ABSTRACT
Background
Tumor necrosis factor (TNF)-α, a proinflammatory cytokine, is involved in the pathogenesis of rheumatoid arthritis (RA). The omega-3 unsaturated fatty acid-derived metabolites resolvin (Rv) D1, RvE1, and maresin-1 (MaR1) have been reported as anti-inflammatory lipid mediators and are known as specialized pro-resolving mediators (SPMs). In this study, we aimed to investigate the anti-inflammatory effects of SPMs on TNF-α-induced responses in synovial fibroblasts.
Methods
We investigated the effects of SPMs on gene expression and/or production of cyclooxygenase-2 (COX-2), microsomal prostaglandin E synthase-1 (mPGES-1), interleukin (IL)-6, and matrix metalloproteinase (MMP)-3, which are involved in TNF-α-induced synovitis in RA or OA synovial fibroblasts, by quantitative real-time PCR. We also investigated the effects of SPMs on the mitogen-activated protein kinase (MAPK) signaling pathway by western blotting. Anti-inflammatory effects of SPMs were evaluated by applying SPMs to cultured synovial fibroblasts, followed by TNF-α stimulation.
Results
The induction of COX-2, mPGES-1, IL-6, and MMP-3 by TNF-α in synovial fibroblasts was not suppressed by omega 3-derived SPMs regardless of their origin such as RA or OA. SPMs had no effect on lipid mediator receptor gene expression induce by TNF-α and did not inhibit the TNF-α-activated MAPK signaling pathway. The production of COX-2 and IL-6 protein was significantly decreased by p38 inhibitor.
Conclusion
Despite reports on the anti-inflammatory effect of omega 3-derived SPMs, its anti-inflammatory effect on TNF-α-induced responses was not observed in synovial fibroblasts. The reason may be that SPMs have no suppressive effect on p38 activation, which plays an important role in the production of inflammatory cytokines in synovial fibroblasts.
Keywords: fatty acids, unsaturated, resolvin D1, rheumatoid arthritis, synoviocytes, tumor necrosis factor-α
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized primarily by synovitis of the joint synovium. In addition, subsequent progressive bone and cartilage destruction greatly reduces a patient’s quality of life. The major pathological condition is inflammation caused by tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 derived from macrophages and synovial cells in synovial tissues. Prostaglandins (PGs), the expression of which is enhanced by the stimulation of these cytokines, play an important role in RA pathogenesis.1,2,3,4,5 PGE2 strongly promotes pathology, including processes related to synovial cell proliferation, angiogenesis, and osteoclast activation, in RA.6 Synovial fibroblasts are key players in the progression of RA, inducing inflammation via the secretion of various cytokines and chemokines, such as IL-8 or regulated on activation, normal T cell expressed and secreted (RANTES), which act as chemoattractants, promoting the invasion of immune cells, such as macrophages or neutrophils. Furthermore, synovial fibroblasts contribute to cartilage degradation, as well as inducing angiogenesis, pannus hyperplasia, and bone erosion.1, 7 The production of proinflammatory cytokines and receptor activator of nuclear factor-κB ligand (RANKL) by synovial fibroblasts directly promotes osteoclastogenesis.
PGs are produced via the arachidonic acid cascade. The fatty acid arachidonic acid is converted to PGH2 by cyclooxygenase (COX). Then, PGH2 is transformed by specific synthase into PGs (PGE2, PGD2, PGI2, PGF2α) and thromboxane (TX) A2.8 PGE2 is converted from PGH2 by microsomal prostaglandin E synthase-1 (mPGES-1). Based on these factors, COX-2 and mPGES-1 have a strong influence on the onset of synovitis.9
Polyunsaturated fatty acids (PUFAs) consist of omega (ω)-3 and -6 types, depending on the position of the double bond in the molecule. ω-6 PUFA-derived lipid metabolites, such as PGs and leukotrienes, play a central role in the early stages of the inflammatory response. ω-3 PUFAs, which are contained in many fish oils, represented by ω-3 eicosapentaenoic acid and docosahexaenoic acid, have anti-inflammatory and cardiovascular protective effects.10,11,12,13 In addition, ω-3 PUFA-derived metabolites are transiently produced via the activation of leukocytes at the site of inflammation, and exert a local anti-inflammatory effect.14 Resolvin (Rv) E1, RvD1, and maresin-1 (MaR1) are biosynthesized from ω-3 essential fatty acids, respectively, and collectively termed specialized pro-resolving mediators (SPMs) based on their potent pro-resolving actions. These SPMs act as potent regulators of neutrophil infiltration, cytokine and chemokine production, and the clearance of apoptotic neutrophils by macrophages, which promote a return to tissue homeostasis via their specific receptors.15 These SPMs produced at the site of inflammation resolve inflammation.13, 16,17,18,19,20,21 In addition, it has been reported that RvE1 suppresses osteoclast differentiation in joint regions.22,23,24 We found that RvE1 suppresses the IL-17-induced receptor activator of nuclear factor kappa-B ligand (RANKL) expression in osteoblasts, and further suppresses RANKL-induced osteoclast and cell differentiation to inhibit osteoclast formation and bone resorption.25 In an adjuvant-induced arthritis model rat, the intraperitoneal administration of 17 (R)-hydroxydocosahexaenoic acid (HDoHE), which is a precursor of RvD1, exerted a pain-relieving effect and a decrease in TNF-α and IL-1β locally in the joint.26 RvD1 levels decreased while connective tissue growth factor (CTGF) levels, which promotes synovial fibroblasts, pannus formation, and the damage of cartilage as well as bone, increased in the serum of patients with RA, and RvD1 suppresses pannus formation via decreasing CTGF by upregulation of miRNA-146a-5p in collagen-induced arthritis.27 In another study, patients with RA showed lower lipoxin A4 (LXA4), RvD1, and RvE1 levels compared to healthy individuals; however, the levels of these SPMs are not related RA activites.28 Randomized controlled trials in patients with early RA have indicated that groups administered fish oil had longer remissions times and fewer transitions to second-line treatment than the untreated groups.29, 30 On the other hand, the intake of dietary long-chain ω-3 PUFAs was found to decrease the risk of developing RA.31 Proudman et al.29 reported that a high intake of fish oil increased the rate of American College of Rheumatology remission compared to a low intake of fish oils. However, no difference was observed in the Disease Activity Score-28 for RA with erythrocyte sedimentation rate (ESR) scores between the control and fish oil groups. In a meta-analysis, Goldberg et al.32 reported on the effects of ω-3 PUFAs on RA or joint pain secondary to inflammatory bowel disease and dysmenorrhea. Dietary supplementation with PUFAs reduced patient-reported joint pain intensity, morning stiffness duration, painful and/or tender joints, and the use of non-steroidal anti-inflammatory drugs (NSAIDs), whereas physician-assessed pain did not change. The results of another meta-analysis indicated a reduction in NSAID consumption with ω-3 PUFA use.33 However, the tender and swollen joint count, morning stiffness, and physical function did not improve in a statistically significant manner.33
Negative large-scale test results have been reported regarding the anti-inflammatory effects of SPMs derived from ω-3 fatty acids in recent years. The intake of ω-3 fatty acids derived from marine organisms was not found to have a significant protective effect on the incidence of cardiovascular disease and cancer.13, 34 Another study found that ω-3 fatty acids did not significantly change the incidence of cardiovascular disease compared to placebo.35
Thus, although SPMs are expected to promote the resolution of inflammation and may help to prevent the progression of an acute inflammatory response to chronic inflammation in patients with arthritis, their anti-inflammatory effects remain controversial. Furthermore, the effects of SPMs on synovial fibroblasts, key players in RA patients, remain unclear. In this study, we investigated the effects of ω-3 lipid mediators on TNF-α-induced synthesis of inflammatory mediators in synovial fibroblasts.
MATERIALS AND METHODS
Cell culture and reagents
MH7A (Riken Bio Resource Research Center, Tsukuba, Japan) is a cell line isolated from intra-articular soft tissues of the knee joints of patients with RA and established by transfection with the SV40 T antigen.36 HT91989516 (National Institute of Biomedical Innovation, Ibaraki, Japan) was extracted during artificial knee joint replacement surgery in patients with knee osteoarthritis (OA). MH7A cells were cultured in RPMI-1640 (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan) with 10% fetal bovine serum (FBS), 100 µg/mL penicillin, and 100 µg/mL streptomycin at 37°C in a humidified atmosphere containing 5% carbon dioxide. OA fibroblasts were cultured in D-MEM (Fujifilm Wako Pure Chemical Corporation) with 10% FBS, 100 µg/mL penicillin, and 100 µg/mL streptomycin at 37°C in a humidified 5% carbon dioxide atmosphere. RvD1 and MaR1 were purchased from Cayman Chemical Company (Ann Arbor, MI), and RvE1 was purchased from Toronto Research Chemicals (Toronto, Canada). These were pre-added 1 h before recombinant human TNF-α (R&D systems, Minneapolis, MN) stimulation, according to previous reports.16, 37,38,39 MEK inhibitor (U0126) was purchased from Cell Signaling Technology (Danvers, MA). JNK inhibitor (SP600125) was purchased from Selleck Chemicals (Houston, TX), and a p38 inhibitor (SB202190) was purchased from Sigma-Aldrich (Saint Louis, MO, USA). These were pre-added 1 h before TNF-α stimulation.
Quantitative real-time PCR
MH7A cells or OA fibroblasts were plated in 6-well plates at a density of 1.0 × 105 cells/mL. Total RNA was isolated from cultured cells using the RNeasy Plus mini kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions. The mRNA was reverse-transcribed into cDNA using the Super Script VILO Master Mix (Invitrogen, Carlsbad, CA). The resultant cDNA was subjected to real-time PCR using TaqMan Fast Advanced Master Mix (Thermo Fisher Scientific, Waltham, MA). Specific primers (Table 1) were purchased from Thermo Fisher Scientific. PCR was conducted using the TaKaRa PCR Thermal Cycler Dice system (Takara Bio, Kusatsu, Japan) under the following conditions: initial holding at 25°C for 10 min, 42°C for 60 min, and 85°C for 5 min. Real-time PCR was performed on a ViiA7 Real-Time PCR system (Thermo Fisher Scientific) for 40 cycles at 95°C for 1 s and 60°C for 20 s. The expression levels of COX-2, mPGES-1, IL-6, and MMP-3 were normalized to glyceraldehyde 3- phosphate dehydrogenase (GAPDH). All real-time PCR experiments were conducted in triplicate and analyzed using the comparative 2-ΔΔCt relative quantification cation method.
Table 1. Primers for real-time PCR.
| Genes | Assay IDa | RefSeq | Exon boundary | Product length (bp) |
| COX-2 | Hs00153133 | NM_000963 | 5–6 | 75 |
| mPGES-1 | Hs01115610 | NM_004878.4 | 2–3 | 136 |
| IL-6 | Hs00985639 | NM_000600.4 | 2–3 | 66 |
| MMP-3 | Hs00968305 | NM_002422.4 | 6–7 | 126 |
| CMKLR1 | Hs01081979 | NM_001142343.1 | 4 | 73 |
| FRP2 | Hs02759175 | NM_001005738.1 | 2 | 98 |
| GAPDH | Hs02758991 | NM_001256799.2 | 6–7 | 93 |
CMKLR1, Chemokine like receptor 1; COX-2, Cyclooxygenase-2; FRP2, N-formyl peptide receptor 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL-6, Interleukin-6; MMP-3, matrix metalloproteinase-3; mPGES-1, microsomal prostaglandin E synthetase-1. a: TaqMan Gene Expression Assay (Applied Biosystems).
Enzyme-linked immunosorbent assay
The amounts of PGE2 and IL-6 in the culture medium were determined using a commercially available enzyme-linked immunosorbent assay kit (Enzo Life Sciences, Farmingdale, NY) (R&D Systems, Minneapolis, MN), according to the manufacturer’s instructions. The data were converted to pg/mL. Finally, duplicate assays were conducted on each sample and the absorbance was recorded at 405 nm.
Western blotting
MH7A cells or OA fibroblasts were cultured in 60-mm dishes at a density of 1.0 × 106 cells/mL. TNF-α was added after culturing to 70–80% confluency. After washing with cold phosphate buffered saline, radioimmunoprecipitation assay buffer [50 mmol/L Tris-HCl, pH 7.4, 1% Triton-X100, 0.25% sodium deoxycholate, 150 mmol/L sodium chloride, 1 mmol/L ethyleneglycol-bis (β-aminoethyleter)-N, N, N, N-tetraacetic acid, 0.1% SDS, 0.5 mmol/L sodium orthovanadate, 1 mmol/L sodium fluoride, and protease inhibitor cocktail (Merck Kgaa, Darmstadt, Germany)] and collected using a cell lifter. The collected cells were subjected to rolling at 4°C for 10 min and then centrifuged at 13,000 rpm and 4°C for 5 min. The protein concentration in the supernatant was measured using a standard assay (Bio-Rad Laboratories, Irvine, CA). The samples were adjusted in concentration with radioimmunoprecipitation assay buffer (Fujifilm Wako Pure Chemical Corporation) and 4× sample buffer (Bio-Rad Laboratories) before separating by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Thereafter, the protein was transferred to a polyvinylidene difluoride (PVDF) blotting membrane (GE Healthcare Life Sciences, Marlborough, MA) at 100 V for 75 min. To prevent the nonspecific reaction of the transferred PVDF membrane, blocking was conducted for 1 h with 5% skim milk. The primary antibody, diluted with 5% skim milk, was incubated overnight at 4°C. The following primary antibodies were used: COX-2 antibody (1:200; Cayman Chemical), mPGES-1 antibody (1:200; Cayman Chemical), IL-6 antibody (1:1000; Cell Signaling Technology, Danvers, MA), MMP-3 antibody (1:200; Santa Cruz Biotechnology, Dallas, TX), β-actin antibody (1 µg/mL) (Medical and Biological Laboratories, Nagoya, Japan), chemokine-like receptor 1 (CMCKLR1) antibody (1:100; Cayman Chemical), leukotriene B4 receptor 1 (BLT1) antibody (1:200; Cayman Chemical), and formyl peptide receptor 2 (FPR2) antibody (1:200; Santa Cruz). To study signal transduction, the primary antibodies used were phospho-p42/p44 [p-extracellular signal-regulated kinase (ERK)1/2] antibody (1:2000; Cell Signaling Technology), p42/p44 (ERK1/2) antibody (1:1000; Cell Signaling Technology), p-p38 antibody (1:1000, Cell Signaling Technology), p38 antibody (1:1000; Cell Signaling Technology), p- stress-activated protein kinase (SAPK)/c-Jun N-terminal kinase (JNK) antibody (1:1000; Cell Signaling Technology), SAPK/JNK antibody (1:1000; Cell Signaling Technology), p-Akt antibody (1:1000; Cell Signaling Technology), Akt antibody (1:1000; Cell Signaling Technology), p-NF-κB p65 antibody (1:1000; Cell Signaling Technology), and NF-κB p65 antibody (1:1000; Cell Signaling Technology). After washing the PVDF membrane three times with Tris-buffered saline-Tween (TBS-T), a secondary antibody diluted with 5% skim milk was reacted for 1 h at room temperature. The secondary antibodies used were: goat anti-mouse immunoglobulin G (IgG) antibodies (1:2000; Santa Cruz Biotechnology) and anti-rabbit IgG antibodies (1:2000, Cell Signaling Technology). After washing the PVDF membrane three times with TBS-T, enhanced chemiluminescence was conducted using Amersham ECL Prime kit (GE Healthcare Life Science) and detected with Image Quant LAS4000 (GE Healthcare Life Science).
Statistical analysis
Data were analyzed using GraphPad Prism 7 (GraphPad Software, San Diego, CA). Multiple groups were compared using one-way analysis of variance (ANOVA) and Dunn’s multiple comparison test. Differences were considered statistically significant at P < 0.05.
RESULTS
TNF-α induced COX-2, mPGES-1, IL-6, and MMP-3 mRNA expressions as well as PGE2 and IL-6 release in MH7A cells
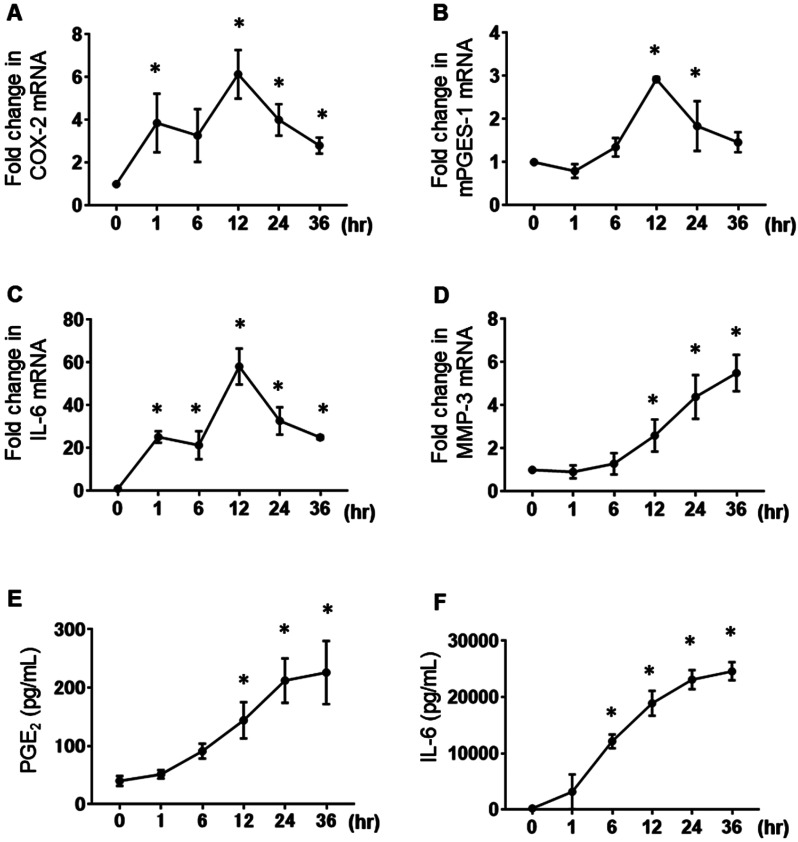
Since TNF-α is an important cytokine that induces PGE2 release via increasing COX-2 and mPGES-1 expression in synovial fibroblasts,40 we examined the effects of TNF-α on the expression of COX-2, mPGES-1, IL-6, and MMP-3 mRNA using real-time reverse transcription PCR (RT-PCR), and PGE2 and IL-6 protein release using enzyme-linked immunosorbent assay in MH7A cells. Treatment with TNF-α (10 ng/mL) enhanced the expression of COX-2, mPGES-1, and IL-6 mRNA in MH7A cells (Figs. 1A–C). The maximal TNF-α-enhancing effects were observed after 12 h of culture. TNF-α increased the MMP-3 mRNA expression in a time-dependent manner (Fig. 1D). When the cells were treated with TNF-α (10 ng/mL) for 36 h, the PGE2 and IL-6 concentrations in the culture medium increased in a time-dependent manner (Figs. 1E and F).
Fig. 1.
Effects of TNF-α on MH7A cells. MH7A cells were stimulated with 10 ng/mL TNF-α for 1, 6, 12, 24, or 36 h. The mRNA expression levels of COX-2 (A), mPGES-1 (B), IL-6 (C), and MMP-3 (D) were determined by real-time PCR. Data are presented as the mean ± SEM of three independent experiments (*P < 0.05 vs. 0 h). The PGE2 (E) and IL-6 (F) production levels in MH7A cells stimulated with 10 ng/mL TNF-α for 1, 6, 12, 24, or 36 h were determined using enzyme-linked immunosorbent assay. Data are expressed as the mean ± SD (n = 3; *P < 0.05 vs. 0 h).
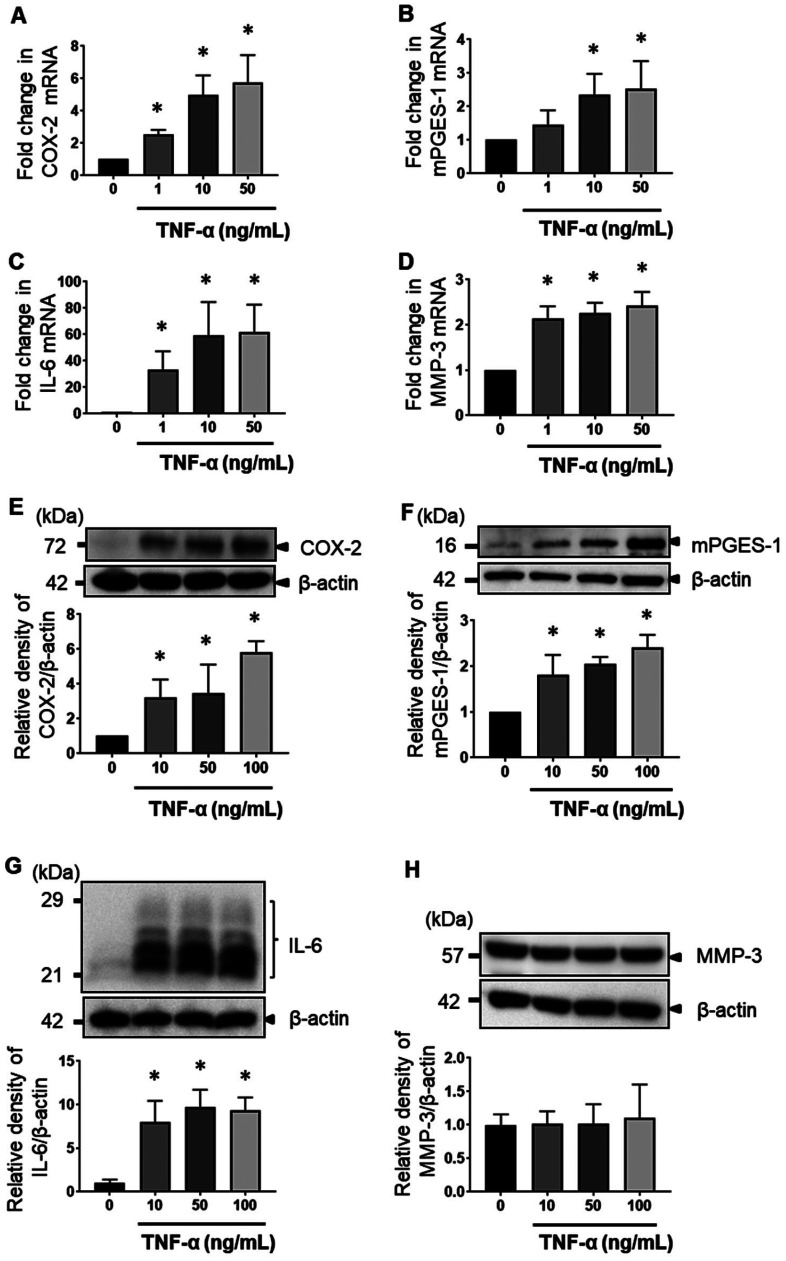
When the MH7A cells were stimulated with TNF-α at various concentrations (0–100 ng/mL) for 12 h, the COX-2, mPGES-1, IL-6, and MMP-3 mRNA levels were enhanced in a concentration-dependent manner (Figs. 2A–D). COX-2, mPGES-1, and IL-6 proteins in the MH7A cells were enhanced by treatment with TNF-α for 24 h in a concentration-dependent manner, according to the results of western blot analysis (Figs. 2E, F, and G). By contrast, MMP-3 protein was not enhanced (Fig. 2H).
Fig. 2.
MH7A cell response with increasing dose of TNF-α. MH7A cells were cultured in the presence of 1, 10, 50, and 100 ng/mL TNF-α. The mRNA expression levels of COX-2 (A), mPGES-1 (B), IL-6 (C), and MMP-3 (D) were determined by real-time PCR. The expression of COX-2 (E), mPGES-1 (F), IL-6 (G), and MMP-3 (H) in cell lysates was determined by western blot analysis. The data are expressed as the relative protein expression of targets/β-actin. Data are presented as the mean ± SEM of three independent experiments (*P < 0.05 vs. untreated).
Effects of ω-3 PUFA-derived lipid mediators on COX-2, mPGES-1, IL-6, and MMP-3 mRNA expressions in MH7A cells
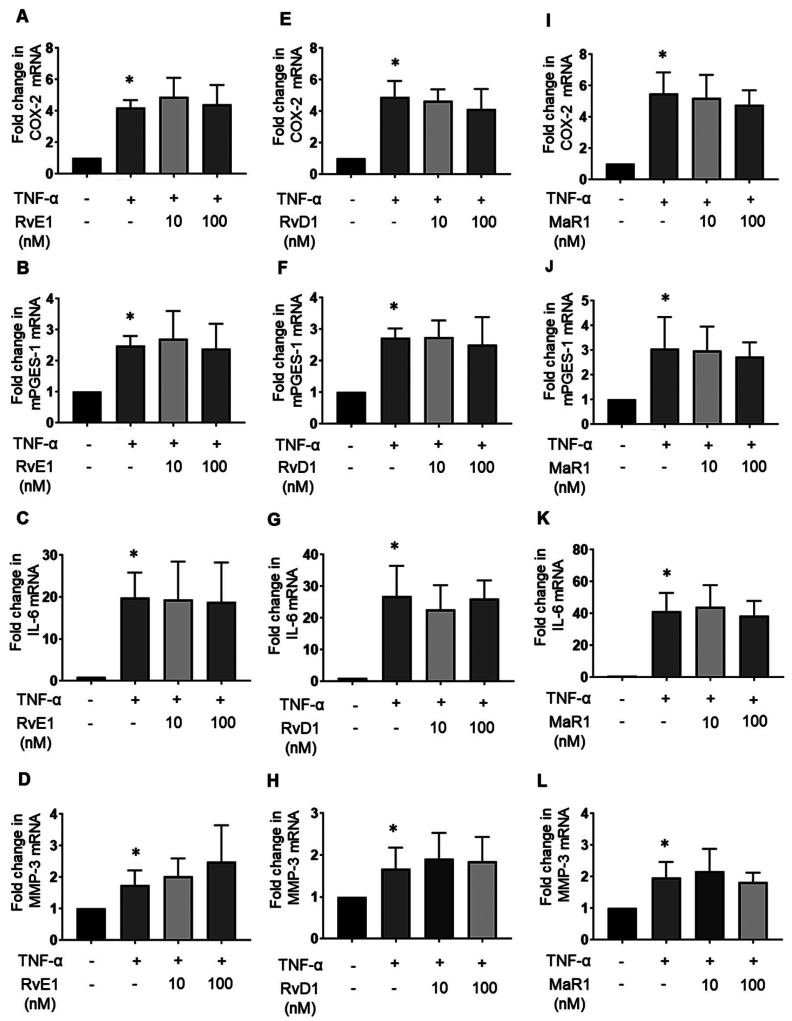
SPMs have been reported to exert pro-resolving and cartilage-protective actions in response to inflammatory arthritis, as well as a reduction of pro-inflammatory cytokine production in vivo.17, 19, 26, 41 Therefore, we evaluated the effects of SPMs on TNF-α stimulation in synovial fibroblasts. To this end, MH7A cells were cultured with or without 10 nM and 100 nM RvE1, RvD1, and MaR1 in the presence of 10 ng/mL TNF-α for 12 h. The mRNA expression levels of COX-2, mPGES-1, IL-6, and MMP-3 were determined by real-time PCR. The mRNA expression levels of COX-2 (Fig. 3A), mPGES-1 (Fig. 3B), IL-6 (Fig. 3C), MMP-3 (Fig. 3D) were increased by TNF-α; however, RvE1 failed to decrease the expression of these inflammatory markers (Figs. 3A–D). Similar results were observed for RvD1 (Figs. 3E–H) and MaR1 (Figs. 3I–L). These mRNA expression levels by SPMs without TNF-α were not increased in MH7A cells (Supplementary Fig. S1).
Fig. 3.
Effects of SPMs on TNF-α-treated MH7A cells. MH7A cells were cultured with or without 10 nM and 100 nM RvE1, RvD1, and maresin-1 (MaR1) in the presence of 10 ng/mL TNF-α for 12 h. The effects of RvE1 on the following substances were examined using real-time PCR: the mRNA expression levels of COX-2 (A), mPGES-1 (B), IL-6 (C), and MMP-3 (D) or RvD1 on COX-2 (E), mPGES-1 (F), IL-6 (G), and MMP-3 (H) or MaR1 on COX-2 (I), mPGES-1 (J) and IL-6 (K), and MMP-3 (L). The plots represent three independent experiments. Data are presented as the mean ± SEM of three independent experiments (*P < 0.05 vs. untreated).
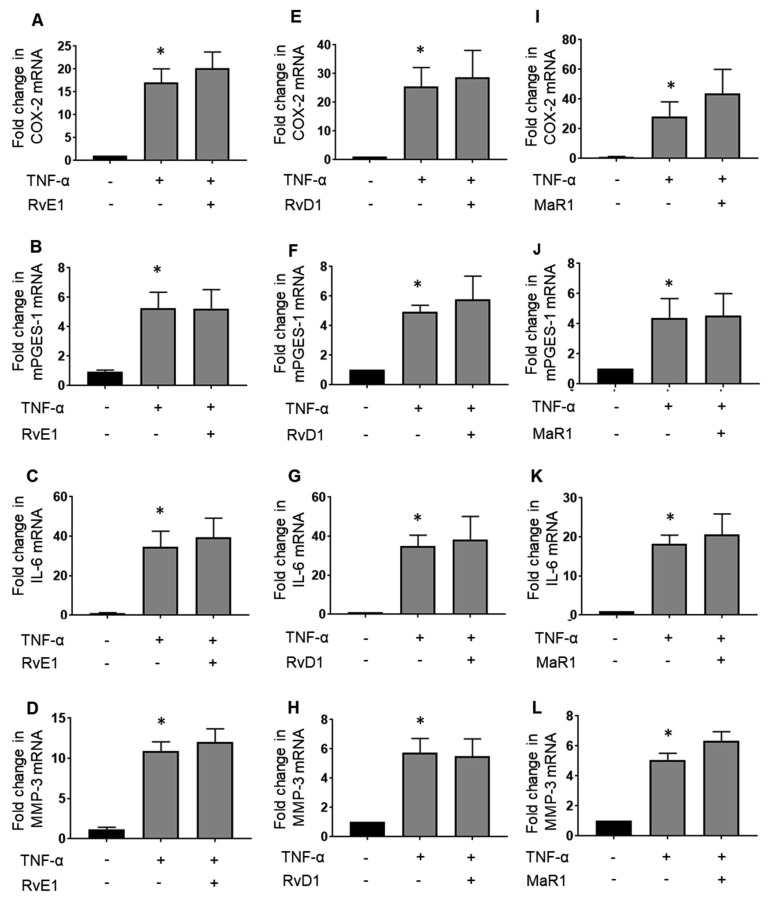
Effects of ω-3 PUFA-derived lipid mediators on COX-2, mPGES-1, IL-6, and MMP-3 mRNA expressions in synovial fibroblasts derived from OA
Since MH7A cells were derived from RA patients, we examined the effects of SPMs on synovial fibroblasts from patients with OA. The mRNA expression levels of COX-2 (Fig. 4A), mPGES-1 (Fig. 4B), IL-6 (Fig. 4C), and MMP-3 (Fig. 4D) were increased by TNF-α stimulation. However, these inflammatory markers were not inhibited by RvE1 (Figs. 4A–D). Similar results were observed for RvD1 (Figs. 4E–H) and MaR1 (Figs. 4I–L). Similar to MH7A cells, the anti-inflammatory effect of ω-3 SPMs was not observed in OA synovial fibroblasts. These mRNA expression levels by SPMs without TNF-α were not increased in OA fibroblasts (Supplementary Fig. S2). Thus, this result suggests that SPMs do not have an anti-inflammatory effect in synovial fibroblasts regardless of their origin such as RA or OA.
Fig. 4.
Effects of SPMs on osteoarthritis-derived synovial fibroblasts treated with TNF-α. Osteoarthritis-derived synovial fibroblasts were cultured with or without 100 nM RvE1, RvD1, and MaR1 in the presence of 10 ng/mL TNF-α for 12 h. The effects of RvE1 on the following substances were examined using real-time PCR: the mRNA expression levels of COX-2 (A), mPGES-1 (B), IL-6 (C), MMP-3 (D), or RvD1 on COX-2 (E), mPGES-1 (F), IL-6 (G), and MMP-3 (H) or MaR1 on COX-2 (I), mPGES-1 (J), IL-6 (K), and MMP-3 (L). The plots represent three independent experiments. Data are presented as the mean ± SEM of three independent experiments (*P < 0.05 vs. untreated).
Effects of TNF-α on expression of SPM receptors in MH7A cells
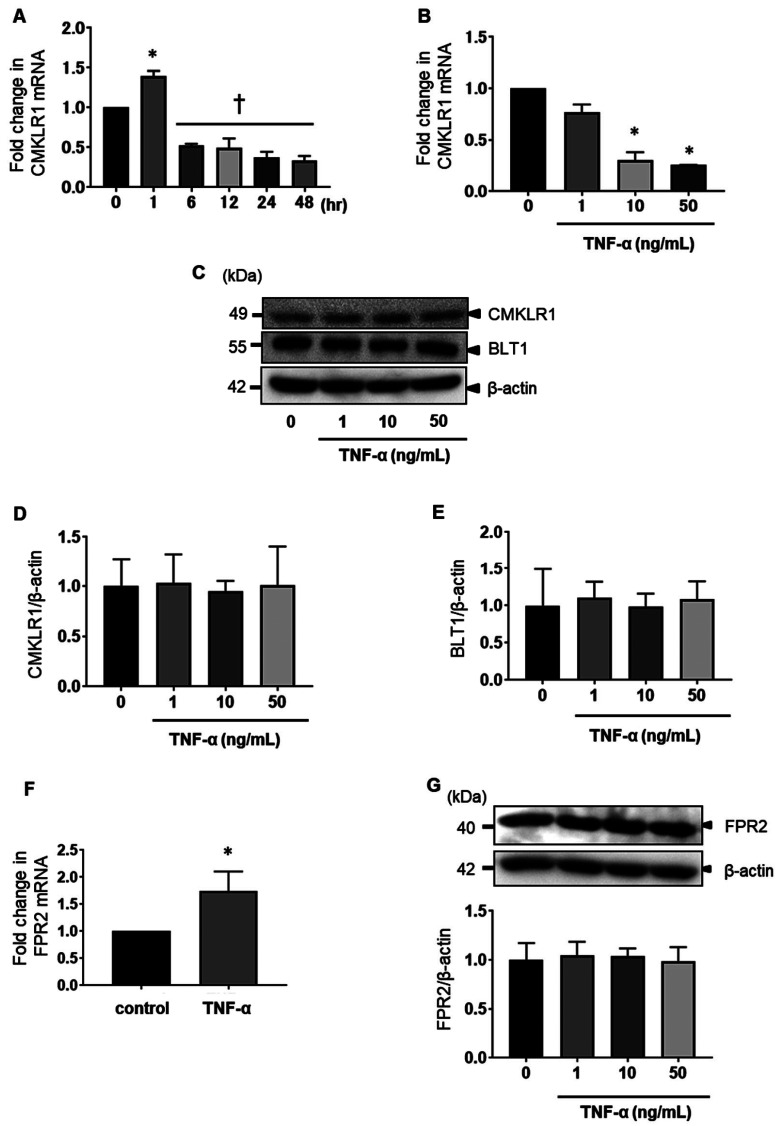
SPMs bind to G-protein-coupled receptors (GPCRs) and induce specialized biological actions.42, 43 To elucidate the mechanism underlying the anti-inflammatory effect of SPMs, the expression of chemerin chemokine-like receptor 1 (CMKLR1), an RvE1 receptor, was evaluated using real-time PCR and western blotting. MH7A cells were stimulated with 10 ng/mL TNF-α for 1, 6, 12, 24, 36, or 48 h. The expression of CMKLR1 mRNA was increased after stimulation with TNF-α for 1 h, according to the results of real-time PCR. However, no difference was observed at 6–48 h (Fig. 5A). CMKLR1 mRNA expression was further reduced by stimulation with 10–50 ng/mL TNF-α, according to real-time PCR (Fig. 4B). The expression of CMKLR1 and another RvE1 receptor, BLT1, did not change after 24 h of stimulation with TNF-α (Figs. 5C–E). The mRNA expression of N-formyl peptide receptor 2 (FPR2), an RvD1 receptor, was increased after stimulation with TNF-α, according to real-time PCR (Fig. 5F); however, the expression of FPR2 did not change after 24 h of stimulation with TNF-α, according to western blotting (Fig. 5G). The mRNA expression level of CMKLR1 was decreased after stimulation with TNF-α for 6-48 h; however, the protein levels of CMKLR1, BLT1, and FPR2 in MH7A cells were not affected by TNF-α. Therefore, these results suggest that SPMs can act via SPMs receptors.
Fig. 5.
Effects of TNF-α on the expression of chemerin chemokine-like receptor 1 (CMKLR1), leukotriene B4 receptor 1 (BLT1), and FPR2 in MH7A cells. MH7A cells were stimulated with 10 ng/mL TNF-α for 1, 6, 12, 24, or 48 h (A) and in the presence of 1, 10, and 50 ng/mL TNF-α for 12 h (B). The mRNA levels of CMKLR1 were determined using real-time PCR. Data are presented as the mean ± SEM of three independent experiments (*P < 0.05, **P < 0.01 vs. 0 h). MH7A cells were incubated for 24 h with 0–50 ng/mL TNF-α. The expression of CMKL1 and BLT1 was determined by western blot analysis (C, D, and E). The mRNA level of FPR2 was determined in the presence of 10 ng/ml TNF-α for 12 h using real-time PCR (F). The expression of FPR2 in the presence of 10 ng/ml TNF-α for 24 h was determined by western blot analysis (G). Data are presented as the mean ± SEM of three independent experiments (*P < 0.05 vs. control. †P < 0.05 vs. TNF-α).
Effect of ω-3 PUFA-derived lipid mediators on TNF-α-induced MAPK signal activation in MH7A cells
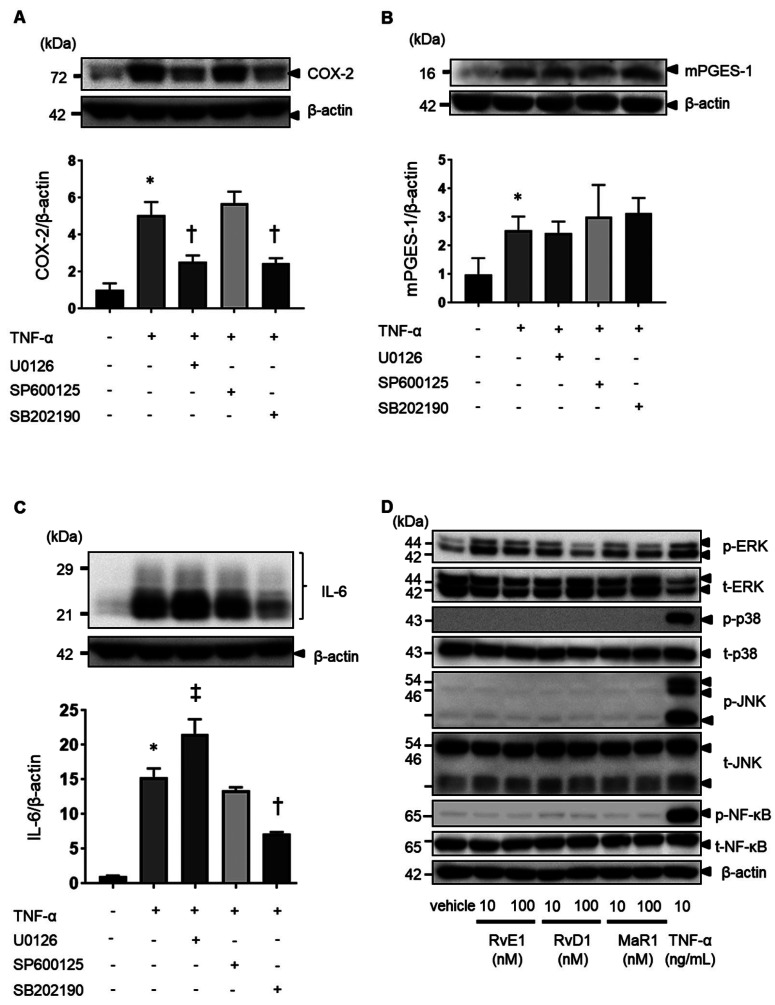
In synovial fibroblasts, MAPK signaling plays an important role in TNF-α-induced inflammation responses.42 Three MAPK signaling pathways have been characterized: MEK/ERK1/2, JNK, and p38 MAPK signaling pathways. We examined the contribution of MAPK signaling pathways to TNF-α-induced COX-2, mPGES-1, and IL-6 expression in MH7A cells using MAPK inhibitors. TNF-α-induced COX-2 expression was inhibited in the presence of the MEK inhibitor U0126 and the p38 inhibitor SB202190 (Fig. 6A). By contrast, mPGES-1 expression was not inhibited (Fig. 6B). TNF-α-induced IL-6 expression was inhibited in the presence of the p38 inhibitor SB202190 and enhanced in the presence of U0126 (Fig. 6C). Next, we evaluated the bioactivity of SPMs in the MH7A cells. SPMs are known to activate the PI3K/AKT and ERK signaling pathways via receptors.16, 44 To evaluate the bioactivity of SPMs in MH7A cells, we investigated the signal activity of SPMs in the absence of TNF-α. As a result, RvE1, RvD1, and MaR1 were found to enhance ERK activation (Fig. 6D), while RvD1 and MaR1 enhanced Akt activation (Supplementary Fig. S3).
Fig. 6.
Effects of ERK1/2, JNK, and p38 inhibitors on TNF-α-induced COX-2, mPGES-1, and IL-6 expression in MH7A cells. When MH7A cells were pretreated with the MEK inhibitor U0126 (10 µM), the JNK inhibitor SP600125 (10 µM), and the p38 inhibitor SB202190 (1 µM) for 1 h, the expression of TNF-α-induced COX2 (A), mPGES-1 (B), and IL-6 (C) in cell lysates was determined using western blot analysis. The data are expressed as the relative protein expression of targets/β-actin. Data are presented as the mean ± SEM of three independent experiments (*P < 0.05 vs. untreated. †P < 0.05 vs. TNF-α, ‡P < 0.05 vs. TNF-α). Effects of SPMs on the MAPK signaling pathway without TNF-α in MH7A cells (D). MH7A cells were cultured with 10 nM and 100 nM RvE1, RvD1, and MaR1 without TNF-α for 15 min. The expression of ERK, p38, JNK, and NF-κB in cell lysates was determined by western blot analysis.
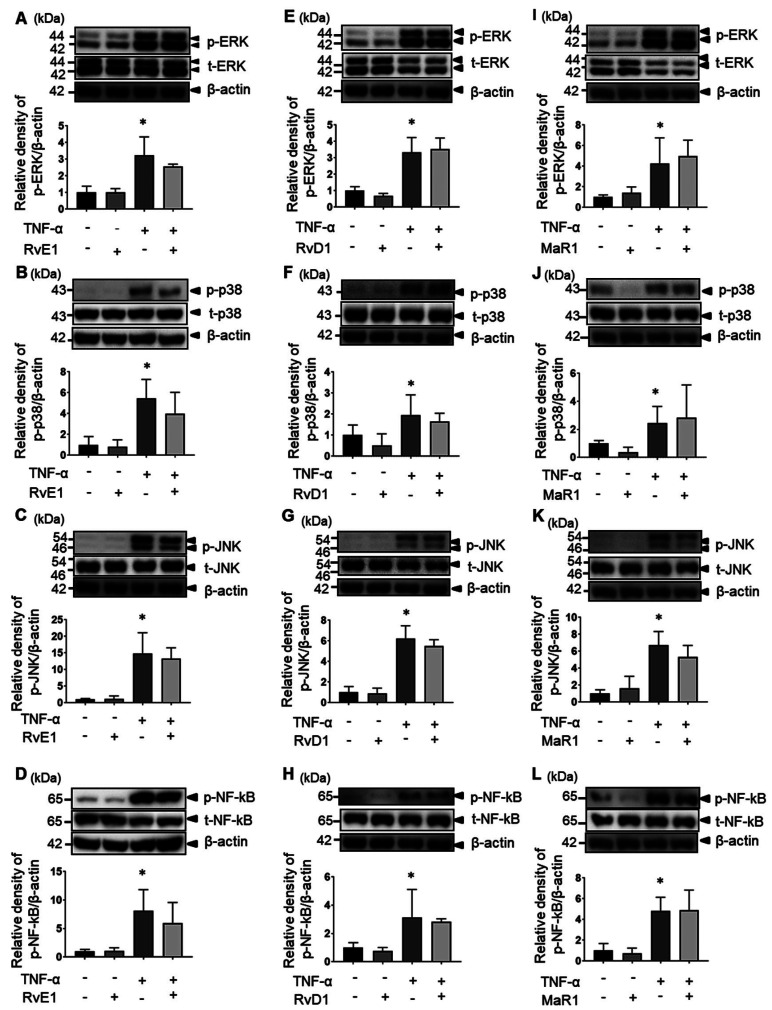
Next, we examined the effects of SPMs on the MAPK signaling pathway in MH7A cells. The expression of p-ERK, p-p38, p-JNK, and p-NF-κB was enhanced by 15–30 min after TNF-α stimulation; however, the expression of p-ERK, p-p38, p-JNK, and p-NF-κB was not inhibited by RvE1 (Figs. 7A–D). Similar results were observed for RvD1 (Figs. 7E–H) and MaR1 (Figs. 7I–L).
Fig. 7.
Effects of SPMs on signaling pathways after stimulation of MH7A cells with TNF-α. MH7A cells were pretreated with SPMs for 1 h and then stimulated with 10 ng/mL TNF-α for 15 min. The effects of RvE1 on the following substances were examined: extracellular signal-regulated kinase (ERK) (A), p38 (B), c-Jun N-terminal kinase (JNK) (C), and transcription factor nuclear factor-κB (NF-κB) (D) or RvD1 on ERK (E), p38 (F), JNK (G), NF-kB (H), and maresin-1 (MaR1) on ERK (I), p38 (J), JNK (K), and transcription factor nuclear factor-κB (NF-κB) (L). The plots represent three independent experiments (*P < 0.05 vs. untreated).
No significant effect of SPMs (RvE1, RvD1, and MaR1) on these signaling systems by TNF-α stimulation was observed. These results indicate that SPMs have no inhibitory effect on TNF-α-stimulated MAPK signaling and NF-κB phosphorylation in synovial fibroblasts.
DISCUSSION
In this study, we investigated the effects of SPMs on TNF-α-induced inflammatory responses in synovial fibroblasts. We found that SPMs exhibited a non-inhibitory effect on the TNF-α-induced inflammatory response in synovial fibroblasts. We also found that the expression of RvE1 receptors was not affected by TNF-α; however, the signaling pathways activated by TNF-α were not affected. The activation of MAPKs is involved in TNF-α-induced synovitis. In synovial fibroblasts, TNF-α enhances the expression of COX-2, mPGES-1, IL-6, and MMP-3 by the activation of MAPKs and NF-κB.21, 40, 45,46,47,48,49,50,51 In our study, SPMs did not inhibit the activation of MAPKs and NF-κB, which may explain why SPMs are incapable of inhibiting the synthesis of COX-2, mPGES-1, IL-6, and MMP-3 induced by TNF-α. It has been reported that prophylactic and therapeutic RvE1 regimens did not ameliorate the incidence or severity of collagen-induced arthritis in mice, including the histopathological scores of synovial inflammation, chondrocyte death, cartilage erosion, bone erosion, proteoglycan depletion, and proinflammatory cytokine production.52 These results suggest that SPM has a limited anti-inflammatory response in patients with RA.
Furthermore, it has been reported that p38 plays an important role in the production of inflammatory cytokines.53,54,55 Our study suggests that p38 phosphorylation plays an important role in the expression of COX-2 and IL-6, but that SPMs could not suppress p38 phosphorylation, thus failing to suppress inflammatory cytokine production.
As a receptor, RvE1 uses CMKLR1 and BLT1, which are expressed on the cell surface of inflammatory cells and synovial fibroblasts.56, 57 RvD1 and LXA4, an ω-6 derived lipid mediator, use FPR2 as a receptor.58, 59 MaR1 has been reported to be mediated by the receptors for retinoic acid-related orphan receptor alpha (RORα) and leucine-rich repeat-containing G-protein coupled receptor 6 (LGR6); however, the mechanisms by which this regulation occurs remain poorly understood.60 In our study, the protein levels of CMKLR1, BLT1 and FPR2 in synovial fibroblasts were not affected by TNF-α while the expression level of FPR2 mRNA was increased after stimulation with TNF-α. Ubiquitination is one of the mechanism for degradation of proteins. Connor et al. reported that TNF-α is not only capable of inducting expression of E3 ubiquitin ligases involved in the ubiquitination pathway but may also stimulate the proteasome itself in RA synovial fibroblasts.61 Y. Zhang et al. reported that the inhibitory receptor, leucocyte-associated immunoglobulin (Ig)-like receptor-1 (LAIR-1), on cell surface could be shed from RA synovial fibroblasts following TNF-α stimulation.62 The further study of leading to strengthened ubiquitination of SPMs receptors and shedding from the cell surface in response to TNF-α should be conducted.
Many types of cells are involved in the pathogenesis of RA, and the effects of SPMs have been investigated in several inflammatory cells. SPMs are able to modulate the inflammatory response of macrophages and neutrophils.63 Furthermore, we previously reported on the inhibitory effect of RvE1 on osteoclasts.25 We found that synovial fibroblasts originating from RA express CMKLR, BLT1, and FPR2; however, SPMs on TNF-α-induced inflammatory responses was not elucidated in MH7A cells and synovial fibroblasts originating from OA. Given the positive effects of SPMs on other inflammatory cells, our results may be due to the cell specificity of synovial fibroblasts to SPMs.
In conclusion, our study shows that the anti-inflammatory effect of ω-3-derived SPMs on TNF-α-induced response was not observed in synovial fibroblasts. This may come from non-inhibitory effects of SPMs on p38 activation induced by TNF-α. Further studies will be needed to elucidate the precise effects of SPMs in the synovial tissue of patients with RA, where many types of cells, including neutrophils, macrophages, lymphocytes, osteoclasts, chondrocytes, and synovial fibroblasts, exist and cause inflammation. It will also be necessary to investigate the effects of SPMs on other inflammatory cytokines such as RANTES, IL-8, and IL-17.
Supplementary materials
Supplementary Fig. S1.
Effects of SPMs on MH7A cells. MH7A cells were cultured with or without 10 nM and 100 nM RvE1, RvD1, and maresin-1 (MaR1) without TNF-α for 12 h. The effects of RvE1 on the following substances were examined using real-time PCR: the mRNA expression levels of COX-2 (A), mPGES-1 (B), IL-6 (C), MMP-3 (D), or RvD1 on COX-2 (E), mPGES-1 (F), IL-6 (G), and MMP-3 (H) or MaR1 on COX-2 (I), mPGES-1 (J), IL-6 (K), and MMP-3 (L). The plots represent three independent experiments. Data are presented as the mean ± SEM of three independent experiments.
Supplementary Fig. S2.
Effects of SPMs on osteoarthritis-derived synovial fibroblasts. OA fibroblasts were cultured with or without 10 nM and 100 nM RvE1, RvD1, and maresin-1 (MaR1) without TNF-α for 12 h. The effects of RvE1 on the following substances were examined using real-time PCR: the mRNA expression levels of COX-2 (A), mPGES-1 (B), IL-6 (C), MMP-3 (D), or RvD1 on COX-2 (E), mPGES-1 (F), IL-6 (G), and MMP-3 (H) or MaR1 on COX-2 (I), mPGES-1 (J), IL-6 (K), and MMP-3 (L). The plots represent three independent experiments. Data are presented as the mean ± SEM of three independent experiments.
Supplementary Fig. S3.
Effects of SPMs on Akt in MH7A cells. Effects of SPMs on Akt without TNF-α in MH7A cells. MH7A cells were cultured with 10 nM and 100 nM RvE1, RvD1, and MaR1 without TNF-α for 15 min. The expression of Akt in cell lysates was determined by western blot analysis.
Acknowledgments
Acknowledgements: We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Bartok B,Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233-55. 10.1111/j.0105-2896.2009.00859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ospelt C. Synovial fibroblasts in 2017. RMD Open. 2017;3:e000471. 10.1136/rmdopen-2017-000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulissen SM,van Hamburg JP,Davelaar N,Asmawidjaja PS,Hazes JM,Lubberts E. Synovial fibroblasts directly induce Th17 pathogenicity via the cyclooxygenase/prostaglandin E2 pathway, independent of IL-23. J Immunol (Baltimore, Md: 1950). 2013;191:1364-72. [DOI] [PubMed]
- 4.Falconer J,Murphy AN,Young SP,Clark AR,Tiziani S,Guma M,et al. . Review: Synovial Cell Metabolism and Chronic Inflammation in Rheumatoid Arthritis. Arthritis Rheumatol. 2018;70:984-99. 10.1002/art.40504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashizume M,Hayakawa N,Mihara M. IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF- and IL-17. Rheumatology. 2008;47:1635-40. 10.1093/rheumatology/ken363 [DOI] [PubMed] [Google Scholar]
- 6.Sano H. The Role of Lipid Mediators in the Pathogenesis of Rheumatoid Arthritis. Inflamm Regen. 2011;31:151-6. [Google Scholar]
- 7.Yoon HY,Lee EG,Lee H,Cho IJ,Choi YJ,Sung MS,et al. . Kaempferol inhibits IL-1β-induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of COX-2, PGE2 and MMPs. Int J Mol Med. 2013;32:971-7. 10.3892/ijmm.2013.1468 [DOI] [PubMed] [Google Scholar]
- 8.Frederick ED,Hausburg MA,Thomas GW,Rael LT,Brody E,Bar-Or D. The low molecular weight fraction of human serum albumin upregulates COX2, prostaglandin E2, and prostaglandin D2 under inflammatory conditions in osteoarthritic knee synovial fibroblasts. Biochem Biophys Rep. 2016;8:68-74. 10.1016/j.bbrep.2016.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pillinger MH,Rosenthal PB,Tolani SN,Apsel B,Dinsell V,Greenberg J,et al. Cyclooxygenase-2-derived E prostaglandins down-regulate matrix metalloproteinase-1 expression in fibroblast-like synoviocytes via inhibition of extracellular signal-regulated kinase activation. J Immunol (Baltimore, Md: 1950). 2003;171:6080-9. [DOI] [PubMed]
- 10.Dyerberg J,Bang HO,Stoffersen E,Moncada S,Vane JR. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978;312:117-9. 10.1016/S0140-6736(78)91505-2 [DOI] [PubMed] [Google Scholar]
- 11.Fredman G,Serhan CN. Specialized proresolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolution. Biochem J. 2011;437:185-97. 10.1042/BJ20110327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang D,Zhao Q,Liu H,Guo Y,Xu H. PPAR-α Agonist WY-14643 Inhibits LPS-Induced Inflammation in Synovial Fibroblasts via NF-kB Pathway. J Mol Neurosci. 2016;59:544-53. 10.1007/s12031-016-0775-y [DOI] [PubMed] [Google Scholar]
- 13.Manson JE,Cook NR,Lee IM,Christen W,Bassuk SS,Mora S,et al. ; VITAL Research Group. Marine n−3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N Engl J Med. 2019;380:23-32. 10.1056/NEJMoa1811403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiram R,Rizcallah E,Marouan S,Sirois C,Sirois M,Morin C,et al. . Resolvin E 1 normalizes contractility, Ca 2+ sensitivity and smooth muscle cell migration rate in TNF-α- and IL-6-pretreated human pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2015;309:L776-88. 10.1152/ajplung.00177.2015 [DOI] [PubMed] [Google Scholar]
- 15.Morin C,Blier PU,Fortin S. Eicosapentaenoic acid and docosapentaenoic acid monoglycerides are more potent than docosahexaenoic acid monoglyceride to resolve inflammation in a rheumatoid arthritis model. Arthritis Res Ther. 2015;17:142. 10.1186/s13075-015-0653-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohira T,Arita M,Omori K,Recchiuti A,Van Dyke TE,Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem. 2010;285:3451-61. 10.1074/jbc.M109.044131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnardottir HH,Dalli J,Norling LV,Colas RA,Perretti M,Serhan CN. Resolvin D3 Is Dysregulated in Arthritis and Reduces Arthritic Inflammation. J Immunol (Baltimore, Md: 1950). 2016;197:2362-8. 10.4049/jimmunol.1502268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcon R,Bento AF,Dutra RC,Bicca MA,Leite DF,Calixto JB. Maresin 1, a proresolving lipid mediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. J Immunol (Baltimore, Md: 1950). 2013;191:4288-98. [DOI] [PubMed]
- 19.Norling LV,Headland SE,Dalli J,Arnardottir HH,Haworth O,Jones HR,et al. . Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI Insight. 2016;1:e85922. 10.1172/jci.insight.85922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Q,Wu Y,Zhao F,Wang J. Maresin 1 inhibits transforming growth factor-β1-induced proliferation, migration and differentiation in human lung fibroblasts. Mol Med Rep. 2017;16:1523-9. 10.3892/mmr.2017.6711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu MH,Tsai CH,Huang YL,Fong YC,Tang CH. Visfatin Promotes IL-6 and TNF-α Production in Human Synovial Fibroblasts by Repressing miR-199a-5p through ERK, p38 and JNK Signaling Pathways. Int J Mol Sci. 2018;19:190. 10.3390/ijms19010190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gyurko R,Van Dyke TE. The role of polyunsaturated ω-3 fatty acid eicosapentaenoic acid-derived resolvin E1 (RvE1) in bone preservation. Crit Rev Immunol. 2014;34:347-57. 10.1615/CritRevImmunol.2014009982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao L,Faibish D,Fredman G,Herrera BS,Chiang N,Serhan CN,et al. Resolvin E1 and chemokine-like receptor 1 mediate bone preservation. J Immunol (Baltimore, Md: 1950). 2013;190:689-94. PMCID: PMC3538964 [DOI] [PMC free article] [PubMed]
- 24.Herrera BS,Ohira T,Gao L,Omori K,Yang R,Zhu M,et al. . An endogenous regulator of inflammation, resolvin E1, modulates osteoclast differentiation and bone resorption. Br J Pharmacol. 2008;155:1214-23. 10.1038/bjp.2008.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Funaki Y. Resolvin E1 Inhibits Osteoclastogenesis and Bone Resorption by Suppressing IL-17-induced RANKL Expression in Osteoblasts and RANKL-induced Osteoclast Differentiation. Yonago Acta Med. 2018;61:008-018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu ZZ,Ji RR. Resolvins are potent analgesics for arthritic pain. Br J Pharmacol. 2011;164:274-7. 10.1111/j.1476-5381.2011.01348.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun W,Ma J,Zhao H,Xiao C,Zhong H,Ling H,et al. . Resolvin D1 suppresses pannus formation via decreasing connective tissue growth factor caused by upregulation of miRNA-146a-5p in rheumatoid arthritis. Arthritis Res Ther. 2020;22:61. 10.1186/s13075-020-2133-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Özgül Özdemir RB,Soysal Gündüz Ö,Özdemir AT,Akgül Ö. Low levels of pro-resolving lipid mediators lipoxin-A4, resolvin-D1 and resolvin-E1 in patients with rheumatoid arthritis. Immunol Lett. 2020;227:34-40. 10.1016/j.imlet.2020.08.006 [DOI] [PubMed] [Google Scholar]
- 29.Proudman SM,Cleland LG,Metcalf RG,Sullivan TR,Spargo LD,James MJ. Plasma n -3 fatty acids and clinical outcomes in recent-onset rheumatoid arthritis. Br J Nutr. 2015;114:885-90. 10.1017/S0007114515002718 [DOI] [PubMed] [Google Scholar]
- 30.Proudman SM,James MJ,Spargo LD,Metcalf RG,Sullivan TR,Rischmueller M,et al. . Fish oil in recent onset rheumatoid arthritis: a randomised, double-blind controlled trial within algorithm-based drug use. Ann Rheum Dis. 2015;74:89-95. 10.1136/annrheumdis-2013-204145 [DOI] [PubMed] [Google Scholar]
- 31.Di Giuseppe D,Crippa A,Orsini N,Wolk A. Fish consumption and risk of rheumatoid arthritis: a dose-response meta-analysis. Arthritis Res Ther. 2014;16:446. 10.1186/s13075-014-0446-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg RJ,Spencer FA,Okolo J,Lessard D,Yarzebski J,Gore JM. Retraction notice to “Long-Term Trends (1986-2003)in the Use of Coronary Reperfusion Strategies in Patients Hospitalized With Acute Myocardial Infarction in Central Massachusetts” [Int. J Cardiol. 131 (2008) (83-89)] [Int. J Cardiol. 131 (2008) (83-89)]. Int J Cardiol. 2017;235:207. 10.1016/j.ijcard.2014.07.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee YH,Bae SC,Song GG. Omega-3 polyunsaturated fatty acids and the treatment of rheumatoid arthritis: a meta-analysis. Arch Med Res. 2012;43:356-62. 10.1016/j.arcmed.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 34.Aung T,Halsey J,Kromhout D,Gerstein HC,Marchioli R,Tavazzi L,et al. ; Omega-3 Treatment Trialists’ Collaboration. Associations of Omega-3 Fatty Acid Supplement Use With Cardiovascular Disease Risks. JAMA Cardiol. 2018;3:225-34. 10.1001/jamacardio.2017.5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowman L,Mafham M,Wallendszus K,Stevens W,Buck G,Barton J,et al. ; ASCEND Study Collaborative Group. Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus. N Engl J Med. 2018;379:1529-39. 10.1056/NEJMoa1804988 [DOI] [PubMed] [Google Scholar]
- 36.Miyazawa K,Mori A,Okudaira H. Establishment and characterization of a novel human rheumatoid fibroblast-like synoviocyte line, MH7A, immortalized with SV40 T antigen. J Biochem. 1998;124:1153-62. 10.1093/oxfordjournals.jbchem.a022233 [DOI] [PubMed] [Google Scholar]
- 37.Yoshihiro Funaki YH, Ryota Okazaki, Akira Yamasaki, Yuriko Sueda, Akihiro Yamamoto,, Masaaki Yanai TF, Tomoya Harada, Haruhiko Makino and Eiji Shimizu. Resolvin E1 Inhibits Osteoclastogenesis and Bone Resorption by Suppressing IL-17-induced RANKL Expression in Osteoblasts and RANKL-induced Osteoclast Differentiation. Yonago Acta Med. 2018;61:008-018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao D,Pi J,Shan Y,Tang Y,Zhou P. Anti‑inflammatory effect of Resolvin D1 on LPS‑treated MG‑63 cells. Exp Ther Med. 2018;16:4283-8. 10.3892/etm.2018.6721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiang N,Libreros S,Norris PC,de la Rosa X,Serhan CN. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J Clin Invest. 2019;129:5294-311. 10.1172/JCI129448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitanaka T,Nakano R,Kitanaka N,Kimura T,Okabayashi K,Narita T,et al. JNK activation is essential for activation of MEK/ERK signaling in IL-1β-induced COX-2 expression in synovial fibroblasts. Sci Rep. 2017;7:39914. 10.1038/srep39914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benabdoun HA,Kulbay M,Rondon EP,Vallières F,Shi Q,Fernandes J,et al. . In vitro and in vivo assessment of the proresolutive and antiresorptive actions of resolvin D1: relevance to arthritis. Arthritis Res Ther. 2019;21:72. 10.1186/s13075-019-1852-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Im DS. Omega-3 fatty acids in anti-inflammation (pro-resolution) and GPCRs. Prog Lipid Res. 2012;51:232-7. 10.1016/j.plipres.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 43.Cash JL,Norling LV,Perretti M. Resolution of inflammation: targeting GPCRs that interact with lipids and peptides. Drug Discov Today. 2014;19:1186-92. 10.1016/j.drudis.2014.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang D,Fu G,Li W,Sun P,Loughran PA,Deng M,et al. . Maresin 1 protects the liver against ischemia/reperfusion injury via the ALXR/Akt signaling pathway. Mol Med. 2021;27:18. 10.1186/s10020-021-00280-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou SM,Hou CH,Liu JF. CX3CL1 promotes MMP-3 production via the CX3CR1, c-Raf, MEK, ERK, and NF-κB signaling pathway in osteoarthritis synovial fibroblasts. Arthritis Res Ther. 2017;19:282. 10.1186/s13075-017-1487-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huber LC,Distler O,Tarner I,Gay RE,Gay S,Pap T. Synovial fibroblasts: key players in rheumatoid arthritis. Rheumatology. 2006;45:669-75. 10.1093/rheumatology/kel065 [DOI] [PubMed] [Google Scholar]
- 47.Jones DS,Jenney AP,Joughin BA,Sorger PK,Lauffenburger DA. Inflammatory but not mitogenic contexts prime synovial fibroblasts for compensatory signaling responses to p38 inhibition. Sci Signal. 2018;11:eaal1601. 10.1126/scisignal.aal1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Julovi SM,Shen K,McKelvey K,Minhas N,March L,Jackson CJ. Activated protein C inhibits proliferation and tumor necrosis factor α-stimulated activation of p38, c-Jun NH2-terminal kinase (JNK) and Akt in rheumatoid synovial fibroblasts. Mol Med. 2013;19:324-31. 10.2119/molmed.2013.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li F,Dai M,Wu H,Deng R,Fu J,Zhang Z,et al. . Immunosuppressive Effect of Geniposide on Mitogen-Activated Protein Kinase Signalling Pathway and Their Cross-Talk in Fibroblast-Like Synoviocytes of Adjuvant Arthritis Rats. Molecules. 2018;23:91. 10.3390/molecules23010091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luan L,Ma Y,Zhang L. HOXD10 silencing suppresses human fibroblast‑like synoviocyte migration in rheumatoid arthritis via downregulation of the p38/JNK pathway. Exp Ther Med. 2018;16:1621-8. 10.3892/etm.2018.6432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Namba S,Nakano R,Kitanaka T,Kitanaka N,Nakayama T,Sugiya H. ERK2 and JNK1 contribute to TNF-α-induced IL-8 expression in synovial fibroblasts. PLoS One. 2017;12:e0182923. 10.1371/journal.pone.0182923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Molon RS,Thurlings RM,Walgreen B,Helsen MM,van der Kraan PM,Cirelli JA,et al. . Systemic Resolvin E1 (RvE1) Treatment Does Not Ameliorate the Severity of Collagen-Induced Arthritis (CIA) in Mice: A Randomized, Prospective, and Controlled Proof of Concept Study. Mediators Inflamm. 2019;2019:1-14. 10.1155/2019/5689465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zauberman A,Zipori D,Krupsky M,Ben-Levy R. Stress activated protein kinase p38 is involved in IL-6 induced transcriptional activation of STAT3. Oncogene. 1999;18:3886-93. 10.1038/sj.onc.1202738 [DOI] [PubMed] [Google Scholar]
- 54.Suzuki M,Tetsuka T,Yoshida S,Watanabe N,Kobayashi M,Matsui N,et al. . The role of p38 mitogen-activated protein kinase in IL-6 and IL-8 production from the TNF-α- or IL-1β-stimulated rheumatoid synovial fibroblasts. FEBS Lett. 2000;465:23-7. 10.1016/S0014-5793(99)01717-2 [DOI] [PubMed] [Google Scholar]
- 55.Nishikai-Yan Shen T,Kanazawa S,Kado M,Okada K,Luo L,Hayashi A,et al. . Interleukin-6 stimulates Akt and p38 MAPK phosphorylation and fibroblast migration in non-diabetic but not diabetic mice. PLoS One. 2017;12:e0178232. 10.1371/journal.pone.0178232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaneko K,Miyabe Y,Takayasu A,Fukuda S,Miyabe C,Ebisawa M,et al. . Chemerin activates fibroblast-like synoviocytes in patients with rheumatoid arthritis. Arthritis Res Ther. 2011;13:R158. 10.1186/ar3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mariani F,Roncucci L. Chemerin/chemR23 axis in inflammation onset and resolution. Inflamm Res. 2015;64:85-95. 10.1007/s00011-014-0792-7 [DOI] [PubMed] [Google Scholar]
- 58.Maderna P,Cottell DC,Toivonen T,Dufton N,Dalli J,Perretti M,et al. FPR2/ALX receptor expression and internalization are critical for lipoxin A 4 and annexin‐derived peptide‐stimulated phagocytosis. FASEB J. 2010;24:4240-9. 10.1096/fj.10-159913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hodges RR,Li D,Shatos MA,Bair JA,Lippestad M,Serhan CN,et al. . Lipoxin A4 activates ALX/FPR2 receptor to regulate conjunctival goblet cell secretion. Mucosal Immunol. 2017;10:46-57. 10.1038/mi.2016.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Im DS. Maresin-1 resolution with RORα and LGR6. Prog Lipid Res. 2020;78:101034. 10.1016/j.plipres.2020.101034 [DOI] [PubMed] [Google Scholar]
- 61.Connor AM,Mahomed N,Gandhi R,Keystone EC,Berger SA. TNFα modulates protein degradation pathways in rheumatoid arthritis synovial fibroblasts. Arthritis Res Ther. 2012;14:R62. 10.1186/ar3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y,Wang S,Dong H,Yi X,Zhang J,Liu X,et al. . LAIR-1 shedding from human fibroblast-like synoviocytes in rheumatoid arthritis following TNF-α stimulation. Clin Exp Immunol. 2018;192:193-205. 10.1111/cei.13100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perretti M,Cooper D,Dalli J,Norling LV. Immune resolution mechanisms in inflammatory arthritis. Nat Rev Rheumatol. 2017;13:87-99. 10.1038/nrrheum.2016.193 [DOI] [PubMed] [Google Scholar]