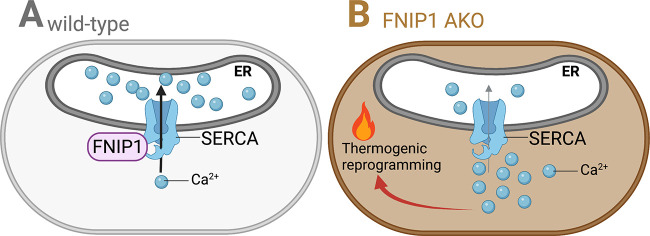
In this issue of Journal of Experimental Medicine, Yin et al. (2022) discover that loss of FNIP1 is associated with browning of white adipose tissue, which they propose is driven by decreased calcium uptake into the ER.
Abstract
In this issue of Journal of Experimental Medicine, Yin et al. (2022. J. Exp. Med. https://doi.org/10.1084/jem.20212491) discover that loss of FNIP1 is associated with browning of white adipose tissue, which they propose is driven by decreased calcium uptake into the ER.
White adipocytes are the main cells in the body that store chemical energy in large single lipid droplets. In contrast, brown and beige adipocytes convert macronutrient energy into heat by elevating mitochondrial respiration through both proton leak and ATP-consuming futile cycles (Wolfrum and Gerhart-Hines, 2022). In response to external signals, the capacity for energy expenditure by thermogenic adipose tissue is promoted by transcriptional reconfiguration of existing adipocytes and through the emergence of newly formed thermogenic adipocytes from adipocyte precursor cells (Hussain et al., 2020). While the transcriptional outputs arising from cAMP signaling are well-characterized (Shao and Gupta, 2019), the transcriptional control over thermogenic gene expression that is regulated by other second messengers in adipocytes is relatively poorly understood.

Insights from Jakub Bunk and Lawrence Kazak.
Calcium (Ca2+) influx into thermogenic adipocytes is regulated by extrinsic adrenergic control (Chen et al., 2017), but a deep mechanistic understanding for how Ca2+ signaling regulates adaptive thermogenesis is far from complete. Yin et al. (2022) identify one piece of the adaptive thermogenesis puzzle by providing evidence that folliculin interacting protein 1 (FNIP1) negatively regulates the browning of white fat by dampening cytosolic Ca2+ levels. The sarcoendoplasmic reticulum calcium transport ATPase (SERCA) pump is the regulator of Ca2+ uptake from the cytosol to the ER. The authors found that FNIP1 physically interacts with SERCA, which elevates both SERCA activity and SERCA protein abundance, leading to rapid removal of Ca2+ from the cytosol to the lumen of the ER. In the absence of FNIP1, the duration of adrenergically stimulated intracellular Ca2+ signals is extended, which causes transcriptional thermogenic reprogramming of adipocytes (see figure), ultimately resulting in improved whole-body glucose tolerance and reduced hepatic steatosis in the setting of dietary obesity. The findings of Yin and colleagues complement a growing list of recent studies implicating the beneficial role that adipocyte thermogenesis plays in metabolic health, particularly systemic glucose homeostasis and insulin resistance centered on the liver. Specifically, mice displaying altered levels of beige fat through either genetic modulation of the key thermogenic transcriptional regulator, PR domain containing 16 (Cohen et al., 2014; Seale et al., 2011), or following implantation of human beige adipocytes (Min et al., 2016), the regulation of systemic glucose homeostasis by beige fat outweighs its effects on whole-body energy expenditure and obesity. The molecular underpinnings through which thermogenic adipocytes promote glucose clearance and insulin sensitivity are not completely understood, and might stem from their regulation over energy expenditure, inflammation, fibrosis, or endocrine-mediated cross-talk. Intriguingly, insulin-stimulated AKT (also known as protein kinase B) activation was elevated in muscle of adipocyte-specific FNIP1 KO (FNIP1 AKO) mice, suggesting a cross-talk between adipose tissue and muscle. Many additional questions remain that could be the focus of future research efforts.
Cartoon of SERCA-mediated Ca2+transfer in wild-type (left) and FNIP1-deficient (right) adipocytes. Ca2+ is pumped into the lumen of the ER by the SERCA pump. (A) FNIP1 binds SERCA and increases its activity and stabilizes its protein levels, ultimately promoting Ca2+ transport to the ER. (B) Upon deletion of FNIP1, SERCA-mediated Ca2+ transport is reduced, which results in prolonged cytosolic Ca2+ transients. Through an unknown mechanism, cytosolic Ca2+ triggers thermogenic gene expression in adipocytes within white adipose tissue.
What are the transcriptional regulators that are required for the thermogenic reprogramming by Ca2+ signaling? Application of an inducible adipocyte-selective FNIP1 KO model could establish the kinetics of adipocyte browning and facilitate the identification of the molecules that are required for regulating the early transcriptional response that precedes the beneficial metabolic outputs that arise from FNIP1 loss.
Is the elevation of cytosolic Ca2+ sufficient on its own to promote adipocyte thermogenesis, or is the coordination with parallel pathways required? If the elevated cytosolic Ca2+ that occurs following FNIP1 deletion is sufficient to promote the expression of thermogenic genes, this should occur in the absence of a superimposing adrenergic tone. Perhaps comparing the extent of adipocyte thermogenic reprogramming in white adipose tissue (WAT) and brown adipose tissue (BAT) at both sub-thermoneutral and thermoneutral temperatures may yield some insight.
Yin et al. (2022) observed that the binding between FNIP1 and SERCA2 was decreased upon adrenergic stimulation. These experiments hint at the idea that loss of FNIP1 binding to SERCA is regulated under physiological settings that promote thermogenic gene expression. Future studies should focus on defining the regulatory mechanism(s) underlying this change in binding, such as possible allosteric and covalent modifiers of FNIP1 or SERCA2. Moreover, a more detailed understanding of how FNIP1 protein abundance is regulated by physiological interventions that promote adipocyte thermogenesis would be informative. For example, is FNIP1 expression decreased upon cold exposure? If FNIP1 protein abundance is not reduced, why not? Might it have a function that is independent from its role in supporting SERCA-dependent Ca2+ pumping?
Yin and coauthors confirmed that noradrenaline increases intracellular Ca2+ (Wilcke and Nedergaard, 1989; Chen et al., 2017), and that elevated intracellular Ca2+ levels can reconfigure transcriptional networks to promote thermogenic gene expression. Future studies should establish the quantitative contribution between the cellular influx of Ca2+ compared to the release of Ca2+ intracellularly toward thermogenic reprogramming. Furthermore, the authors use an unbiased approach (RNA sequencing) to explore subcutaneous WAT reprogramming upon FNIP1 loss; however, only a select number of genes were examined in BAT. It would be informative to use similar unbiased methods to investigate possible Ca2+-mediated changes in thermogenic gene expression in BAT. Indeed, some experiments point to possible changes in thermogenesis within BAT. For example, FNIP1 AKO mice maintained a higher body temperature compared to control mice upon rapid transition to severe cold only when fasted, a context that has been shown to require the delivery of metabolic fuel to tissues from WAT lipolysis (Schreiber et al., 2017; Shin et al., 2017). Could adipocyte browning in FNIP1-deficient adipocytes be secondary to elevated lipolytic signaling? Indeed, Ca2+ has been demonstrated to regulate lipolysis (Schimmel, 1976; Chen et al., 2017), and lipolytic signaling regulates the expression of thermogenic genes (Sveidahl Johansen et al., 2021). If true, the cold-resistance exhibited by FNIP1 AKO mice in the context of fasting may be explained by Ca2+-stimulated sensitization of WAT lipolysis, which would support BAT-mediated thermogenesis. These mechanistic details could help refine our understanding of the role of FNIP1 in thermogenic gene expression and its role in whole body energy metabolism.
The studies on the cell autonomous respiratory effects following FNIP1 loss lay the groundwork for further experiments. Using saponin-permeabilized WAT, application of respiratory substrate alone was sufficient to demonstrate a higher respiratory rate in FNIP1 AKO versus controls. However, the respiratory buffer contained ∼6 mM ATP, a quantity of purine nucleotide that is expected to completely inhibit uncoupling protein 1 (UCP1)–mediated leak. These data suggest that the elevated respiratory rate was at least partly independent of UCP1. Consistent with this idea, the addition of ADP increased respiration in an oligomycin-sensitive manner, demonstrating respiratory effects linked to ATP synthesis. Furthermore, even though loss of FNIP1 caused elevated protonophore-stimulated cellular respiration, Ucp1 abundance was reduced, indicating that alternative effector pathways may underlie the observed enhanced respiratory effects of FNIP1 AKO adipocytes. The pathway responsible for the respiratory gains remains to be determined.
Interestingly, in addition to the diminution of SERCA activity by FNIP1 deletion, Yin and colleagues show that FNIP1-deficient cells display reduced SERCA protein levels. This raises the question about the quantitative contribution of SERCA interaction with FNIP1 compared to reduced SERCA protein abundance toward cytosolic Ca2+ clearance by the ER.
In sum, Yin et al. demonstrate a connection between intracellular Ca2+ signals and thermogenic gene expression using a series of well-executed genetic and in vivo approaches. They identify a thermogenic brake mechanism mediated by FNIP1–SERCA interaction, which promotes Ca2+ pumping into the ER. Impairing this interaction renders adipocytes within WAT more prone to browning and improves systemic glucose homeostasis in the context of obesity. A further understanding of the mechanisms regulating Ca2+-dependent thermogenic reprogramming will provide fundamental insight into how adipose tissue influences physiology and could bridge the knowledge gap toward therapeutically targeting adipocyte thermogenesis in the context of impaired metabolic homeostasis.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (PJT-159529 and PJT-180557 to L. Kazak). L. Kazak is a Canada Research Chair in Adipocyte Biology.
References
- Chen, Y., et al. 2017. Cell. 10.1016/j.cell.2017.09.015 [DOI] [Google Scholar]
- Cohen, P., et al. 2014. Cell. 10.1016/j.cell.2013.12.021 [DOI] [Google Scholar]
- Hussain, M.F., et al. 2020. FEBS J. 10.1111/febs.15331 [DOI] [Google Scholar]
- Min, S.Y., et al. 2016. Nat. Med. 10.1038/nm.4031 [DOI] [Google Scholar]
- Schimmel, R.J. 1976. Horm. Metab. Res. 10.1055/s-0028-1093659 [DOI] [Google Scholar]
- Schreiber, R., et al. 2017. Cell Metab. 10.1016/j.cmet.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale, P., et al. 2011. J. Clin. Invest. 10.1172/JCI44271 [DOI] [Google Scholar]
- Shao, M., and Gupta R.K.. 2019. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 10.1016/j.bbalip.2018.05.010 [DOI] [Google Scholar]
- Shin, H., et al. 2017. Cell Metab. 10.1016/j.cmet.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveidahl Johansen, O., et al. 2021. Cell. 10.1016/j.cell.2021.04.037 [DOI] [Google Scholar]
- Wilcke, M., and Nedergaard J.. 1989. Biochem. Biophys. Res. Commun. 10.1016/0006-291x(89)92134-7 [DOI] [PubMed] [Google Scholar]
- Wolfrum, C., and Gerhart-Hines Z.. 2022. Science. 10.1126/science.abl7108 [DOI] [PubMed] [Google Scholar]
- Yin, Y., et al. 2022. J. Exp. Med. 10.1084/jem.20212491 [DOI] [Google Scholar]