Abstract
Context
Polycystic ovary syndrome (PCOS) is common and diagnosis requires an elevated testosterone. The clinical importance of adrenal 11-oxyandrogens in PCOS is unclear.
Objective
We sought to determine if 11-oxyandrogens 1) better identify PCOS diagnosis compared to testosterone, 2) predict clinical comorbidities of PCOS, and 3) are altered with an combined oral contraceptive pill (COCP) or metformin therapy.
Methods
Data from 200 adolescent female participants aged 12 to 21 years, most with obesity, enrolled across 6 studies in pediatric endocrinology were included: 70 non-PCOS controls, 115 untreated PCOS, 9 PCOS + obesity treated with COCP, and 6 PCOS + obesity treated with metformin. 11-Hydroxyandrostenedione (11-OHA4), 11-hydroxytestosterone (1-OHT), 11-ketotestosterone (11-KT), and testosterone were measured with liquid chromatography–tandem mass spectrometry. Data between 1) untreated PCOS and controls and 2) untreated PCOS and the 2 treatment groups were compared.
Results
Untreated girls with PCOS had higher 11-OHA4 (P = .003) and 11-OHT (P = .005) compared to controls, but not 11-KT (P = .745). Elevated 11-OHA4 remained statistically significant after controlling for obesity. Testosterone better predicted PCOS status compared to 11-oxyandrogens (receiver operating characteristic curve analysis: 11-OHA4 area under the curve [AUC] = 0.620, 11-OHT AUC = 0.638; testosterone AUC = 0.840). Among untreated PCOS patients, all 3 11-oxyandrogens correlated with hirsutism severity. 11-KT (P = .039) and testosterone (P < .006) were lower in those on COCP treatment compared to untreated PCOS. Metformin treatment had no effect on 11-oxyandrogens, although testosterone was lower (P = .01).
Conclusion
Although 11-oxyandrogens do not aid in the diagnosis of PCOS, they relate to excess hair growth. COCP treatment may related to 11-KT; however, further work is needed to determine causality, relationship with metabolic outcomes, and the clinical utility of measuring these androgens in PCOS.
Keywords: 11-oxyandrogens, polycystic ovary syndrome, adolescents
Polycystic ovary syndrome (PCOS) is common, clinically presents during adolescence, and is associated with an increased risk for obesity. Diagnostic criteria have changed in recent years in acknowledgement of the heterogeneity of the clinical phenotype [1]. In adolescents, an elevated serum testosterone or clinical signs consistent with excess testosterone are required for diagnosis under both the classic and latest diagnostic criteria [2, 3]. Clinical phenotypic characteristics of androgen excess in PCOS include hirsutism, acne, and androgenic alopecia. PCOS is also associated with obesity and metabolic syndrome, which may manifest as dysglycemia, hyperlipidemia, hepatic steatosis, and cardiovascular disease [2]. The severity of metabolic disease is thought to relate to testosterone concentrations and the presence of and degree of obesity [4].
Recently, there has been a renewed interest in quantifying the adrenal-derived 11-oxygenated androgens (11-oxyandrogens for short) in hyperandrogenic disorders because of their newly realized potency and potential to serve as biomarkers [5]. Most adrenal androgens are synthesized in the zona reticularis, with dehydroepiandrosterone (DHEA) and its sulfate, DHEAS, being the most abundant [6]. The enzyme 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) converts DHEA to androstenedione, followed by aldo-keto reductase 1C3 (AKR1C3) modulation of androstenedione to testosterone. Androstenedione and testosterone emerge as substrates for the adrenal enzyme cytochrome P450 11β-hydroxylase to yield 11-hydroxyandrostenedione (11-OHA4) and 11-hydroxytestosterone (11-OHT) [6]. In addition, 11-OHA4 can be oxidized to 11-ketoandrostenedione (11-KA4), and 11-OHT can be oxidized to 11-ketotestosterone (11-KT) primarily in peripheral tissue such as in the skin and to a lesser extent in the adrenal glands [6]. These C19 steroids have emerged as major compounds in several androgen excess disorders [6]. Androgen excess often manifests during adrenarche, between ages 6 and 8 years, secondary to expansion of the zona reticularis; this results in increased synthesis of adrenal androgens, which allows overproduction (hyperandrogenemia) if enzymatic regulators become inhibited [7]. For instance, 11-KT has been found to be elevated in girls with premature adrenarche, indicating it plays a role in clinical symptoms of hyperandrogenism in females [7].
The ovaries have traditionally been considered the primary source of androgen excess in females with PCOS [6], with minimal adrenal contribution; however, limited studies have been conducted to determine the contribution of 11-oxyandrogens [8-11]. Rege et al [7] found that 11-KT is the dominant circulating bioactive androgen during normal and premature adrenarche and suggested this androgen may be responsible for a subset of girls with premature adrenarche being more susceptible to the development of PCOS and insulin resistance. Owen et al [12] measured 11-OHA4 concentrations in plasma and follicular fluid from the ovary and found that raised plasma concentrations may reflect extra-adrenal contribution to hyperandrogenism due to follicular fluid concentrations being lower in women with PCOS than normally cycling women. Another potential cause of adrenal androgen excess could be ovarian metabolism of androstenedione into testosterone, and the authors conclude that 11-OHA4 cannot be regarded as a biomarker for PCOS [12]. Owing to 11-KT having the same relative androgenicity as testosterone and suggestions that 11-OHA4 concentrations may be elevated because of adrenal contributions, further examination into the role 11-oxyandrogens play in adolescents with PCOS is needed [6].
The aims of this project were primarily to assess if 11-oxyandrogens better identified PCOS diagnosis compared to testosterone, as well as to assess the relationship between 11-oxyandrogens and clinical symptoms and comorbidities, and to measure the effects of common pharmaceutical treatments for PCOS on 11-oxyandrogens hormone concentrations in adolescents with PCOS.
Materials and Methods
Participants included were enrolled in 1 of 6 previously conducted pediatric endocrine studies at Children’s Hospital Colorado who had stored frozen serum samples and consented to blood banking for future endocrine testing. Three studies were focused on understanding metabolic disease in PCOS: Androgens and Insulin Resistance Study (AIRS, prior to NCT, N = 76, principal investigator [PI]: M. Cree-Green [4, 13]); Liver and Fat Regulation in Overweight Adolescent Girls (APPLE, NCT02157974, N = 92, PI: M. Cree-Green [14]) and Post-Prandial Liver Glucose Metabolism in PCOS (PLUM, NCT03041129, N = 17, PI: M. Cree-Green [15]). All studies recruited females between ages 12 and 21 years, described as having a sedentary activity level (< 3 h/wk of physical activity), and had a body mass index (BMI) greater than or equal to the 90th percentile or between the 10th and 85th percentile. PCOS status was met based on the National Institutes of Health definition: irregular menstrual cycles, at least 1.5 years post menarche, and either clinical (cystic acne or hirsutism) or biochemical (elevated testosterone) markers of hyperandrogenism [2]. Other causes of oligomenorrhea (thyroid, prolactin, ovarian failure) and hyperandrogenism (congenital adrenal hyperplasia and adrenal tumor) were ruled out with standard laboratory tests. Exclusion criteria included currently pregnant or breastfeeding, implanted medical devices not compatible with magnetic resonance imaging, evidence of diabetes, and use of medication such as combined oral contraceptive pills (COCPs) and those known to affect insulin sensitivity both for PLUM and AIRS; however, for the PCOS group in APPLE, COCPs and treatment with metformin was allowed. Additional healthy controls without PCOS were included from Characterization of Glucose Variability by Continuous Glucose Monitoring (CGM) (NCT02211235, PI: C. Chan [16, 17]), Combined Influence of Puberty and Obesity on Insulin Resistance in Adolescents (HIPS, NCT01775813, PI: M. M. Kelsey [18]), and Microbiome Insulin Sensitivity Study (MISS, NCT03120871, PI: M. M. Kelsey [19]).
For each of the studies, participants had at least 2 visits: one for consent and screening (eligibility labs and history) and one for a metabolic and hormonal visit. A physical was performed by a pediatric endocrinologist (M.C.G., C.L.C., M.M.K., or K.J.N.). Hirsutism was assessed with the modified Ferriman-Gallwey score (FGS) and acne graded in severity from none, mild (comedomes or small pustules), moderate (pustules and some inflammation), or severe (nodulcytic). Blood pressure was measured after at least 5 minutes of rest with the appropriately sized cuff. Weight was measured without shoes in light clothes and height assessed with a calibrated stadiometer. Fasting laboratory measures included hormones (estradiol, progesterone, testosterone, free testosterone, sex hormone binding–globulin [SHBG], and DHEAS) and metabolic measures (glucose, insulin, alanine aminotransferase [ALT], aspartate aminotransferase [AST], lipid panel, and glycated hemoglobin A1c [HbA1c]). Insulin and glucose were also frequently sampled during a 2-hour oral glucose tolerance test in AIRS, PLUM, and APPLE. Hepatic fat percentage was assessed via magnetic resonance imaging proton density fat fraction in AIRS, PLUM, and APPLE participants, as previously described [4, 13].
Eleven-oxyandrogen concentrations were measured from frozen serum samples by LabCorp Esoterix via liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis. Other measurements were performed as previously described [4, 13]. Briefly, total testosterone was analyzed using LC-MS/MS and free testosterone with equilibrium dialysis (Esoterix); DHEA-S and SHBG by electrochemiluminescence immunoassay by Esoterix; estradiol and progesterone via chemiluminescent immunoassay (Beckman Coulter); AST and ALT were measured on a Vitros 5600 (Ortho Clinical Diagnostics). Serum insulin was analyzed by radioimmunoassay (Millipore); free fatty acids (Wako Chemicals Inc) were assessed enzymatically. HbA1c was measured by DCCT-calibrated ion-exchange high-performance liquid chromatography (Bio-Rad Laboratories). Total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglyceride assays were performed enzymatically on a Hitachi 917 autoanalyzer (Boehringer Mannheim Diagnostics). Low-density lipoprotein cholesterol (LDL-C) concentrations were calculated by the Friedewald equation.
Calculations performed included the free androgen index (FAI), calculated from total testosterone and SHBG [2]. This formula was also used to calculate a “free 11-oxyandrogen index” for each of the 3 metabolites. Measures of insulin sensitivity included the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) calculated as fasting plasma glucose [mg/dL] × fasting plasma insulin [mg/dL]/405 [13], the Matsuda index as 10 000/(sqrt(fasting glucose*fasting insulin*mean glucose*mean insulin) [20], and the oral minimal model calculated with a 2-hour exponential model using SAAM II software (SAAM II software v 2.2, The Epsilon Group) [21].
The distributions of all variables were examined before analysis. Descriptive statistics included means and SD if normally distributed, or medians and 25th to 75th percentile for nonnormally distributed data. T tests or Wilcoxon rank sum tests were performed to compare groups, and multiple linear regression was performed to control for the effect of obesity in the androgens only. Associations were evaluated via simple linear regression. Logistic regression and receiver operating characteristic (ROC) curve analysis was used to evaluate sensitivity and specificity of androgens for PCOS status using the Youden index as a cutoff (sensitivity + sensitivity – 1). Analysis of variance was used to compare differences in outcomes between untreated PCOS and the 2 treatment groups, and not controlled for BMI, because it was similar between the groups. All analysis was performed in GraphPad Prism 9.1.0.
Results
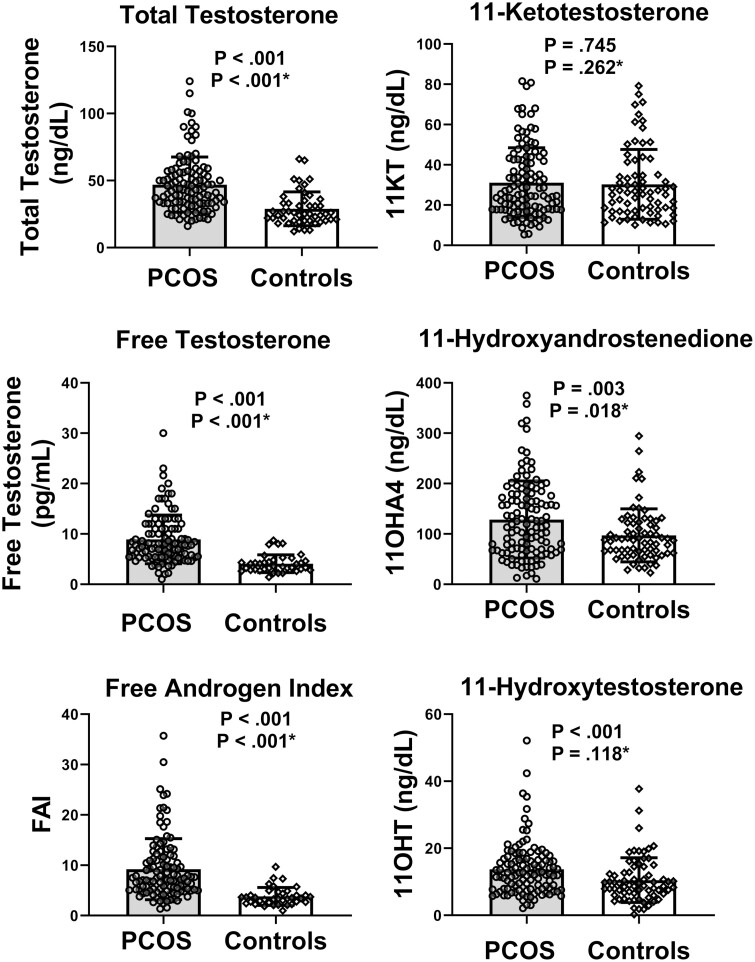
A total of 200 participants had samples available for analysis. This included 115 with untreated PCOS (13 lean [BMI = 22.6 ± 2.2] and 102 with overweight/obesity [BMI = 35.4 ± 5.3]), 70 controls (18 normal weight [BMI = 20.2 ± 1.4], 52 with obesity [BMI = 35.5 ± 10.4]), 9 girls with PCOS and obesity (BMI = 33.7 ± 5.7) treated with COCPs, and 6 girls with PCOS and obesity (BMI = 36.4 ± 5.3) treated with metformin. Demographic and physical exam data for the untreated PCOS and control groups are shown in Table 1. The untreated PCOS group was older and had higher BMI percentiles (P < .001), as well as more participants in the category of obesity (P = .24). As expected, FGS hirsutism scores were statistically significantly higher in the PCOS cohort (P < .001). Laboratory data for the untreated PCOS and control groups are shown in Table 2. The medians of total testosterone, (P < .001), FAI (P < .001), and free testosterone (P < .001) were statistically significantly higher in those with untreated PCOS. Two of the 11-oxyandrogens, 11O-HA4 (P = .003) and 11-OHT (P < .001) were statistically significantly higher in PCOS, with data graphed in Fig. 1. When these comparisons were adjusted for obesity, the following results were found: total testosterone, (P < .001 for PCOS, β = 18.4 ± 3.3 ng/dL), free testosterone (P < .001 for PCOS, β = 5.38 ± 0.78 pg/mL), FAI (P < .001 for PCOS, β = 6.1 ± 1.0, no units), 11-KT (P = .262 for PCOS, β = 3.2 ± 2.8 ng/dL), 11-OHA (P = .018 for PCOS, β = 28.5 ± 12.0 ng/dL), 11-OHT (P = 0.118 for PCOS, β = 2.0 ± 1.3 ng/DL). However, there was no difference between PCOS and controls for 11-KT. Table 2 also shows descriptive data for other comorbidity measures that are associated with PCOS. Statistically significant findings between the 2 groups include SHBG, FAI, cholesterol, LDL-C, triglycerides, HbA1c, 2-hour glucose, fasting insulin, and 2-hour insulin, ALT, and AST. There were no statistically significant differences in hypertension measures.
Table 1.
Demographic variables and physical exam findings for participants with and without polycystic ovary syndrome
| Untreated PCOS (n = 115) | Controls (n = 70) | P | |
|---|---|---|---|
| Age, y | 16 (15-17) | 14 (13-16) | < .001 |
| Race, n (%) | .146 | ||
| White | 98 (85) | 46 (73) | |
| Black | 15 (13) | 15 (23) | |
| Asian | 2 (2) | 1(2) | |
| > 1 Race | 0 | 1 (2) | |
| Hispanic ethnicity, n (%) | 61 (53) | 31 (46) | .447 |
| BMI | 34.3 (30.2-37.8) | 30.9 (22.4-36.1) | .071 |
| BMI percentile | 98.1 (96.0-99.0) | 96.7 (67.0-98.6) | < .001 |
| Normal BMI (BMI percentile < 90, No. [%]) | 13 (11) | 18 (26) | .024 |
| Menarchea,b | 12 (11, 12) | 11 (11, 12) | .172 |
| Frequency of menses, n (%)c | < .001 | ||
| 4-6 wk | 3 (3) | 34 (92) | |
| 1-2 mo | 33 (30) | 1 (3) | |
| 3-6 mo | 41 (37) | 2 (5) | |
| 7-12 mo | 22(20) | 0 | |
| > 1 y | 11(10) | 0 | |
| Hirsutismd FGS | 6.0 (3.0-11.0) | 0 (0-3.0) | < .001 |
| Acne severity, n (%)d | < .001 | ||
| None | 8 (7) | 18 (50) | |
| Mild | 52 (46) | 11(31) | |
| Moderate | 45 (40) | 6 (17) | |
| Severe | 8 (7) | 1 (2) |
Data are expressed as median (25th-75th percentile), mean ± SD, or number (% of total; includes all study controls).
Abbreviations: BMI, body mass index; CGM, continuous glucose monitoring study; FGS, Ferriman-Gallwey score; HIPS, Combined Influence of Puberty and Obesity on Insulin Resistance in Adolescents; MISS, Microbiome Insulin Sensitivity Study; PCOS, polycystic ovary syndrome.
a Missing CGM study values, n = 6.
b Missing HIPS values, n = 12.
c Missing MISS data, n = 17.
d Missing CF study values, HIPS values, and MISS values, n = 35.
Table 2.
Biochemical measures for participants with and without polycystic ovary syndrome
| Untreated PCOS (n = 115) | Controls (n = 70) | P | |
|---|---|---|---|
| Androgens | |||
| Total testosterone, ng/dLc,d | 43.0 (33.0-56.0) | 25.0 (20.0-34.5) | < .001 |
| Free testosterone, pg/mLe | 7.8 (5.4-11.0) | 3.6 (2.9-4.7) | < .001 |
| DHEAS, μg/dLc,d | 196 (125-311) | 147 (75-263) | .061 |
| Progesterone, ng/mLe | 0.80 (0.50-1.20) | 0.80 (0.60-1.27) | .007 |
| Estradiol, pg/mLe | 45.0 (35.0-56.0) | 53.0 (31.5-91.5) | .002 |
| SHBG, nmol/L c ,d | 19.0 (13.4-27.1) | 27.2 (20.9-40.5) | .001 |
| FAI e | 7.3 (5.1-11.5) | 3.4 (2.6-4.5) | < .001 |
| 11-OH androgens | |||
| 11-KT, ng/dLb | 25.5 (17.8-42.5) | 26.3 (17.1-36.6) | .745 |
| 11-OHA4, ng/dL b | 117 (68-179) | 88 (62-123) | .003 |
| 11-OHT, ng/dL b | 12.7 (7.8-16.9) | 8.9 (6.3-13.2) | .001 |
| Lipids | |||
| Cholesterol, mg/dL c | 151 (129-175) | 133 (121-148) | .003 |
| LDL, mg/dL c | 88.0 (69.5-116.5) | 78.6 (67.0-89.8) | .008 |
| HDL, mg/dLc | 37.0 (32.0-44.0) | 38.0 (32.6-47.0) | .052 |
| Triglycerides, mg/dL c | 109 (77-150) | 89 (70-118) | .016 |
| Glycemia | |||
| HbA1c, %b | 5.3 (5.2-5.6) | 5.2 (5.1-5.5) | .060 |
| Fasting glucose, mg/dLa,b | 88 ± 8 | 87 ± 5 | .977 |
| 2-h glucose, mg/dL a | 132 ± 25 | 114 ± 29 | .002 |
| Insulin resistance | |||
| Fasting insulin, mIU/L a ,b | 21(15-31) | 16 (10-22) | .009 |
| 2-h insulin, mIU/L a ,b | 172 (98-338) | 105 (21-189) | .001 |
| Peak insulin, mIU/La,d | 250 (112-427) | 210 (142-330) | .294 |
| HOMA-IRa,d | 5.0 (3.3-7.1) | 4.2 (3.4-5.2) | .033 |
| Matsudad | 1.6 (0.9-2.1) | 1.8 (1.5-2.1) | .343 |
| OMMa,d | 1.5 × 10–4 (8.4 × 10–5 to 3.3 × 10–4) | 2.2 × 10–4(1.4 × 10–4 to 4.0 × 10–4) | .543 |
| Hypertension | |||
| Systolic BP, mm Hgd | 121 ± 12 | 121 ± 12 | .859 |
| Diastolic BP, mm Hgd | 66 (63-72) | 66 (61-72) | .652 |
| Fatty liver disease | |||
| ALT, IU/L c | 34 (28-42) | 28 (24-35) | .002 |
| AST, IU/L c | 38 (32-46) | 34 (29-40) | .017 |
| Liver fatd, % | 4.5 (2.5-8.7) | 3.2 (2.6-5.8) | .102 |
| Hepatic steatosis, n (%) d | 53 (47) | 9 (25) | .0182 |
Data are expressed as median (25th-75th percentile), mean ± SD, or number (% of total).
Abbreviations: 11-KT, 11-ketotestosterone; 11-OH, 11β-hydroxylase; 11-OHA4, 11β-hydroxyandrostenedione; 11-OHT, 11-OH testosterone; ALT, alanine transaminase; AST, aspartate transaminase; BP, blood pressure; CGM, continuous glucose monitoring study; DHEAS, dehydroepiandrosterone sulfate; FAI, free androgen index; HbA1c, glycated hemoglobin A1c; HDL, high-density lipoprotein; HIPS, Combined Influence of Puberty and Obesity on Insulin Resistance in Adolescents; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; LDL, low-density lipoprotein; MISS, Microbiome Insulin Sensitivity Study; OMM, oral minimal model; PCOS, polycystic ovary syndrome; SHBG, sex hormone–binding globulin.
a Ten exenatide participants excluded.
b Has all study controls.
c Missing CGM study values, n = 6.
d Missing MISS data, n = 17.
e Missing CGM study values, HIP values, and MISS values, n = 35.
Figure 1.
Androgen concentration distributions in untreated polycystic ovary syndrome (PCOS) compared to non-PCOS controls. Individual points, means, and 25th to 75th percentile bars are shown for each of the androgen concentrations. Statistical analysis is controlled for obesity status.
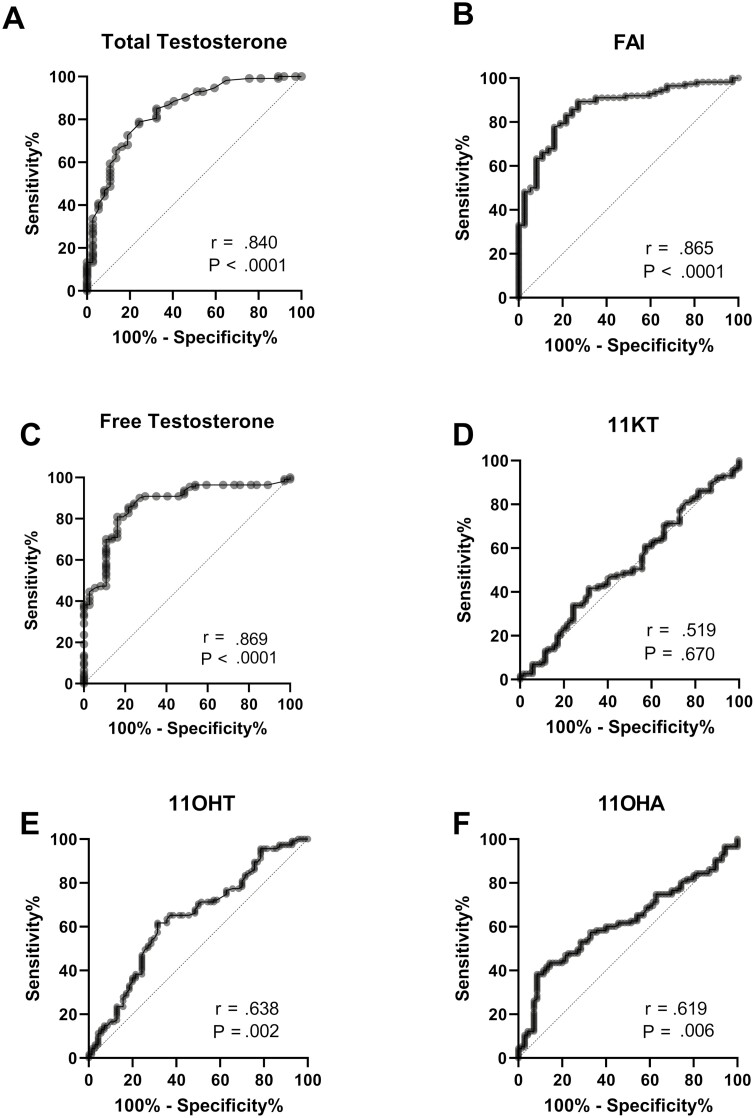
ROC analysis was performed to determine sensitivity and specificity of the various androgen measures for diagnosing PCOS and are shown in Fig. 2. As expected, total testosterone (Fig. 2A, sensitivity 79%, specificity, at Younden index, AUC = 0.840), free testosterone (Fig. 2C, sensitivity 85%, specificity 78%, AUC = 0.869), and calculated FAI (Fig. 2E, sensitivity 83%, specificity 78%, AUC = 0.865) performed well for diagnosing PCOS (AUC > 0.84 for all). 11-KT did not perform well at identifying individuals with PCOS (AUC = 0.519). The Younden index for 11-OHA4 for PCOS (Fig. 2D, sensitivity 75%, specificity 31%, AUC = 0.620) and 11-OHT (Fig. 2F, sensitivity 69%, specificity 51%, AUC = 0.638), respectively, were not as strong as testosterone or FAI.
Figure 2.
Predictive value of different androgen concentrations for polycystic ovary syndrome (PCOS) diagnosis. Receiver operating characteristic curves of each of the androgen concentrations as related to PCOS diagnosis are shown. P values are from unadjusted comparisons.
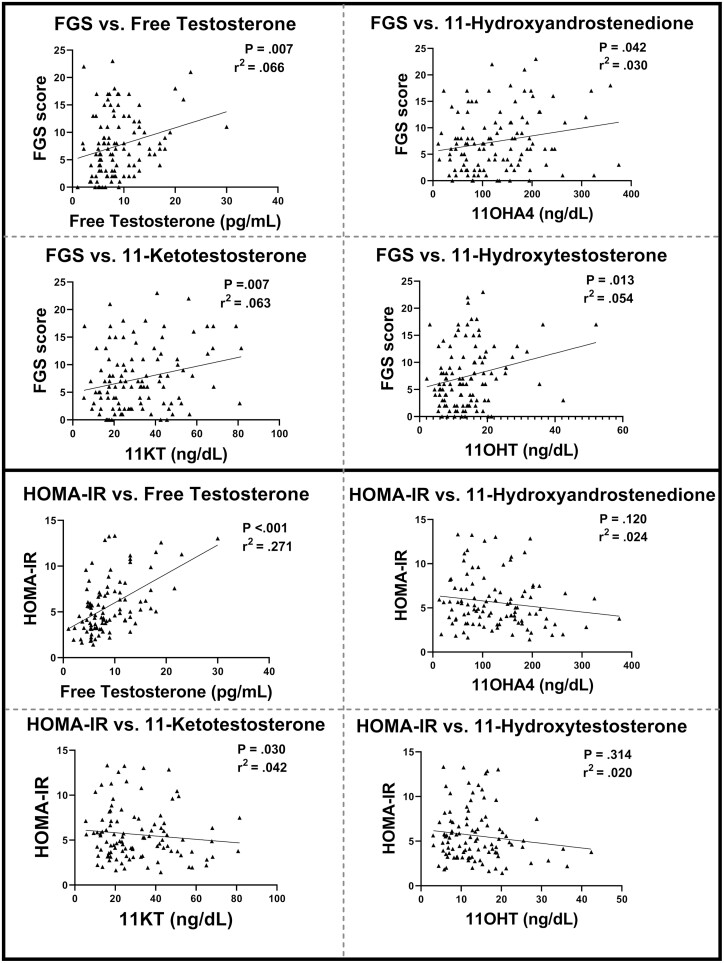
Since FGS hirsutism score and insulin sensitivity (HOMA-IR) were highly different between PCOS and controls and of clinical importance, we examined the relationship between these 2 measures with 11-oxyandrogens and free testosterone (Fig. 3). There was a statistically significant correlation between all 3 11-oxyandrogens ([11-OHA4, P = .030], [11-OHT, P = .013], [11-KT, P = .008], free testosterone (P = .007), and FGS score. Whereas there was a strong relationship between higher free testosterone and worse HOMA-IR, there was no relationship between HOMA-IR and any of the 11-oxyandrogens. We also calculated a “free 11-oxyandrogen index” for each metabolite, to determine if there was a role of SHBG in altering the measurement of the androgens and affecting correlations. We did not find that any of the correlations improved with a calculated free index (data not shown).
Figure 3.
Correlations for androgen, metabolic, and dermatological measures. Individual points, lines of best fit of each of the androgen concentrations related to Ferriman-Gallwey score (FGS), and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) calculated value.
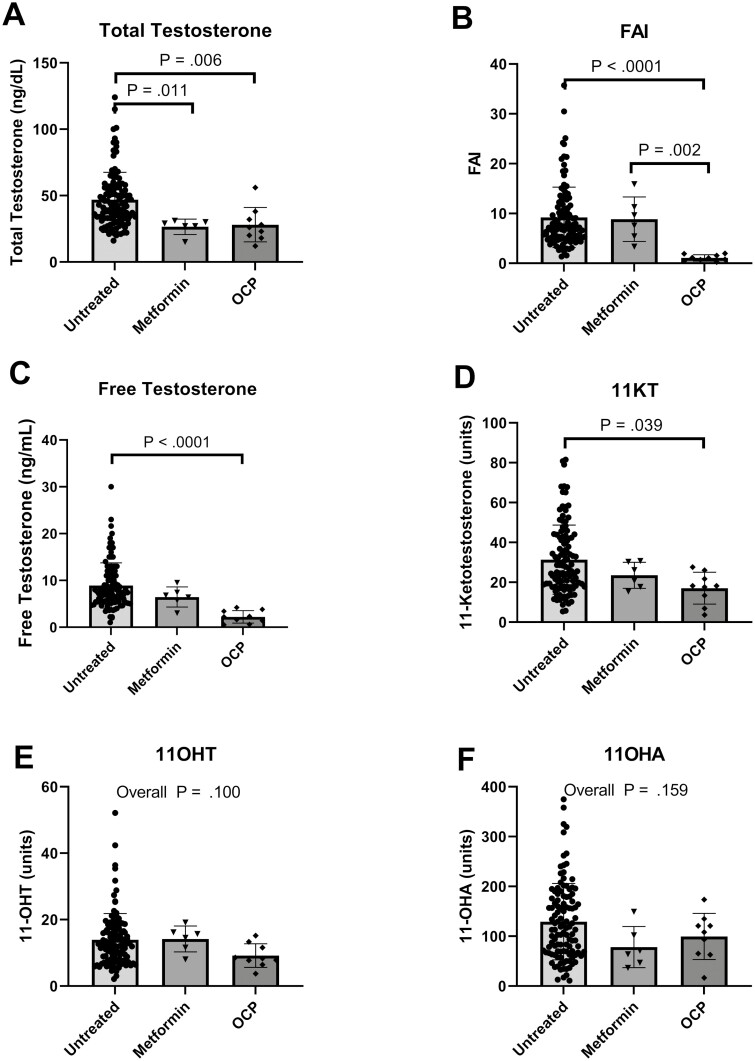
Demographic information for participants on pharmaceutical medical treatment (COCPs or metformin) for PCOS are shown in Table 3. Hirsutism and SHBG scores were statistically different (P = .007 and P < .001), which was expected as COCPs are known for improving excess hair growth and increasing SHBG to bind testosterone. Fig. 4 demonstrates the differences between the untreated group and the 2 treatment arms. If initial overall analysis of variance was statistically significant, then pairwise comparisons are shown in the graph. Between the untreated PCOS group and the COCP-treated group, there was a statistical decrease in total testosterone (median difference = –17.0 ng/dL, P = .006), FAI (median difference = –6.3 units, P ≤ .001), free testosterone (median difference = –5.6 units, P < .001), and 11-KT (median difference = –7.4 units, P = .040). Statistical differences between the untreated PCOS group and the metformin-treated group were seen in total testosterone (median difference = –15.0 ng/dL, P = .011), and in FAI between metformin and COCPs (mean difference = –7.6, P = .002).
Table 3.
Demographic variables and physical exam findings for participants with untreated and pharmaceutically treated polycystic ovary syndrome
| Untreated PCOS(N = 115) | COCP (N = 9) | Metformin (N = 6) | P | |
|---|---|---|---|---|
| Age, y | 15 ± 2 | 15 ± 1 | 15 ± 1 | .845 |
| Race, n (%) | .636 | |||
| White | 98 (85) | 9 (100) | 6 (100) | |
| Black | 15 (13) | 0 | 0 | |
| Asian | 2 (2) | 0 | 0 | |
| > 1 Race | 0 | 0 | 0 | |
| Hispanic ethnicity, n (%) | 61 (53) | 6 (67) | 4 (67) | .723 |
| BMI | 33.9 ± 6.4 | 33.7 ± 5.7 | 36.4 ± 5.3 | .621 |
| BMI percentile | 94.1 ± 12.2 | 97.1 ± 1.5 | 98.5 ± 0.6 | .454 |
| Menarche, ya | 12 ± 2 | 12 ± 1 | 11 ± 1 | .291 |
| Duration of mensesa | < .001 | |||
| Normal | 3 (3) | 8 (88) | 1 (17) | |
| 1-2 mo | 33 (30) | 0 | 3 (50) | |
| 3-6 mo | 41 (37) | 0 | 1 (17) | |
| 7-12 mo | 22 (20) | 1 (13) | 1 (17) | |
| > 1 y | 11 (10) | 0 | 0 | |
| Hirsutism a | 6.0 (3.0-11.0) | 3.0 (0.5-5.5) | 1.5 (0.8-5.5) | .007 |
| Acne severity, n (%)a | .245 | |||
| None | 8 (7) | 2 (22) | 1 (17) | |
| Mild | 52 (46) | 4 (45) | 5 (83) | |
| Moderate | 45 (40) | 3 (33) | 0 | |
| Severe | 8 (7) | 0 | 0 | |
| DHEASa | 226.0 ± 135.4 | 209.6 ± 60.1 | 142.0 ± 60.8 | .263 |
| Progesteronea | 0.99 ± 0.86 | 1.16 ± 0.61 | 1.77 ± 3.06 | .240 |
| Estradiola | 50.7 ± 30.8 | 46.4 ± 49.4 | 72.3 ± 55.2 | .153 |
| SHBG a | 19.0 (13.4-27.1) | 102.2 (65.5-123.3) | 12.0 (8.2-16.4) | < .001 |
Data are expressed as median (25th-75th percentile), mean ± SD, or number (% of total).
Abbreviations: BMI, body mass index; COCP, combined oral contraceptive pill; DHEAS, dehydroepiandrosterone sulfate; PCOS, polycystic ovary syndrome; SHBG, sex hormone–binding globulin.
a Missing Microbiome Insulin Sensitivity Study; PCOS values, n = 2.
Figure 4.
Androgen concentration distributions in untreated polycystic ovary syndrome (PCOS) compared with PCOS treated with metformin and combined oral contraceptive pill (COCP). Individual points, means, and 25th to 75th percentile bars are shown for each of the androgen concentrations. The overall analysis of variance is reported if not statistically significant, and if statistically significant, only statistically significant pairwise P values are shown.
Discussion
We found that adolescent girls with PCOS had higher 11-OHA4 and 11-OHT values than non-PCOS controls. However, 11-oxyandrogens did not appear to have any clinical utility when it came to aiding in the diagnosis of PCOS compared to serum testosterone, determined by the statistical relationship shown in the ROC curve analysis. While 11-oxyandrogens correlated with hirsutism severity, they did not correlate well with many measures of dysmetabolism. An interesting finding was that COCPs did lower androgens and testosterone concentrations, but not androstenedione (see Fig. 3). These findings further strengthen the argument that ovarian testosterone, not androgenic testosterone derivatives, are the primary source of excess androgens in PCOS. The data in this study suggest that 11-oxyandrogen production does correlate with excess hair growth in PCOS but does not seem to be a driving force behind PCOS or aid in diagnosis.
Results from existing studies that look at 11-oxyandrogen concentrations in PCOS vary greatly, especially between patient populations. Our data are consistent with Pretorius et al [5] in regard to 11-OHA4 being elevated in PCOS and relating to hyperandrogenic signs associated with PCOS. The association of 11-oxyandrogens and dermatological manifestations of androgen excess, namely hirsutism, are consistent with findings from Hudson et al [22] on the clinical role of 11-oxyandrogens. Yoshida et al [11] found that, in a cohort of 28 participants with PCOS, 4 had increased serum concentrations of 11-oxyandrogens but normal levels of testosterone, and 20 had increased serum concentrations of testosterone, indicating that both play an important role in the phenotype of PCOS. These combined results suggest that 11-oxygenated steroids are unlikely to be of high utility as clinical biomarkers in PCOS.
Because PCOS first manifests during puberty, it is important to understand at what age and stage in development 11-oxyandrogens begin to increase, and in what settings these may be altered. Holownia et al [23] found that the rise in concentrations of 11-OHA4 in children appears to reflect 3 phenomena: the maturation of the adrenal gland in adrenarche; progression through puberty shown by steadily increasing levels of DHEAS with a sharp rise in estradiol in girls; and, last, stress, which is likely the cause of the wide variation of concentrations with chronological age. Abnormalities are noted with premature adrenarche, including a 3.5-fold elevation in 11-OHA4, 11-OHT, and 11-KT rather than testosterone in girls with premature adrenarche [7]. Davio et al [24] found that 11-KT is the dominant circulating bioactive androgen during normal and premature adrenarche and that concentrations do not seem to decline in women as they age. Ovarian-derived testosterone is higher in peripubertal girls with obesity [25]. However, in premenarchal daughters of women with PCOS, 11-oxyandrogens were not elevated relative to age-matched controls [9]. Our results in older adolescents were in direct contrast to those of O’Reilly and colleagues [8], who found that serum concentrations of 11-KT were statistically significantly higher than those of testosterone in women with PCOS and in female controls. We speculate that 11-KT concentrations may be lower in adolescents with PCOS because of incomplete zona reticularis growth with submaximal adrenal hormone release.
Some of the discrepancy in findings regarding 11-oxyandrogens in health and disease may be due to measurement methodology. LC-MS/MS allows for accurate and simultaneous measurement of multiple adrenal and gonadal steroids [26]. However, even with an accurate and reliable assay, the interpretation of androgens can be confounded by alterations in SHBG concentrations and diurnal variation. As androgens peak in the morning, morning measurement is preferable and is the standard for our protocols (6-8 am). Some of the variability in results across studies may be explained by a lack of standardization in timing of the androgen blood draw. Of greater importance is the effect of SHBG on total testosterone concentrations—roughly 65% of circulating testosterone is bound to SHBG [27]. Women with low SHBG and PCOS can have normal total testosterone levels but elevated bioavailable circulating testosterone, and this can lead to a PCOS diagnosis potentially being missed [27]. Indeed, the calculated FAI, or free testosterone, measured by equilibrium dialysis in our study had greater associations with PCOS clinical findings, hirsutism, and metabolic measures [26]. However, unlike testosterone, when we calculated a free 11-oxyandrogen index for each of the 3 11-oxyandrogen metabolites, it did not change the diagnostic utility or relationship to metabolic disease. Further, in prepubertal girls, 11-KT concentrations did not relate to SHBG [9]. The combination of these findings suggests that SHBG may not be as important for these androgen metabolites, and that uncorrected “total” values can be measured.
While the role of COCPs and metformin in treating hormonal and metabolic abnormalities in PCOS is well established, knowledge surrounding the effect of treatments on adrenal and 11-oxyandrogens is limited. Kjøtrød et al [28] found that during 12 to 14 weeks of metformin pretreatment for women with PCOS about to undergo fertility treatments, DHEAS levels increased in the metformin group compared to controls, whereas other adrenal androgens such as DHEA and androstenedione were unaffected. In our small group of participants on treatment, we found a slight decrease in total and free testosterone, but no changes in other measures with metformin. It is known that COCPs decrease testosterone production and also decrease bioavailable testosterone by increasing SHBG, consistent with our results [2]. We were unable to find any published information on COCPs and 11-oxyandrogens, although COCPs have been shown to decrease other adrenal androgens, such as DHEAS. We did find that 11-KT was statistically significantly lower and 11-OHA4 and 11-OHT were nonsignificantly slightly lower in those treated with a COCP. These findings support the data in this study that while COCPs are of further interest in studying to determine their exact relationship with 11-oxyandrogens in perhaps adrenal disease, particularly 11-KT, metformin has little effect on 11-oxyandrogens, where adrenal DHEAS was similarly unaffected by metformin.
It is estimated that 20% to 30% of women with PCOS have increased adrenal androgens [29, 30]. For example, androgen levels remain higher in PCOS patients compared to healthy women even after ovarian suppression with gonadotropin-releasing hormone agonists [5]. However, Moran et al [31] reported that in normal adult women, testosterone is derived 25% from ovaries, 25% from the adrenals, and 50% from peripheral conversion of androstenedione, and in hirsute women with PCOS the predominant source of androgen overproduction is primarily ovarian. This is further supported by findings that adrenal and ovarian stimulation data do not fully account for endogenous testosterone or 11-KT observed in girls with hyperandrogenism and suggests the possibility of peripheral production via AKR1C3 in adipose tissue [32]. Alpañés et al [33] also noted that adrenal hyperandrogenism in patients with PCOS ranged from 20% to 60% in early studies depending on the definition used, age, BMI, and race. Our results support that the primary source of androgens that affect adolescents with PCOS is the ovaries, but a more diverse group of participants may lead to different conclusions.
We found a relationship between adrenal androgens and hirsutism, but not acne or acanthosis (see Fig. 4). This finding is consistent with the argument by Hudson and colleagues [22] that 11-OHA4 can be used as a marker to assess the adrenal contribution to hirsutism. However, the 11-oxyandrogens correlated with so few metabolic measures that they likely do not have a role in the manifestation of dysmetabolism in PCOS.
While this study has many strengths, further investigation is needed on the topic of 11-oxyandrogens and their importance in PCOS. Highlights of this study include a large adolescent sample size of varied weight, with limited data on the role of the 2 most common treatments for PCOS, metformin and COCPs. Although 11-oxyandrogens were not better than testosterone in diagnosing PCOS, this is confounded by the fact that testosterone is part of the diagnosis of PCOS. This study was cross-sectional and longitudinal data are needed. Furthermore, our racial and ethnic demographics reflect that of the local community of Colorado, and a broader, more diverse sample would be needed to determine whether heritage affects 11-oxyandrogen concentrations.
Understanding the role 11-oxyandrogens play in androgen disorders is complex. We did not find that 11-oxyandrogens aid in diagnosing PCOS, and they did not relate to measures of metabolic disease. However, they do relate to clinical signs of hyperandrogenism, and should perhaps be considered in the workup of hirsutism. Metformin does not seem to have a substantial effect on these adrenal androgens and effects of COCPs appear to be modest, though longitudinal randomized studies would be needed to confirm this hypothesis. Finally, these results confirm that ovarian androgen excess is the prevalent contributor to clinical features of PCOS, even in adolescents with pubertal adrenal stimulation.
Acknowledgments
We would like to thank the clinical research nurses for their assistance in performing these protocols, the participants for lending us their time for these important studies, and our research coordinators Rebekah Miller, Allison Hilkin, Lindsey Newnes, and Gregory Coe.
Glossary
Abbreviations
- 11-KT
11-ketotestosterone
- 11-OH
11β-hydroxylase
- 11-OHA4
11β-hydroxyandrostenedione
- 11-OHT
11-OH testosterone
- AIRS
Androgens and Insulin Resistance Study
- ALT
alanine transaminase
- APPLE
Liver and Fat Regulation in Overweight Adolescent Girls
- AST
aspartate transaminase
- AUC
area under the curve
- CGM
continuous glucose monitoring study
- COCP
combined oral contraceptive pill
- DHEAS
dehydroepiandrosterone sulfate
- FAI
free androgen index
- FGS
Ferriman-Gallwey score
- HbA1c
glycated hemoglobin A1c
- HDL-C
high-density lipoprotein cholesterol
- HIPS
Combined Influence of Puberty and Obesity on Insulin Resistance in Adolescents
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- LDL-C
low-density lipoprotein cholesterol
- MISS
Microbiome Insulin Sensitivity Study
- PCOS
polycystic ovary syndrome
- PI
principal investigator
- PLUM
Post-Prandial Liver Glucose Metabolism in PCOS
- ROC
receiver operating characteristic
- SHBG
sex hormone–binding globulin
Contributor Information
Anya E Taylor, Department of Pediatrics, Division of Pediatric Endocrinology, University of Colorado Anschutz Medical Campus, Aurora, Colorado 80045, USA.
Meredith A Ware, Department of Pediatrics, Division of Pediatric Endocrinology, University of Colorado Anschutz Medical Campus, Aurora, Colorado 80045, USA.
Emily Breslow, Department of Pediatrics, Division of Pediatric Endocrinology, University of Colorado Anschutz Medical Campus, Aurora, Colorado 80045, USA.
Laura Pyle, Department of Pediatrics, University of Colorado Anschutz Medical Campus, Aurora, Colorado 80045, USA; Department of Biostatistics and Informatics, Colorado School of Public Health, Aurora, Colorado 80045, USA.
Cameron Severn, Department of Pediatrics, University of Colorado Anschutz Medical Campus, Aurora, Colorado 80045, USA; Department of Biostatistics and Informatics, Colorado School of Public Health, Aurora, Colorado 80045, USA.
Kristen J Nadeau, Department of Pediatrics, Division of Pediatric Endocrinology, University of Colorado Anschutz Medical Campus, Aurora, Colorado 80045, USA; Center for Women’s Health Research, Aurora, Colorado, USA.
Christine L Chan, Department of Pediatrics, Division of Pediatric Endocrinology, University of Colorado Anschutz Medical Campus, Aurora, Colorado 80045, USA.
Megan M Kelsey, Department of Pediatrics, Division of Pediatric Endocrinology, University of Colorado Anschutz Medical Campus, Aurora, Colorado 80045, USA; Center for Women’s Health Research, Aurora, Colorado, USA.
Melanie Cree-Green, Email: Melanie.Green@childrenscolorado.org, Department of Pediatrics, Division of Pediatric Endocrinology, University of Colorado Anschutz Medical Campus, Aurora, Colorado 80045, USA; Center for Women’s Health Research, Aurora, Colorado, USA.
Financial Support
This work was supported by the American Diabetes Association Junior Faculty Award (1-11-JF-23), Children’s Hospital Colorado Research Institute Research Scholar Award Building Interdisciplinary Research Careers in Women’s Health NIH/NICHD BIRCWH K12 (HD057022-06), NIH/NCATS Colorado CTSA UL1 TR001082, Nutrition and Obesity Research Center Pilot Award NIH/NIDDK DK048520-13.
Disclosures
The authors have nothing to disclose.
Clinical Trial Information
ClinicalTrials.gov numbers NCT02157974 (registered June 6, 2014), NCT03041129 (registered February 2, 2017), NCT02211235 (registered August 7, 2014), NCT01775813 (registered January 25, 2013) and NCT03120871 (registered April 19, 2017).
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. O’Reilly MW, Taylor AE, Crabtree NJ, et al. . Hyperandrogenemia predicts metabolic phenotype in polycystic ovary syndrome: the utility of serum androstenedione. J Clin Endocrinol Metab. 2014;99(3):1027-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Teede HJ, Misso ML, Costello MF, et al. . International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodin A, Thakkar H, Taylor N, Clayton R. Hyperandrogenism in polycystic ovary syndrome. Evidence of dysregulation of 11beta-hydroxysteroid dehydrogenase. N Engl J Med. 1994;330(7):460-465. [DOI] [PubMed] [Google Scholar]
- 4. Cree-Green M, Bergman BC, Coe GV, et al. . Hepatic steatosis is common in adolescents with obesity and PCOS and relates to de novo lipogenesis but not insulin resistance. Obesity (Silver Spring). 2016;24(11):2399-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pretorius E, Arlt W, Storbeck KH.A new dawn for androgens: novel lessons from 11-oxygenated C19 steroids. Mol Cell Endocrinol. 2017;441:76-85. [DOI] [PubMed] [Google Scholar]
- 6. Turcu AF, Rege J, Auchus RJ, Rainey WE. 11-Oxygenated androgens in health and disease. Nat Rev Endocrinol. 2020;16(5):284-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rege J, Turcu AF, Kasa-Vubu JZ, et al. . 11-Ketotestosterone is the dominant circulating bioactive androgen during normal and premature adrenarche. J Clin Endocrinol Metab. 2018;103(12):4589-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Reilly MW, Kempegowda P, Jenkinson C, et al. . 11-Oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(3):840-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Torchen LC, Sisk R, Legro RS, Turcu AF, Auchus RJ, Dunaif A. 11-Oxygenated C19 steroids do not distinguish the hyperandrogenic phenotype of PCOS daughters from girls with obesity. J Clin Endocrinol Metab. 2020;105(11):e3903-e3909. https://pubmed.ncbi.nlm.nih.gov/32797203/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keevil B. Steroid mass spectrometry for the diagnosis of PCOS. Med Sci (Basel). 2019;7(7):78. https://pubmed.ncbi.nlm.nih.gov/31295971/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoshida T, Matsuzaki T, Miyado M, et al. . 11-Oxygenated C19 steroids as circulating androgens in women with polycystic ovary syndrome. Endocr J. 2018;65(10):979-990. [DOI] [PubMed] [Google Scholar]
- 12. Owen EJ, Holownia P, Conway GS, Jacobs HS, Honour JW. 11 Beta-hydroxyandrostenedione in plasma, follicular fluid, and granulosa cells of women with normal and polycystic ovaries. Fertil Steril. 1992;58(4):713-718. [PubMed] [Google Scholar]
- 13. Cree-Green M, Rahat H, Newcomer BR, et al. . Insulin resistance, hyperinsulinemia, and mitochondria dysfunction in nonobese girls with polycystic ovarian syndrome. J Endocr Soc. 2017;1(7):931-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carreau AM, Pyle L, Garcia-Reyes Y, et al. . Clinical prediction score of nonalcoholic fatty liver disease in adolescent girls with polycystic ovary syndrome (PCOS-HS index). Clin Endocrinol (Oxf). 2019;91(4):544-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carreau AM, Jin ES, Garcia-Reyes Y, et al. . A simple method to monitor hepatic gluconeogenesis and triglyceride synthesis following oral sugar tolerance test in obese adolescents. Am J Physiol Regul Integr Comp Physiol. 2019;317(1):R134-R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan CL, McFann K, Newnes L, Nadeau KJ, Zeitler PS, Kelsey M. Hemoglobin A1c assay variations and implications for diabetes screening in obese youth. Pediatr Diabetes. 2014;15(8):557-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan CL, Pyle L, Kelsey M, Newnes L, Zeitler PS, Nadeau KJ. Screening for type 2 diabetes and prediabetes in obese youth: evaluating alternate markers of glycemia—1,5-anhydroglucitol, fructosamine, and glycated albumin. Pediatr Diabetes. 2016;17(3):206-211. [DOI] [PubMed] [Google Scholar]
- 18. Kelsey MM, Severn C, Hilkin AM, Pyle L, Nadeau KJ, Zeitler PS. Puberty is associated with a rising hemoglobin A1c, even in youth with normal weight. J Pediatr. 2021;230:244-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jobira B, Frank DN, Pyle L, et al. . Obese adolescents with PCOS have altered biodiversity and relative abundance in gastrointestinal microbiota. J Clin Endocrinol Metab. 2020;105(6):e2134-e2144. doi: 10.1210/clinem/dgz263. PMID: 31970418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462-1470. [DOI] [PubMed] [Google Scholar]
- 21. Bartlette K, Carreau AM, Xie D, et al. . Oral minimal model-based estimates of insulin sensitivity in obese youth depend on oral glucose tolerance test protocol duration. Metabol Open. 2021;9:100078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hudson RW, Lochnan HA, Danby FW, Margesson LJ, Strang BK, Kimmett SM. 11 Beta-hydroxyandrostenedione: a marker of adrenal function in hirsutism. Fertil Steril. 1990;54(6):1065-1071. [PubMed] [Google Scholar]
- 23. Holownia P, Owen EJ, Conway GS, Round J, Honour JW. Studies to confirm the source of 11 beta-hydroxyandrostenedione. J Steroid Biochem Mol Biol. 1992;41(3-8):875-880. [DOI] [PubMed] [Google Scholar]
- 24. Davio A, Woolcock H, Nanba AT, et al. . Sex differences in 11-oxygenated androgen patterns across adulthood. J Clin Endocrinol Metab. 2020;105(8):e2921-e2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burt Solorzano CM, Helm KD, Patrie JT, et al. . Increased adrenal androgens in overweight peripubertal girls. J Endocr Soc. 2017;1(5):538-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keefe CC, Goldman MM, Zhang K, Clarke N, Reitz RE, Welt CK. Simultaneous measurement of thirteen steroid hormones in women with polycystic ovary syndrome and control women using liquid chromatography-tandem mass spectrometry. PLoS One. 2014;9(4):e93805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karakas SE. New biomarkers for diagnosis and management of polycystic ovary syndrome. Clin Chim Acta. 2017;471:248-253. [DOI] [PubMed] [Google Scholar]
- 28. Kjøtrød SB, Sunde A, von Düring V, Carlsen SM. Possible metformin effect on adrenal androgens during pretreatment and IVF cycle in women with polycystic ovary syndrome. Fertil Steril. 2009;91(2):500-508. [DOI] [PubMed] [Google Scholar]
- 29. Goodarzi MO, Carmina E, Azziz R. DHEA, DHEAS and PCOS. J Steroid Biochem Mol Biol. 2015;145:213-225. [DOI] [PubMed] [Google Scholar]
- 30. Luque-Ramírez M, Escobar-Morreale HF. Adrenal hyperandrogenism and polycystic ovary syndrome. Curr Pharm Des. 2016;22(36):5588-5602. [DOI] [PubMed] [Google Scholar]
- 31. Moran C, Arriaga M, Arechavaleta-Velasco F, Moran S. Adrenal androgen excess and body mass index in polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100(3):942-950. [DOI] [PubMed] [Google Scholar]
- 32. Burt Solorzano CM, Beller JP, Abshire MY, Collins JS, McCartney CR, Marshall JC. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids. 2012;77(4):332-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alpañés M, Luque-Ramírez M, Martínez-García MÁ, Fernández-Durán E, Álvarez-Blasco F, Escobar-Morreale HF. Influence of adrenal hyperandrogenism on the clinical and metabolic phenotype of women with polycystic ovary syndrome. Fertil Steril. 2015;103(3):795-801.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.