Abstract
Electrical disturbances, such as atrial fibrillation (AF), dyssynchrony, tachycardia, and premature ventricular contractions (PVCs), are present in most patients with heart failure (HF). While these disturbances may be the consequence of HF, increasing evidence suggests that they may also cause or aggravate HF. Animal studies show that longer-lasting left bundle branch block, tachycardia, AF, and PVCs lead to functional derangements at the organ, cellular, and molecular level. Conversely, electrical treatment may reverse or mitigate HF. Clinical studies have shown the superiority of atrial and pulmonary vein ablation for rhythm control and AV nodal ablation for rate control in AF patients when compared with medical treatment. Ablation of PVCs can also improve left ventricular function. Cardiac resynchronization therapy (CRT) is an established adjunct therapy currently undergoing several interesting innovations. The current guideline recommendations reflect the safety and efficacy of these ablation therapies and CRT, but currently, these therapies are heavily underutilized. This review focuses on the electrical treatment of HF with reduced ejection fraction (HFrEF). We believe that the team of specialists treating an HF patient should incorporate an electrophysiologist in order to achieve a more widespread use of electrical therapies in the management of HFrEF and should also include individual conditions of the patient, such as body size and gender in therapy fine-tuning.
Keywords: heart failure, tachycardia, atrial fibrillation, premature ventricular contractions, ventricular dyssynchrony, resynchronization therapy, ablation
Graphical Abstract
Graphical Abstract.
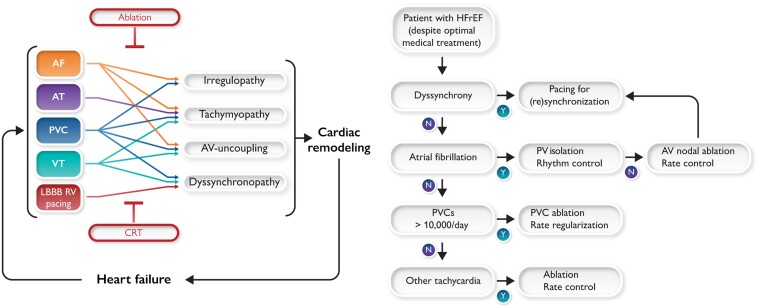
Upper panel: Schematic representation of the interaction between the various arrhythmia and conduction abnormalities with four electrical abnormalities and their consequences for remodelling and developing heart failure. Red text in boxes indicates the therapeutic approaches that treat the electrical abnormalities and thereby also heart failure. CRT, cardiac resynchronization therapy. Lower panel: Flow chart of recommended checks for the eligibility of heart failure with reduced ejection fraction patients for the various electrical therapies based on the evidence presented in the upper panel and guidelines.
Introduction
Heart failure (HF) has many causes, the most commonly considered being volume overload, inflammation, ischaemia, valvular dysfunction, or genetic derangements. Treatment is largely based on restoring coronary blood flow, treatment of valvular abnormalities, and use of HF medications.
Electrical disorders are frequent in patients with HF. Approximately one-third of HF patients has ventricular conduction abnormalities,1,2 one-third to half has atrial fibrillation (AF),3 and almost a half may suffer from premature ventricular contractions (PVCs).4 These electrical disturbances may contribute to, or are the primary cause of, the HF syndrome. This also implies that treatment of them could be either first line or adjunct therapy for HF.
In this review, we consider four different kinds of electrical cardiomyopathy:
Tachymyopathy: reversible cardiac dysfunction solely due to an increase in ventricular rates occurring during frequent atrial tachycardia (AT) (more commonly) and ventricular tachycardia (VT).
Irregulopathy: cardiac dysfunction caused by irregular heart rhythm occurring in AF, frequent PVCs and premature atrial contractions (PAC's).
Atrioventricular (AV) dissociation (lack or low contribution of atrial contraction to filling): clinically occurring in significantly prolonged PR interval, ventricular paced beats retrogradely conducted to the atria and during VT, PVC, and AF.
Cardiac dysfunction caused by non-synchronous ventricular activation and contraction: clinically, this is most prominent in left bundle branch block (LBBB), but also right bundle branch block (RBBB), and intraventricular conduction delay (IVCD), chronic right ventricular (RV) pacing, and during VT and PVC.
Figure 1 depicts the putative mechanism to the depression of cardiac pump function by LBBB, frequent PVC, AT, VT, and AF, thus resulting in one of these cardiomyopathies. However, in a given patient, a different combination of these mechanisms could be the causative condition of HF. The clearest example of an electrical therapy that improves patient outcomes is cardiac resynchronization therapy (CRT). CRT is an effective therapy for patients with HF with reduced ejection fraction (HFrEF) and electrical dyssynchrony and results in considerable improvement of quality of life, reverse myocardial remodelling, as well as lower morbidity and mortality. Correction of the other electrical disturbances provides similar although less well-proven benefits. Catheter ablation is a well-established option for AF and other supraventricular tachycardias (SVTs). Similarly, ablation of myocardial substrate (areas that give rise to the origin of PVCs, VT, and SVTs) may contribute to the treatment of HF.
Figure 1.
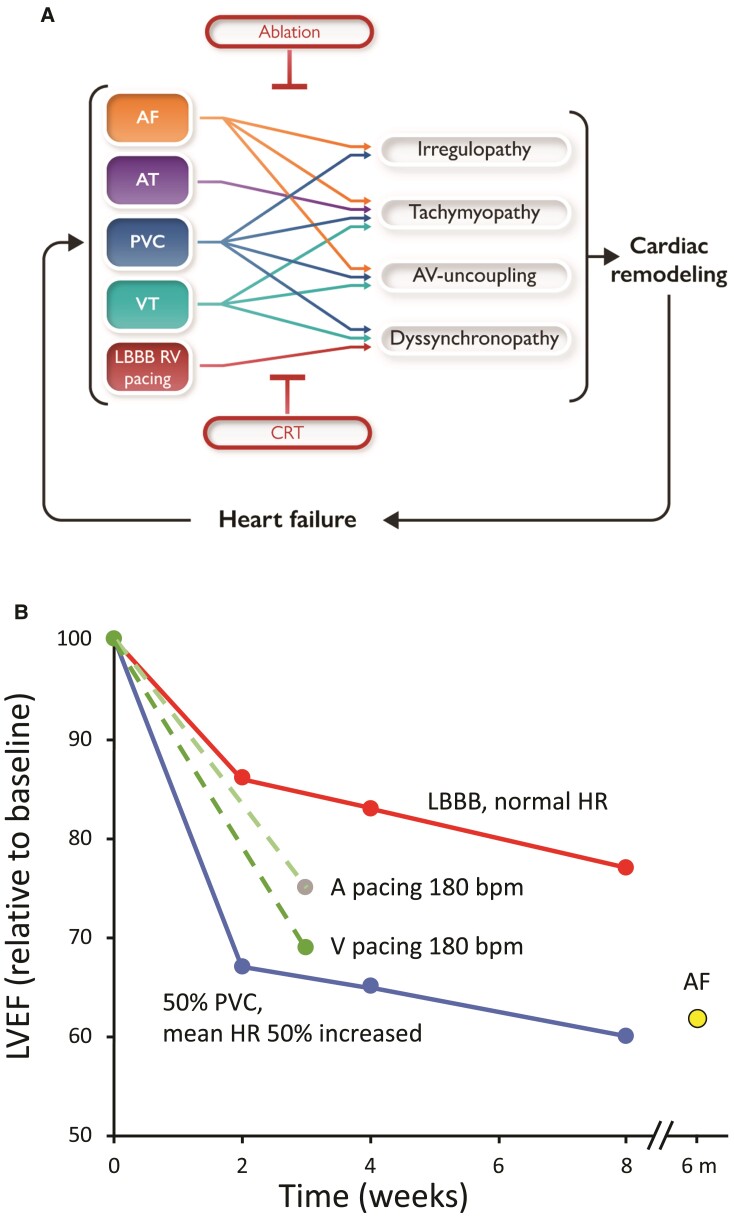
Upper panel (A): Schematic representation of the interaction between the various arrhythmia and conduction abnormalities with the four pathophysiological mechanisms / triggers and their consequences for remodelling and developing heart failure. Red text in boxes indicate the therapeutic approaches that primarily treat the electrical abnormalities, but thereby also of failure. CRT, cardiac resynchronization therapy. Lower panel (B): Relative reduction in left ventricular ejection fraction after five electrophysiological interventions in experimental studies in dogs: left bundle branch block by radiofrequency ablation and maintained normal heart rate,5 atrial (A) and ventricular (V) pacing at 180 b.p.m. for 3 weeks,6 a pacing protocol simulating premature ventricular contractions with an average premature ventricular contraction burden of ∼50%7 and chronic atrial fibrillation for 6 months.8
This review paper aims to explain from a pathophysiological perspective how electrical disorders and HFrEF intertwine to better understand the potential value of ‘electrical therapies’ for HFrEF. Selection of the best therapy/ies for electrical abnormalities in HFrEF patients must occur in the context of other HF interventions and comorbidities (Graphical Abstract). As recently suggested by the position paper jointly developed by the Heart Failure Association of the European Society of Cardiology (ESC), the growing HF treatment armamentarium requires the setting of a multidisciplinary HF team at each centre and the early referral/evaluation.9 Continued and better coordination of the complex care of such patients in daily clinical practice is a challenge yet essential to deliver effective and timely management. We hope to contribute to better coordination between the various subspecialities within cardiology by emphasizing the considerable potential for electrical therapies in the treatment of HFrEF.
Pathobiology
The pathophysiology of cardiomyopathies associated with arrhythmias or electrical disturbances frequently has overlapping intrinsic cardiac triggers, primarily including irregular rhythm associated with post-extrasystolic potentiation (PESP), abnormal ventricular mechanics due to ventricular dyssynchrony, tachycardia, and AV uncoupling (Figure 1A).4 Experimental models to understand the consequences of these triggers have shown common cardiac remodelling at the organ, tissue, and cellular level (especially oxidative and metabolic stress, eccentric hypertrophy, calcium mishandling) (Table 1) that leads to contractile dysfunction and autonomic remodelling.10–15 Figure 1B shows data from several experimental studies in dogs. Data from these studies indicate that ventricular dyssynchrony (LBBB) without an essential change in heart rate causes a ∼20% relative decrease in left ventricular ejection fraction (LVEF), while also rapid atrial and ventricular pacing and a 50% burden of PVCs cause 25–33% decreases in LVEF.
Table 1.
Pathophysiological mechanisms of cardiomyopathies associated with arrhythmias or electrical disturbances
| Tachy-cardiomyopathy | PVC-mediated cardiomyopathy | AF-mediated cardiomyopathy | LBBB-mediated cardiomyopathy | ||
|---|---|---|---|---|---|
| Triggers | Rhythm | Fast but regular | Irregular | Irregular | Regular |
| Post-extrasystolic potentiation | Absent | Presenta | Variable | Absent | |
| AV coupling | Preservedb | Dissociatedc,d | Non-existent | Preservede | |
| LV dyssynchrony | Only present in VT | Intermittentc,f | None | Continuous | |
| Myocardial blood flow | Reducedf | ?? | Likely reduced | reduced (septum) | |
| Haemodynamic compromise | Present, low EF | (?) Likely present | (?) Likely present | Present, low EF | |
| Intrinsic autonomic nerve activity | (?) Unchanged | Significantly increasedf | g | g | |
| Cardiac intrinsic effects | |||||
| Tissue | Inflammation | Presenta | Absent | g | g |
| Fibrosis | Increaseda | Mildf | g | Variable | |
| Oxidative, metabolic stress | Present | (?) Likely present | (?) Likely present | Present | |
| Cellular | Ventricular electrical remodelling | Present | Presentf | g | Present |
| Ca2+ transient | Reducedf | Reducedf | g | Reducedf | |
| Action potential duration | Increasedf | Prolongedf (heterogeneous) | g | Heterogeneousf | |
| β-adrenergic signalling | Decreasedf | g | g | Decreased | |
| Organ | Hypertrophy | Eccentrica | Eccentricf | g | Asymmetric, eccentric |
| Ejection fraction | Reduceda | Reduceda | Reducedd | Reduceda | |
| Extrinsic (non-cardiac) effects | |||||
| Neurohumoral | +; BNP; Symp; RAAS | +; BNP; Symp; RAAS | +; BNP; Symp; RAAS | +; BNP; Symp; RAAS | |
| Recovery | LV ejection fraction | Normalizeda | Normalizeda | Normalizedd | Normalizeda |
| Dimensions | Partially dilateda | Normalizeda | g | Normalizedd | |
| Diastolic dysfunction | Persistentd | g | g | g | |
| Electrical remodelling | g | g | g | Partial reversal | |
| Hypertrophy | Reactivea | g | g | Partial reversal | |
| Fibrosis | Reactive/persistenta | (?) Persistentf | g | g | |
AF, atrial fibrillation; AV, atrioventricular; BNP, B-type natriuretic peptide; EF, ejection fraction; LBBB, left bundle branch bock; LV, left ventricular; PVC, premature ventricular contraction; Symp, sympathetic tone; RAAS, renin–angiotensin–aldosterone system; VT, ventricular tachycardia.
Patient and animal data.
Ustained VT will frequently have AV dissociation.
During PVCs only.
Patient data.
AV delay is frequently prolonged.
Animal data.
Unknown.
Irregular rhythm
Irregular rhythm is the result of PVCs, premature atrial contractions (PACs), and AF.4,16–18 An important aspect of irregular rhythm is PESP, a phenomenon that refers to an increase in contractility that is associated with Ca2+ overload.16,19,20 The role of PESP as a trigger for the development of HF is supported by a study in isolated myocytes and intact myocardium showing that irregular cycles of excitation and contraction induce an altered profile of gene and protein expression including down-regulation of sarcoplasmic reticulum ATPase 2a pump (SERCA) and an abnormal ratio of phosphorylated to total phospholamban (PLB).21 Using a combination of echocardiographic imaging and computer simulations, Lyon et al.22 showed that during AF, left ventricular (LV) peak strain is larger with larger RR interval in the preceding heartbeat. Importantly, this relationship is more variable at fast than at slow heart rates, because of insufficient reserve capacity of LV diastolic filling time. Therefore, the effect of cardiac loading may vary depending on both acute beat-to-beat changes in RR interval and mean preceding heart rate.23
Ventricular dyssynchrony
Ventricular dyssynchrony refers to an uncoordinated contraction within and between the two ventricles and is present when ventricular activation occurs outside the normal conduction system such as in LBBB, VT, chronic RV pacing, pre-excitation syndrome, and PVCs. Dyssynchrony causes disruption and progression of dyssynergic wall motion, resulting in contractile dysfunction and HF, most extensively studied in LBBB. Studies in animal models of dyssynchronous HF have reported changes in Ca2+ dynamics (SERCA and PLB) and gap junction remodelling, particularly in the late-activated, high-stress LV free wall24–26 that could partly explain the deterioration of LV function and propensity to arrhythmias.10
Tachycardia
Animal and clinical studies have shown that the mechanism of tachymyopathy is multifactorial, including subclinical ischaemia due to underperfusion caused by short diastolic periods and reduced blood pressure combined with increased demands, abnormalities in cellular energetics, redox stress, and calcium overload.6,27 These stressors lead to a cascade of mechanisms that ultimately trigger a wide range of maladaptive reprogramming, leading to prolongation of the action potential, abnormal excitation–contraction coupling, and depression of contractile function.10–13 Tachycardia plays a role in HFrEF in patients with sustained AT or VT and AF without adequate rate control. Rapid rates may be accompanied by rate-dependent bundle branch block (dyssynchrony) that further reduces pump efficiency and worsens haemodynamics.
Atrioventricular dissociation
The optimal coupling between atria and ventricles implies the completion of atrial contraction and subsequent atrial filling of the ventricles before the onset of systole. Atrioventricular dissociation can be complete (random intervals between the atrial and ventricular contraction) or a constantly prolonged interval between the two (in the case of prolonged PR interval on the ECG). Atrioventricular dissociation can result in increased atrial pressure,28 inadequate ventricular filling, and/or diastolic mitral regurgitation,29 all factors that decrease ventricular stroke volume and, therefore, may contribute to the development of HF. Ventricularly paced beats with retrograde conduction to the atrium, a cause of pacemaker syndrome, may have similar effects. Finally, a prolonged PR interval causes a long pause between the end of atrial contraction and onset of ventricular contraction, causing diastolic mitral regurgitation and suboptimal ventricular filling.30,31
Understanding the role of other potential triggers of these cardiomyopathies, including haemodynamic compromise, decreased myocardial flow, and intrinsic autonomic nerve activity, is challenging due to the overlap with the primary triggers described above.
Tachycardia-mediated cardiomyopathy
The time to develop and the severity of tachycardia-mediated cardiomyopathy (T-CM) are dependent on the type, rate, and duration of tachycardia.32 Although likely underestimated, the prevalence of T-CM has been reported in close to 3% of all patients referred for catheter ablation.33 Although less common, AT and permanent junctional reciprocating tachycardia are also frequently associated with T-CM with a prevalence as high as 59 and 23%, respectively.34,35 Nevertheless, AF and atrial flutter are some of the most frequent causes of T-CM due to their high prevalence in the adult population.36 Tachycardia-mediated cardiomyopathy typically presents with palpitations, HF symptoms, and severe LV systolic dysfunction. Tachycardia-mediated cardiomyopathy should be strongly considered in patients with poor LV systolic function and persistent or frequent paroxysmal tachycardia without other obvious aetiology. Importantly, T-CM diagnosis can only be confirmed if LV recovery is documented within few weeks or months after treatment.
The main treatment of T-CM is the elimination of tachycardia with either antiarrhythmics and/or catheter ablation. In the ESC guidelines, catheter ablation has a IB indication to reverse LV dysfunction in AF patients when T-CM is probable.37 However, standard HF medical therapy should not be ignored to maximize LV recovery. While eliminating tachycardia will resolve LV systolic dysfunction and HF symptoms, persistent myocardial fibrosis will remain and in part contribute to an 8–12% risk of sudden cardiac death (SCD) in patients with VT despite resolution of T-CM.4,32,38 Surveillance of recurrent tachycardia after treatment is key, since its recurrence can result in a more rapid and severe clinical presentation.4,38 Therefore, catheter ablation should be strongly considered in patients with arrhythmias known to have a high success rate (e.g. atrial flutter, AT, AV reciprocating tachycardia).
Premature ventricular contraction-mediated cardiomyopathy
Premature ventricular contractions are the most frequent ventricular arrhythmia and commonly associated with HF, ventricular arrhythmias, and SCD.4,39,40 Frequent PVCs are recognized as a reversible cause of LV systolic dysfunction referred to as ‘PVC-mediated cardiomyopathy’ (PVC-CM), where PVC suppression will improve and even normalize LV function. The prevalence of PVC-CM is estimated between 10 and 29% in patients with frequent PVCs (>5–10%).4,40,41 While PVC burden is the most consistent predictor of PVC-M, various factors, including genetics, comorbidities, cardiac phenotype, or PVC features and length of exposure, play a role in the susceptibility or resilience to develop PVC-CM.42,43 In addition, PVCs with a longer QRS duration and epicardial location are more frequently associated with PVC-CM,4,41,44 supporting the role of LV dyssynchrony in PVC-CM (Table 2 and Figure 1).
Table 2.
Clinical and premature ventricular contraction features to identify premature ventricular contraction-mediated cardiomyopathy
| CM resulting in PVCs | PVCs causing CM | |
|---|---|---|
| Patient characteristics | Older with known heart disease | Healthy otherwise |
| Comorbidities | CAD, myocarditis, RV dysplasiaa | No prior cardiac hx |
| Echocardiogram | Segmental hypokinesis, LVEF <25% | Global hypokinesis, LVEF 35 ± 10%b |
| Cardiac MRI (late gadolinium enhancement) | Significant scar | Absence or minimal scar burden (≤9 g) |
| PVC frequency | <5000/24 h (<5%) | ≥10 000/24 h (≥10%) |
| PVC pattern | Multifocal | Monomorphic |
| QRS morphology | Non-specific | RVOT/LVOT/epicardial |
| Response to PVC suppression | No change in LV function | Improvement of LV function |
CAD, coronary artery disease; CM, cardiomyopathy; LVEF, left ventricular ejection fraction; RV, right ventricular; RVOT, right ventricular outflow tract; LV, left ventricular; LVOT, left ventricular outflow tract; MRI, magnetic resonance imaging.
PVCs can cause a superimposed PVC-mediated cardiomyopathy even patients with other comorbidities.
While PVC-mediated cardiomyopathy does not typically present with severe left ventricular systolic dysfunction (LVEF <25%), LVEF alone should not exclude the diagnosis of PVC-mediated cardiomyopathy. Reproduced with permission from Huizar et al.17
Premature ventricular contraction-mediated cardiomyopathy can present with or without fatigue, HF symptoms, syncope, and SCD.4,17,39,45 Frequently, patients are referred for bradycardia due to bigeminy where heart rate is underestimated (i.e. pseudo-bradycardia) due to the lack of pulse pressure generated by PVC. The time to develop PVC-CM is unknown, but probably months or years of exposure to frequent PVCs are needed.17,44
Premature ventricular contraction-mediated cardiomyopathy diagnosis should be suspected if PVC burden is >5–10% and other causes of HF are excluded, and confirmed only if PVC suppression improves and even normalizes LV function. A major diagnosis challenge is when PVCs cannot be successfully eliminated. A frequent dilemma is to determine whether the PVCs are a bystander or the cause of CM. Some PVCs and clinical features (e.g. absence of scar) can assist to differentiate these two scenarios (Table 2).4,17
Treatment
Successful treatment of PVC-CM requires at least an 80% reduction in PVCs due to a significant day-to-day PVC variability.4,46 While PVC ablation or antiarrhythmic drugs offer overall a good long-term suppression, standard HF guideline-directed medical therapy is essential and should be optimized, but beta-blockers and antiarrhythmic drugs are often unsuccessful in suppressing PVCs or not tolerated.4,17,41,44,47 Premature ventricular contraction ablation is, therefore, preferred due to higher PVC reduction rate and low recurrence.47,48 Premature ventricular contraction ablation is a low-risk procedure (1.5–2.8%) with estimated acute and long-term suppression between 80–90% and 60–90%, respectively.4,41,44 However, PVC ablation can be challenging due to the inability to reach PVC origin [e.g. intramural location, LV summit (most basal part of the septum and LV wall)] or catheter instability. Specific technologies, such as cryoablation and contact force sensor catheters, can assist in improving success and overcome some limitations.49 Moreover, intracardiac echocardiography frequently assist PVC ablation within papillary muscle origin.50
Premature ventricular contraction ablation has been reported to reverse remodelling with the improvement of LV and mitral valve function, B-type natriuretic peptide levels, and renal function.4,41,47 Factors that predict the PVC-CM diagnosis also forsee response to PVC ablation.4 Importantly, a scar mass <9 g (cardiac magnetic resonance) predicts LV recovery.51 Besides improving LV function, PVC ablation may also improve survival or long-term outcomes as suggested by the Congestive heart failure: Survival trial of antiarrhythmic therapy (CHF-STAT) study and the Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA) trial.40,52 Future clinical studies are clearly needed to better understand the clinical impact of PVC-CM and its treatment.
Atrial fibrillation
Atrial fibrillation and HF often coexist, and are accompanied by worse adverse outcomes. Several options are available to treat AF divided into rate and rhythm control. Both strategies have invasive (ablation) and non-invasive (pharmacological) approaches.
Although rate control is a management strategy in AF,53 the optimal heart rate target in AF patients is unclear yet. Rate control is the background treatment for all AF patients, including those receiving treatment with a rhythm control strategy. Randomized trials did not show a difference in the composite endpoint of clinical events, New York Heart Association class, or hospitalizations between the strict (target heart rate <80 b.p.m. at rest and <110 b.p.m. during moderate exercise) and lenient (heart rate target <110 b.p.m.) arm irrespective of activity level.54,55 Notably, only a small portion of patients included in these studies had HFrEF. Still, the ESC HF guidelines recommend ‘lenient rate control’ (meaning <110 b.p.m. at rest) as the initial approach, regardless of HF status (with the exception of tachycardia-induced cardiomyopathy).56
Pharmacological approaches for rhythm and rate control appear equivalent strategies with respect to outcome.57–59 Available studies showed a low success in maintenance of sinus rhythm, but the presence of sinus rhythm was associated with better LV function57,59,60 and survival.61 The slight increase in LVEF and beneficial consequences of maintaining sinus rhythm may not translate into long-term effect on mortality and morbidity due to the adverse effects associated with antiarrhythmics.
Electrophysiological approaches for rate and rhythm control provide more specific and definitive solutions. The rhythm control option of catheter ablation has been tested in several prospective randomized controlled trials (RCTs).62–64 All these relatively small studies consistently showed greater improvement of LV function, and quality of life in the catheter ablation arm compared with medical therapy. Moreover, in HF in patients with AF and LVEF ≤35%, catheter ablation provided a significantly lower composite endpoint of mortality and hospitalization than pharmacological therapy. Notably, the recent long-term results of the Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Heart Failure-An MRI-Guided Multicenter Randomized Controlled Trial (CAMERA-MRI) study showed an absolute increase in LVEF with catheter ablation of 16.4 ± 13.3% compared with 8.6 ± 7.6% in medical therapy (P = 0.001).65 At 4.0 ± 0.9 years of follow-up in the catheter ablation group, the absence of ventricular late gadolinium enhancement was associated with a greater improvement in absolute LVEF (19 ± 13 vs. 10 ± 11%; P = 0.04) and LVEF normalization in 19 patients (58%) vs. 4 patients (18%; P = 0.008) compared with the late gadolinium enhancement-positive group. Therefore, recent clinical practice guidelines recommend catheter ablation as an alternative to pharmacological therapy at Level of evidence I for patients with paroxysmal or persistent AF and HF.37
When medication for rate control fails, ablation of the AV node and pacemaker implantation can be considered. The procedure is relatively simple and has a low complication rate and low long-term mortality risk, especially when the pacemaker is implanted a few weeks before AV node ablation and the initial pacing rate after ablation is set at >70 b.p.m. While this will not restore sinus rhythm and AV coupling, the procedure does not worsen LV function and may even improve LVEF in selected patients.66 Most studies have included older patients with limited life expectancy. For younger patients, ablation of the AV node should only be considered if there is urgent need for rate control and all other pharmacological and non-pharmacological treatment options have been carefully considered. The choice of pacing therapy (RV or biventricular pacing) depends on patient characteristics. The results of the APAF-CRT study, which was performed in severely symptomatic patients with permanent AF and within 1 year of HF hospitalization irrespective of LVEF, showed that AV node ablation combined with CRT is preferred.67,68 In this study, patients with narrow QRS complex had a 62% lower risk for the combined endpoint of mortality, HF hospitalization or worsening of HF, and a 72% lower risk of all-cause mortality and HF hospitalization in the ablate and pace group when compared with the pharmacological rate control group. Forty per cent of the study patients had LVEF ≤35%. The combination of biventricular pacing and AV node ablation also shows superior outcomes in patients with a conventional CRT indication who obtain the suboptimal level of biventricular stimulation due to AF with intrinsic conduction.69 More physiological pacing, such as His bundle pacing (HBP) and left bundle branch pacing (LBBP) (see below), may evolve as an attractive alternative pacing mode, as currently tested in ongoing clinical trials.
Dyssynchrony and resynchronization therapy
Disease prevalence
In a Swedish registry, LBBB and IVCD were found in ∼25 and ∼15% of patients with HFrEF, respectively.2 The presence of both LBBB and IVCD increased the risk of all-cause mortality by ∼30%. In transcutaneous aortic valve implantation procedure, new-onset LBBB may even increase the risk of mortality by ∼50%.70 The pathophysiology paragraph describes the serious consequences of ventricular dyssynchrony. Therefore, ventricular dyssynchrony is an important therapeutic target in patients with HFrEF. It has been estimated that at least 400 patients per million of the population would be eligible for CRT, but in practice, only Germany and Italy at least approach such implantation numbers in Europe.71
Cardiac resynchronization therapy
According to current guidelines,71 good candidates for CRT are those with HFrEF and an abnormal QRS complex (LBBB morphology and/or QRS duration >130 ms). Likewise, because activation sequence in RV pacing mimicks that of LBBB, CRT is indicated for patients who are RV paced for a significant portion and have LVEF <35% and patients with LVEF <40% with high-degree AV block who have an indication for ventricular pacing.71
As reviewed in the guideline documents, randomized trials have clearly and consistently shown the benefit of CRT with regard to HF symptoms, HF hospitalization, and survival.71 Many studies report that approximately one-third of patients receiving CRT are non-responders in terms of LV reverse remodelling, clinical improvement, or both. Lately, however, the response definitions have been challenged since recent evidence indicates that patients who are stable during CRT also benefit from this therapy.72 Therefore, perception of lack of response such as in patients with ischaemic heart disease who more commonly present with IVCD should not preclude patients from this potentially life-saving treatment.
Patient selection
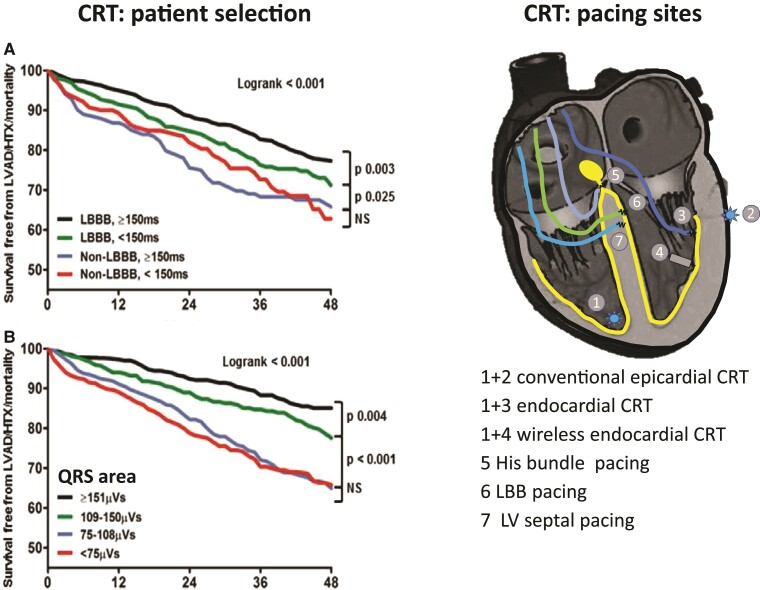
The most important factor that determines the CRT effect is the presence of an ‘electrical substrate’ in the patient, i.e. the amount of viable tissue that can be resynchronized. The largest benefit is commonly observed in patients with a ‘true’ LBBB and no evidence of ischaemic heart disease, and commonly women (see below). However, currently used ECG criteria (QRS duration and LBBB) have weaknesses. QRS duration poorly expresses the amount of resynchronizable tissue and its critical value is body size-dependent.73 The definition of LBBB based on ECG criteria is complicated by its subjective assessment and multiple ECG criteria of LBBB that lead to widely different percentages of LBBB patients in cohorts of CRT patients.74,75 Recent studies indicate that the electrical substrate may be better identified when using the area under the QRS complex (Figure 2).76,80,81 These promising results may be explained by the fact that QRS area reflects late LV activation, independent of QRS morphology.82 QRS area is also inversely related to the presence of ischaemic heart disease and scar size,83,84 factors that reduce CRT response. A randomized study is required to provide final proof of this approach, while standard addition of the calculation of QRS area to ECG equipment would greatly enhance the use of this parameter.
Figure 2.
Upper panels: Electrocardiographic selection criteria for cardiac resynchronization therapy and their relation to outcome (combined endpoint of survival free from left ventricular assist device implant, heart transplant, or death) in a study on ∼1500 patients. (A) Conventional criteria (left bundle branch block and QRS duration >150 ms), (B) area under the QRS complex (QRSarea). The presence of left bundle branch block is a determinant of cardiac resynchronization therapy outcome, in particular if QRS duration is >150 ms, but QRSarea >109 µV s is a stronger determinant of cardiac resynchronization therapy outcome than left bundle branch block.76 Lower panels: Schematic overview of the current options for cardiac resynchronization therapy. Positions 1 and 2 indicate the conventional right ventricular and left ventricular pacing locations. Endocardial cardiac resynchronization therapy can be achieved by introducing a conventional pacing lead through the foramen ovale and the mitral valve into the left ventricle (3) or using a wireless pacing electrode (4) that is stimulated using an ultrasound transducer.77 His bundle (5), left bundle branch (6), and deep left ventricular septal pacing (7) is performed using a 4 Fr lead introduced transvenously and screwed in the septum. Small studies also investigated the combination of His bundle pacing (HOT-CRT)78 or left bundle branch pacing with left ventricular pacing (LOT-CRT).79
A previously proposed approach for improving patient selection was the use of markers of mechanical dyssynchrony in addition to ECG criteria, in particular using speckle tracking echocardiography. After initial promising results, large randomized trials have not been able to show a consistent benefit when using time-to-peak shortening as a measure of dyssynchrony.85,86 Simpler markers, such as apical rocking and septal flash, are improving the prediction of CRT response in single-centre evaluation,87 but suffer from relatively low intercentre agreement.88 Interesting new developments are the use of deformation patterns in addition to electrical dyssynchrony criteria89 and myocardial work,88 but these need to be confirmed in randomized studies.
Atrial fibrillation patients do not derive the same benefit from CRT, which may be due to several factors such as insufficient amount of biventricular stimulation (as discussed above), fusion or pseudofusion beats without proper biventricular stimulation, and lack of atrial contribution to ventricular filling.
Right ventricular pacing even in a percentage as low as 20% may induce RV dyssynchrony and by time HF.71 Therefore, patients with LVEF ≤40% in need of RV pacing such as those with high-degree AV block should be given CRT pacing (Recommendation IA in pacing guidelines). This recommendation includes patients in AF.71
Therapy delivery
Apart from delivery of at least 95% biventricular stimulation, a second factor determining the outcome of CRT is the position of the LV lead. The optimal LV position appears to differ between patients, therefore requiring a personalized approach. There is general agreement that the best (epicardial) LV lead position is the viable region with the latest intrinsic activation. However, two studies attempting to validate this tailored approach were small and results were not clearly better than the default use of the (postero) lateral vein, because the choice of veins to implant the lead is limited (usually two veins, not all of them being suitable for most leads).90 A larger study, comparing strategies of LV lead positioning using electrical determination of the latest activated region with that of mechanically latest activated region outside a scar, found no significant difference between these strategies.91 Multipoint pacing (MPP) has been studied in an RCT setting, but its use was not superior to conventional CRT for reverse remodelling, despite earlier promising findings in smaller studies. However, because most LV leads implanted are quadripolar, reprogramming the device to MPP may be an option in case of poor response. The SMART-MSP study showed that of the patients that did not respond to CRT after 6 months, half could be converted to responders by activating MPP. These patients had a significantly lower risk of HF decompensation at the subsequent 6-month follow-up.92
Several approaches have been employed to deliver LV pacing at the endocardium in order to create more physiological activation in CRT. However, implantation of a lead, using a trans-atrial septal approach, resulted in transient ischaemic attacks and non-disabling strokes in 6.8 and 3.8% of cases.93 The conceptually novel approach of implanting encapsuled piezo-electric (receiver) crystals to the LV endocardium that is activated using ultrasound seems more promising in the prevention of coagulation problems. The WiSE-CRT94 and SELECT-LV studies77 showed the feasibility and efficacy of this approach, although peri-procedural/device-related events occurred in ∼8% of patients, and additional 10% events in the post-procedural phase. Currently, this novel technology is tested in a large prospective study.95
Recently evolving and physiologically superior approaches to resynchronization are endocardial CRT,96 HBP,97 LBBP,97 and deep LV septal pacing (LVSP)98,99 (Figure 2).
The excellent electrocardiographic and functional benefit of resynchronization using a single ventricular lead (HBP,100,101 LBBP,102 and LVSP)99 is explained by the increasing evidence that in about two-thirds of CRT candidates, the ‘LBBB’ is actually located very proximal in the left bundle branch or even His bundle103 in combination with a more physiological sequence of activation (septum > LV free wall and endocardium > epicardium).104 These single-lead resynchronization approaches may expand the acceptance and application of CRT, using a simple dual-chamber pacemaker. Of these three approaches, HBP is practically more difficult and long-term reliability has come into question.105 In that regard, LBBP and LVSP appear more promising, because of lower dislodgement rate and lower and more stable lead performance, but long-term data are even less than for HBP.
Device optimization
Device optimization implies programming of the interval between atrial and ventricular stimulation (AV delay) and, for biventricular pacing, between RV and LV stimulation (VV delay) and multi-site LV stimulation (LV1–LV2) in quadripolar leads. Conventionally, such optimization is performed a single time using echocardiography (if any). However, studies showed limited to no benefit of such one-time optimization compared with using the default setting.106 More promising results have been obtained in studies where device-based algorithms were used that perform optimization in an automated and ambulatory fashion. Adaptive CRT adjusts AV delay and withholds RV pacing to create a fusion of LV stimulation with intrinsic conduction. This approach was shown to increase device longevity and to reduce the risk of developing AF.107 Also the SyncAV algorithm dynamically adjusts AV delays to the intrinsic conduction, but also has the option of a programmable offset, aiming at creating a triple wavefront of activation. This algorithm improved electrical resynchronization,108 but until now reports on clinical benefits have been published. The SONR algorithm employs an accelerometer integrated into the RV or RA pacing lead. Using the amplitude of the SonR1 signal (equivalent to the first heart sound), the SONR algorithm automatically determines the optimal combination of AV and VV delay in an ambulatory fashion. The RESPOND-CRT trial randomized almost a thousand patients to SONR or echocardiography optimization. While SONR marginally increased CRT response (NYHA class) by 5% points (P = 0.13), and lowered combined risk of death and hospitalization (P = 0.12), hospitalization alone had a 35% risk reduction (P = 0.01).109
Other electrical therapies
Cardiac contractility modulation (CCM) consists of the delivery of non-excitatory electrical signals in the absolute ventricular refractory period to the RV septum.110 The FIX trials were performed in patients with New York Heart Association (NYHA) Class III–IV HF, with an LVEF ≥25 to ≤45% and QRS duration <130 ms. In these studies, CCM was associated with a small improvement in exercise tolerance and quality of life.111
The poor cardiac function creates imbalances in the autonomic nervous system. During recent years, several approaches for modulations of the autonomic nervous system have been investigated in patients not eligible for CRT, in particular vagal nerve stimulation, renal (sympathetic) nerve ablation, and baroreceptor stimulation. Despite promising results in preclinical models, clinical trials showed only limited or no benefits. A randomized clinical trial failed to show significant reverse remodelling by vagal nerve stimulation over a 6-month follow-up.112 There is no randomized clinical trial showing a significant benefit of renal denervation in HF patients. Baroreceptor stimulation, performed with a novel electrical stimulation device with electrodes in the vicinity of the carotid baroreceptors, was shown to significantly improve the quality of life and exercise capacity and to reduce N-terminal pro-B-type natriuretic peptide in HF patients,113 but studies indicating significant improvements in hard clinical endpoints are yet lacking.
Recommendation and implementation of electrical therapies for heart failure
Current ESC/European Heart Rhythm Association (EHRA) guidelines recommend catheter ablation of AF to reverse LV dysfunction, independent of their symptom status (Class I) and in selected AF patients with HF to improve survival and reduce HF hospitalization (Class IIa).37 Catheter ablation for SVTs is recommended (Class IB) in patients with reduced LV function and frequent or persistent elevated heart rate above 100 b.p.m. consistent with T-CM.114
Catheter ablation for PVCs is recommended in symptomatic or asymptomatic patients with frequent monomorphic PVCs and suspected PVC-CM (Class I), as well as non-responders of CRT due to suboptimal biventricular pacing (Class IIa).115
Similarly, CRT is recommended (on the top of optimal medical therapy) in symptomatic HFrEF patients in sinus rhythm and QRS duration ≥130 ms (Class I–II depending on QRS width and morphology).71 Other groups that may be considered for CRT include NYHA Class III–IV HFrEF patients in AF and a QRS duration ≥130 ms, provided a strategy to ensure biventricular capture is in place, and occasionally as an upgrade to a conventional pacemaker or an implantable cardioverter defibrillator in patients who develop worsening systolic function with >20% RV pacing.71
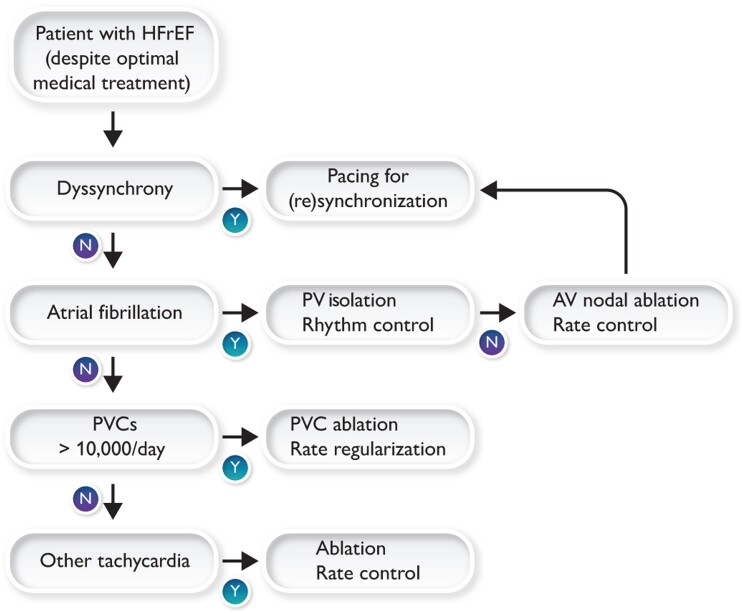
Based on the abovementioned evidence and guideline recommendation, we propose the decision schedule presented in Figure 3. Each HFrEF patient should be checked for treatment of dyssynchrony, persistent tachycardia, AF, and PVCs.
Figure 3.
Schematic representation of judgement that is recommended for optimal treatment of heart failure patients, based on the evidence presented in this article.
However, clinical practice is far from optimal.
In the case of CRT, the optimal combination of medical therapy and CRT is performed in a minority of patients who qualify for CRT. European data suggest that only one in three eligible patients actually receives a CRT device.116 Moreover, only 20–30% of patients implanted with CRT are on maximal guideline recommended doses before CRT.116 But a delay in CRT implementation may be suboptimal since HF medication is less effective in achieving reverse remodelling in patients with LBBB, when compared with HFrEF patients with narrow QRS.117
A recent joint position statement from several cardiac societies of the ESC described in-depth theoretical and practical strategies to achieve more comprehensive CRT referral and post-procedural care by focusing on four actionable domains: (i) overcoming CRT under-utilization, (ii) better understanding of pre-implant characteristics, (iii) abandoning the term ‘non-response’ and replacing this by the concept of disease modification, and (iv) implementing a dedicated post-implant CRT care pathway.118
Similarly, in old frail patients with HFrEF and AF, the utilization of medical therapy to preserve sinus rhythm has shown to be detrimental compared with AF ablation or AV node ablation plus CRT.118 Therefore, it seems wise to more frequently consider device therapy and/or ablation as adjunctive therapies with a synergistic effect rather than consecutive therapies to be used only if medical therapy fails.
Unfortunately, the diagnosis of T-CM and PVC-CM is frequently missed due to the lack of using long-term ambulatory monitors to identify subclinical and intermittent arrhythmias. Appropriate treatment of T-CM and PVC-CM does not only improve LV systolic function but likely to impact morbidity and mortality.32,40 Therefore, implementing care pathways, comparable to that for CRT, for the aforementioned ablation treatments seem advisable. Finally, the implementation of electrical therapies can be further improved by better integration of cardiological and non-specialist care, leading to better post-implant management.119,120
Gender differences in electrical heart failure and its treatment
Personalized electrical management of HF should include gender consideration. Currently, device therapy and ablation are especially underused in women with HF. Striking is that while women are less likely to receive CRT, they derive more benefit from this therapy than men.1 This difference can partly be explained by differences in risk factors (more non-ischaemic cardiomyopathy, lower scar burden, less LV dilatation, more LBBB). A meta-analysis of three randomized studies by the Food and Drug Administration showed CRT benefit in women with QRS duration >130 ms while the benefit for men only started at QRS duration >150 ms.121 This difference may be explained by the fact that women are generally smaller, including their heart and body size.122,123
The lower referral for CRT in women may be related to sex/gender bias in referral patterns and women more often having HF with preserved ejection fraction than HFrEF.1 In addition, the shorter QRS duration in women make them (unjustified) fall into the weaker recommendations of the CRT guidelines.71,73 Alternatively, there is a fear that women derive more complications from CRT therapy than men. In the CRT Survey II comprising 11.088 new CRT implantations, women did have had a higher procedural complication rate, in particular related to vascular access as evidenced by pneumothorax (1.4%), coronary sinus dissection (2.1%), and pericardial tamponade (0.3%).1 The probable cause is the smaller dimensions of vessels in women compared with men. Nonetheless, women should not be withheld CRT, and an individual approach in assessing CRT eligibility based on body size is probably helpful in increasing access to CRT across gender and ethnicity.
Female patients are also under-referred for AF ablation124 and receive significantly less ICD therapy than men.124 While only a single study suggests that PVC-CM is more prevalent in males,125 it is not clear that there are gender differences in the treatment of PVC-CM or T-CM.
Conclusions
A review of the literature provides evidence that ‘electrical’ diseases like AF, AT, PVCs, LBBB, and AV uncoupling may seriously impact cardiac pump function and may cause or contribute to worsening HF.
Likewise, electrical therapies, like ablation and resynchronization pacing, have been shown and are guideline recommended to contribute to the treatment of HF patients with any of these electrical diseases.
Despite all this, there is a considerable underutilitzation of these therapies in the treatment of HF patients. Therefore, we recommend that an electrocardiologist becomes part of the treatment team of the HF patient. Besides the promotion of the electrical therapies, such a treatment should also pay attention to personal aspects of the patient such as sex and body size.
Contributor Information
Frits W Prinzen, Department of Physiology, Cardiovascular Research Institute Maastricht, Maastricht University, PO Box 616, 6200 MD Maastricht, The Netherlands.
Angelo Auricchio, Division of Cardiology, Istituto Cardiocentro Ticino, Lugano, Switzerland.
Wilfried Mullens, Ziekenhuis Oost Limburg, Genk, Belgium; Biomedical Research Institute, Faculty of Medicine and Life Sciences, University Hasselt, Hasselt, Belgium.
Cecilia Linde, Department of Medicine, Karolinska Institutet, Solna, Sweden; Department of Cardiology, Karolinska University Hospital, Stockholm, Sweden.
Jose F Huizar, Cardiology Division, Virginia Commonwealth University/Pauley Heart Center, Richmond, VA, USA; Cardiology Division, Hunter Holmes McGuire Veterans Affairs Medical Center, Richmond, VA, USA.
Funding
Research of J.F.H. was funded by NIH/NHLBI (1R01HL139874-01; PI: J.F.H.), Department of Veteran Affairs Merit Grant (BX004861-01; PI: J.F.H.). Research of C.L. was funded by the Swedish Heart-Lung Foundation, Swedish Research Council, and Stockholm County Council.
Conflict of interest: F.W.P. received research grants from Medtronic, Abbott Inc., Biotronik, MicroportCRM, and EBR Systems. J.F.H. received research support from Abbott Inc. A.A. is a consultant to Boston Scientific, Cairdac, Corvia, MicroportCRM, EPD Philips, Radcliffe Publishers, received speaker fees from Boston Scientific, Medtronic, and Microport, participates in clinical trials sponsored by Boston Scientific, Medtronic, EPD Philips, and has intellectual properties with Boston Scientific, Biosense Webster, and Microport CRM. C.L. received consulting fees from AstraZeneca, Roche diagnostics, Bayer and speaker honoraria from Novartis, Astra, Bayer, Medtronic, Impulse Dynamics, and Vifor. W.M. has received research grants/consultancy fees from Novartis, Vifor, Medtronic, Abbott, AstraZenica, and Boehringer Ingelheim.
References
- 1. Dickstein K, Normann C, Auricchio A, Bogale N, Cleland JG, Gitt AK, et al. CRT Survey II: an ESC survey of cardiac resynchronization therapy in 11088 patients—who is doing what to whom and how? Eur J Heart Fail 2018;20:1039–1051. [DOI] [PubMed] [Google Scholar]
- 2. Lund LH, Benson L, Ståhlberg M, Braunschweig F, Edner M, Dahlström U, et al. Age, prognostic impact of QRS prolongation and left bundle branch block, and utilization of cardiac resynchronization therapy: findings from 14 713 patients in the Swedish Heart Failure Registry. Eur J Heart Fail 2014;16:1073–1081. [DOI] [PubMed] [Google Scholar]
- 3. Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol 2003;91:2D–8D. [DOI] [PubMed] [Google Scholar]
- 4. Huizar JF, Tan AY, Kaszala K, Ellenbogen KA. Clinical and translational insights on premature ventricular contractions and PVC-induced cardiomyopathy. Prog Cardiovasc Dis 2021;66:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vernooy K, Verbeek XA, Peschar M, Crijns HJGM, Arts T, Cornelussen RNM, et al. Left bundle branch block induces ventricular remodelling and functional septal hypoperfusion. Eur Heart J 2005;26:91–98. [DOI] [PubMed] [Google Scholar]
- 6. Zupan I, Rakovec P, Budihna N, Brecelj A, Kozelj M. Tachycardia induced cardiomyopathy in dogs; relation between chronic supraventricular and chronic ventricular tachycardia. Int J Cardiol 1996;56:75–81. [DOI] [PubMed] [Google Scholar]
- 7. Huizar JF, Kaszala K, Potfay J, Minisi AJ, Lesnefsky EJ, Abbate A, et al. Left ventricular systolic dysfunction induced by ventricular ectopy: a novel model for premature ventricular contraction-induced cardiomyopathy. Circ Arrhythm Electrophysiol 2011;4:543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dosdall DJ, Ranjan R, Higuchi K, Kholmovski E, Angel N, Li L, et al. Chronic atrial fibrillation causes left ventricular dysfunction in dogs but not goats: experience with dogs, goats, and pigs. Am J Physiol Heart Circ Physiol 2013;305:H725–H731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019;21:1169–1186. [DOI] [PubMed] [Google Scholar]
- 10. Aiba T, Hesketh GG, Barth AS, Liu T, Daya S, Chakir K, et al. Electrophysiological consequences of dyssynchronous heart failure and its restoration by resynchronization therapy. Circulation 2009;119:1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang M, Zhang M, Howren M, Wang Y, Tan A, Balijepalli RC, et al. JPH-2 interacts with Cai-handling proteins and ion channels in dyads: contribution to premature ventricular contraction-induced cardiomyopathy. Heart Rhythm 2016;13:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Eltit JM, Kaszala K, Tan A, Jiang M, Zhang M, et al. Cellular mechanism of premature ventricular contraction-induced cardiomyopathy. Heart Rhythm 2014;11:2064–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Torrado J, Kowlgi GN, Ramirez RJ, Balderas-Villalobos J, Jovin D, Parker C, et al. Eccentric hypertrophy in an animal model of mid- and long-term premature ventricular contraction-induced cardiomyopathy. Heart Rhythm 2021;2:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta S, Figueredo VM. Tachycardia mediated cardiomyopathy: pathophysiology, mechanisms, clinical features and management. Int J Cardiol 2014;172:40–46. [DOI] [PubMed] [Google Scholar]
- 15. Mueller KAL, Heinzmann D, Klingel K, Fallier-Becker P, Kandolf R, Kilias A, et al. Histopathological and immunological characteristics of tachycardia-induced cardiomyopathy. J Am Coll Cardiol 2017;69:2160–2172. [DOI] [PubMed] [Google Scholar]
- 16. Cooper MW. Postextrasystolic potentiation. Do we really know what it means and how to use it? Circulation 1993;88:2962–2971. [DOI] [PubMed] [Google Scholar]
- 17. Huizar JF, Ellenbogen KA, Tan AY, Kaszala K. Arrhythmia-induced cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:2328–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takada H, Takeuchi S, Ando K, Kaito A, Yoshida S, Hisada S, et al. Experimental studies on myocardial contractility and hemodynamics in extrasystoles. Jpn Circ J 1970;34:419–430. [DOI] [PubMed] [Google Scholar]
- 19. Cooper MW, Lutherer LO, Lust RM. Postextrasystolic potentiation and echocardiography: the effect of varying basic heart rate, extrasystolic coupling interval and postextrasystolic interval. Circulation 1982;66:771–776. [DOI] [PubMed] [Google Scholar]
- 20. Ling L-h, Khammy O, Byrne M, Amirahmadi F, Foster A, Li G, et al. Irregular rhythm adversely influences calcium handling in ventricular myocardium. Implications for the interaction between heart failure and atrial fibrillation. Circ Heart Fail 2012;5:786–793. [DOI] [PubMed] [Google Scholar]
- 21. Lipshultz SE, Rifai N, Sallan SE, Lipsitz SR, Dalton V, Sacks DB, et al. Predictive value of cardiac troponin T in pediatic patients at risk for myocardial injury. Circulation 1997;96:2641–2648. [DOI] [PubMed] [Google Scholar]
- 22. Lyon A, van Mourik M, Cruts L, Heijman J, Bekkers SCAM, Schotten U, et al. Both beat-to-beat changes in RR-interval and left ventricular filling time determine ventricular function during atrial fibrillation. Europace 2021;23:i21–i28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mase M, Ravelli F. Understanding the effects of heartbeat irregularity on ventricular function in human atrial fibrillation: simulation models may help to untie the knot. Europace 2021;23:1868. [DOI] [PubMed] [Google Scholar]
- 24. Kirk JA, Kass DA. Electromechanical dyssynchrony and resynchronization of the failing heart. Circ Res 2013;113:765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spragg DD, Leclercq C, Loghmani M, Faris OP, Tunin RS, DiSilvestre D, et al. Regional alterations in protein expression in the dyssynchronous failing heart. Circulation 2003;108:929–932. [DOI] [PubMed] [Google Scholar]
- 26. Nguyên UC, Verzaal NJ, van Nieuwenhoven FA, Vernooy K, Prinzen FW. Pathobiology of cardiac dyssynchrony and resynchronization therapy. Europace 2018;20:1898–1909. [DOI] [PubMed] [Google Scholar]
- 27. Moe GW, Armstrong P. Pacing-induced heart failure: a model to study the mechanism of disease progression and novel therapy in heart failure. Cardiovasc Res 1999;42:591–599. [DOI] [PubMed] [Google Scholar]
- 28. Kuroki K, Tada H, Seo Y, Ishizu T, Igawa M, Yamasaki H, et al. Prediction and mechanism of frequent ventricular premature contractions related to haemodynamic deterioration. Eur J Heart Fail 2012;14:1112–1120. [DOI] [PubMed] [Google Scholar]
- 29. Appleton CP, Basnight MA, Gonzalez MS. Diastolic mitral regurgitation with atrioventricular conduction abnormalities: relation of mitral flow velocity to transmitral pressure gradients in conscious dogs. J Am Coll Cardiol 1991;18:843–849. [DOI] [PubMed] [Google Scholar]
- 30. Auricchio A, Ding J, Spinelli JC, Kramer AP, Salo RW, Hoersch W, et al. Cardiac resynchronization therapy restores optimal atrioventricular mechanical timing in heart failure patients with ventricular conduction delay. J Am Coll Cardiol 2002;39:1163–1169. [DOI] [PubMed] [Google Scholar]
- 31. Salden FCWM, Huntjens PR, Schreurs R, Willemen E, Kuiper M, Wouters P, et al. Pacing therapy for atrioventricular dromotropathy: a combined computational-experimental-clinical study. Europace 2022;euab248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Watanabe H, Okamura K, Chinushi M, Furushima H, Tanabe Y, Kodama M, et al. Clinical characteristics, treatment, and outcome of tachycardia induced cardiomyopathy. Int Heart J 2008;49:39–47. [DOI] [PubMed] [Google Scholar]
- 33. Donghua Z, Jian P, Zhongbo X, Feifei Z, Xinhui P, Hao Y, et al. Reversal of cardiomyopathy in patients with congestive heart failure secondary to tachycardia. J Interv Card Electrophysiol 2013;36:27–32. [DOI] [PubMed] [Google Scholar]
- 34. Medi C, Kalman JM, Haqqani H, Vohra JK, Morton JB, Sparks PB, et al. Tachycardia-mediated cardiomyopathy secondary to focal atrial tachycardia: long-term outcome after catheter ablation. J Am Coll Cardiol 2009;53:1791–1797. [DOI] [PubMed] [Google Scholar]
- 35. Moore JP, Patel PA, Shannon KM, Albers EL, Salerno JC, Stein MA, et al. Predictors of myocardial recovery in pediatric tachycardia-induced cardiomyopathy. Heart Rhythm 2014;11:1163–1169. [DOI] [PubMed] [Google Scholar]
- 36. Gentlesk PJ, Sauer WH, Gerstenfeld EP, Lin D, Dixit S, Zado E, et al. Reversal of left ventricular dysfunction following ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2007;18:9–14. [DOI] [PubMed] [Google Scholar]
- 37. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 38. Ling LH, Kalman JM, Ellims AH, Iles LM, Medi C, Sherratt C, et al. Diffuse ventricular fibrosis is a late outcome of tachycardia-mediated cardiomyopathy after successful ablation. Circ Arrhythm Electrophysiol 2013;6:697–704. [DOI] [PubMed] [Google Scholar]
- 39. Agarwal SK, Simpson RJJ, Rautaharju P, Alonso A, Shahar E, Massing M, et al. Relation of ventricular premature complexes to heart failure (from the Atherosclerosis Risk In Communities [ARIC] Study). Am J Cardiol 2012;109:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huizar JF, Fisher SG, Ramsey FV, Kaszala K, Tan AY, Moore H, et al. Outcomes of premature ventricular contraction-cardiomyopathy in the veteran population: a secondary analysis of the CHF-STAT study. JACC Clin Electrophysiol 2021;7:380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Latchamsetty R, Yokokawa M, Morady F, Kim HMa, Mathew S, Tilz R, et al. Multicenter outcomes for catheter ablation of idiopathic premature ventricular complexes. JACC Clin Electrophysiol 2015;1:116–123. [DOI] [PubMed] [Google Scholar]
- 42. Altintas B, Ozkalayci F, Cinier G, Kaya İ, Aktan A, Küp A, et al. The effect of idiopathic premature ventricular complexes on left ventricular ejection fraction. Ann Noninvasive Electrocardiol 2020;25:e12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tan AY, Hu YL, Potfay J, Kaszala K, Howren M, Sima AP, et al. Impact of ventricular ectopic burden in a premature ventricular contraction-induced cardiomyopathy animal model. Heart Rhythm 2016;13:755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hamon D, Blaye-Felice MS, Bradfield JS, Chaachoui N, Tung R, Elayi CS, et al. A new combined parameter to predict premature ventricular complexes induced cardiomyopathy: impact and recognition of epicardial origin. J Cardiovasc Electrophysiol 2016;27:709–717. [DOI] [PubMed] [Google Scholar]
- 45. Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, et al. Ventricular ectopy as a predictor of heart failure and death. J Am Coll Cardiol 2015;66:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Loring Z, Hanna P, Pellegrini CN. Longer ambulatory ECG monitoring increases identification of clinically significant ectopy. Pacing Clin Electrophysiol 2016;39:592–597. [DOI] [PubMed] [Google Scholar]
- 47. Zhong L, Lee YH, Huang XM, Asirvatham SJ, Shen WK, Friedman PA, et al. Relative efficacy of catheter ablation vs antiarrhythmic drugs in treating premature ventricular contractions: a single-center retrospective study. Heart Rhythm 2014;11:187–193. [DOI] [PubMed] [Google Scholar]
- 48. Ling Z, Liu Z, Su L, Zipunnikov V, Wu J, Du H, et al. Radiofrequency ablation versus antiarrhythmic medication for treatment of ventricular premature beats from the right ventricular outflow tract: prospective randomized study. Circ Arrhythm Electrophysiol 2014;7:237–243. [DOI] [PubMed] [Google Scholar]
- 49. Rivera S, de la Paz Ricapito M, Espinoza J, Belardi D, Albina G, Giniger A, et al. Cryoablation for ventricular arrhythmias arising from the papillary muscles of the left ventricle guided by intracardiac echocardiography and image integration. JACC Clin Electrophysiol 2015;1:509–516. [DOI] [PubMed] [Google Scholar]
- 50. Rivera S, Ricapito MLP, Tomas L, Parodi J, Bardera Molina G, Banega R, et al. Results of cryoenergy and radiofrequency-based catheter ablation for treating ventricular arrhythmias arising from the papillary muscles of the left ventricle, guided by intracardiac echocardiography and image integration. Circ Arrhythm Electrophysiol 2016;9:e003874. [DOI] [PubMed] [Google Scholar]
- 51. Penela D, Martinez M, Fernandez-Armenta J, Aguinaga L, Tercedor L, Ordóñez A, et al. Influence of myocardial scar on the response to frequent premature ventricular complex ablation. Heart 2019;105:378–383. [DOI] [PubMed] [Google Scholar]
- 52. Doval HC, Nul DR, Grancelli HO, Perrone SV, Bortman GR, Curiel R. Randomised trial of low-dose amiodarone in severe congestive heart failure. Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA). Lancet 1994;344:493–498. [DOI] [PubMed] [Google Scholar]
- 53. Van Gelder IC, Rienstra M, Crijns HJGM, Olshansky B. Rate control in atrial fibrillation. Lancet 2016;388:818–828. [DOI] [PubMed] [Google Scholar]
- 54. Groenveld HF, Crijns HJ, Van den Berg MP, Van Sonderen E, Alings AM, Tijssen JGP, et al. The effect of rate control on quality of life in patients with permanent atrial fibrillation: data from the RACE II (Rate Control Efficacy in Permanent Atrial Fibrillation II) study. J Am Coll Cardiol 2011;58:1795–1803. [DOI] [PubMed] [Google Scholar]
- 55. Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JGP, Alings AM, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 2010;362:1363–1373. [DOI] [PubMed] [Google Scholar]
- 56. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 57. Hagens VE, Crijns HJ, Van Veldhuisen DJ, Van Den Berg MP, Rienstra M, Ranchor AV, et al. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J 2005;149:1106–1111. [DOI] [PubMed] [Google Scholar]
- 58. Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. A Comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]
- 59. Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med 2008;358:2667–2677. [DOI] [PubMed] [Google Scholar]
- 60. Shelton RJ, Clark AL, Goode K, Rigby AS, Houghton T, Kaye GC, et al. A randomised, controlled study of rate versus rhythm control in patients with chronic atrial fibrillation and heart failure: (CAFE-II Study). Heart 2009;95:924–930. [DOI] [PubMed] [Google Scholar]
- 61. Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004;109:1509–1513. [DOI] [PubMed] [Google Scholar]
- 62. Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol 2014;7:31–38. [DOI] [PubMed] [Google Scholar]
- 63. MacDonald MR, Connelly DT, Hawkins NM, Steedman T, Payne J, Shaw M, et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart 2011;97:740–747. [DOI] [PubMed] [Google Scholar]
- 64. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 65. Sugumar H, Prabhu S, Costello B, Chieng D, Azzopardi S, Voskoboinik A et al. Catheter ablation versus medication in atrial fibrillation and systolic dysfunction: late outcomes of CAMERA-MRI Study. JACC Clin Electrophysiol 2020;6:1721–1731. [DOI] [PubMed] [Google Scholar]
- 66. Vernooy K, Dijkman B, Cheriex EC, Prinzen FW, Crijns HJ. Ventricular remodeling during long-term right ventricular pacing following His bundle ablation. Am J Cardiol 2006;97:1223–1227. [DOI] [PubMed] [Google Scholar]
- 67. Brignole M, Pokushalov E, Pentimalli F, Palmisano P, Chieffo E, Occhetta E, et al. A randomized controlled trial of atrioventricular junction ablation and cardiac resynchronization therapy in patients with permanent atrial fibrillation and narrow QRS. Eur Heart J 2018;39:3999–4008. [DOI] [PubMed] [Google Scholar]
- 68. Brignole M, Pentimalli F, Palmisano P, Landolina M, Quartieri F, Occhetta E, et al. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: the APAF-CRT mortality trial. Eur Heart J 2021;42:4731–4739. [DOI] [PubMed] [Google Scholar]
- 69. Gasparini M, Leclercq C, Lunati M, Landolina Mo, Auricchio A, Santini M, et al. Cardiac resynchronization therapy in patients with atrial fibrillation: the CERTIFY study (Cardiac Resynchronization Therapy in Atrial Fibrillation Patients Multinational Registry). JACC Heart Fail 2013;1:500–507. [DOI] [PubMed] [Google Scholar]
- 70. Houthuizen P, Van Garsse LA, Poels TT, de Jaegere P, van der Boon RMA, Swinkels BM, et al. Left bundle-branch block induced by transcatheter aortic valve implantation increases risk of death. Circulation 2012;126:720–728. [DOI] [PubMed] [Google Scholar]
- 71. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2021;42:3427–3520. [DOI] [PubMed] [Google Scholar]
- 72. Gold MR, Rickard J, Daubert J, Zimmerman P, Linde C. Redefining the classifications of response to cardiac resynchronization therapy: results from the REVERSE Study. JACC Clin Electrophysiol 2021;7:871–880. [DOI] [PubMed] [Google Scholar]
- 73. Linde C, Ståhlberg M, Benson L, Braunschweig F, Lund LH, Edner M, et al. Gender and utilization of cardiac resynchronization therapy, and prognostic impact of QRS prolongation and left bundle branch block in heart failure. Europace 2015;17:424–431. [DOI] [PubMed] [Google Scholar]
- 74. Caputo ML, van Stipdonk A, Illner A, D’Ambrosio G, Regoli F, Conte G, et al. The definition of left bundle branch block influences the response to cardiac resynchronization therapy. Int J Cardiol 2018;269:165–169. [DOI] [PubMed] [Google Scholar]
- 75. van Stipdonk AMW, Hoogland R, Ter Horst I, Kloosterman M, Vanbelle S, Crijns HJGM, et al. Evaluating electrocardiography-based identification of cardiac resynchronization therapy responders beyond current left bundle branch block definitions. JACC Clin Electrophysiol 2020;6:193–203. [DOI] [PubMed] [Google Scholar]
- 76. van Stipdonk AMW, Ter Horst I, Kloosterman M, Engels EB, Rienstra M, Crijns HJGM, et al. QRS area is a strong determinant of outcome in cardiac resynchronization therapy. Circ Arrhythm Electrophysiol 2018;11:e006497. [DOI] [PubMed] [Google Scholar]
- 77. Reddy VY, Miller MA, Neuzil P, Søgaard P, Butter C, Seifert M, et al. Cardiac resynchronization therapy with wireless left ventricular endocardial pacing: the SELECT-LV Study. J Am Coll Cardiol 2017;69:2119–2129. [DOI] [PubMed] [Google Scholar]
- 78. Vijayaraman P, Herweg B, Ellenbogen KA, Gajek J. His-optimized cardiac resynchronization therapy to maximize electrical resynchronization: a feasibility study. Circ Arrhythm Electrophysiol 2019;12:e006934. [DOI] [PubMed] [Google Scholar]
- 79. Jastrzębski M, Moskal P, Huybrechts W, Curila K, Sreekumar P, Rademakers LM, et al. Left bundle branch-optimized cardiac resynchronization therapy (LOT-CRT): results from an international LBBAP collaborative study group. Heart Rhythm 2022;19:13–21. [DOI] [PubMed] [Google Scholar]
- 80. Emerek K, Friedman DJ, Sørensen PL, Hansen SM, Larsen JM, Risum N, et al. Vectorcardiographic QRS area is associated with long-term outcome after cardiac resynchronization therapy. Heart Rhythm 2019;16:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Okafor O, Zegard A, van Dam P, Stegemann Berthold, Qiu Tian, Marshall Howard, et al. Changes in QRS area and QRS duration after cardiac resynchronization therapy predict cardiac mortality, heart failure hospitalizations, and ventricular arrhythmias. J Am Heart Assoc 2019;8:e013539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rad MM, Wijntjens GW, Engels EB, Blaauw Y, Luermans JGLM, Pison L, et al. Vectorcardiographic QRS area identifies delayed left ventricular lateral wall activation determined by electroanatomic mapping in candidates for cardiac resynchronization therapy. Heart Rhythm 2016;13:217–225. [DOI] [PubMed] [Google Scholar]
- 83. van Deursen CJ, Vernooy K, Dudink E, et al. Vectorcardiographic QRS area as a novel predictor of response to cardiac resynchronization therapy. J Electrocardiol 2015;48:45–52. [DOI] [PubMed] [Google Scholar]
- 84. Nguyên UC, Claridge S, Vernooy K, Engels EB, Razavi R, Rinaldi CA, et al. Relationship between vectorcardiographic QRS area, myocardial scar quantification, and response to cardiac resynchronization therapy. J Electrocardiol 2018;51:457–463. [DOI] [PubMed] [Google Scholar]
- 85. Beshai JF, Grimm RA, Nagueh SF, Baker JH, Beau SL, Greenberg SM, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med 2007;357:2461–2471. [DOI] [PubMed] [Google Scholar]
- 86. Ruschitzka M, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 2013;369:1395–1405. [DOI] [PubMed] [Google Scholar]
- 87. Beela AS, Ünlü S, Duchenne J, Ciarka A, Daraban AM, Kotrc M, et al. Assessment of mechanical dyssynchrony can improve the prognostic value of guideline-based patient selection for cardiac resynchronization therapy. Eur Heart J Cardiovasc Imaging 2019;20:66–74. [DOI] [PubMed] [Google Scholar]
- 88. Aalen JM, Donal E, Larsen CK, Duchenne J, Lederlin M, Cvijic M, et al. Imaging predictors of response to cardiac resynchronization therapy: left ventricular work asymmetry by echocardiography and septal viability by cardiac magnetic resonance. Eur Heart J 2020;41:3813–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lumens J, Tayal B, Walmsley J, Delgado-Montero A, Huntjens PR, Schwartzman D, et al. Differentiating electromechanical from non–electrical substrates of mechanical discoordination to identify responders to cardiac resynchronization. Circ Cardiovasc Imaging 2015;8:e003744. [DOI] [PubMed] [Google Scholar]
- 90. Behar JM, Mountney P, Toth D, Reiml S, Panayiotou M, Brost A, et al. Real-time X-MRI-guided left ventricular lead implantation for targeted delivery of cardiac resynchronization therapy. JACC Clin Electrophysiol 2017;3:803–814. [DOI] [PubMed] [Google Scholar]
- 91. Stephansen C, Sommer A, Kronborg MB, Jensen JM, Nørgaard BL, Gerdes C, et al. Electrically vs. imaging-guided left ventricular lead placement in cardiac resynchronization therapy: a randomized controlled trial. Europace 2019;21:1369–1377. [DOI] [PubMed] [Google Scholar]
- 92. Saba S, Nair D, Ellis C, Ciuffo A, Cox M, Gupta N, et al. Usefulness of multisite ventricular pacing in nonresponders to cardiac resynchronization therapy. Am J Cardiol 2022;164:86–92. [DOI] [PubMed] [Google Scholar]
- 93. Morgan JM, Biffi M, Gellér L, Leclercq C, Ruffa F, Tung S, et al. ALternate Site Cardiac ResYNChronization (ALSYNC): a prospective and multicentre study of left ventricular endocardial pacing for cardiac resynchronization therapy. Eur Heart J 2016;37:2118–2127. [DOI] [PubMed] [Google Scholar]
- 94. Auricchio A, Delnoy PP, Butter C, Brachmann J, Van Erven L, Spitzer S, et al. Feasibility, safety, and short-term outcome of leadless ultrasound-based endocardial left ventricular resynchronization in heart failure patients: results of the Wireless Stimulation Endocardially for CRT (WiSE-CRT) study. Europace 2014;16:681–688. [DOI] [PubMed] [Google Scholar]
- 95. Singh JP, Abraham WT, Auricchio A, et al. Design and rationale for the Stimulation Of the Left Ventricular Endocardium for Cardiac Resynchronization Therapy in non-responders and previously untreatable patients (SOLVE-CRT) trial. Am Heart J 2019;217:13–22. [DOI] [PubMed] [Google Scholar]
- 96. Strik M, Rademakers LM, van Deursen CJM, van Hunnik A, Kuiper M, Klersy C, et al. Endocardial left ventricular pacing improves cardiac resynchronization therapy in chronic asynchronous infarction and heart failure models. Circ Arrhythm Electrophysiol 2012;5:191–200. [DOI] [PubMed] [Google Scholar]
- 97. Narula OS. Longitudinal dissociation in the His bundle. Bundle branch block due to asynchronous conduction within the His bundle in man. Circulation 1977;56:996–1006. [DOI] [PubMed] [Google Scholar]
- 98. Mills RW, Cornelussen RN, Mulligan LJ, Strik M, Rademakers LM, Skadsberg ND, et al. Left ventricular septal and left ventricular apical pacing chronically maintain cardiac contractile coordination, pump function and efficiency. Circ Arrhythm Electrophysiol 2009;2:571–579. [DOI] [PubMed] [Google Scholar]
- 99. Salden FCWM, Luermans JGLM, Westra SW, Weijs B, Engels EB, Heckman LIB, et al. Short-term hemodynamic and electrophysiological effects of cardiac resynchronization by left ventricular septal pacing. J Am Coll Cardiol 2020;75:347–359. [DOI] [PubMed] [Google Scholar]
- 100. Upadhyay GA, Vijayaraman P, Nayak HM, Verma N, Dandamudi G, Sharma PS, et al. On-treatment comparison between corrective His bundle pacing and biventricular pacing for cardiac resynchronization: a secondary analysis of the His-SYNC pilot trial. Heart Rhythm 2019;16:1797–1807. [DOI] [PubMed] [Google Scholar]
- 101. Arnold AD, Shun-Shin MJ, Keene D, Howard JP, Sohaib SMA, Wright IJ, et al. His resynchronization versus biventricular pacing in patients with heart failure and left bundle branch block. J Am Coll Cardiol 2018;72:3112–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Huang W, Wu S, Vijayaraman P, Su L, Chen X, Cai B, et al. Cardiac resynchronization therapy in patients with nonischemic cardiomyopathy using left bundle branch pacing. JACC Clin Electrophysiol 2020;6:849–858. [DOI] [PubMed] [Google Scholar]
- 103. Upadhyay GA, Cherian T, Shatz DY, Beaser AD, Aziz Z, Ozcan C, et al. Intracardiac delineation of septal conduction in left bundle-branch block patterns. Circulation 2019;139:1876–1888. [DOI] [PubMed] [Google Scholar]
- 104. Rademakers LM, van Hunnik A, Kuiper M, Vernooy K, van Gelder B, Bracke FA, et al. A possible role for pacing the left ventricular septum in cardiac resynchronization therapy. JACC Clin Electrophysiol 2016;2:413–422. [DOI] [PubMed] [Google Scholar]
- 105. Teigeler T, Kolominsky J, Vo C, Shepard RK, Kalahasty G, Kron J, et al. Intermediate-term performance and safety of His-bundle pacing leads: a single-center experience. Heart Rhythm 2021;18:743–749. [DOI] [PubMed] [Google Scholar]
- 106. Ellenbogen KA, Gold MR, Meyer TE, Fernndez Lozano I, Mittal S, Waggoner AD, et al. Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation 2010;122:2660–2668. [DOI] [PubMed] [Google Scholar]
- 107. Birnie D, Hudnall H, Lemke B, Aonuma K, Lee KLF, Gasparini M, et al. Continuous optimization of cardiac resynchronization therapy reduces atrial fibrillation in heart failure patients: results of the adaptive cardiac resynchronization therapy trial. Heart Rhythm 2017;14:1820–1825. [DOI] [PubMed] [Google Scholar]
- 108. Thibault B, Ritter P, Bode K, Calò L, Mondésert B, Mangual JO, et al. Dynamic programming of atrioventricular delay improves electrical synchrony in a multicenter cardiac resynchronization therapy study. Heart Rhythm 2019;16:1047–1056. [DOI] [PubMed] [Google Scholar]
- 109. Brugada J, Delnoy PP, Brachmann J, Reynolds D, Padeletti L, Noelker G, et al. Contractility sensor-guided optimization of cardiac resynchronization therapy: results from the RESPOND-CRT trial. Eur Heart J 2017;38:730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lyon AR, Samara MA, Feldman DS. Cardiac contractility modulation therapy in advanced systolic heart failure. Nat Rev Cardiol 2013;10:584–598. [DOI] [PubMed] [Google Scholar]
- 111. Abraham WT, Kuck KH, Goldsmith RL, Lindenfeld J, Reddy VY, Carson PE, et al. A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation. JACC Heart Fail 2018;6:874–883. [DOI] [PubMed] [Google Scholar]
- 112. Zipes DP, Neuzil P, Theres H, Caraway D, Mann DL, Mannheimer C, et al. Determining the feasibility of spinal cord neuromodulation for the treatment of chronic systolic heart failure: the DEFEAT-HF study. JACC Heart Fail 2016;4:129–136. [DOI] [PubMed] [Google Scholar]
- 113. Zile MR, Lindenfeld J, Weaver FA, Zannad F, Galle E, Rogers T, et al. Baroreflex activation therapy in patients with heart failure with reduced ejection fraction. J Am Coll Cardiol 2020;76:1–13. [DOI] [PubMed] [Google Scholar]
- 114. Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomström-Lundqvist C, et al. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia: the Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J 2020;41:655–720. [DOI] [PubMed] [Google Scholar]
- 115. Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri N, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace 2019;21:1143–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Raatikainen MJP, Arnar DO, Zeppenfeld K, Merino JL, Levya F, Hindricks G, et al. Statistics on the use of cardiac electronic devices and electrophysiological procedures in the European Society of Cardiology countries: 2014 report from the European Heart Rhythm Association. Europace 2016;17:i1–i75. [DOI] [PubMed] [Google Scholar]
- 117. Sze E, Samad Z, Dunning A, Campbell KB, Loring Z, Atwater BD, et al. Impaired recovery of left ventricular function in patients with cardiomyopathy and left bundle branch block. J Am Coll Cardiol 2018;71:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mullens W, Auricchio A, Martens P, Witte K, Cowie MR, Delgado V, et al. Optimized implementation of cardiac resynchronization therapy: a call for action for referral and optimization of care: a joint position statement from the Heart Failure Association (HFA), European Heart Rhythm Association (EHRA), and European Association of Cardiovascular Imaging (EACVI) of the European Society of Cardiology. Eur J Heart Fail 2020;22:2349–2369. [DOI] [PubMed] [Google Scholar]
- 119. Daubert JC, Saxon L, Adamson PB, Auricchio A, Berger RD, Beshai JF, et al. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow-up recommendations and management. Europace 2012;14:1236–1286. [DOI] [PubMed] [Google Scholar]
- 120. Varma N, Boehmer J, Bhargava K, Yoo D, Leonelli F, Costanzo M, et al. Evaluation, management, and outcomes of patients poorly responsive to cardiac resynchronization device therapy. J Am Coll Cardiol 2019;74:2588–2603. [DOI] [PubMed] [Google Scholar]
- 121. Zusterzeel R, Selzman KA, Sanders WE, Caños DA, O’Callaghan KM, Carpenter JL, et al. Cardiac resynchronization therapy in women: US food and drug administration meta-analysis of patient-level data. JAMA Intern Med 2014;174:1340–1348. [DOI] [PubMed] [Google Scholar]
- 122. Linde C, Cleland JG, Gold MR, Claude Daubert J, Tang ASL, Young JB, et al. The interaction of sex, height, and QRS duration on the effects of cardiac resynchronization therapy on morbidity and mortality: an individual-patient data meta-analysis. Results from a case based meta-analysis. Eur J Heart Fail 2018;20:780–791. [DOI] [PubMed] [Google Scholar]
- 123. Varma N, Lappe J, He J, Niebauer M, Manne M, Tchou P. Sex-specific response to cardiac resynchronization therapy: effect of left ventricular size and QRS duration in left bundle branch block. JACC Clin Electrophysiol 2017;3:844–853. [DOI] [PubMed] [Google Scholar]
- 124. Grecu M, Blomström-Lundqvist C, Kautzner J, Laroche C, Van Gelder IC, Jordaens L, et al. In-hospital and 12-month follow-up outcome from the ESC-EORP EHRA Atrial Fibrillation Ablation Long-Term registry: sex differences. Europace 2020;22:66–73. [DOI] [PubMed] [Google Scholar]
- 125. Park KM, Im SI, Park SJ, Kim JS, On YK. Risk factor algorithm used to predict frequent premature ventricular contraction-induced cardiomyopathy. Int J Cardiol 2017;233:37–42. [DOI] [PubMed] [Google Scholar]