Abstract
Leucine-rich repeat receptor-like kinases (LRR-RLKs) are ubiquitous in higher plants and act as receptors of extracellular signals to trigger multiple physiological processes. However, the functions of the majority of LRR-RLKs remain largely unknown, especially in tomato (Solanum lycopersicum L.). Here, we found that MRK1 (Multiple resistance-associated kinase 1), encoding a novel tomato LRR-RLK, was significantly induced by temperature stresses and bacterial pathogen attacks. Knocking out MRK1 impaired tolerance to both cold and heat stress, accompanied by decreased transcript levels of the master regulators C-repeat binding factor 1 (CBF1) and Heat shock transcription factor a-1a (HsfA1a), respectively. In addition, mrk1 mutants were hypersensitive to Pseudomonas syringae pv. tomato DC3000 and Ralstonia solanacearum and showed compromised pattern-triggered immunity (PTI) responses, as evidenced by decreased production of reactive oxygen species and reduced upregulation of PTI marker genes. Moreover, bimolecular fluorescence complementation, split-luciferase assays, and co-immunoprecipitation supported the formation of a complex of MRK1, FLS2, and Somatic embryogenesis receptor kinase (SERK3A/SERK3B) in a ligand-independent manner. This work demonstrates that tomato MRK1 is a novel positive regulator of multiple stress responses and may be a potential breeding target for improving crop stress resistance.
Introduction
Climate change increases the frequency of various stresses such as extreme temperatures and microbial pathogen attacks. As sessile organisms, plants have to manage all such biotic and abiotic stresses at the same time. Over the course of evolution, plants have acquired multiple strategies to deal with adverse environmental conditions. These usually consist of three steps: stress signal perception, signal transduction, and activation of related gene expression, which contribute to protective or adaptive physiological responses [1]. Accurate signal perception is the first step by which plants stimulate appropriate defense responses. Plants employ a set of receptor-like kinases (RLKs) on the cell surface to detect diverse signals in the apoplast between the cells. The leucine-rich repeat RLKs (LRR-RLKs) are well studied and are one of the largest classes of RLKs. Nearly half of the estimated receptor kinases in Arabidopsis genomes possess LRR receptor domains, and there are 234 LRR-RLK genes in the tomato genome [2, 3]. LRR-RLKs contain three functional domains: an extracellular LRR region responsible for signal recognition, a transmembrane domain to immobilize the protein on the membrane, and a cytoplasmic protein kinase domain that participates in signal transduction by autophosphorylation and subsequent phosphorylation of specific substrates [4].
LRR-RLKs are ubiquitous in plants and play critical roles in responses to biotic and abiotic stresses [5, 6]. Several LRR-RLKs have been shown to participate in pathogen recognition, functioning as pattern recognition receptors (PRRs) to sense diverse pathogen- or damage-associated molecular patterns (PAMPs or DAMPs). Upon recognition, these PRRs trigger basal immunity and non-host resistance [7, 8]. In sensitive host plants, PRR-triggered immunity can effectively repulse the majority of virulent pathogens, contributing to basal immunity, which is also called pattern-triggered immunity (PTI) [7, 8]. PTI responses involve the production of reactive oxygen species (ROS), the activation of mitogen-activated protein kinase (MAPK), and the transcriptional reprogramming of immunity-associated genes [9–11]. The well-studied PRRs contain the bacterial flagellin receptors FLS2 (Flagellin sensing 2) and FLS3, the bacterial elongation factor Tu receptor EFR, the peptide Pep receptors PEPR1 and PEPR2, and the peptide phytosulfokine (PSK) receptor PSKR1 [12–15]. Interestingly, LRR-RLKs form complexes with each other to contribute to plant immunity. For example, the Arabidopsis LRR-RLK receptor BRASSINOSTEROID INSENSITIVE1-ASSOCIATED RECEPTOR KINASE (BAK1) functions as a co-receptor, forming complexes with FLS2 or EFR to promote ligand perception, which plays a role in resistance to Pseudomonas syringae and Hyaloperonospora arabidopsidis [16–18]. Similarly, RXEG1 (Receptor-like protein response to XEG1) forms a complex with BAK1 and SOBIR1 (Suppressor of BIR1–1) to transduce defense signals against Phytophthora sojae [19]. In addition, some LRR-RLKs have been found to participate in abiotic stress defense responses. Several studies have reported that LRR-RLKs can sense the change in membrane fluidity caused by abiotic stresses like cold and heat and activate the expression of stress-responsive genes [20–22]. For instance, GsLRPK from Glycine soja functions as a key regulator in cold tolerance [23]; OsCTB4a enhances rice cold tolerance [24]; and OsGIRL1 negatively regulates rice heat tolerance [25]. Despite these examples, the vast majority of LRR-RLKs have not been investigated, and their possible contributions to plant defense responses remain largely unknown, especially for those that function in both biotic and abiotic stresses.
Tomato (Solanum lycopersicum L.) is an economically important vegetable crop worldwide, and its production is threatened by temperature fluctuations and pathogen attacks, causing severe crop losses. Because tomato is a temperature-sensitive plant, temperatures lower than 12°C or higher than 35°C significantly impair its growth, resulting in up to 70% yield losses [26, 27]. Global climate change has markedly increased the frequency of temperature fluctuations, which adversely affect tomato productivity. Moreover, diseases caused by various bacterial pathogens frequently occur in tomato cultivation. In particular, P. syringae pv. tomato (Pst DC3000) and Ralstonia solanacearum are the two most important bacterial pathogens and cause bacterial leaf speck disease and root-borne bacterial wilt disease, respectively [28–30]. Characterization of the functions of LRR-RLKs in tomato is a prerequisite for breeding tomato cultivars with enhanced resistance to various stresses. In this study, through analyzing the expression patterns of 234 tomato LRR-RLK genes in response to temperature stress and bacterial pathogen inoculation, we identified a novel LRR-RLK from tomato, Solyc01g105080, and named it Multiple resistance-associated kinase 1 (MRK1). To gain deeper insight into the function of MRK1 in response to multiple stresses, we generated two homozygous mrk1 mutant lines using CRISPR/Cas9 and MRK1 overexpression plants using transgenic overexpression approaches. Our results showed that MRK1 plays a positive role in the resistance to both cold and heat stresses, as well as the PTI response, and it functions as a component in a complex with FLS2 and the co-receptor Somatic embryogenesis receptor kinase (SERK3A/SERK3B) to trigger immunity signaling. This study not only broadens our understanding of the role of LRR-RLKs in defense against multiple stresses but also highlights MRK1 as an ideal gene for breeding tomato with resistance to multiple biotic and abiotic stresses.
Results
MRK1 is induced by multiple stresses
The expression patterns of all tomato genes under temperature stress and bacterial pathogen attacks were inferred from tomato gene transcriptomic studies [31, 32]. We found that a gene (Solyc01g105080) annotated as a putative kinase was significantly upregulated in response to multiple stresses, and we subsequently named it Multiple resistance-associated kinase 1 (MRK1) (Table S1). The MRK1 protein is predicted to be a transmembrane LRR-RLK by SMART and TMHMM V2.0 (Fig. 1a–d). We confirmed the expression of MRK1 using quantitative real-time polymerase chain reaction (qRT-PCR). MRK1 transcript levels increased 5- and 2.5-fold after exposure to cold and heat stress, respectively (Fig. 1e). Meanwhile, inoculation with the bacterial pathogens Pst DC3000 and R. solanacearum also significantly enhanced the expression of MRK1 at 12 hours post-inoculation (hpi) and 3 days post-inoculation (dpi), respectively (Fig. 1f). However, the expression of MRK1 was not affected by inoculation with the necrotrophic fungal pathogen Botrytis cinerea (Fig. S1a).
Figure 1.
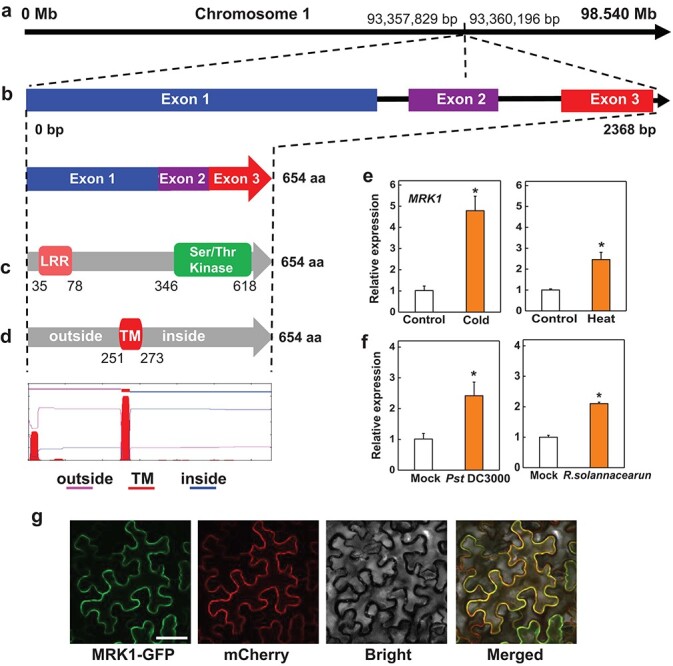
Bioinformatics and expression characteristics of tomato Multiple resistance-associated kinase 1 (MRK1). a, b The chromosomal locus and exon-intron structure of MRK1. Data were retrieved from the Solanaceae Genomics Network. c The functional domains of MRK1 determined using Pfam. d Illustration of the putative transmembrane region of MRK1 predicted by TMHMM V2.0. The expression profiles of MRK1 in response to cold (4°C) and heat (45°C) stress (e) and Pst DC3000 and R. solanacearum inoculation (f) were quantified using qRT-PCR with ACTIN2 as a normalization control. Samples were collected at 6 hours after exposure to cold or heat stress, at 12 hours after Pst DC3000 infection, and at 3 days after R. solanacearum inoculation. h Subcellular localization of MRK1. The tomato MRK1-GFP plasmid was transiently expressed in N. benthamiana leaves. The GFP and mCherry (a plasma membrane marker) signals were visualized by confocal microscopy at 48 h after infiltration. Bars = 50 μm. An asterisk indicates a significant difference between treatments (P < 0.05, Tukey’s test). The results in e and f are presented as mean values ± SD; n = 3. The experiment was performed three times with similar results.
We also analyzed the subcellular localization of tomato MRK1 by transiently expressing an MRK1-GFP fusion protein driven by the cauliflower mosaic virus 35S promoter in Nicotiana benthamiana leaves. As shown in Fig. 1g, the fluorescence signal was present mainly on the plasma membrane (Fig. 1g), suggesting that MRK1 is probably localized in the plasma membrane.
Generation of mrk1 mutants and MRK1 overexpression plants in tomato
To study the potential role of MRK1 in plant resistance, mutations in MRK1 were generated by CRISPR/Cas9. The CRISPR-P web tool was used to design a guide RNA, MRK1-gRNA (AAGTTGACTGATTAAAACCG; Fig. 2a), that targeted the first exon of the MRK1 gene. After transformation of the cultivar Condine Red, we obtained two homozygous mutant lines (mrk1#2 and mrk1#9) (Fig. 2a). Line mrk1#2 had a 1-bp insertion in MRK1 that resulted in an early stop codon at the 172nd amino acid of the protein, whereas line mrk1#9 had a 1-bp deletion in MRK1 that resulted in an early stop codon at the 176th amino acid (Fig. 2a). The expression levels of MRK1 and several other well-studied LRR-RLKs were also analyzed in mrk1 mutants by qRT-PCR, and the results showed that knocking out MRK1 only reduced the expression of MRK1 (Fig. S2). In addition, we generated two MRK1 overexpression lines, OE-MRK1#1 and OE-MRK1#3, with the 35S promoter using transgenic overexpression approaches (Fig. S3a). The growth patterns of the two mrk1 mutant lines and the MRK1 overexpression lines were indistinguishable from those of wild-type (WT) plants (Fig. 2b and Fig. S3b). Likewise, there were no obvious differences in plant height and net photosynthetic rate (Pn) between mrk1 mutants and WT plants (Fig. 2c–d).
Figure 2.
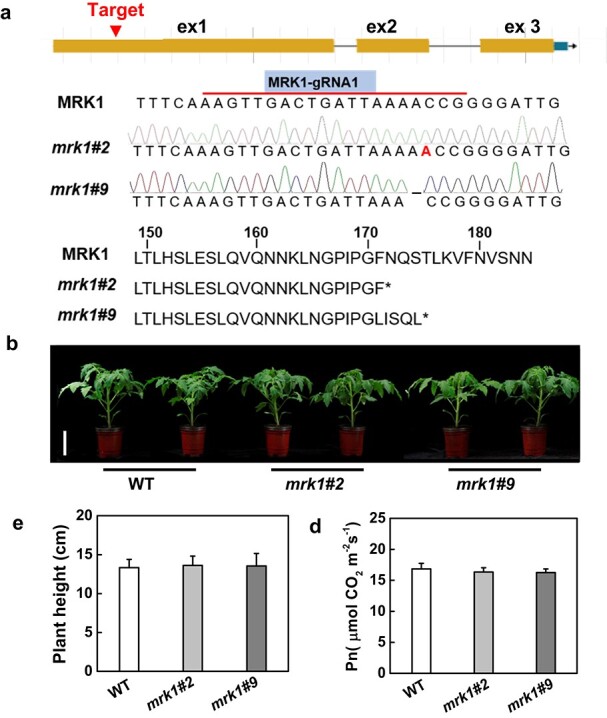
Generation of tomato MRK1 mutants by CRISPR/Cas9. a Schematics show the guide-RNA (gRNA) target site in the first exon (ex) of MRK1 and the missense mutations present in two mrk1 mutant lines (mrk1#2 and mrk1#9). The mrk1#2 line has a 1-bp insert, and the mrk1#9 line has a 1-bp deletion. The mrk1 mutant lines have a premature stop codon at the 172nd or 176th amino acid of the MRK1 protein. b Plant phenotypes of WT plants and mrk1 mutants at 4 weeks after germination. Bars = 8 cm. Plant height (c) and net photosynthetic rate, Pn, (d) were measured at the same time. An asterisk indicates a significant difference between treatments (P < 0.05, Tukey’s test). The results in c and d are presented as mean values ± SD; n = 8.
MRK1 is critical for tomato tolerance to temperature stress
To investigate the function of MRK1 in cold tolerance, mrk1 mutants and WT plants were exposed to 4°C. We found that the mrk1 mutants exhibited increased sensitivity to cold stress, as shown by changes in plant phenotype, reduced maximum photochemical efficiency of PSII (Fv/Fm), and increased relative electrolyte leakage (REL, an indicator of membrane permeability) (Figs. 3a–d). Previous studies have shown that the transcription factor C-repeat binding factor (CBF) pathway plays a critical role in cold stress response [33]. In this study, transcripts of CBF1 increased significantly in WT plants but were greatly suppressed in mrk1 mutants upon exposure to cold stress (Fig. 3e). These results suggest that MRK1 has a positive role in cold tolerance and is associated with the CBF pathway.
Figure 3.
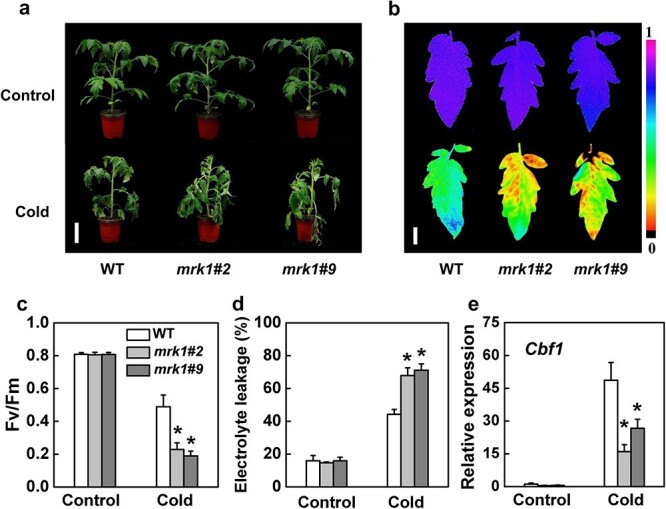
MRK1 positively regulates tomato cold tolerance. a Representative image of mrk1 mutants and WT plants after exposure to control temperature (Control, 25°C) or cold temperature (Cold, 4°C) for 7 days. Bars = 8 cm. b, c The maximum photochemical efficiency of PSII (Fv/Fm) of WT plants and mrk1 mutants after 7 days at different temperatures. The color gradient scale (b) at the right indicates the magnitude of the fluorescence signal represented by each color. Bars = 1 cm. d Relative electrolyte leakage of WT and mrk1 leaves after 7 days at 25°C or 4°C. e CBF1 expression in tomato leaves was assessed by qRT-PCR after 6 h at 4°C. An asterisk indicates a significant difference between treatments (P < 0.05, Tukey’s test). The results in c to e are presented as mean values ± SD; n = 5. These experiments were performed twice with similar results.
Next, mrk1 mutants and WT plants were exposed to 45°C to investigate the role of MRK1 in thermotolerance. Crucially, mrk1 mutants also showed a higher susceptibility to heat stress. Compared with WT plants, mrk1 mutants exhibited a notable decrease in Fv/Fm and a significant increase in REL (Fig. 4a–c). Heat shock transcription factor a-1a (HsfA1a) has been identified as a master regulator of the heat-shock response in tomato plants [34]. The induction of HsfA1a expression by heat stress was largely suppressed in mrk1 mutants (Fig. 4e). These results demonstrate that MRK1 is critical for plant tolerance to extreme temperature stress.
Figure 4.
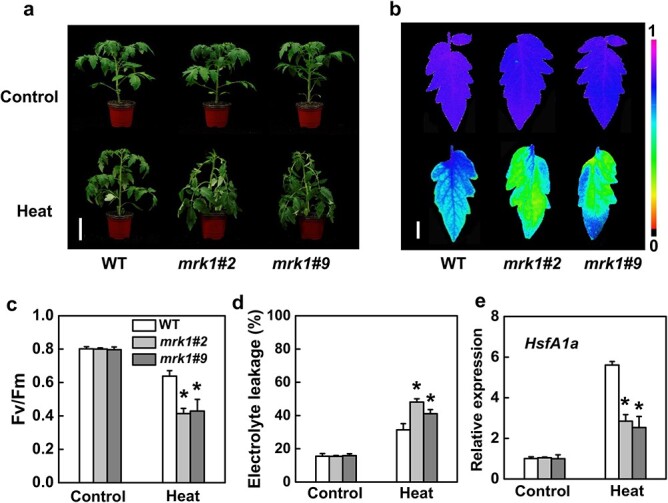
MRK1 positively regulates tomato thermotolerance. a Representative image of mrk1 mutants and WT plants after exposure to control temperature (Control, 25°C) or high temperature (Heat, 45°C) for 12 h. Bars = 8 cm. b, c The maximum photochemical efficiency of PSII (Fv/Fm) of WT plants and mrk1 mutants after 12 h at different temperatures. The color gradient scale (b) at the right indicates the magnitude of the fluorescence signal represented by each color. Bars = 1 cm. d Relative electrolyte leakage of WT and mrk1 leaves after 12 h at 25°C or 45°C. e HsfA1a expression in tomato leaves was assessed by qRT-PCR after 12 h at 45°C. An asterisk indicates a significant difference between treatments (P < 0.05, Tukey’s test). The results in c and e are presented as mean values ± SD; n = 5. These experiments were performed twice with similar results.
The role of MRK1 in response to extreme temperature stress was further evaluated by analyzing the tolerance of OE-MRK1 plants. However, the overexpression of MRK1 did not further enhance tolerance to either cold or heat stress (Fig. S3c–d).
MRK1 is required for resistance to bacterial disease
The expression of MRK1 was strongly induced by inoculation with the bacterial pathogens Pst DC3000 and R. solanacearum (Fig. 1f). Both mrk1 mutant lines exhibited increased disease symptoms compared with WT plants at 5 dpi with Pst DC3000, as reflected by increased chlorosis and necrosis of tomato leaves (Fig. 5a). This phenotype was consistent with greatly increased bacterial growth in mrk1 mutant leaves compared with WT leaves (Fig. 5b). Similarly, mrk1 mutants were more susceptible to R. solanacearum. The mrk1 mutants showed more severe disease symptoms than WT plants at 10 dpi with R. solanacearum (Fig. 5c), with significantly higher bacterial growth on leaves (Fig. 5d). The role of MRK1 in anti-bacterial immunity was further evaluated by analyzing the susceptibility of OE-MRK1 plants to Pst DC3000 and R. solanacearum. However, overexpression of MRK1 was unable to improve the resistance to these bacterial diseases compared with that of wild-type controls (Fig. S3e–h).
Figure 5.
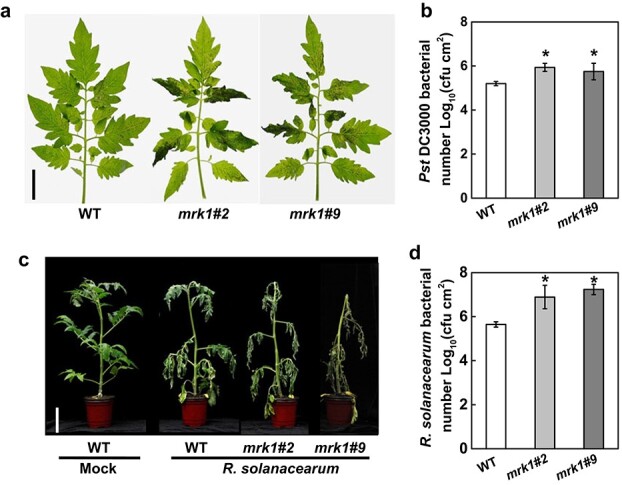
MRK1 positively regulates tomato disease resistance to P. syringae pv. tomato (Pst DC3000) and R. solanacearum pathogens. a Disease symptoms of mrk1 mutants and WT plants photographed at 5 days post inoculation (dpi) with Pst DC3000. Bars = 2 cm. b Pst DC3000 bacterial populations were assessed at 3 dpi. c The phenotypes of tomato plants at 10 dpi with R. solanacearum and R. solanacearum bacterial populations (d) in plants assessed at 10 dpi. Bars = 8 cm. An asterisk indicates a significant difference between treatments (P < 0.05, Tukey’s test). The results in b and d are presented as mean values ± SD; n = 5. These experiments were performed three times with similar results.
To investigate the function of MRK1 in plant immunity to pathogens other than bacteria, mrk1 mutants were used to evaluate the role of MRK1 in resistance to the necrotrophic fungal pathogen B. cinerea. The mrk1 mutants were as sensitive as WT plants to B. cinerea, suggesting that MRK1 has no evident function in defense against this necrotrophic fungus (Fig. S1b–c). Together, these results indicate that MRK1 plays a positive role in tomato anti-bacterial immunity.
MRK1 modulates PTI responses
PTI response is an important component of plant basal immunity, which can repel most virulent pathogens. To assess whether MRK1 is related to PTI responses, we monitored the expression of MRK1 after treatment with 100 nM flg22. The results showed that MRK1 transcripts increased at 1 h after treatment (Fig. S4). Notably, ROS production and activation of MAPK cascades are two typical early PTI-related responses in plants [7, 10]. To assess whether MRK1 is involved in early PTI events, we performed ROS assays with flg22 in mrk1 and WT leaves. Because both of the mrk1 mutant lines showed similar susceptibility to Pst DC3000 and R. solanacearum, most subsequent experiments focused on the mrk1#2 line. PAMP-triggered ROS production was strongly reduced in mrk1 mutants compared with WT plants (Fig. 6a). However, mrk1 mutants displayed the same level of MAPK activation as the WT after treatment with flg22 (Fig. 6b). Together, these results illustrate that MRK1 is required for the ROS burst but not for MAPK activation.
Figure 6.
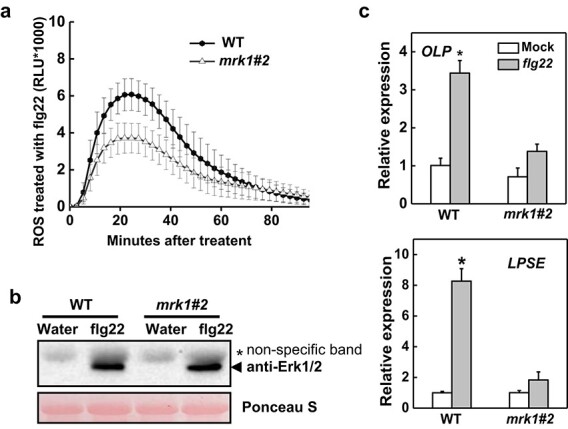
Effects of MRK1 knockout on the pathogen-associated molecular pattern (PAMP)-induced immunity (PTI) response. a ROS production in mrk1 mutants. Leaf discs from mrk1 mutants and WT plants were treated with 100 nM flg22, and the production of ROS is expressed as relative light units (RLU) over a period of 80 minutes after elicitation. Values are means ± SD of three independent experiments, each with six leaf discs. One-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference post hoc test (p < 0.05) was performed at 23 min (peak readout) after elicitation. There was an obvious decrease in ROS production of mrk1 mutants compared with WT plants. b MAPK activation in mrk1 mutants. Leaf discs from mrk1 mutants and WT plants were treated with water or 100 nM flg22 for 10 min. Proteins were extracted and subjected to immunoblotting using an anti-pMAPK antibody, which detects the phosphorylated MAPKs. Ponceau S staining was used to estimate equal loading in each lane. Similar results were observed in a second independent experiment. c Upregulation of the PTI-responsive genes Osmotin-like protein (OLP) and Lipid particle serine esterase (LPSE). Relative expression of OLP and LPSE was evaluated at 1 h post treatment with 100 nM flg22 in mrk1 mutants and WT plants. Relative expression levels were compared with those of the WT mock control (set to 1) using qRT-PCR analysis with ACTIN2 as a normalization control. Values are means ± SD of three independent experiments, each with three samples from six plants. An asterisk indicates a significant difference between treatments (P < 0.05, Tukey’s test). These experiments were performed twice with similar results.
Genes encoding Osmotin-like protein (OLP) and Lipid particle serine esterase (LPSE) are well known as PTI-specific markers in tomato [35]. To further examine the function of MRK1 in late PTI responses, we observed the expression of OLP and LPSE after treatment with flg22 (Fig. 6c). At 1 h after PAMP treatment, mrk1 mutants showed less change in OLP and LPSE expression levels than WT plants. Together, these results indicate that MRK1 positively regulates several PTI responses.
MRK1 associates with FLS2 and SERK3A/SERK3B in a ligand-independent manner
The above results indicate that MRK1 acts upstream of the ROS burst in the PTI responses triggered by flg22. Thus, we considered the possibility that MRK1 associates with FLS2 as a complex, similar to previous reports on FLS2 and WAK1 in tomato [36]. First, bimolecular fluorescence complementation (BiFC) assays were used to verify the molecular interactions of MRK1 with FLS2 or the co-receptor SERK3A/SERK3B (an ortholog of Arabidopsis BAK1). YFP fluorescence signals showed that MRK1 interacted with FLS2 at the plasma membrane and interacted with SERK3A/SERK3B in the cytoplasm (Fig. 7a). Next, the interactions were evaluated by a split-luciferase assay, and the luciferase signal showed that MRK1 associated with both FLS2 and SERK3A/SERK3B (Fig. 7b).
Figure 7.
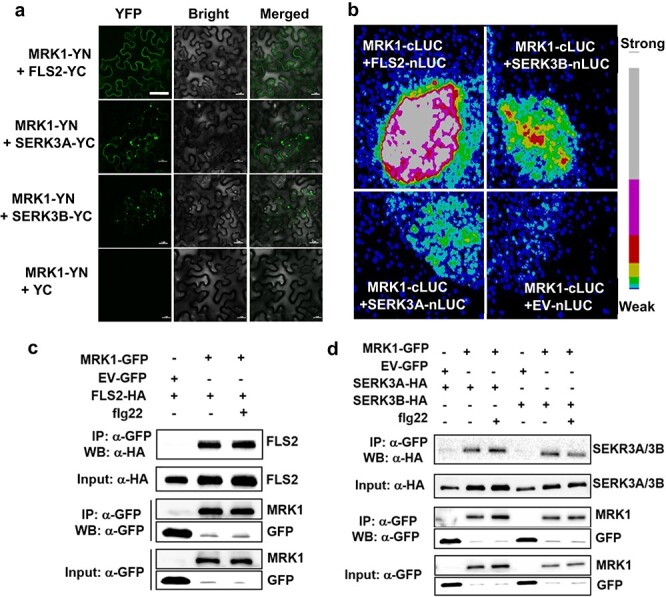
MRK1 associates with FLS2 and SERK3A/SERK3B in a complex. a Bimolecular fluorescence complementation (BiFC) assays showing MRK1 interaction with FLS2 and SERK3A/SERK3B. MRK1-YFPN and FLS2-YFPC or SERK3A/SERK3B-YFPC were co-transfected into N. benthamiana leaves. The YFP fluorescence was visualized under a confocal microscope at 2 d after transfection. At least two independent experiments were performed with similar results. Bars = 50 μm. b Split-luciferase (LUC) assays showing the interactions of MRK1 with FLS2 and SERK3A/SERK3B. MRK1-nLUC and FLS2-cLUC or SERK3A/SERK3B-cLUC were co-transfected into N. benthamiana leaves. The signal was visualized using a Photek camera. The pseudocolor bar indicates the range of luminescence intensity. These experiments were repeated three times with similar results. c, d Co-immunoprecipitation of MRK1 with FLS2 or SERK3A/SERK3B proteins in N. benthamiana. Proteins were extracted from N. benthamiana leaves expressing MRK1-GFP in combination with FLS2-HA (c) or SERK3A/SERK3B-HA (d) and subjected to immunoprecipitation (IP) with GFP-trap beads followed by immunoblotting with anti-HA antibody. EV-GFP was used as a negative control. These experiments were performed twice with similar results.
Next, co-immunoprecipitation (CoIP) assays were used to determine whether the molecular interaction of MRK1 with FLS2 or SERK3A/SERK3B was affected by the presence of flg22. MRK1-GFP was co-expressed with HA epitope-tagged FLS2 or SERK3A/SERK3B in N. benthamiana. Equal amounts of samples were used for immunoprecipitation with GFP-Trap beads, and FLS2-HA or SERK3A/SERK3B-HA was detected by anti-HA immunoblotting. FLS2 was detected in both mock- and flg22-treated samples (Fig. 7c). Likewise, SERK3A/SERK3B was also detected in the MRK1 immunoprecipitate with and without flg22 (Fig. 7d). These observations demonstrate that MRK1 forms a complex with FLS2 and SERK3A/SERK3B in a ligand-independent manner.
Discussion
In the natural environment, plants are frequently exposed to a range of environmental challenges involving biotic and abiotic stress, and they must therefore cope with multiple stresses at the same time. In plants, LRR-RLKs comprise a large gene family and modulate multiple plant processes, including responses to biotic and abiotic stress [5]. Genetic analyses have identified the roles of a small fraction of LRR-RLKs, but most have yet to be functionally defined, especially those that are involved in multiple signaling pathways [5, 37]. Here, we identified a novel LRR-RLK gene MRK1 from tomato that was upregulated by temperature stress and bacterial pathogen inoculation. Our results demonstrate that MRK1 positively regulates tolerance to cold and heat by regulating the transcripts of master transcription factors in temperature stress. MRK1 also associates in a complex with FLS2 and SERK3A/SERK3B to modulate the flagellin-induced PTI response.
In this study, a reverse genetics approach identified two independent mrk1 mutants with hypersensitivity to cold and heat stress and to the bacterial pathogens Pst DC3000 and R. solanacearum (Figs. 3–5) but with a sensitivity to the fungal pathogen B. cinerea similar to that of the WT (Fig. S1). However, overexpression of MRK1 with the 35S promoter did not further enhance resistance to these stresses. One possibility is that the expression of MRK1 in plants has been upregulated by various stresses, and its overexpression cannot further increase plant resistance. It is also possible that transgenic overexpression with the 35S promoter is an artificial process that cannot completely simulate the native physiological process in plants; the gene needs to be studied further with its native promoter. These results indicate that MRK1 is critical for tolerance to cold and heat and for resistance to hemi-biotrophic bacteria but not for tolerance to the necrotrophic fungus B. cinerea. Similarly, the Arabidopsis LRR-RLK gene PHLOEM INTERCALATED WITH XYLEM-like 1 (AtPXL1) has been shown to play a positive role in the regulation of tolerance to both cold and heat stress [38], but its function in immunity remains unclear. Several LRR-RLKs have been shown to participate in responses to stress in tomato. For instance, FLS2 recognizes the flg22 peptide derived from P. syringae to trigger the immunity response [13], and EFR and PSKR1 positively regulate tomato immunity against R. solanacearum and B. cinerea, respectively [12, 39]. Based on the studies above, it appears that these LRR-RLKs only regulate the response to a single stress in tomato, whereas MRK1 identified here regulates the responses to multiple stresses. Furthermore, we found that mutation of MRK1 did not impair the growth of tomato plants (Fig. 2). In conclusion, tomato LRR-RLK MRK1 appears to be a potentially ideal candidate for genetic engineering programs aimed at generating multiply resistant materials.
Plants have evolved a complex adaptation process in response to temperature stress [40]. Previous studies have shown that CBFs are master transcription factors in response to cold stress, activating the transcription of cold-regulated (COR) genes like WRKY6 and PYL6 [41]. HsfA1a can regulate most heat shock genes by binding to the heat shock element (HSE) motif under heat stress [42]. The LRR-RLK gene GsLRPK enhances plant cold tolerance by triggering the expression of CBFs [23]. Therefore, MRK1 possibly regulates plant cold and heat tolerance by modulating the expression of cold- and heat-inducible marker genes. Consistent with this assumption, we found that knocking out MRK1 suppressed the upregulation of CBF1 and HsfA1a, leading to compromised cold and heat tolerance, respectively (Figs. 3–4). However, the mechanisms by which transmembrane MRK1 regulates the expression of these two nuclear-localized master transcription factors remain unknown. It is possible that MRK1 may regulate the expression of these two genes through phosphorylation or transcription events, which need to be addressed in the future.
In this study, we found that the increased sensitivity of mrk1 mutants to bacterial disease was related to a defective PTI response (Fig. 6). The flg22-induced production of ROS was greatly reduced in mrk1 mutants. Moreover, upregulation of the PTI-responsive genes OLP and LPSE was greatly suppressed in mrk1 mutants compared with WT plants. By contrast, the activation of MAPKs upon flg22 elicitation occurred at the same level in mrk1 mutants and WT plants. These observations demonstrate that MRK1 is essential for the complete activation of both early and late PTI responses. Likewise, the LRR-RLK gene IMPAIRED OOMYCETE SUSCEPTIBILITY1 (IOS1) is essential for activation of some early and late PTI responses in Arabidopsis [43]. However, ios1 mutants exhibited reduced MAPK activation and reduced upregulation of FLG22-INDUCED RECEPTOR-LIKE KINASE1 (FRK1), one of the PTI marker genes, but showed no defects in ROS production upon PAMP treatment, indicating that these two positive regulators of PTI have different mechanisms.
MRK1 is an LRR-RLK gene whose importance in flg22-triggered PTI upstream of the ROS burst and PTI marker gene expression was genetically clarified in the current study. These findings raised the possibility that MRK1 might form a part of PRR complexes for the recognition of bacterial PAMPs. We therefore tested this hypothesis and found that MRK1 does interact with FLS2 and the co-receptor SERK3A/SERK3B, but in an flg22-independent manner (Fig. 7). This is reminiscent of IOS1, an LRR-RLK from Arabidopsis that was found to associate with FLS2 and BAK1 to regulate the PTI response; elicitation by flg22 also does not significantly affect this association [43]. It is possible that MRK1, like IOS1, may play a positive role in FLS2-BAK1 complex formation. Based on our observations, we propose that MRK1 acts as a component of the FLS2 and SERK3A/SERK3B complex to modulate PTI responses.
In addition, salicylic acid (SA)-mediated signaling is one of the most important components of plant immunity for repelling microbial pathogens [44]. However, in the present study of plant defense against Pst DC3000 in tomato, knockout of MRK1 did not impair SA accumulation or expression of the SA-responsive defense genes PR1 and PR4, suggesting that MRK1-mediated defense may be independent of SA (Fig. S5). A previous study also showed that silencing CaLRR-RLK1 in pepper did not affect the expression of the SA signaling-associated genes CaPR1 and CaNPR1 during R. solanacearum infection [45]. By contrast, negative regulation of cell death (an important response in plant immunity) by BAK1 and BAK1-LIKE1 (BKK1) was dependent on the SA pathway in Arabidopsis [46], and PSKR1 negatively regulated plant resistance to Pst DC3000 via the SA-mediated signaling pathway [47]. The above results suggest that the mechanisms by which LRR-RLKs regulate immunity differ among different plant pathosystems.
In conclusion, we identified the novel LRR-RLK MRK1, which was induced by several abiotic and biotic stresses. We demonstrated that MRK1 positively regulated cold and heat tolerance, perhaps by modulating the expression of the master regulator genes CBF1 and HsfA1a, respectively. In addition, MRK1 also plays a positive role in anti-bacterial immunity and acts as a component of an FLS2 and SERK3A/SERK3B complex. This research provides insights into the complex structure of LRR-RLK-mediated signaling systems and provides a new target for the breeding of tomato with enhanced resistance to multiple stresses.
Materials and methods
Plant materials and growth conditions
To knock out the MRK1 gene in tomato, a guide RNA (AAGTTGACTGATTAAAACCG) targeting the first exon of MRK1 was designed using CRISPR-P (http://crispr.hzau.edu.cn/CRISPR2/). Next, the gRNA was cloned into a Cas 9-expression binary vector (pCAMBIA1301) as described previously [31]. Agrobacterium cells containing the target construct were used for transformation of the tomato cultivar Condine Red (CR). Genomic DNA was extracted from each transgenic plant by the CTAB method, and the genomic regions spanning the guide RNA were amplified and then sequenced at Zhejiang Sunya Biotech Co., Ltd. MRK1 overexpression tomato lines were generated by PCR-amplifying the coding sequence of MRK1 and inserting it into the pGWB417 vector under the control of the CaMV 35S promoter with an MYC epitope tag at the C terminus. The confirmed plasmids were transformed into tomato CR by Agrobacterium tumefaciens-mediated cotyledon tissue culture. After confirmation by western blotting, two independent overexpression lines were selected for experiments.
Tomato plants were grown in a plant growth room under a 12-h light/dark photoperiod, 500 μmol m−2 s−1 photosynthetic photon flux density, 25/21°C (day/night) temperatures, and 85% relative humidity. Tomato plants were used for experiments at the five-leaf stage. N. benthamiana plants were grown under similar conditions. The net photosynthetic rate (Pn) was assessed with an LI-6400 photosynthesis system (LI-COR, Lincoln, NE, USA).
Subcellular localization
Agrobacterium GV3101 strains carrying the 35S:MRK1-GFP vector and the FLS2-mCherry vector (as a plasma membrane marker) were used to perform transient expression in N. benthamiana as previously described [12]. At 48 h after infiltration, the fluorescence signals were detected with a Zeiss LSM 780 confocal microscope (Zeiss, Germany) according to a previously described method [48].
Cold and heat tolerance assays
For the cold and heat stress treatments, tomato plants were exposed to temperatures of 4°C and 45°C, respectively, under 500 μmol m−2 s−1 photosynthetic photon flux density with a 12/12 h (light/dark) cycle and 85% relative humidity in controlled-environment growth chambers (Zhejiang Qiushi Artificial Environment, China).
Relative electrolyte leakage (REL), which indicates membrane permeability, was evaluated as described previously [49]. The maximum quantum yield of PSII (Fv/Fm) was measured with an Imaging-PAM (IMAG-MAXI; Heinz Walz).
Pathogen inoculation and sensitivity assays
P. syringae pv. tomato DC3000 (Pst DC3000) was cultured at 28°C in King’s B solid medium with 25 mg mL−1 rifampicin. The bacterial infection with Pst DC3000 was carried out according to previously described methods [50]. Disease severity was evaluated by assessing the bacterial population at 3 d post-inoculation (dpi) and evaluating the plant phenotypes at 5 dpi. R. solanacearum was cultured in Casamino Peptone Agar (CPG) at 28°C. The inoculation with R. solanacearum was performed as described previously [51]. In planta stem colonization with R. solanacearum was measured at 10 dpi as described previously [51].
RNA extraction and transcript analysis
Total RNA was extracted from leaves using RNA extraction kits (Easy-do Biotech Co., Ltd., China) and reverse transcribed using a HiScript II Q RT SuperMix for qPCR kit (Vazyme Biotech Co., Ltd., China) following the manufacturer’s instructions. The AceQ qPCR SYBR Green Master Mix Kit (Vazyme Biotech Co., Ltd., China) was used to perform real time-quantitative PCR (RT-qPCR) assays on a LightCycler 480 II detection system (Roche, Germany) as described previously [52]. The housekeeping gene ACTIN2 was used as the internal reference gene. Sequences of primer pairs are listed in Table S1.
Reactive oxygen species (ROS) assay
ROS production was measured according to a previously described method [36]. After treatment with water or flg22, ROS production was then measured with a Synergy 2 microplate reader (BioTek).
Mitogen-activated protein kinase (MAPK) phosphorylation assay
The activation of MAPKs was measured according to a previously described method [36]. Leaf discs were used to perform the experiment after allowing the wound response to subside, and samples were collected after treatment with 10 nM flg22. Next, the total protein was extracted, and MAPK phosphorylation was assessed with an anti-phospho-p44/42 MAPK (Erk1/2) antibody (anti-pMAPK; Cell Signaling).
Bimolecular fluorescence complementation assay
Bimolecular fluorescence complementation (BiFC) assays were carried out as described previously [12]. In brief, MRK1, FLS2, and SERK3A/SERK3B genes were cloned into the BiFC vectors p2YN and p2YC, which were generously provided by C. Mao (Zhejiang University, China). At 48 h after infiltration of N. benthamiana leaves with Agrobacterium strains, samples were examined using a Zeiss LSM 780 confocal microscope (520 to 560 nm wavelengths).
Split-luciferase assay
pCAMBIA-GW-nLUC and pCAMBIA-GW-cLUC were used for the split-luciferase assay and were provided by Y. Liang (Zhejiang University, China). At 48 h after infiltration with Agrobacterium strains, N. benthamiana leaves were incubated with 1 mM luciferin (MedChemExpress, USA) for 10 min. A Photek camera (HRPCS5, Photek) was then used to capture signals and images.
Co-immunoprecipitation
Co-immunoprecipitation (CoIP) was performed as described previously [36]. Agrobacterium strains carrying the given vectors with MRK1, FLS2 and SERK3A/SERK3B or GFP were infiltrated into N. benthamiana leaves. About 48 h later, leaves were treated with 100 nM flg22 or buffer for 10 minutes before harvest, and the total protein was then extracted from the N. benthamiana tissue using extraction buffer.
Soluble proteins of each sample were incubated with GFP-Trap beads (Chromotek) for 2 h at 4°C, then washed three times with extraction buffer. In order to analyze the immunoprecipitated proteins, immunoblotting was performed with anti-GFP or anti-HA antibodies.
Supplementary Material
Acknowledgments
This work was supported by the Natural Science Foundation of Zhejiang Province for Distinguished Young Scholars (LR19C150001), the National Natural Science Foundation of China (31822046, 32172650), the Key Research and Development Program of Zhejiang Province (2021C02040), and the Starry Night Science Fund of Zhejiang University Shanghai Institute for Advanced Study (SN-ZJU-SIAS-0011).
Contributor Information
Qiaomei Ma, Department of Horticulture, Zhejiang University, 866 Yuhangtang Road, Hangzhou 310058, China.
Zhangjian Hu, Department of Horticulture, Zhejiang University, 866 Yuhangtang Road, Hangzhou 310058, China.
Zhuo Mao, Department of Horticulture, Zhejiang University, 866 Yuhangtang Road, Hangzhou 310058, China.
Yuyang Mei, Department of Horticulture, Zhejiang University, 866 Yuhangtang Road, Hangzhou 310058, China.
Shuxian Feng, Department of Horticulture, Zhejiang University, 866 Yuhangtang Road, Hangzhou 310058, China.
Kai Shi, Department of Horticulture, Zhejiang University, 866 Yuhangtang Road, Hangzhou 310058, China.
Statistical analysis
At least three independent biological replicates from different plants were performed for each experiment. Unless otherwise stated, experiments were performed three times independently. The data obtained were subjected to analysis of variance using SAS software version 8 (SAS Institute) and tested for significance using one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference post hoc test.
Accession numbers
Sequence data from this article can be found at the Sol genomics network database (http://solgenomics.net/) under the following accession numbers: MRK1 (Solyc01g105080), FLS2 (Solyc02g070890), SERK3A (Solyc10g047140), SERK3B (Solyc01g104970), CBF1 (Solyc03g026280), HsfA1a (Solyc08g005170), OLP (Solyc11g044390), LPSE (Solyc04g077180), PAL4 (Solyc09g007890), PR1 (Solyc01g106620), PR4 (Solyc00g174340) and ACTIN2 (Solyc03g078400).
Author contributions
K.S. conceived the research; Q.M. and Z.H. designed the experiments; Q.M., Z.M., Y.M., and S.F. performed the research and analyzed the data; Q.M. and K.S. wrote the article with contributions from other authors.
Data availability
Data sharing is not applicable to this article because no data sets were generated or analyzed in this study.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Supplementary data
Supplementary data is available at Horticulture Research Journal online.
References
- 1. Ahanger MA, Akram N, Ashraf M et al. Signal transduction and biotechnology in response to environmental stresses. Biol Plant. 2017;61:401–16. [Google Scholar]
- 2. Wei Z, Wang J, Yang S et al. Identification and expression analysis of the LRR-RLK gene family in tomato (Solanum lycopersicum) Heinz 1706. Genome. 2015;58:121–34. [DOI] [PubMed] [Google Scholar]
- 3. Shiu SH, Bleecker AB. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. PNAS. 2001;98:10763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. He X, Feng T, Zhang D et al. Identification and comprehensive analysis of the characteristics and roles of leucine-rich repeat receptor-like protein kinase (LRR-RLK) genes in sedum alfredii Hance responding to cadmium stress. Ecotoxicol Environ Saf. 2019;167:95–106. [DOI] [PubMed] [Google Scholar]
- 5. Ye Y, Ding Y, Jiang Q et al. The role of receptor-like protein kinases (RLKs) in abiotic stress response in plants. Plant Cell Rep. 2017;36:235–42. [DOI] [PubMed] [Google Scholar]
- 6. Oh E, Lee Y, Chae WB et al. Biochemical analysis of the role of leucine-rich repeat receptor-like kinases and the carboxy-terminus of receptor kinases in regulating kinase activity in Arabidopsis thaliana and Brassica oleracea. Molecules. 2018;23:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Couto D, Zipfel C. Regulation of pattern recognition receptor signalling in plants. Nat Rev Immunol. 2016;16:537–52. [DOI] [PubMed] [Google Scholar]
- 8. Yu X, Feng B, He P et al. From chaos to harmony: responses and signaling upon microbial pattern recognition. Annu Rev Phytopathol. 2017;55:109–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jia Y, Martin GB. Rapid transcript accumulation of pathogenesis-related genes during an incompatible interaction in bacterial speck disease-resistant tomato plants. Plant Mol Biol. 1999;40:455–65. [DOI] [PubMed] [Google Scholar]
- 10. Zipfel C. Plant pattern-recognition receptors. Trends Immunol. 2014;35:345–51. [DOI] [PubMed] [Google Scholar]
- 11. Li B, Meng X, Shan L et al. Transcriptional regulation of pattern-triggered immunity in plants. Cell Host Microbe. 2016;19:641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang H, Hu Z, Lei C et al. A plant phytosulfokine peptide initiates auxin-dependent immunity through cytosolic Ca2+ signaling in tomato. Plant Cell. 2018;30:652–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roberts R, Liu AE, Wan L et al. Molecular characterization of differences between the tomato immune receptors flagellin sensing 3 and flagellin sensing 2. Plant Physiol. 2020;183:1825–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitre LK, Teixeira-Silva NS, Rybak K et al. The Arabidopsis immune receptor EFR increases resistance to the bacterial pathogens Xanthomonas and Xylella in transgenic sweet orange. Plant Biotechnol J. 2021;19:1294–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamaguchi Y, Huffaker A, Bryan AC et al. PEPR2 is a second receptor for the pep1 and pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell. 2010;22:508–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chinchilla D, Zipfel C, Robatzek S et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. [DOI] [PubMed] [Google Scholar]
- 17. Heese A, Hann DR, Gimenze-Ibanz S et al. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. PNAS. 2007;104:12217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roux M, Schwessinger B, Albrecht C et al. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell. 2011;23:2440–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Xu Y, Sun Y et al. Leucine-rich repeat receptor-like gene screen reveals that Nicotiana RXEG1 regulates glycoside hydrolase 12 MAMP detection. Nat Commun. 2018;9:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Falcone DL, Ogas JP, Somerville CR. Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biol. 2004;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu J. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sangwan V, Orvar BL, Beyerly J et al. Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J. 2002;31:629–38. [DOI] [PubMed] [Google Scholar]
- 23. Yang L, Wu K, Gao P et al. GsLRPK, a novel cold-activated leucine-rich repeat receptor-like protein kinase from Glycine soja, is a positive regulator to cold stress tolerance. Plant Sci. 2014;215–216:19–28. [DOI] [PubMed] [Google Scholar]
- 24. Zhang Z, Li J, Pan Y et al. Natural variation in CTB4a enhances rice adaptation to cold habitats. Nat Commun. 2017;8:14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park S, Moon JC, Park YC et al. Molecular dissection of the response of a rice leucine-rich repeat receptor-like kinase (LRR-RLK) gene to abiotic stresses. J Plant Physiol. 2014;171:1645–53. [DOI] [PubMed] [Google Scholar]
- 26. Zhang L, Guo X, Zhang Z et al. Cold-regulated gene LeCOR413PM2 confers cold stress tolerance in tomato plants. Gene. 2021;764:145097. [DOI] [PubMed] [Google Scholar]
- 27. Ruggieri V, Calafiore R, Schettini C et al. Exploiting genetic and genomic resources to enhance heat-tolerance in tomatoes. Agronomy. 2019;9:22. [Google Scholar]
- 28. Xin X, He SY. Pseudomonas syringae pv. Tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants. Annu Rev Phytopathol. 2013;51:473–98. [DOI] [PubMed] [Google Scholar]
- 29. French E, Kim B, Rivera-Zuluaga K et al. Whole root transcriptomic analysis suggests a role for auxin pathways in resistance to Ralstonia solanacearum in tomato. Mol Plant-Microbe Interact. 2018;31:432–44. [DOI] [PubMed] [Google Scholar]
- 30. Mansfield J, Genin S, Magori S et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol. 2012;13:614–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu ZJ, Ma Q, Foyer CH et al. High CO2- and pathogen-driven expression of the carbonic anhydrase βCA3 confers basal immunity in tomato. New Phytol. 2021;229:2827–43. [DOI] [PubMed] [Google Scholar]
- 32. Fragkostefanakis S, Mesihovic A, Simm S et al. HsfA2 controls the activity of developmentally and stress-regulated heat stress protection mechanisms in tomato male reproductive tissues. Plant Physiol. 2016;170:2461–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jia Y, Ding Y, Shi Y et al. The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 2016;212:345–53. [DOI] [PubMed] [Google Scholar]
- 34. Fragkostefanakis S, Simm S, El-Shershaby A et al. The repressor and co-activator HsfB1 regulates the major heat stress transcription factors in tomato. Plant Cell Environ. 2019;42:874–90. [DOI] [PubMed] [Google Scholar]
- 35. Pombo MA, Zheng Y, Fernandez-Pozo N et al. Transcriptomic analysis reveals tomato genes whose expression is induced specifically during effector-triggered immunity and identifies the Epk1 protein kinase which is required for the host response to three bacterial effector proteins. Genome Biol. 2014;15:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang N, Pombo MA, Rosli HG et al. Tomato wall-associated kinase SlWak1 depends on Fls2/Fls3 to promote apoplastic immune responses to pseudomonas syringae. Plant Physiol. 2020;183:1869–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin F, Li S, Wang K et al. A leucine-rich repeat receptor-like kinase, OsSTLK, modulates salt tolerance in rice. Plant Sci. 2020;296:110465. [DOI] [PubMed] [Google Scholar]
- 38. Jung CG, Hwang SG, Park YC et al. Molecular characterization of the cold- and heat-induced Arabidopsis PXL1 gene and its potential role in transduction pathways under temperature fluctuations. J Plant Physiol. 2015;176:138–46. [DOI] [PubMed] [Google Scholar]
- 39. Kunwar S, Iriate F, Fan Q et al. Transgenic expression of EFR and Bs2 genes for field management of bacterial wilt and bacterial spot of tomato. Phytopathology. 2018;108:1402–11. [DOI] [PubMed] [Google Scholar]
- 40. Ding Y, Shi Y, Yang S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019;222:1690–704. [DOI] [PubMed] [Google Scholar]
- 41. Li H, Ye K, Shi Y et al. BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in Arabidopsis. Mol Plant. 2017;10:545–59. [DOI] [PubMed] [Google Scholar]
- 42. Yoshida T, Ohama N, Nakajima J et al. Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Mol Gen Genomics. 2011;286:321–32. [DOI] [PubMed] [Google Scholar]
- 43. Yeh Y, Panzeri D, Kadota Y et al. The Arabidopsis malectin-like/LRR-RLK IOS1 is critical for BAK1-dependent and BAK1-independent pattern-triggered immunity. Plant Cell. 2016;28:1701–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang Y, Li X. Salicylic acid: biosynthesis, perception, and contributions to plant immunity. Curr Opin Plant Biol. 2019;50:29–36. [DOI] [PubMed] [Google Scholar]
- 45. Mou S, Gao F, Shen L et al. CaLRR-RLK1, a novel RD receptor-like kinase from Capsicum annuum and transcriptionally activated by CaHDZ27, act as positive regulator in Ralstonia solanacearum resistance. BMC Plant Biol. 2019;19:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gao Y, Wu Y, Du J et al. Both light-induced SA accumulation and ETI mediators contribute to the cell death regulated by BAK1 and BKK1. Front Plant Sci. 2017;8:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mosher S, Seybold H, Rodriguez P et al. The tyrosine-sulfated peptide receptors PSKR1 and PSY1R modify the immunity of Arabidopsis to biotrophic and necrotrophic pathogens in an antagonistic manner. Plant J. 2013;73:469–82. [DOI] [PubMed] [Google Scholar]
- 48. Hu C, Wei C, Ma Q et al. Ethylene response factors 15 and 16 trigger jasmonate biosynthesis in tomato during herbivore resistance. Plant Physiol. 2021;185:1182–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hu Z, Li J, Ding S et al. The protein kinase CPK28 phosphorylates ascorbate peroxidase and enhances thermotolerance in tomato. Plant Physiol. 2021;186:1302–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ma Q, Liu Y, Fang H et al. An essential role of mitochondrial α-ketoglutarate dehydrogenase E2 in the basal immune response against bacterial pathogens in tomato. Front Plant Sci. 2020;11:579772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ding S, Shao X, Li J et al. Nitrogen forms and metabolism affect plant defence to foliar and root pathogens in tomato. Plant Cell Environ. 2021;44:1596–610. [DOI] [PubMed] [Google Scholar]
- 52. Sun M, Xu Y, Huang J et al. Global identification, classification, and expression analysis of MAPKKK genes: functional characterization of MdRaf5 reveals evolution and drought-responsive profile in apple. Sci Rep. 2017;7:13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed in this study.


