a-deficiency/ (2019).
- 8.Harjes CE, et al. Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Science. 2008;319:330–333. doi: 10.1126/science.1150255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan J, et al. Rare genetic variation at Zea mays crtRB1 increases β-carotene in maize grain. Nat. Genet. 2010;42:322–327. doi: 10.1038/ng.551. [DOI] [PubMed] [Google Scholar]
- 10.Muthusamy V, et al. Genetic variability for kernel β-carotene and utilization of crtRB1 3'TE gene for biofortification in maize (Zea mays L.) Indian J. Genet. Plant Breed. 2012;72:189–194. [Google Scholar]
- 11.Babu R, Rojas NP, Gao S, Yan J, Pixley K. Validation of the effects of molecular marker polymorphisms in LcyE and CrtRB1 on provitamin A concentrations for 26 tropical maize populations. Theor. Appl. Genet. 2013;126:389–399. doi: 10.1007/s00122-012-1987-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mertz ET, Bates LS, Nelson OE. Mutant gene that changes protein composition and increases lysine content of maize endosperm. Science. 1964;145:279–280. doi: 10.1126/science.145.3629.279. [DOI] [PubMed] [Google Scholar]
- 13.Nelson OE, Mertz ET, Bates LS. Second mutant gene affecting the amino acid pattern of maize endosperm proteins. Science. 1965;150:1469–1470. doi: 10.1126/science.150.3702.1469. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Nelson OE. Amino acid composition and storage proteins in two new high-lysine mutants in maize. Cereal Chem. 1975;52:412. [Google Scholar]
- 15.McWhirter KS. A floury endosperm, high-lysine locus on chromosome 10. Maize Genet. Coop. News Letter. 1971;45:184. [Google Scholar]
- 16.Habben JE, Kirleis AW, Larkins BA. The origin of lysine-containing proteins in opaque-2 maize endosperm. Plant Mol. Bio. 1993;23:825–838. doi: 10.1007/BF00021537. [DOI] [PubMed] [Google Scholar]
- 17.Vasal SK. The quality protein maize story. Food Nutr. Bull. 2000;21:445–450. doi: 10.1177/156482650002100420. [DOI] [Google Scholar]
- 18.Prasanna BM, Vasal SK, Kassahun B, Singh NN. Quality protein maize. Curr. Sci. 2001;81:1308–1319. [Google Scholar]
- 19.Feng F, Wang Q, Liang C, Yang R, Li X. Enhancement of tocopherols in sweet corn by marker-assisted backcrossing of ZmVTE4. Euphytica. 2015;206:513–521. doi: 10.1007/s10681-015-1519-8. [DOI] [Google Scholar]
- 20.Lungaho MG, et al. Genetic and physiological analysis of iron biofortification in maize kernels. PLoS ONE. 2011;6(6):e20429. doi: 10.1371/journal.pone.0020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chilimba ADC, et al. Agronomic biofortification of maize with selenium (Se) in Malawi. Field Crops Res. 2012;125:118–128. doi: 10.1016/j.fcr.2011.08.014. [DOI] [Google Scholar]
- 22.Pixley, K. et al. Biofortification of maize with provitamin A carotenoids. in Carotenoids and Human Health, 271–292. (eds. Tanumihardjo, S.) (Humana Press, 2013).
- 23.Zhang YQ, et al. Zinc fertilizer placement affects zinc content in maize plant. Plant Soil. 2013;372:81–92. doi: 10.1007/s11104-013-1904-9. [DOI] [Google Scholar]
- 24.Gupta HS, et al. Accelerated development of quality protein maize hybrid through marker-assisted introgression of opaque-2 allele. Plant Breed. 2013;132:77–82. doi: 10.1111/pbr.12009. [DOI] [Google Scholar]
- 25.Yang L, Wang W, Yang W, Wang M. Marker-assisted selection for pyramiding the waxy and opaque-16 genes in maize using cross and backcross schemes. Mol. Breed. 2013;31:767–775. doi: 10.1007/s11032-012-9830-8. [DOI] [Google Scholar]
- 26.Muthusamy V, et al. Development of β-carotene rich maize hybrids through marker-assisted introgression of β-carotene hydroxylase allele. PLoS ONE. 2014;9(12):e113583. doi: 10.1371/journal.pone.0113583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muthusamy V, et al. Molecular characterization of exotic and indigenous maize inbreds for biofortification with kernel carotenoids. Food Biotechnol. 2015;29:276–295. doi: 10.1080/08905436.2015.1059768. [DOI] [Google Scholar]
- 28.Zunjare RU, et al. Development of biofortified maize hybrids through marker-assisted stacking of β-carotene hydroxylase, lycopene-ε-cyclase and opaque2 genes. Front. Plant Sci. 2018;9:178. doi: 10.3389/fpls.2018.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pukalenthy B, et al. Incorporation of opaque-2 into ‘UMI 1200’, an elite maize inbred line, through marker-assisted backcross breeding. Biotechnol. Biotechnol. Equip. 2019;33:144–153. doi: 10.1080/13102818.2018.1556121. [DOI] [Google Scholar]
- 30.Natesan S, et al. Enhancing β-carotene concentration in parental lines of CO6 maize hybrid through marker-assisted backcross breeding (MABB) Front. Nutr. 2020;7:134. doi: 10.3389/fnut.2020.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L, et al. Introgression of the crtRB1 gene into quality protein maize inbred lines using molecular markers. Mol. Breed. 2015;35:1–12. doi: 10.1007/s11032-015-0202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarika K, et al. Marker-assisted pyramiding of opaque2 and novel opaque16 genes for further enrichment of lysine and tryptophan in sub-tropical maize. Plant Sci. 2018;272:142–152. doi: 10.1016/j.plantsci.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Chandran S, et al. Marker-assisted selection to pyramid the opaque-2 (o2) and β-carotene (crtRB1) genes in maize. Front. Genet. 2019;10:859. doi: 10.3389/fgene.2019.00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sagare DB, Shetti P, Surender M, Reddy SS. Marker-assisted backcross breeding for enhancing β-carotene of QPM inbreds. Mol. Breed. 2019;39:1–12. doi: 10.1007/s11032-019-0939-x. [DOI] [Google Scholar]
- 35.Veldboom LR, Lee M. Molecular-marker-facilitated studies of morphological traits in maize. II: Determination of QTLs for grain yield and yield components. Theor. Appl. Genet. 1994;89:451–458. doi: 10.1007/BF00225380. [DOI] [PubMed] [Google Scholar]
- 36.Lu H, Romero-Severson J, Bernardo R. Chromosomal regions associated with segregation distortion in maize. Theor. Appl. Genet. 2002;105:622–628. doi: 10.1007/s00122-002-0970-9. [DOI] [PubMed] [Google Scholar]
- 37.Goswami R, et al. Marker-assisted introgression of rare allele of β-carotene hydroxylase (crtRB1) gene into elite quality protein maize inbred for combining high lysine, tryptophan and provitamin A in maize. Plant Breed. 2019;138:174–183. doi: 10.1111/pbr.12676. [DOI] [Google Scholar]
- 38.Vivek, B. S. Breeding quality protein maize (QPM): Protocols for developing QPM cultivars. CIMMYT, pp. 8–11 (2008).
- 39.Hossain F, et al. Marker-assisted introgression of opaque2 allele for rapid conversion of elite hybrids into quality protein maize. J. Genet. 2019;97:287–298. doi: 10.1007/s12041-018-0914-z. [DOI] [PubMed] [Google Scholar]
- 40.Surender M, et al. Development of QPM version of DHM117 maize hybrid using marker assisted selection. Int. J. Curr. Microbiol. Appl. Sci. 2017;6:3275–3289. doi: 10.20546/ijcmas.2017.610.384. [DOI] [Google Scholar]
- 41.Gibbon BC, Larkins BA. Molecular genetic approaches to developing quality protein maize. Trends Genet. 2005;21:227–233. doi: 10.1016/j.tig.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4326. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anonymous. “IBPGR. Descriptors for Maize,” in International Wheat and Maize Improvement Center (Mexico City: International Board for Plant Genetic) (1991).
- 44.Kurilich AC, Juvik JA. Quantification of carotenoid and tocopherol antioxidants in Zea mays. J. Agric. Food Chem. 1999;47:1948–1955. doi: 10.1021/jf981029d. [DOI] [PubMed] [Google Scholar]
- 45.Galicia, L. N., Rosales, E. & A Palacios Rojas, N. Laboratory protocols 2008: Maize nutrition quality and plant tissue analysis laboratory. CIMMYT, pp. 10–14 (2008).
- 46.Moro GL, Habben JE, Hamaker BR, Larkins BA. Characterization of the variability in lysine content for normal and opaque2 maize endosperm. Crop Sci. 1996;36:1651–1659. doi: 10.2135/cropsci1996.0011183X003600060039x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.