Abstract
Biomarkers for selecting gastric cancer (GC) patients likely to benefit from sequential paclitaxel treatment followed by fluorinated-pyrimidine-based adjuvant chemotherapy (sequential paclitaxel) were investigated using tissue samples of patients recruited into SAMIT, a phase III randomized controlled trial. Total RNA was extracted from 556 GC resection samples. The expression of 105 genes was quantified using real-time PCR. Genes predicting the benefit of sequential paclitaxel on overall survival, disease-free survival, and cumulative incidence of relapse were identified based on the ranking of p-values associated with the interaction between the biomarker and sequential paclitaxel or monotherapy groups. Low VSNL1 and CD44 expression predicted the benefit of sequential paclitaxel treatment for all three endpoints. Patients with combined low expression of both genes benefitted most from sequential paclitaxel therapy (hazard ratio = 0.48 [95% confidence interval, 0.30–0.78]; p < 0.01; interaction p-value < 0.01). This is the first study to identify VSNL1 and CD44 RNA expression levels as biomarkers for selecting GC patients that are likely to benefit from sequential paclitaxel treatment followed by fluorinated-pyrimidine-based adjuvant chemotherapy. Our findings may facilitate clinical trials on biomarker-oriented postoperative adjuvant chemotherapy for patients with locally advanced GC.
Subject terms: Tumour biomarkers, Gastric cancer
Introduction
In Japan, 134,650 patients were diagnosed with gastric cancer (GC) in 2019, out of which 25,850 had stage II/III disease, according to the Union for TNM 8th edition1,2. The standard treatment for patients with stage II/III GC in Japan is curative D2 gastrectomy followed by postoperative adjuvant chemotherapy3, based on the results of the Japanese Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer (ACTS-GC) and Korean Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC) randomized phase III trials4–7. However, despite the improved overall survival (OS) with adjuvant chemotherapy, the five-year OS rate of patients with pathological stage III (pStage III) GC remains unsatisfactory. Hence, there is an urgent clinical need to develop new more effective regimens and personalized adjuvant chemotherapy treatments based on biomarkers.
It has been reported recently that patients with curatively resected pathological (p) stage III GC treated with adjuvant docetaxel and S-1 had significantly longer 3-year recurrence-free survival than those treated with adjuvant S-1 monotherapy chemotherapy (JACCRO GC-07 study) 8. Based on the results, chemotherapy with S-1 and docetaxel after D2 gastrectomy was recommended as the new standard of care for patients with pStage III GC in Japan.
Biomarkers for personalized adjuvant chemotherapy have been investigated in resected cancer tissue specimens from the ACTS-GC and CLASSIC trials 9–13. Although several novel GC biomarkers were discovered in ACTS-GC, none of the biomarkers showed a significant interaction with S-1 treatment 9–12. In a post-hoc analysis of resection specimens from the CLASSIC trial, it was reported that the combined RNA expression levels of three genes (granzyme B [GZMB], WARS, and caudal-related homeobox [CDX1]) were able to predict the benefit of adjuvant chemotherapy with capecitabine plus oxaliplatin compared to no adjuvant chemotherapy13.
In addition to fluorinated-pyrimidine plus platinum-based anticancer drugs such as capecitabine plus oxaliplatin, fluorinated-pyrimidines plus taxanes such as paclitaxel or docetaxel have been considered for GC treatment 14. Taxane-based anticancer drugs have lower incidences of nephrotoxicity or neuropathy than platinum-based compounds, such as cisplatin or oxaliplatin, and can be administered safely in an outpatient setting. Both the JACCRO GC-07 trial and SAMIT have demonstrated improved outcomes in the subgroup of patients with pathological stage III GC treated with postoperative adjuvant chemotherapy using fluorinated-pyrimidine and taxane-based anticancer drugs 8,14. However, the disease recurrence rate within 2 years after surgery was 75.3% in the JACCRO GC-07 trial and 55.3% in the SAMIT trial. Therefore, adjuvant chemotherapy using fluorinated-pyrimidine plus taxane-based anticancer drugs may only be effective in a subset of GC patients. If such patients can be identified by biomarker assessment in the gastrectomy specimens, adjuvant chemotherapy regimens could be personalized, and patient outcomes could be improved.
In the present study, we performed a post-hoc analysis of tissue samples collected from patients recruited in the SAMIT using a comprehensive panel of mRNA expression-based biomarkers. The aim of the present study was to identify genes suitable for selecting patients likely to benefit more from adjuvant chemotherapy with sequential paclitaxel followed by fluorinated-pyrimidine (sequential paclitaxel).
Results
Patients and sample collection
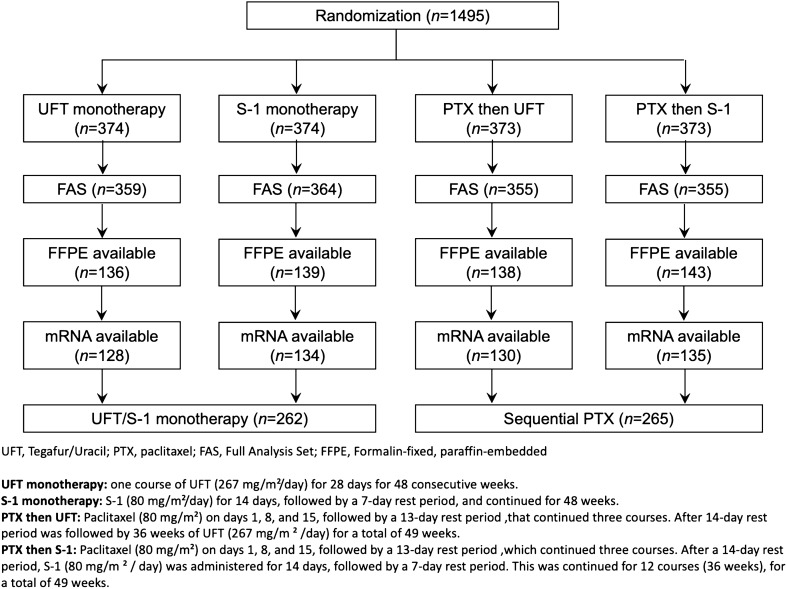
Formalin-fixed paraffin-embedded (FFPE) samples were retrospectively collected from 556 SAMIT patients. Twenty-nine patients had to be excluded subsequently due to insufficient RNA, leaving 527 patients for biomarker analysis (Fig. 1). The clinicopathological characteristics of the patients included in the current study were representative of the entire SAMIT population (Table 1). Except for sex (there were more males in the sequential paclitaxel treatment group [p = 0.04]), the clinical and pathological characteristics were well balanced between the sequential paclitaxel treatment and fluorinated-pyrimidine monotherapy subgroups (Supplementary Table S1, Online Resource 1). The median follow-up times from randomization were 56.8 (interquartile range [IQR] = 45.3–69.8 months) and 59.1 months (IQR = 46.2–72.8 months) for patients in the fluorinated-pyrimidine monotherapy and sequential paclitaxel arms, respectively.
Figure 1.
Flowchart of SAMIT patients available for primary analysis and subsequent biomarker analysis. Formalin-fixed, paraffin-embedded (FFPE) samples were available from 556 SAMIT patients. Twenty-nine patients had to be excluded owing to insufficient RNA.
Table 1.
Clinical and pathological characteristics of patients included in the biomarker analysis compared to the entire SAMIT patient cohort.
| Biomarker analysis cohort (n = 527) | Entire SAMIT cohort (n = 1433) | p-value | |||
|---|---|---|---|---|---|
| No. of patients | % | No. of patients | % | ||
| Arms | 0.980 | ||||
| S-1 only | 128 | 24.3 | 359 | 25.1 | |
| UFT only | 134 | 25.4 | 364 | 25.4 | |
| Paclitaxel then UFT | 130 | 24.7 | 355 | 24.8 | |
| Paclitaxel then S-1 | 135 | 25.6 | 355 | 24.8 | |
| Age | 1.00 | ||||
| < 65 years | 243 | 46.7 | 670 | 46.8 | |
| ≥ 65 years | 284 | 53.3 | 763 | 53.2 | |
| Sex | 1.00 | ||||
| Male | 361 | 68.5 | 980 | 68.4 | |
| Female | 166 | 31.5 | 453 | 31.6 | |
| PS | 0.211 | ||||
| 0 | 442 | 83.9 | 1234 | 86.1 | |
| 1 | 85 | 16.1 | 199 | 13.9 | |
| 2 or 3 | 0 | 0 | 0 | 0 | |
| Tumor location | |||||
| T | 12 | 2.3 | 43 | 3.0 | 0.785 |
| U | 131 | 24.9 | 366 | 25.5 | |
| M | 176 | 33.4 | 482 | 33.6 | |
| L | 208 | 39.5 | 542 | 37.8 | |
| Tumor diameter | 0.554 | ||||
| < 65 | 278 | 52.8 | 686 | 47.9 | |
| ≥65 | 249 | 47.2 | 747 | 52.1 | |
| Surgery | 0.432 | ||||
| Total gastrectomy | 241 | 45.7 | 696 | 48.6 | |
| Proximal gastrectomy | 1 | 0.2 | 5 | 0.3 | |
| Distal gastrectomy | 284 | 53.9 | 728 | 50.8 | |
| Lymph node dissection | 0.252 | ||||
| D1 | 1 | 0.2 | 2 | 0.1 | |
| D1 + | 22 | 4.2 | 92 | 6.4 | |
| D2 | 496 | 94.1 | 1311 | 91.5 | |
| D3 | 8 | 1.5 | 28 | 2.0 | |
| Lauren’s classification | 0.791 | ||||
| Intestinal type | 212 | 40.2 | 567 | 39.6 | |
| Diffuse type | 315 | 59.8 | 866 | 60.4 | |
| pT | 0.051 | ||||
| 1 | 7 | 1.3 | 12 | 0.8 | |
| 2 | 161 | 30.6 | 366 | 25.5 | |
| 3 | 339 | 64.3 | 966 | 67.4 | |
| 4 | 20 | 3.8 | 89 | 6.2 | |
| pN | 0.167 | ||||
| 0 | 109 | 20.7 | 268 | 18.7 | |
| 1 | 90 | 17.1 | 296 | 20.6 | |
| 2 | 119 | 22.6 | 350 | 24.4 | |
| 3 | 209 | 40.0 | 519 | 36.2 | |
| pTNM stage | 0.080 | ||||
| I | 37 | 7.0 | 77 | 5.4 | |
| IIA | 107 | 20.3 | 266 | 18.6 | |
| IIB | 106 | 20.1 | 318 | 22.2 | |
| IIIA | 105 | 19.9 | 344 | 24.0 | |
| IIIB | 101 | 19.2 | 291 | 20.3 | |
| IIIC | 71 | 13.5 | 147 | 10.3 | |
UFT tegafur/uracil, T total stomach, U upper third of stomach, M medium third of stomach, D distal third of stomach, T pathological tumor depth, pN pathological lymph node metastasis, M distant metastasis, PS performance status.
Predictive biomarkers for selecting patients likely to benefit from sequential paclitaxel therapy
We conducted multivariable Cox regression analysis to assess the potential relationships between gene expression level and overall survival (OS), disease-free survival (DFS), or cumulative incidence of relapse after sequential paclitaxel therapy; the genes were ranked based on the interaction-related p-values. Visinin-like 1 (VSNL1) and CD44 were the only genes with mRNA expression levels that were statistically significant as predictive biomarkers of sequential paclitaxel treatment for all three endpoints (Supplementary Table S2, Online Resource 1).
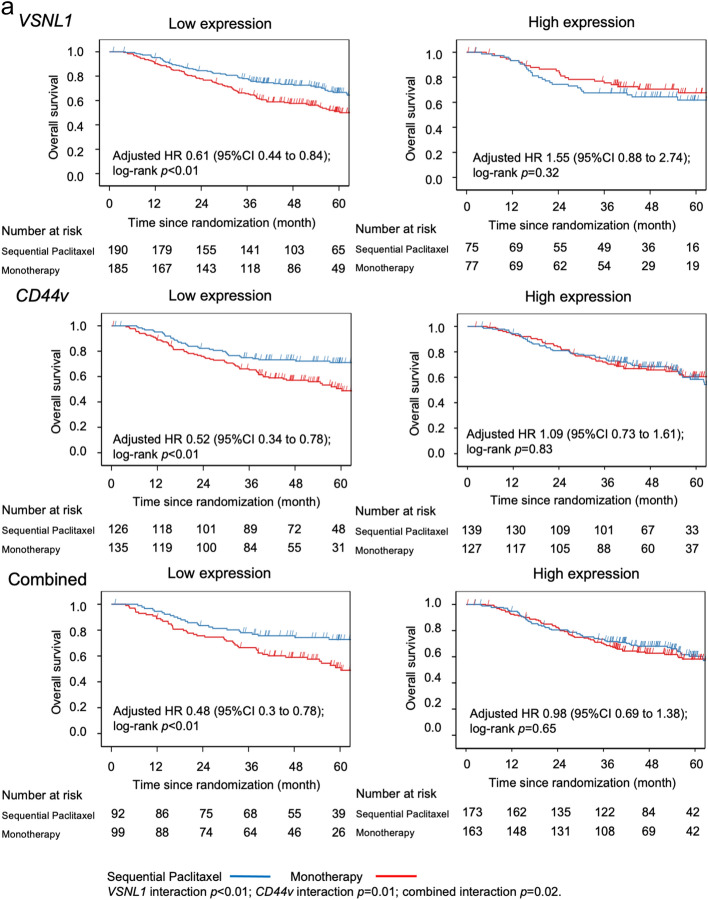
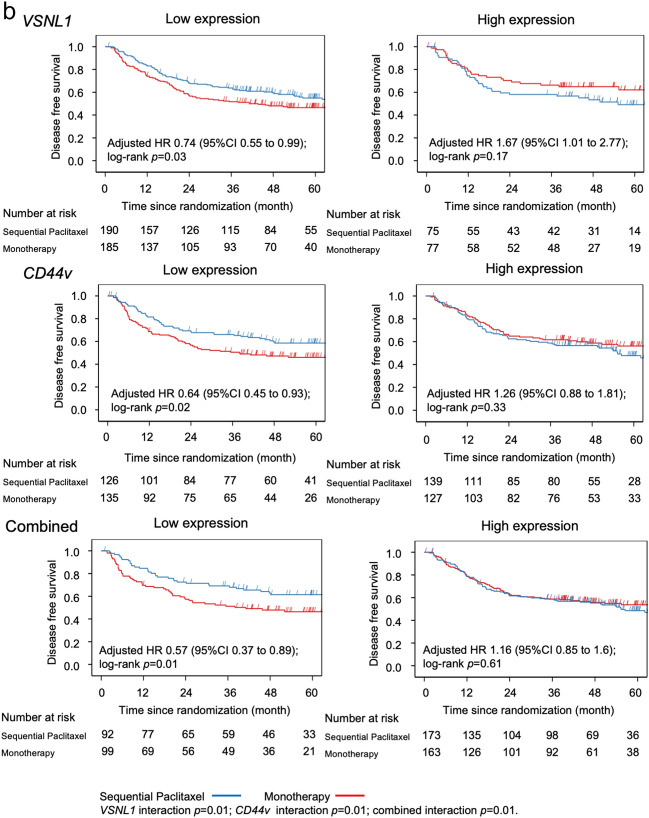
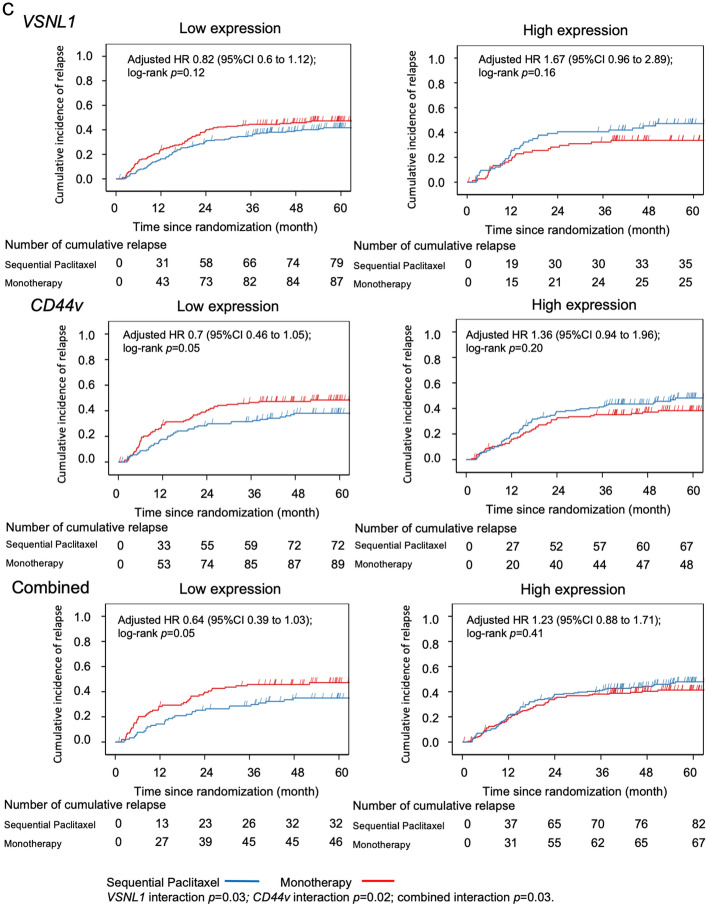
A total of 191 (36.2%) patients showed combined low expression of both genes, which was associated with the greatest benefit from sequential paclitaxel treatment compared to fluorinated-pyrimidine monotherapy (Table 2). Patients with low levels of expression of VSNL1, CD44v, or both, had significantly longer OS and DFS after sequential paclitaxel treatment than after monotherapy (Fig. 2a,b). However, no such effect was observed in the cumulative incidence of relapse (Fig. 2c).
Table 2.
Effects of sequential paclitaxel followed by UFT or S-1 on overall survival, disease-free survival, and cumulative incidence of relapse, based on gene expression levels.
| Subgroups | Comparison of sequential paclitaxel and monotherapy over time | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | Main effect p | Interaction p-value | |||
| Overall survival | ||||||
| Total | (n = 527) | 0.76 | 0.57 | 1.01 | 0.05 | |
| VSNL1 | Low expression (n = 375) | 0.61 | 0.44 | 0.84 | < 0.01 | < 0.01 |
| High expression (n = 152) | 1.55 | 0.88 | 2.74 | 0.13 | ||
| CD44 | Low expression (n = 261) | 0.52 | 0.34 | 0.78 | < 0.01 | 0.01 |
| High expression (n = 266) | 1.09 | 0.73 | 1.61 | 0.67 | ||
| Combined | low expression of both genes (n = 191) | 0.48 | 0.3 | 0.78 | < 0.01 | 0.02 |
| high expression of either gene (n = 336) | 0.98 | 0.69 | 1.38 | 0.89 | ||
| Disease-free survival | ||||||
| Total | (n = 527) | 0.91 | 0.7 | 1.17 | 0.44 | |
| VSNL1 | Low expression (n = 375) | 0.74 | 0.55 | 0.99 | 0.04 | 0.01 |
| High expression (n = 152) | 1.67 | 1.01 | 2.77 | 0.05 | ||
| CD44 | Low expression (n = 261) | 0.64 | 0.45 | 0.93 | 0.02 | 0.01 |
| High expression (n = 266) | 1.26 | 0.88 | 1.81 | 0.21 | ||
| Combined | low expression of both genes (n = 191) | 0.57 | 0.37 | 0.89 | 0.01 | 0.01 |
| high expression of either gene (n = 336) | 1.16 | 0.85 | 1.6 | 0.35 | ||
| Cumulative incidence of relapse | ||||||
| Total | (n = 527) | 0.98 | 0.75 | 1.28 | 0.87 | |
| VSNL1 | Low expression (n = 375) | 0.82 | 0.6 | 1.12 | 0.21 | 0.03 |
| High expression (n = 152) | 1.67 | 0.96 | 2.89 | 0.07 | ||
| CD44 | Low expression (n = 261) | 0.7 | 0.46 | 1.05 | 0.08 | 0.02 |
| High expression (n = 266) | 1.36 | 0.94 | 1.96 | 0.1 | ||
| Combined | low expression of both genes (n = 191) | 0.64 | 0.39 | 1.03 | 0.07 | 0.03 |
| high expression of either gene (n = 336) | 1.23 | 0.88 | 1.71 | 0.22 | ||
HR hazard ratio, CI confidence interval, UFT tegafur/uracil.
Figure 2.
Kaplan–Meier curves based on gene expression level in the sequential paclitaxel and monotherapy arms. Patients with low RNA expression levels of VSNL1, CD44, or both had significantly longer overall survival (a), longer disease-free survival (b), and lower cumulative incidence of relapse (c) after sequential paclitaxel treatment than after monotherapy.
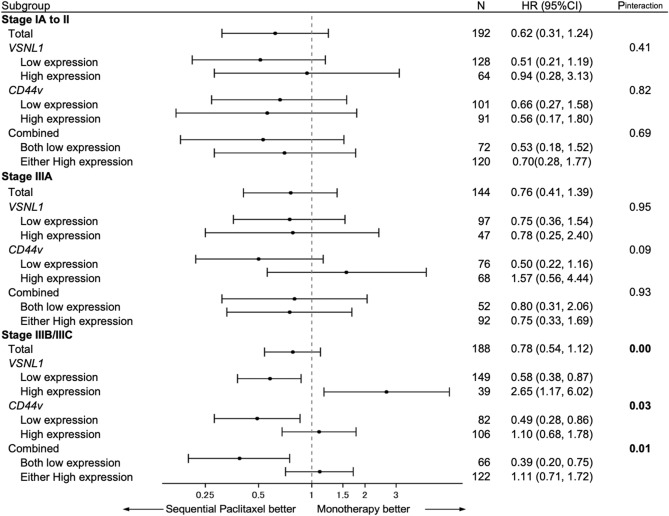
Patient stratification based on pTNM stage showed that OS improvement in response to sequential paclitaxel treatment in patients with low VSNL1 and/or CD44v expression was the greatest in patients with stage IIIB/IIIC GC (Fig. 3).
Figure 3.
Forest plot of the study results. After patient stratification based on the pTNM stage, the survival benefit from sequential paclitaxel treatment was greater among patients with stage IIIB gastric cancer with a low expression of either gene or both. The association between the low expression levels of VSNL1 and CD44 and potential benefits from sequential paclitaxel treatment were significant for disease-free survival and cumulative incidence of relapse.
Internal validation
The overall performances of the different statistical models, including the interactions between VSNL1 mRNA expression and the treatment group, as well as the clinical and pathological factors for OS prediction with C statistics using the bootstrap 0.632 + estimator (0.7111) and apparent estimator (0.7266), were evaluated. The accuracy of OS prediction based on CD44 and VSNL1 mRNA expression levels was comparable when the apparent estimator was used (0.7252), whereas it was not sufficiently accurate when the bootstrap 0.632 + estimator was used (0.7083) (Supplementary Table S3, Online Resource 1).
Relationship between VSNL1 or CD44v mRNA expression and clinicopathological factors
Significant relationships between the expression level of VSLN1 mRNA and age, histopathological type, and pTNM stage were observed. Patients with low expression levels of VSNL1 mRNA in GC tissue had significantly higher rates of age < 65 years, undifferentiated adenocarcinoma, and high pTMN stage compared to those with high expression. In contrast, there was no significant relationship between CD44 mRNA expression and any clinicopathological factors (Supplementary Table S4, Online Resource 1).
Relationship between mRNA expression levels and protein expression levels of VSNL1 and CD44v
Protein expression levels of VSNL1 and CD44 were investigated in a subgroup of patients based on immunohistochemistry (IHC) analyses, and patients were dichotomized into low and high expression groups, based on an immune response scoring system.
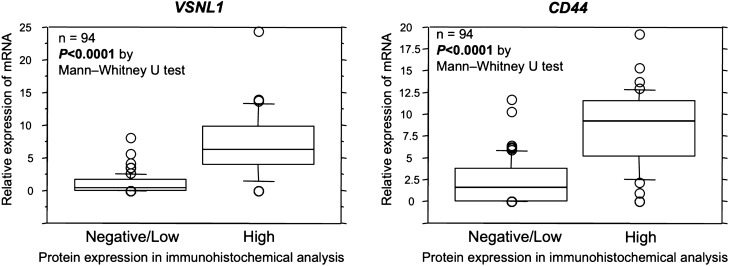
For CD44v IHC, since there are eight variant isoforms (CD44v1-8) created by mRNA splice variants, we analyzed the relationship between CD44v1-8 and CD44 using data from NanoString analysis and found that all CD44v mRNA expression was strongly correlated with that of CD44 mRNA (Supplementary Fig. S1, Online Resource 1). Therefore, CD44 expression in IHC was examined as a representative of CD44 and CD44v1-8. The relationship between VSNL1 and CD44 protein expression levels and mRNA expression levels by IHC analysis showed that mRNA expression levels were significantly higher in the high protein-expression group than in the low-protein expression group, based on the Mann–Whitney U test (Fig. 4; P < 0.0001, P < 0.0001, respectively). In addition, the concordance between high/low mRNA expression levels and high/low protein expression levels were 79.8% and 81.9% for VSNL1 and CD44, respectively (Table 3).
Figure 4.
Relationship between mRNA expression levels and protein expression levels of VSNL1 and CD44. The protein expression levels of VSNL1 and CD44 based on immunohistochemistry (IHC) analysis were divided into low and high expression groups. There was a significant difference in the mRNA expression levels of VSNL1 and CD44 between the low and high protein expression groups of both VSNL1 and CD44 based on IHC analysis.
Table 3.
Relationship between VSNL1 mRNA expression and VSNL1 protein expression, and for the relationship between CD44 mRNA expression and CD44 protein expression.
| VSLN1 | CD44 | ||||||
|---|---|---|---|---|---|---|---|
| IHC high | IHC low | IHC high | IHC low | ||||
| mRNA high | 27 | 13 | 40 | mRNA High | 31 | 11 | 42 |
| mRNA low | 6 | 48 | 54 | mRNA Low | 6 | 46 | 52 |
| 33 | 61 | 37 | 57 | ||||
| Concordance rate: 79.8% | Concordance rate: 81.9% | ||||||
Furthermore, patients were divided into low expression groups of both VSNL1 and CD44 proteins (n = 53) and high expression groups of either VSNL1 or CD44 protein (n = 41), according to the VSNL1 and CD44 protein expression results in the IHC analyses. In each group, the OS of sequential paclitaxel and fluoropyrimidine monotherapy was evaluated using a log-rank test. The results showed that the OS of sequential paclitaxel was significantly better than that of fluoropyrimidine monotherapy in patients with low levels of expression of both VSNL1 and CD44. Conversely, no difference was observed in the high expression groups of either VSNL1 or CD44 (Fig. 4), which was consistent with the mRNA results.
Examination of the usefulness of the algorithm with the four biomarkers (GZMB, WARS, SFRP4, and CDX1) validated in the CLASSIC study sample to stratify the risk of recurrence and select patients who would benefit from adjuvant chemotherapy with paclitaxel followed by sequential pyrimidine fluoride using the sample from this biomarker study
In the sample of the current biomarker study (n = 527), the algorithm based on GZMB, WARS, and SFRP4 mRNA expression levels did not significantly stratify the risk of recurrence (Supplementary Fig. 2a,b, Online Resource 1). Subsequently, when the patients were separated into "chemotherapy benefit group" and "chemotherapy no-benefit group" according to the algorithm based on GZMB, WARS, and CDX1 mRNA expression levels, in the chemotherapy no-benefit group, the survival rates of patients in the chemotherapy-responsive group were the same regardless of the type of adjuvant treatment. However, in the chemotherapy-naive group, characterized by high immunity (GZMB + , WARS +) and low epitheliotropism (CDX1-), patients treated with sequential paclitaxel had significantly longer survival (Supplementary Fig. 2c,d, Online Resource 1).
Discussion
The present study explored biomarkers for identifying gastric cancer (GC) patients that are likely to benefit from sequential paclitaxel treatment followed by fluorinated-pyrimidine-based adjuvant chemotherapy at the mRNA level using clinical samples and data from GC patients treated in a randomized controlled phase III trial of adjuvant chemotherapy, SAMIT15. Although previous studies using clinical samples from the ACTS-GC have revealed several novel molecular GC biomarkers, significant interactions between S-1 treatment and RNA expression levels have not been observed8–11. In a study of clinical samples from the CLASSIC trial, an algorithm based on the RNA expression levels of three genes was able to predict patients who were likely to benefit from adjuvant chemotherapy with capecitabine plus oxaliplatin12.
Although several candidate biomarkers of resistance or sensitivity to paclitaxel, such as Tau, COL4A3BP, UGCG, MCL1, FBW7, SLC31A2, SLC35A5, SLC43A1, SLC41A2, and CCNG1 have previously been suggested16–23, none have been validated in a second independent series. Hence, there remains a clinical need to validate the proposed biomarkers and/or identify new biomarkers that can be used in routine clinical practice to identify patients likely to benefit from paclitaxel therapy24. Moreover, associations between the expression of several genes or proteins and the benefits of paclitaxel, such as CCND1, ABCB1, BCL-2, and SPARC in different tumor types, have been reported in multiple studies 25–29. For example, CCND1 overexpression promotes paclitaxel-induced apoptosis in breast cancer26. BCL-2 family members such as BCL-2, BCl-xL, BAX, and ABCB1, have been reported to be involved in paclitaxel resistance in esophageal cancer27. In addition, SPARC expression in tumor stromal cells is a potential negative predictor of paclitaxel treatment in patients with lung cancer28,29. However, the expression levels of all previously suggested biomarkers were not significantly associated with patient outcomes in the present study. This may be related to the cancer type, sample size, case mix, ethnic differences, or methodological differences.
In the present study, we identified the expression levels of VSNL1 and/or CD44v as potential novel predictive biomarkers to identify patients who could benefit from postoperative adjuvant chemotherapy with sequential paclitaxel followed by a fluorinated-pyrimidine after curative gastrectomy. Although the combined low expression of the two biomarkers predicted the greatest benefits from adjuvant chemotherapy with sequential paclitaxel and a fluorinated-pyrimidine, no clear interaction between VSNL1 and CD44v has been reported to date.
The VSNL1 gene encodes visinin-like protein 1 (VILIP-1), a member of the neuronal calcium sensor protein family that regulates calcium-dependent cells and signaling adenylate cyclase30. In cancers, VSNL1 is overexpressed in various cancers such as GC, colorectal cancer, non-small cell lung cancer, and squamous cell carcinoma31–34, and inhibits cell proliferation, adhesion, and infiltration. In addition, it has been reported to function as a tumor suppressor gene33,34. Deficiency or reduced expression of VSNL1 by knockdown in vitro has been reported to increase the motility of cancer cells, suggesting a potential tumor suppressor function of the protein. VSNL1 regulates SNAIL1, which is a transcription factor with cAMP-dependent function, and SNAIL1 expression prevents epithelial-mesenchymal transition in cancer cells34. In recent years, it has been reported that high expression of VSNL1 promotes the proliferation and migration of GC cells by regulating the expression of P2X3 and P2Y2 receptors, and that high expression of VSNL1 in GC tissue may be a good clinical indicator for poor prognosis in GC patients35. However, in the present study, VSNL1 expression in GC tissue was not a prognostic factor. Regarding the association with chemotherapy, VSNL-1 has been reported to be involved in epithelial-mesenchymal transition (EMT) of cancer cells by regulating the transcription factor Snail1 in a cAMP-dependent manner 34. Therefore, high expression of VSLN1 suppresses EMT by regulating Snail1, which may weaken chemoresistance to anticancer agents, including paclitaxel, and increase chemosensitivity.
The CD44 gene encodes the CD44 protein, an adhesion molecule that uses hyaluronan as a ligand, and there are eight isoforms (CD44v1-8) that are created by mRNA splice variants. In the present study, we initially investigated only CD44v1 mRNA expression and identified it as a biomarker. Additional analysis of the relationship between CD44v1-8 and CD44 using data from NanoString analysis showed that the expression of all CD44v isoforms was strongly correlated with CD44 expression, indicating that CD44 and CD44v1-8 mRNA expression may be biomarkers in the present study. CD44 protein is overexpressed on the cell surface of cancer stem cells in GC tissues, and binding of hyaluronan to CD44 has been reported to affect various downstream signaling pathways, leading to cancer invasion, metastasis, and resistance to chemoradiotherapy36–42. As for paclitaxel resistance, ovarian cancer has been reported to exhibit higher levels of CD44 expression than paclitaxel-sensitive cancer cells43.
To the best of our knowledge, this is the first and most comprehensive study to identify biomarkers for the prediction of patients with survival benefit from sequential paclitaxel followed by fluorinated-pyrimidine adjuvant chemotherapy in GC patients. However, the present study has several limitations. First, although we demonstrated that the study cohort was representative of the entire SAMIT patient cohort, with respect to clinicopathological characteristics, including survival, we were only able to retrieve material from approximately a third of the original SAMIT population. Furthermore, the number of samples in which biomarkers identified at the mRNA level were validated at the protein level was limited. Second, we only analyzed RNA samples from a single tissue block, not whole tumors. Therefore, the intertumoral heterogeneity may not be sufficiently assessed. Third, SAMIT recruited patients with serosal invasion (e.g., cT4 tumors), a major risk for peritoneal recurrence, and randomized them to receive fluorinated pyrimidine monotherapy or sequential paclitaxel, which was hypothesized to reduce postoperative recurrence, such as peritoneal recurrence, and improve prognosis. However, it should be noted that there was a small number of patients with pT4 tumors in the SAMIT.
In conclusion, the biomarkers for selecting patients with GC who would most likely benefit from adjuvant chemotherapy with sequential paclitaxel and fluorinated-pyrimidine treatment after curative gastrectomy were identified. Although the validation of our findings in a second independent series followed by a prospective trial is necessary, personalized adjuvant chemotherapy using these biomarkers may further improve treatment outcomes in patients with locally advanced GC.
Methods
Patients and sample collection
This biomarker study was conducted using GC specimens and clinicopathological data from patients who participated in a phase 3 randomized comparative study (SAMIT) performed using a two × two factorial design of postoperative adjuvant chemotherapy after D2 gastrectomy. SAMIT was performed in 230 hospitals in Japan in patients with GC. Patients aged 20–80 years with an ECOG performance score of 0–1 who were diagnosed with cT4a or T4b GC by preoperative diagnosis were enrolled. The patients were randomly assigned to one of the four postoperative adjuvant chemotherapy groups (tegafur and uracil [UFT] monotherapy, S-1 monotherapy, three courses of paclitaxel followed by UFT, or three courses of paclitaxel followed by S-1) after undergoing D2 gastrectomy.
The completion rate of the trial was 60% in the UFT-only group, 62% in the S-1-only group, 68% in the UFT-treated group after paclitaxel treatment, and 70% in the S-1-treated group after paclitaxel15.
The present study was approved by the Institutional Review Board (IRB) of Kanagawa Cancer Center, the central institute for this study (approval number: 26-42), as well as the IRBs of all institutions that participated in the present study. Representative blocks from formalin-fixed paraffin-embedded (FFPE) gastrectomy specimens were collected retrospectively from participating institutions according to the following inclusion criteria: (1) patients were participants in the SAMIT, (2) FFPE blocks or unstained cut sections were available, and (3) the translational study protocol was approved by the IRB. Samples were collected from the data center of the Kanagawa Cancer Center and shipped to Yokohama City University for RNA extraction and analysis. Sections (each 10-μm thick) were cut from the FFPE blocks and stored at 4 °C until microdissection.
RNA extraction and complementary DNA (cDNA) synthesis
Hematoxylin and eosin-stained slides were reviewed, and the area with the highest tumor content was manually outlined. After manual microdissection, total RNA was isolated using NucleoSpin FFPE RNA XS (Macherey-Nagel GMBH & Co. KG, Düren, Germany). For RNA quality control, the OD260/OD280 ratio was measured using a NanoDrop 2000 (Thermo Fisher Scientific Inc., MA, USA; RRID:SCR_018042). The total RNA integrity number was measured using an Agilent 2100 Bioanalyzer (Agilent Technologies Inc., Waldbronn, Germany, RRID:SCR_018043). To confirm that the total RNA samples were not contaminated with DNA, RNA18S1 expression was evaluated by quantitative real-time PCR (qRT-PCR) in each sample before cDNA preparation. cDNA was prepared from samples that passed all the quality control checks. cDNA was synthesized from 0.4 µg of total RNA using an iScript cDNA Synthesis Kit (BIO-RAD LABORATORIES, Inc., CA, USA), diluted to 0.2 µg/µL with distilled water, and stored at − 20 °C until use.
qRT-PCR
qRT-PCR was performed using the QuantiFast Probe Assay (Qiagen, Venlo, Netherlands) and a QuantiFast Probe PCR (Qiagen) according to the manufacturer’s instructions. The expression of each gene was quantified in triplicate. A standard curve was plotted for each run using three fixed concentrations of human control cDNA synthesized using Xpress Ref Universal Total RNA (Qiagen) with an iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Inc.) to measure the mRNA expression levels in all samples. The concentration of each sample was determined based on the point of intersection of the sample value with the standard curve. β-actin and RNA18S1 were used as the internal controls.
Gene selection
The RNA expression levels of 105 genes were quantified in the present study (Table 4). Fifty-eight genes were selected from a previous DNA microarray study44. An additional 47 genes were selected from 14 categories previously linked to tumor progression or survival in GC patients, along with 14 genes that did not overlap with the 58 genes mentioned above. The 14 categories are described in Table 4 (categories 1–14).
Table 4.
Genes investigated (n = 105).
| 1. Genes encoding proteins related to the metabolism or activation of anticancer agents | |||||||
| TYMS | DPYD | UMPS | UPP1 | TYMP | GGH | DUT | MTHFR |
| RRM1 | RRM2 | FPGS | DHFR | TOP1 | ERCC1 | TOP2A | MAPT |
| 2. Genes encoding growth factors and receptor tyrosine kinases | |||||||
| EGF | AREG | EREG | VEGFA | IGF2 | HGF | MET | FGFR2 |
| EGFR | ERBB2 | ERBB3 | KDR | IGF1R | PDGFRB | ||
| 3. Genes encoding proteins related to the p13K-AKT, RAS, and RAP1 signaling pathways | |||||||
| PIK3CA | JAK2 | PTEN | ITGB3 | PLA2G2A | THBS1 | ||
| 4. Tumor suppressor protein-encoding genes | |||||||
| SEMA3B | RUNX3 | MLH1 | APC | DAPK1 | MGMT | CDKN2A | |
| 5. Genes encoding apoptosis-related proteins | |||||||
| E2F1 | BCL-2 | GADD45 | FAS | BIRC5 | BCL-xL | BAX | CCND1 |
| 6. Genes related to cancer stem cells | |||||||
| LGR5 | PROM1 | CD44v | NANOG | MSI1 | |||
| 7. Genes related to anticancer drug resistance | |||||||
| ABCG2 | ABCB1 | ABCC1 | CAV1 | ||||
| 8. Genes encoding members of the MMP family | |||||||
| MMP2 | MMP7 | MMP9 | MMP10 | MMP11 | MMP14 | TIMP1 | |
| 9. Genes encoding cell adhesion factor and ECM | |||||||
| CDH17 | LGALS4 | VCAM1 | HPSE | DSG2 | CDX2 | ||
| 10. Genes encoding members of the claudin family | |||||||
| CLDN3 | CLDN4 | CLDN7 | CLDN18.2 | ||||
| 11. Genes encoding chemokine receptors | |||||||
| CCR7 | CXCR4 | ||||||
| 12. Genes related to immune checkpoint regulation | |||||||
| PDL1 | PDL2 | ||||||
| 13. Epigenetic repression genes | |||||||
| HDAC1 | EZH2 | ||||||
| 14. Genes identified by SAGE and CAST methods17 | |||||||
| APOE | REG4 | MIA | OLFM4 | SEC11A | TSPAN8 | TM9SF3 | ZDHHC14 |
| 15. Other genes | |||||||
| INHBA | LDHA | PTGS2 | VSNL1 | TGFA | MUC13 | SIRT1 | GZMA |
| ESR1 | MUC2 | SPARC | ANGPT2 | PLAU | PECAM1 | ||
The 105 selected genes included 63 genes analyzed in an exploratory biomarker study of ACTS-GC participants10. Among them, 57 genes have been previously reported as biomarkers of paclitaxel resistance or sensitivity. The functional annotation of each gene carried out using DAVID 6.7 (https://david-d.ncifcrf.gov/), is outlined in Supplementary Table S6 (Online Resource 1).
Defining the predictive value of the biomarkers
The mRNA expression level of each gene was classified as low versus high using the median mRNA expression level as a cut-off point, as described previously44. If the mRNA expression level of a particular gene was below 1.0 × 10–8 ng/μL, the expression level was set to ‘0.00’. The value of a biomarker in predicting the benefit of sequential paclitaxel treatment based on the OS, DFS, and cumulative incidence of relapse was determined by examining the p-values of the interactions between the dichotomized gene expression level and the treatment group (sequential paclitaxel versus monotherapy) after adjusting for clinical and pathological factors using Cox regression or Fine-Gray models45,46. The genes were ranked according to treatment interaction-related p-values. Values were considered significant at p < 0.05. Additionally, we combined the expression levels of selected genes to identify sensitive and non-sensitive patient subsets.
Immunohistochemistry (IHC) of VSLN1 and CD44
IHC of VSLN1 and CD44v was performed using FFPE specimens from 94 patients who participated in SAMIT at the Kanagawa Cancer Center. Tissue sections were deparaffinized and incubated in 10 mM sodium citrate buffer (pH 6.0) at 121 °C for 15 min for antigen retrieval. Sections were incubated with primary antibodies overnight at 4 °C. Anti-VSNL1 (UM870034; ORI GENE TECHNOLOGIES, Inc. MD, USA, diluted at 1:200 with PBS [pH 7.3] containing 1% BSA, 50% glycerol, and 0.02% sodium azide) and anti-CD44 (ab51037; ABCAM PLC, Cambridge, UK, dilution at 1:100 with PBS [pH 7.3] containing 1% BSA, 50% glycerol, and 0.02% sodium azide) were used. Preliminary testing was performed using positive controls to determine the optimal dilution of each antibody. Peroxidase-labeled polymers (EnVision + , Rabbit, DAKO, Glostrup, Denmark) and diaminobenzidine were used for detection. All sections were counterstained with hematoxylin. Immunohistochemical assessments were performed based on the Immune Response Scoring system. Intensity scores were used to classify the strongest positive immunostaining tumor cells as absent (score 0), weak (score 1), moderate (score 2), and strong (score 3). Typical VSNL1 and CD44 intensity score classifications are shown in Supplementary figures S3a, b. Proportion scores were used to classify the proportions of positive immunostained tumor cells into four grades (0, 1, 2, 3, 4, and 5) based on a marker-specific approach (Supplementary Fig. S4). The sum of the scores for the intensity and proportion scores ranges from 0 to 8. A score of 0–4 was defined as negative/low protein expression, and a score of 5–8 was defined as high protein expression, in both VSNL1 and CD44.
Examination of the relationship between VSNL1 and CD44 mRNA expression and those protein expression
We investigated each VSNL1 and CD44 mRNA expression levels in each negative/low protein expression group or high protein expression group. In addition, we investigated the concordance between mRNA expression levels split into two by the median used in the present study and the protein expression levels in immunohistochemical analyses. In addition, patients were divided into low expression groups for both VSNL1 and CD44 and high expression groups of either VSNL1 or CD44, according to VSNL1 and CD44 protein expression in IHC. In each group, the OS of sequential paclitaxel and fluoropyrimidine monotherapy was evaluated.
Internal validation
We adopted an internal validation strategy, as proposed by Wahl et al.47, to address the potential overestimation of the standard error owing to multiple imputations and optimism in the predictive performance. We used Harrell’s C statistics to analyze the predictive performance of the survival data and addressed the optimistic bias by Harrell’s C statistics using the bootstrap 0.632 + method with 20 bootstrap samples from the original dataset with replacement, followed by multiple imputations.
Statistical analysis
The pre-defined statistical analysis plan for this study has been reported previously48. The primary and secondary endpoints were the OS and DFS, respectively. The OS and DFS curves were constructed using the Kaplan–Meier method, and the cumulative incidence curves of relapse were constructed using the Aalen-Johansen method49 to compare sequential paclitaxel and monotherapy, considering the expression levels of the selected genes either individually or in combination. The adjusted hazard ratios (HRs), 95% confidence intervals (CIs), and p-values of the major treatment effects and interactions were estimated for the entire patient population and subgroups according to the Union for International Cancer Control TNM 8th ed stage2. We used multiple imputations to handle missing clinical and pathological factor data and generated 20 multiply imputed datasets for parameter estimates. The reported p-values were two-tailed, and the major effects and interactions were considered statistically significant at p < 0.05. Statistical analyses were performed using SAS version 9.4 (SAS INSTITUTE, Inc., Cary, NC, USA).
Ethical statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute for it was obtained from all patients for inclusion in the study.
Supplementary Information
Acknowledgements
We are grateful to Kazuaki Tanabe (Hiroshima University), Michiya Kobayashi (Kochi University), Shigehumi Yoshino (Yamaguchi University), Masazumi Takahashi (Yokohama Citizens Hospital), Nobuhiro Takiguchi (Chiba Cancer Center), Norio Mitsumori (Tokyo Jikeikai University), Kazumasa Fujitani (Osaka Prefectural General Medical Center), Ryoji Fukushima (Teikyo University), Isao Noguchi (Shikoku Cancer Center), Yoshihiro Kakechi (Kobe University), Naoki Hirabayashi (Hiroshima City Asa Citizens Hospital), Yukihiko Tokunaga (Osaka Kita Teishin Hospital), Akinori Takagane (Hakodate Goryokaku Hospital), and Kazuhiro Nishikawa (Osaka Medical Center) for providing clinical samples from SAMIT participants.
Author contributions
Conceptualization and study design were undertaken by A.T., K.Y., and J.S. Tissue specimens were collected by T.Y. and Y.R. The measurements of mRNA expression in GC tissue were performed by T.O. and Y.M. IHC analyses were performed by H.I.G. and Y.M. Statistical analysis and interpretation were performed by J.G. and S.T. Data were interpreted by all investigators. The article and figures were drafted by T.O., A.T., J.G., S.T., P.T., and H.I.G. This article was revised and approved by all investigators, and all authors actively participated in this study.
Funding
This work was supported by the Epidemiological and Clinical Research Information Network (ECRIN), Kanagawa Standard Anti-Cancer Therapy Support System (KSATSS), JSPS KAKENHI (Grant Numbers 842038 and 26461984), the Japan Agency for Medical Research and Development (AMED) (Grant Number 18lk0201061t0003), and a Grant-in-Aid for Scientific Research in Singapore.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-12439-3.
References
- 1.Cancer Information Service. Cancer Statistics in Japan ’19. https://ganjoho.jp/en/professional/statistics/brochure/2019_en.html.
- 2.Brierley, J.D., Gospodarowicz, M.K., & Wittekind, C. (Eds.) TNM Classification of Malignant Tumors. 8th edn. ISBN:978-1-119-26357-9 (Wiley-Blackwell, 2017).
- 3.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakuramoto S, ACTS-GC Group et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N. Engl. J. Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 5.Sasako M, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 2011;29:4387–4393. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 6.Bang YJ, CLASSIC Trial Investigators et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 7.Noh SH, CLASSIC Trial Investigators et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389–1396. doi: 10.1016/S1470-2045(14)70473-5. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida K, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: Interim analysis of JACCRO GC-07, a randomized controlled trial. J. Clin. Oncol. 2019;37:1296–1304. doi: 10.1200/JCO.18.01138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terashima M, ACTS-GC Group et al. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin. Cancer Res. 2012;18:5992–6000. doi: 10.1158/1078-0432.CCR-12-1318. [DOI] [PubMed] [Google Scholar]
- 10.Sasako M, et al. Erratum to: Impact of the expression of thymidylate synthase and dihydropyrimidine dehydrogenase genes on survival in stage II/III gastric cancer. Gastric Cancer. 2015;18:549. doi: 10.1007/s10120-014-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terashia M, ACTS-GC Group et al. TOP2A, GGH, and PECAM1 are associated with hematogenous, lymph node, and peritoneal recurrence in stage II/III gastric cancer patients enrolled in the ACTS-GC study. Oncotarget. 2017;8:57574–57582. doi: 10.18632/oncotarget.15895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichikawa W, et al. Impact of insulin-like growth factor-1 receptor and amphiregulin expression on survival in patients with stage II/III gastric cancer enrolled in the Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer. Gastric Cancer. 2017;20:263–273. doi: 10.1007/s10120-016-0600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheong JH, et al. Predictive test for chemotherapy response in resectable gastric cancer: A multi-cohort, retrospective analysis. Lancet Oncol. 2018;19:629–638. doi: 10.1016/S1470-2045(18)30108-6. [DOI] [PubMed] [Google Scholar]
- 14.Koizumi W, JACCRO and KCSG Study Group et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: A randomized study (START) J. Cancer Res. Clin. Oncol. 2014;140:319–328. doi: 10.1007/s00432-013-1563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuburaya A, et al. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): A phase 3 factorial randomized controlled trial. Lancet Oncol. 2014;15:886–893. doi: 10.1016/S1470-2045(14)70025-7. [DOI] [PubMed] [Google Scholar]
- 16.Rouzier R, et al. Microtubule-associated protein tau: A marker of paclitaxel sensitivity in breast cancer. Proc. Natl. Acad. Sci. USA. 2005;102:8315–8320. doi: 10.1073/pnas.0408974102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swanton C, et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell. 2007;11:498–512. doi: 10.1016/j.ccr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Weaver BA, Cleveland DW. Decoding the links between mitosis, cancer, and chemotherapy: The mitotic checkpoint, adaptation, and cell death. Cancer Cell. 2005;8:7–12. doi: 10.1016/j.ccr.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Pusztai L, et al. Evaluation of microtubule-associated protein-Tau expression as a prognostic and predictive marker in NSABP-B 28 randomized clinical trials. J. Clin. Oncol. 2009;27:4287–4292. doi: 10.1200/JCO.2008.21.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juul N, et al. Assessment of an RNA interference screen-derived mitotic and ceramide pathway metagene as a predictor of response to neoadjuvant paclitaxel for primary triple-negative breast cancer: A retrospective analysis of five clinical trials. Lancet Oncol. 2010;11:358–365. doi: 10.1016/S1470-2045(10)70018-8. [DOI] [PubMed] [Google Scholar]
- 21.Wertz IE, et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2017;471:110–114. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- 22.Njiaju UO, et al. Whole-genome studies have identified solute carrier transporters in cellular susceptibility to paclitaxel. Pharmacogenet. Genomics. 2012;22:498–507. doi: 10.1097/FPC.0b013e328352f436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell P, et al. Cyclin G1 regulates the outcome of taxane-induced mitotic checkpoint arrest. Oncogene. 2012;31:2450–2460. doi: 10.1038/onc.2011.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weaver BA. How taxol/paclitaxel kills cancer cells. Mol. Biol. Cell. 2014;25:2677–2681. doi: 10.1091/mbc.e14-04-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, et al. ARID1A is downregulated in non-small cell lung cancer and regulates cell proliferation and apoptosis. Tumor Biol. 2014;35:5701–5707. doi: 10.1007/s13277-014-1755-x. [DOI] [PubMed] [Google Scholar]
- 26.Gavressea T, et al. Prognostic value of the immunohistochemical expression of phosphorylated RB and p16 proteins in association with cyclin D1 and the p53 pathway in a large cohort of patients with breast cancer treated with taxane-based adjuvant chemotherapy. Anticancer Res. 2017;37:2947–2957. doi: 10.21873/anticanres.11648. [DOI] [PubMed] [Google Scholar]
- 27.Shi X, et al. Targeting the Bcl-2 family and P-glycoprotein reverses paclitaxel resistance in a human esophageal carcinoma cell line. Biomed. Pharmacother. 2017;90:897–905. doi: 10.1016/j.biopha.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 28.Duan J, et al. Efficacy and safety of weekly intravenous nanoparticle albumin-bound paclitaxel for non-small cell lung cancer patients who have failed at least two prior systemic treatments. Thorac. Cancer. 2017;8:138–146. doi: 10.1111/1759-7714.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen AL, et al. A phase I trial of azacitidine and nanoparticle albumin-bound paclitaxel in patients with advanced or metastatic solid tumors. Oncotarget. 2016;8:52413–52419. doi: 10.18632/oncotarget.14183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ola R, Lefebvre S, Braunewell KH, Sainio K, Sariola H. The expression of Visinin-like 1 during mouse embryonic development. Gene Expr. Patterns. 2012;12:53–62. doi: 10.1016/j.gep.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Ju F, et al. Systematic analysis of gene expression and molecular interactions in cardiac and non-cardiac gastric carcinomas. Hepatogastroenterology. 2014;61:1835–1842. [PubMed] [Google Scholar]
- 32.Pitule P, et al. Differential expression and prognostic role of selected genes in colorectal cancer patients. Anticancer Res. 2013;33:4855–4865. [PubMed] [Google Scholar]
- 33.Fu J, et al. Promoter regulation of the visinin-like subfamily of neuronal calcium sensor proteins by nuclear respiratory factor-1. J. Biol. Chem. 2009;284(40):27577–27586. doi: 10.1074/jbc.M109.049361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schönrath K, Klein-Szanto AJ, Braunewell KH. The putative tumor suppressor VILIP-1 counteracts epidermal growth factor-induced epidermal-mesenchymal transition in squamous carcinoma cells. PLoS ONE. 2012;7:e33116. doi: 10.1371/journal.pone.0033116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiao-Qiong D, et al. VSNL1 promotes gastric cancer cell proliferation and migration by regulating P2X3/P2Y2 receptors and is a clinical indicator of poor prognosis in gastric cancer patients. Gastroenterol. Res. Pract. 2020;9:7241942. doi: 10.1155/2020/7241942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaghobi Z, et al. The role of CD44 in cancer chemoresistance: A concise review. Eur. J. Pharmacol. 2021;903:174147. doi: 10.1016/j.ejphar.2021.174147. [DOI] [PubMed] [Google Scholar]
- 37.Meran S, et al. Hyaluronan facilitates transforming growth factor-beta1-dependent proliferation via CD44 and epidermal growth factor receptor interaction. J. Biol. Chem. 2011;286:17618–17630. doi: 10.1074/jbc.M111.226563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun M, et al. CD44(+) gastric cancer cells with stemness properties are chemoradioresistant and highly invasive. Oncol. Lett. 2013;5:1793–1798. doi: 10.3892/ol.2013.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toole BP. Hyaluronan-CD44 interactions in cancer: Paradoxes and possibilities. Clin. Cancer Res. 2009;15:7462–7468. doi: 10.1158/1078-0432.CCR-09-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morath I, Hartmann TN, Orian-Rousseau V. CD44: More than a mere stem cell marker. Int. J. Biochem. Cell Biol. 2016;81(Pt A):166–173. doi: 10.1016/j.biocel.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Chanmee T, Ontong P, Kimata K, Itano N. Key roles of hyaluronan and its CD44 receptor in the stemness and survival of cancer stem cells. Front. Oncol. 2015;5:180. doi: 10.3389/fonc.2015.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishimoto T, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc- and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 43.Chen M, et al. Chemokine CCL20 promotes the paclitaxel resistance of CD44(+) CD117(+) cells via the Notch1 signaling pathway in ovarian cancer. Mol. Med. Rep. 2021;24:635. doi: 10.3892/mmr.2021.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oshima T, et al. Biomarker analysis to predict the pathological response to neoadjuvant chemotherapy in locally advanced gastric cancer: An exploratory biomarker study of COMPASS, a randomized phase II trial. Oncotarget. 2020;11:2906–2918. doi: 10.18632/oncotarget.27658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 46.Boulesteix AL, Sauerbrei W. Added predictive value of high-throughput molecular data to clinical data and its validation. Brief Bioinform. 2011;12:215–229. doi: 10.1093/bib/bbq085. [DOI] [PubMed] [Google Scholar]
- 47.Oshima T, et al. An ancillary biomarker study in the SAMIT randomized trial: sequential paclitaxel followed by UFT or S-1 versus UFT or S-1 alone as adjuvant chemotherapy for T4a/b gastric cancer. Ann. Cancer Res. 2018;26:39–42. doi: 10.4993/acrt.26.39. [DOI] [Google Scholar]
- 48.Wahl S, et al. Assessment of predictive performance in incomplete data by combining internal validation and multiple imputation. BMC Med. Res. Methodol. 2016;16:144. doi: 10.1186/s12874-016-0239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aalen OO, Johansen S. An empirical transition matrix for nonhomogeneous Markov chains is based on censored observations. Scand. Stat. Theory Appl. 1978;5:141–150. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.