Abstract
Background
Uterine leiomyomas are benign monoclonal tumors originating from the myometrium. Little information exists concerning metabolomics and the presence of leiomyomas.
Objective
The present study evaluated circulating metabolites in the plasma and their correlation with the presence and size of leiomyomas.
Study Design
Cross-sectional observational study, including women divided into 3 groups: 37 with leiomyomas and uterus >500 cm3, 17 with leiomyomas and uterus ≤150 cm3, and 21 leiomyoma-free. Patients underwent peripheral blood collection using untargeted metabolic assessment by gas chromatography coupled to mass spectrometer.
Results
There was no statistical difference between patients’ anthropometric and demographic features and laboratory tests. Statistical differences in uterus volume (P < 0.0001) were found. Forty-six metabolites were identified (35% amino acids and derivatives, 22% fatty acids, and 18% carbohydrates). Statistically significant metabolic distinction (P < 0.05, false discovery rate< 0.05) was observed for 14 metabolites. Most amino acids (L-isoleucine, L-valine, and pyroglutamic acid) were significantly reduced in plasma levels of patients with large leiomyomas. The only exception was L-glutamine, with a significant increase. Fatty acids (arachidonic acid, alfa-tocopherol, palmitic acid, and stearic acid) were similarly reduced in large leiomyomas patients, except for alpha-linolenic acid, which increased. For carbohydrates (myo-inositol, D-threitol, and D-ribose), there was a decrease in the plasma of patients with leiomyomas.
Conclusion
There are different plasma metabolites levels of amino acids, fatty acids, and carbohydrates among patients with leiomyomas, most of them reduced, but some significantly increased in large leiomyomas, compared to leiomyoma-free patients.
Keywords: leiomyoma, metabolomics, lipidomics, biomarkers, plasma metabolites
Uterine leiomyomas are benign monoclonal tumors originating from the smooth muscle of the myometrium. They consist of disordered bundles of smooth muscle and fibroblasts, as well as excess extracellular matrix (ECM) [1, 2]. They are the most frequent genital tumors in women, reaching 80% prevalence in Black women and 70% in White women of reproductive age [1-5]. Leiomyomas, when symptomatic, can lead to critical impact on quality of life, affect fertility and even cause obstetric complications, such as miscarriage and premature birth [2, 6]. In terms of public health, diagnosis, follow-up, treatment, and complications of uterine leiomyoma are costly, reaching up to $34.4 billion annually in the United States [7]. According to the National Cancer Institute data, the costs surpass that of breast, colon, and ovarian cancer, respectively.
The growth patterns of leiomyomas can vary widely, suggesting that the biological mechanism involved in their formation and development is intricate. Factors such as age, race, hormonal status, parity, and family history are well-known predisposing factors for the emergence and growth of leiomyomas [8]. First-degree relatives diagnosed with fibroids are at greater risk of developing the disease compared to women without affected relatives [9]. Studies with African American descendants have shown that these women have pronounced symptoms earlier than White women [10].
Metabolomics is the final product of events arising from a genetic configuration or drive and its subsequent transcriptomic and proteomic result in the organism, added to other external factors, such as diet, environmental variables, and treatments [11]. Metabolomics has been a promising path in diagnostic medicine and characterization of disease biomarkers [12]. Scarce information on metabolomics and leiomyomas is available, and thus far, a profile of biomarkers for the disease has not been well defined.
The search for biomarkers in patients with leiomyomas seems critical, and early and easy diagnostic methods for such a prevalent disease may be promising. There are few studies in the literature showing tissue metabolomic characteristics of leiomyomas [11]. The only global metabolomic tissue profile was conducted by Heinonen et al [13]. The authors evaluated the global metabolomic profile of 25 tissue samples of leiomyomas and correlated each metabolic finding with 3 well-known gene drives of leiomyomas: Mediator Complex Subunit 12 (MED12) mutation, High mobility group AT-hook 2 (HMGA2) upregulation, and fumarate hydratase (FH) inactivation. They observed that these leiomyomas were distinguishable from each other and from the myometrium due to the abundance of different metabolites. There are no studies, however, that evaluate serum biomarkers profiling in leiomyomas. Knowledge of plasma biomarkers could reduce the high cost of complementary exams, and early serum diagnosis could improve early clinical treatment and increase conservative surgical treatments, lowering the expensive cost with hysterectomies worldwide. In addition, the early identification of fibroids with fast-growing profiles may be a promising path in terms of prognosis and follow-up of patients with leiomyomas. Thus, the aim of this novel study involved evaluating whether there is a difference in specific metabolites in the plasma of patients with leiomyomas, which can be used as metabolic signatures compared to patients without leiomyomas. Furthermore, we wanted to check whether there is a differential profile of metabolites in the plasma of patients with large uterine leiomyomas compared to those with smaller leiomyomas. In this way, we also sought to correlate metabolic differences with tumor growth patterns.
Material and Methods
Study Design
This was an observational cross-sectional study that included women aged 18 to 45 years treated at the Uterine Myoma Section, Department of Gynecology at Escola Paulista de Medicina / Universidade Federal de São Paulo (EPM / UNIFESP). Typical age of onset of menopause ranges between 45 and 50 years, with the mean age being close to 50. An age limit of 45 was established to avoid and decrease possible metabolic biases due to menopausal transition. Diagnosis of leiomyoma was confirmed by transvaginal ultrasound for every patient. Patients diagnosed with endometrial diseases or malignant tumors were excluded. All patients were verbally informed about the objectives and methodology. Those who agreed to participate in the study signed an informed consent form.
Patients with leiomyomas were subsequently divided into 2 groups according to the size of the uterus: group 1 included patients with leiomyomas and large uterus [dimensions >500 cm3 on ultrasound (U500)], and group 2 included patients with leiomyomas and smaller uterus [dimensions ≤150 cm3 on ultrasound (U150)]. Patients with uterine volume between 150 and 500 cm3 were arbitrarily omitted to establish 2 remarkably different groups in terms of uterine volume and leiomyoma size, with a considerable size gap between them.
As a control group (C group), patients from the Family Planning Section, Department of Gynecology at EPM/UNIFESP, aged from 18 to 45 years, who did not present leiomyomas on ultrasound were included. Every participant filled out a clinical form at the medical appointment, including demographic, anthropometric, and clinical data. This study was approved on June 13, 2018, by the Research Ethics Committee of the Federal University of Sao Paulo—EPM/UNIFESP (CAAE 91630218.4.0000.5505).
Sample Collection
Sample and data collection took place between June 2018 and November 2019. Each patient included in the study underwent peripheral blood collection in 2 EDTA tubes (4 mL each) at the Myoma Section Outpatient Clinic. There was no requirement for participants to fast prior to blood collection. One of the tubes was sent to the Central Laboratory of EPM/UNIFESP to analyze blood count, kidney and liver function, and hormonal profile. The other tube was sent to the Laboratory of Molecular and Metabolomic Gynecology at EPM/UNIFESP for processing, centrifugation, and blood plasma collection. Plasma samples obtained were stored at −80°C [14, 15] for further metabolomic evaluation by mass spectrometry at the Beneficent Association for Blood Collection (Colsan).
Extraction of Metabolites From Plasma
Extraction of metabolic and lipid fractions followed the protocol for gas chromatography/mass spectrometry (GC-MS) [16, 17]. Quality control samples (QCs) were prepared from aliquots of pooled samples. Due to the high detection sensitivity of the GCMS-QP 2020NX, blank samples were used throughout the procedure to check the linearity of the noise during the analysis. The blank spectra were then discounted from the analysis spectra, eliminating false chromatographic signals. More details about sample extraction are available in the Supplemental Data [18].
Mass Spectrometry Analysis (Untargeted)
Samples were analyzed using a gas chromatography system coupled to quadrupole mass spectrometry (GCMS-QP2020 NX) from Shimadzu Prominence LC System (Kyoto, Japan). For separation, 1 uL of the sample was loaded onto a DB5-MS column (30 m × 0.25 mm, 0.25 um, Restek). The sample was injected in splitless mode at a 20-mL/min Helium gas flow. The carrier gas was conducted at a constant 1.36-mL/minute flow. Temperature of the column was initially maintained at 80°C and gradually increased at a rate of 15°C/minute until the final temperature of 300°C was reached. The column was held at this same temperature for 8 minutes before cooling down. The injector, the transfer line, the source filament, and quadrupole temperature were maintained at 280°C, 200°C, 150°C, and 150°C, respectively. The system operated in full-scan mode (m/z 40-650) at a rate of 3 spectra/second, and the Electron Ionization (EI) was set at 70 eV. Then, a method was applied to reduce the retention time of the entire analysis [16, 19]. Instrument control, data acquisition, and data processing were performed by LabSolutions software (GCMS version 4.5, Shimadzu Co., Japan) for real-time control of each analyzed analyte in SIM and Scan modes.
Identification and Quantification of Detected Metabolites
The detected metabolites were processed for Scan mode analyses to create a unified matrix of variables with different charge states, adducts, and groups of analytes across all samples. For this, GCMS Solution (v.3.30), NIST 17 MASS (v.1.00.1), and GCMS Smart Metabolite (v.3.01) software were used, all developed by Shimadzu Co. Software was configured as efficiently as possible to process all detected peaks, separating them from the equipment noise. Publicly available online databases were used to evaluate the spectra detected in Scan mode (https://www.genome.jp/kegg/, and http://www.hmdb.ca). To analytically validate the repeatability and robustness of the method, the injection precision, and the analytical variation, all metabolites with relative SD > 30% were removed from the analysis.
Metabolomic Statistical Analysis
Statistical analysis for multi- and univariate metabolomics data was performed in Metaboanalyst 5.0 (http://www.metaboanalyst.ca/) following parametric and nonparametric algorithms. The multivariate analysis (MVA) used unsupervised and supervised models. For the unsupervised analysis, logarithmic transformation was created when necessary, and principal component analysis (PCA) was used. These analyses were conducted to confirm data quality, detect outliers and trends, check the sample’s quality, and analyze the separation between groups. Additionally, an analysis using a supervised model was used, and for that, partial least squares (PLS) regression was used to confirm the separation between groups. For the univariate analysis, a nonparametric version of analysis of variance (Kruskal-Wallis) was applied to the data to obtain significant results (P < 0.05), followed by a post-hoc Tukey’s test [false discovery rate (FDR)] to separate false-positive signals (q = 0.05). The interactome analysis of metabolic pathways of the important molecules for the proposed experimental model was conducted in Cytoscape.
Results
The following variables were initially evaluated: anthropometric and demographic history; menstrual, obstetric history, personal, and family history; symptoms; ultrasound data related to leiomyomas; and general laboratory tests.
Anthropometric and Demographic Variables
No statistical differences regarding anthropometric and demographic variables were observed for the 3 experimental groups: U500, U150, and C (Table 1). Participants’ mean age was between 38 and 39 years, body mass index was between 27 and 29 kg/m2, and weight was between 69 and 75 kg (Table 1).
Table 1.
Anthropometric and demographic variables of the different experimental groups of women analyzed
| Control (normal uterus) (n = 21) | Uterus > 500 cm3 (n = 37) | Uterus < 150 cm3(n = 17) | P-valuea | |
|---|---|---|---|---|
| Height, m | 1.60 ± 0.05 | 1.62 ± 0.06 | 1.58 ± 0,06 | 0.068 |
| Weight, kg | 75.50 ± 11.40 | 74.00 ± 11.20 | 69.44 ± 11.32 | 0.52 |
| BMI, kg/m2 | 29.34 ± 5.84 | 28.14 ± 5.83 | 27.91 ± 5.82 | 0.691 |
| Age, years | 38.71 ± 3.97 | 39.00 ± 4.03 | 39.82 ± 3.99 | 0.682 |
| Race, % | ||||
| White | 52 | 43 | 53 | 0.717 |
| Non-White | 48 | 57 | 47 |
Values are presented as mean ± SD.
Abbreviation: BMI, body mass index; C, control group (normal uterus); U150, uterus < 150 cm3; U500, uterus > 500 cm3.
aChi-square test.
Menstrual, Obstetric, Personal, and Family History
Family history factor was significant (P = 0.0004); U500 and U150 women who had a family history of myoma were statistically different from the C group (Table 2). In addition, U500 participants had a significantly lower median number of pregnancies than the other 2 groups (Table 2). Mean parity of the U500 group was also significantly lower than the mean of the other 2 groups (P = 0.002) (Table 2). There was no statistical difference for age at menarche, smoking, and alcohol consumption among participants.
Table 2.
Variables related to the antecedents of the groups of women analyzed (C, U500, and U150)
| Control (normal uterous) (n = 21) | Uterus > 500 cm3 (n = 37) | Uterus < 150 cm3 (n = 17) | P-valuea | |
|---|---|---|---|---|
| Age at menarche, years | 13.14 ± 1.94 | 12.30 ± 1.92 | 12.906 ± 1.91 | 0.169 |
| Number of pregnancies, median | 2 | 1 | 3 | 0.004 |
| Parity, average | 1.81 ± 1.26 | 0.70 ± 1.27 | 1.71 ± 1.26 | 0.002 |
| Smoking, % | ||||
| No | 95 | 92 | 71 | 0.062 |
| Yes | 5 | 8 | 29 | |
| Alcohol consumption, % | ||||
| No | 76 | 84 | 76 | 0.720 |
| Yes | 24 | 16 | 24 | |
| Familiar history, % | 0.0004 | |||
| No | 95 | 49 | 59 | |
| Yes | 5 | 51 | 41 |
Values are presented as mean ± SD or as frequency. Bolded p-value data show statistically significant variables (p<0.05).
aChi square test.
Symptoms
The effect of abnormal uterine bleeding was significant (P = 0.021). U150 women complained significantly more about abnormal uterine bleeding than U500 women and the C group (P = 0.048 for multiple comparisons). The effect of acyclic pelvic pain was also significant (P = 0.0002). The proportion of pelvic pain for the C group was significantly lower than the leiomyoma groups (P = 0.002 for C × U500 and P = 0.043 for C × U150, using multiple comparisons) (Supplemental Table 1 [18]). Dysmenorrhea (P = 0.008) was also significantly lower for the C group compared to U500 women (P = 0.011). On the other hand, the presence of dyspareunia did not show statistical difference between the 3 groups (P = 0.294).
The increase in urinary frequency was also significant (P = 0.006); that is, the percentage of U500 patients (38%) with increased urinary frequency was significantly higher than the C group (10%) and U150 women (6%). Constipation was also significant (P = 0.008) (Supplemental Table 1 (18)), and U500 women showed a higher percentage (30%) of constipation, with statistical significance (Supplemental Table 1 (18)).
Ultrasound Data
As expected, the uterine volume effect was remarkably significant (Chi-square test P < 0.0001) (Table 3). There was a significant difference between the estimated means of the uterus volume of the different groups. The mean uterine volume of U500 participants was 906.9 cm3, which is significantly higher than the estimated mean of the U150 patients (130.7 cm3) and the C group (79.2 cm3). There was no statistical difference, however, between the C group and U150 participants.
Table 3.
Estimated means for each experimental group in relation to uterine volume
| Group | n | Estimated average | Inferior limit | Superior limit | Adjusted P-value |
|---|---|---|---|---|---|
| Control (normal uterus) | 21 | 79.20 | −120.31 | 278.71 | <0.001 (C × U500) |
| Uterus > 500 cm3 | 37 | 906.91 | 756.60 | 1057.22 | 0.9388 (C × U150) |
| Uterus < 150 cm3 | 17 | 130.71 | −91.04 | 352.46 | <0.001 (U500 × U150) |
Chi-square test for uterine volume was significant (P < 0.0001).
The U500 group had significantly higher leiomyoma median frequency than the U150 group (P = 0.0004). In the U150 group, 70.6% of the participants had only 1 fibroid on ultrasound, while in the U500 group, 51.4% had ≥5 fibroids on examination (Table 4). There was also a difference between the 2 leiomyoma groups for the International Federation of Gynecology and Obstetrics (FIGO) classification system (P < 0.0001). The FIGO classification subdivides fibroids into 9 different types (FIGO 0-8), according to their location in the uterus. Fibroids can be submucosal, intramural, subserosal, hybrid, or nonmyometrial. The FIGO classification system has an essential role in describing and classifying leiomyomas uniformly [20]. FIGO 2 to 5 classification was observed in 46% of the U500 group, while in the U150 group, prevalence was 12%. In comparison, FIGO 4 classification was observed in 11% and 47% of U500 and U150 groups, respectively.
Table 4.
Frequency distribution of the number of leiomyomas by experimental group
| Leiomyomas, n | Uterus > 500 cm3 | Uterus < 150 cm3 | ||
|---|---|---|---|---|
| Frequency | % | Frequency | % | |
| 1 | 9 | 24.3 | 12 | 70.6 |
| 2 | 5 | 13.5 | 2 | 11.8 |
| 3 | 4 | 10.8 | 2 | 11.8 |
| ≥5 | 19 | 51.4 | 1 | 5.9 |
Laboratory Tests
Hemoglobin count was the only laboratory test that was significantly different between the experimental groups (P = 0.0179) (Supplemental Table 2 [18]). Although the 3 groups had numbers within laboratory reference values (12.0-15.5 g/dL), the hemoglobin of the C group was significantly higher than that of the U500 group. No statistical differences were observed between the different experimental groups in terms of liver function (glutamic oxaloacetic transaminase and glutamic pyruvic transaminase ), kidney function (creatinine, urea), and hormonal status (follicle-stimulating hormone, luteinizing hormone, estradiol). All were within reference values. Follicle-stimulating hormone, luteinizing hormone, and estradiol confirmed the premenopausal status of all study participants (Supplemental Table 2 [18]).
Metabolic Profile
Chromatographic fingerprint by GC-MS
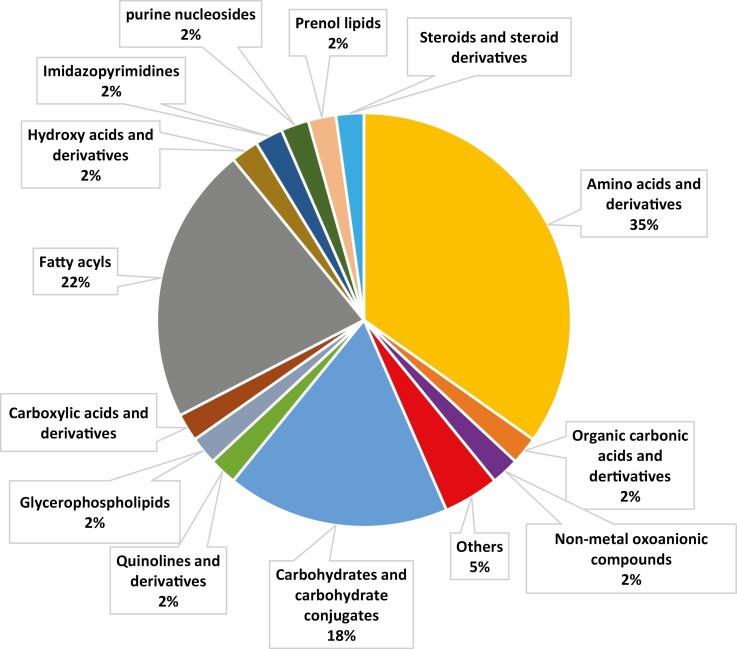
The chromatographic profile of the 75 samples performed by GC-MS generated a matrix of 46 molecules, including the QCs. Metabolites found in the plasma from all serum samples are shown in Figure 1. According to biochemical classification, the polar and nonpolar molecules derivatized and chromatographically detected by GC-MS were separated. The detected classes were amino acids and derivatives (35%), fatty acids (22%), carbohydrates and conjugates (18%), and external metabolites (5%), with other classes making up 2% each. (Fig. 1). The forty-six metabolites were identified and separated according to biochemical classification, identification code, level, and identification key using the Human Metabolome Database (https://hmdb.ca/). Relative SD was calculated from QCs (Supplemental Table 3 [18]). As previously reported, data quality was ensured using QC samples [16, 17]. More information concerning chromatographic fingerprint is also available in the Supplemental Data [18].
Figure 1.
Serum metabolic profile detected according to respective biochemical classification.
Multivariate Analysis
For the MVA, data from the final matrix were submitted to unsupervised chemometric analysis PCA in Metaboanalyst 5.0 to test the analytical reproducibility of the data [19]. The grouping of QCs involves analytical conditions with good robustness and reproducibility (Supplemental Figure 1 [18]). However, the differences between samples/groups from the PCA analysis were minor. The same result was observed for the supervised multivariate analysis (PLS) (Fig. 2A and 2B). The predictive value of Q2 quality was just above 0.4.
Figure 2.
(A) Two-dimensional projection of the metabolite profile of patients in the 3 groups (U500, U150, and C). The partial least squares-discriminant analysis is a supervised multivariate analysis demonstrating grouping of patients in the U500 (blue), U150 (green), and control groups (red), according to the profile of metabolites. (B) Predictive value of quality (Q2) is just above 0.4. C, control group (normal uterus); U150, uterus < 150 cm3; U500, uterus > 500 cm3.
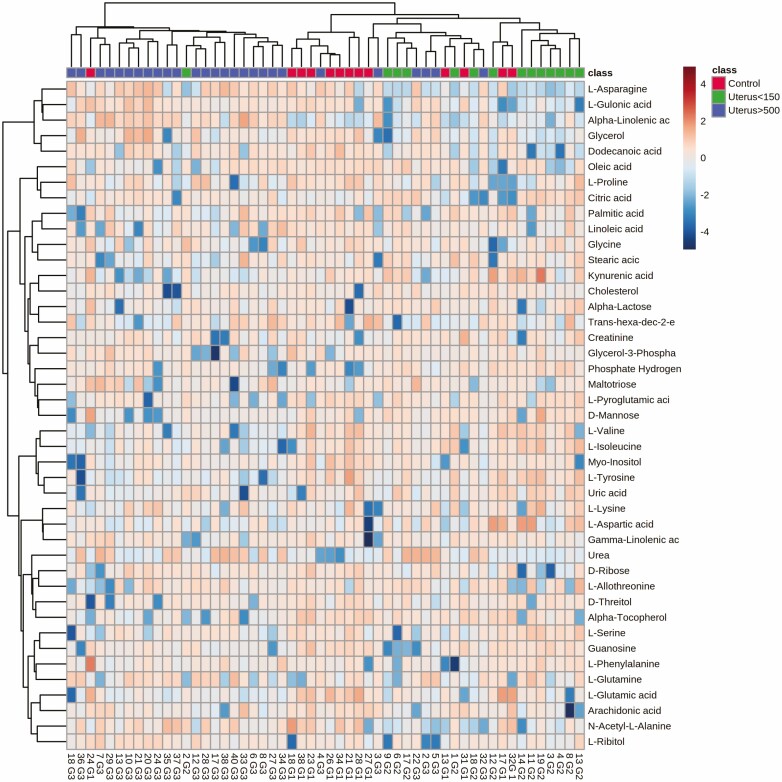
The MVA analysis was complemented using a heat map to identify the pattern and trend of distribution of metabolites according to the 3 groups. No clear separation between the 3 groups was seen. Therefore, the differences observed between the groups were subtle using MVA (Fig. 2A and 2B, Fig. 3).
Figure 3.
Heatmap of the metabolites found: unsupervised analysis contrasting the U500 (blue), U150 (green), and control (red) groups, according to the metabolite profile. The heat map did not show strong separation between the groups. C, control group (normal uterus); U150, uterus < 150 cm3; U500, uterus > 500 cm3.
Univariate Analysis
The Kruskal-Wallis test was applied to test for significant differences between the experimental groups for each metabolite. Fourteen metabolites were found to be statistically significant (Table 5).
Table 5.
Kruskal-Wallis test showing all 18 metabolites with statistically different (P < 0.05) abundances in the plasma of patients for each experimental group
| Metabolites | P-value | FDR |
|---|---|---|
| Amino acids | ||
| L-isoleucine | 0.000599 | 0.001433 |
| L-valine | 0.000121 | 0.000399 |
| L-pyroglutamic acid | 0.000238 | 0.000640 |
| L-Glutamine | 0.008507 | 0.015904 |
| L-asparagine | 0.010485 | 0.188850 |
| L-tyrosine | 0.014210 | 0.872890 |
| Fatty acids | ||
| Alpha-linolenic acid | 0.000068 | 0.029296 |
| Arachidonic acid | 0.000213 | 0.000636 |
| Alpha-tocopherol | 0.000222 | 0.000636 |
| Palmitic acid | 0.002714 | 0.005835 |
| Stearic acid | 0.004203 | 0.008214 |
| Carbohydrates | ||
| Myo-inositol | 0.000110 | 0.0003945 |
| D-ribose | 0.000454 | 0.001148 |
| D-threitol | 0.013159 | 0.023576 |
| D-mannose | 0.021572 | 0.231890 |
| Organic acids, nucleotides, and other classes | ||
| Uric acid | 0.003361 | 0.006882 |
| Hydrogen phosphate | 0.000814 | 0.001842 |
| Kynurenic acid | 0.013175 | 0.188850 |
Metabolites are grouped according to biochemical class. In bold, metabolites with significant FDR are shown. In total, we found 14 metabolites with both statistically significant P-value and FDR.
Abbreviation: FDR, false discovery rate.
Figures 4 to 7 represent the mean quantities of compounds with significant variation (P < 0.05) for all metabolites identified for the 3 groups (U500, U150, and C) of patients. All figures represent qualitative and analytical results estimated from the variance of the areas detected for each metabolite in a box diagram to visualize the sample variation within each group of patients and possible outliers. It is important to notice in Table 5 that L-asparagine, L-tyrosine, D-mannose, and kynurenic acid, despite P < 0.05, have FDR > 0.05 and are therefore nonsignificant.
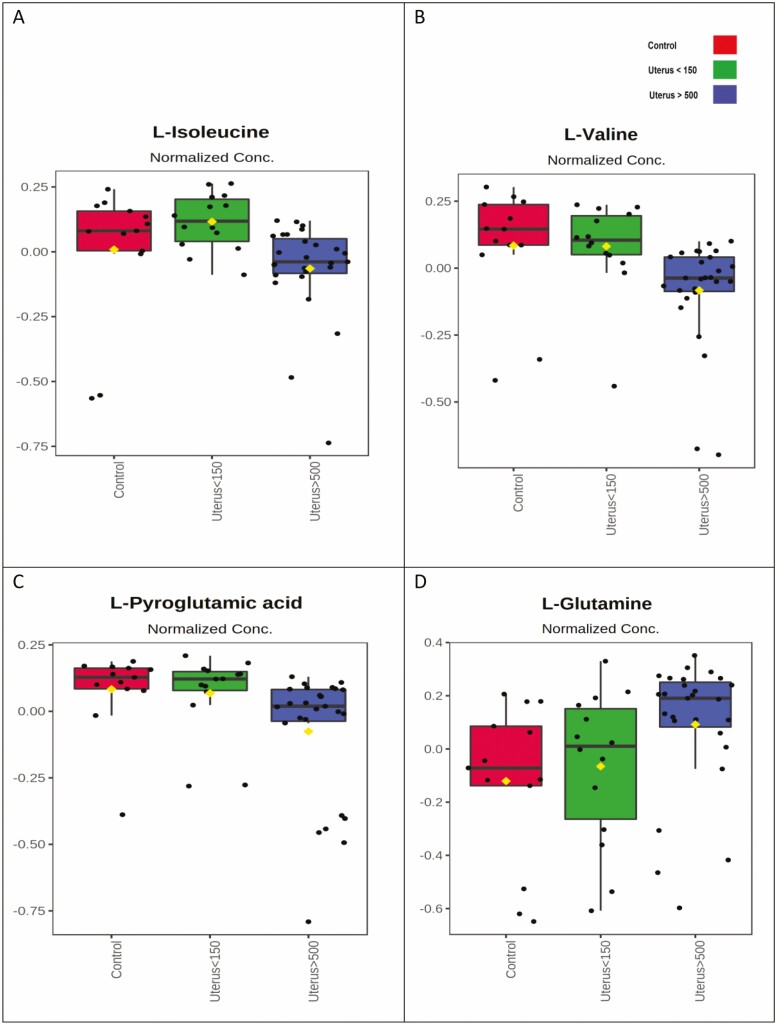
Figure 4.
Qualitative comparison of each experimental group’s amino acid plasma levels with significant differences. Mean abundances for each metabolite and the variance are shown. Control group (red), U150 (green), and U500 (blue). C, control group (normal uterus); U150, uterus < 150 cm3; U500, uterus > 500 cm3.
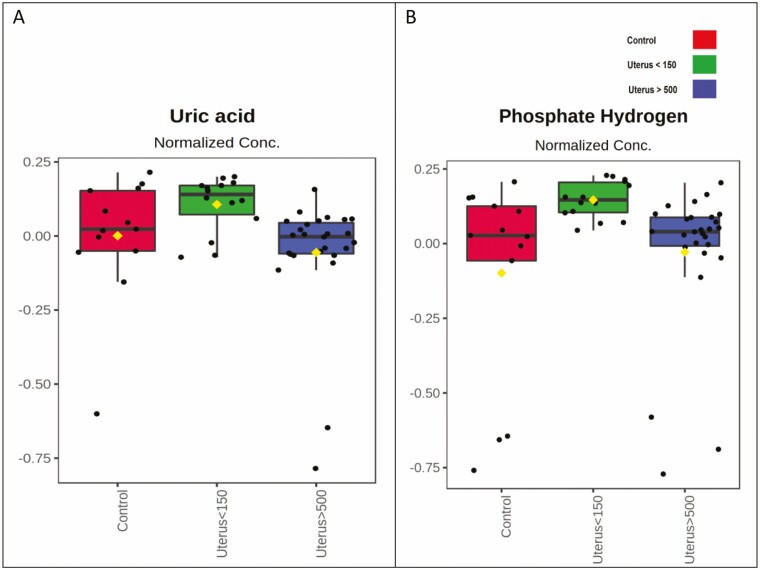
Figure 7.
Qualitative comparison between plasma levels of organic acids, nucleosides, and other classes of metabolites by experimental group with significant differences. The mean abundance for each metabolite and the variance are shown. Control group (red), U150 (green), and U500 (blue). C, control group (normal uterus); U150, uterus < 150 cm3; U500, uterus > 500 cm3.
Qualitative comparison between plasma levels of each amino acid with significant differences can be seen in Figure 4. A decrease in isoleucine, valine, and pyroglutamic acid and an increase in glutamine was observed in the U500 group. The amino acids isoleucine, valine, and pyroglutamate did not vary between the C and U150 groups, compared to the U500 group. Glutamine levels showed a progressive increase for the U150 and U500 groups compared to the C group.
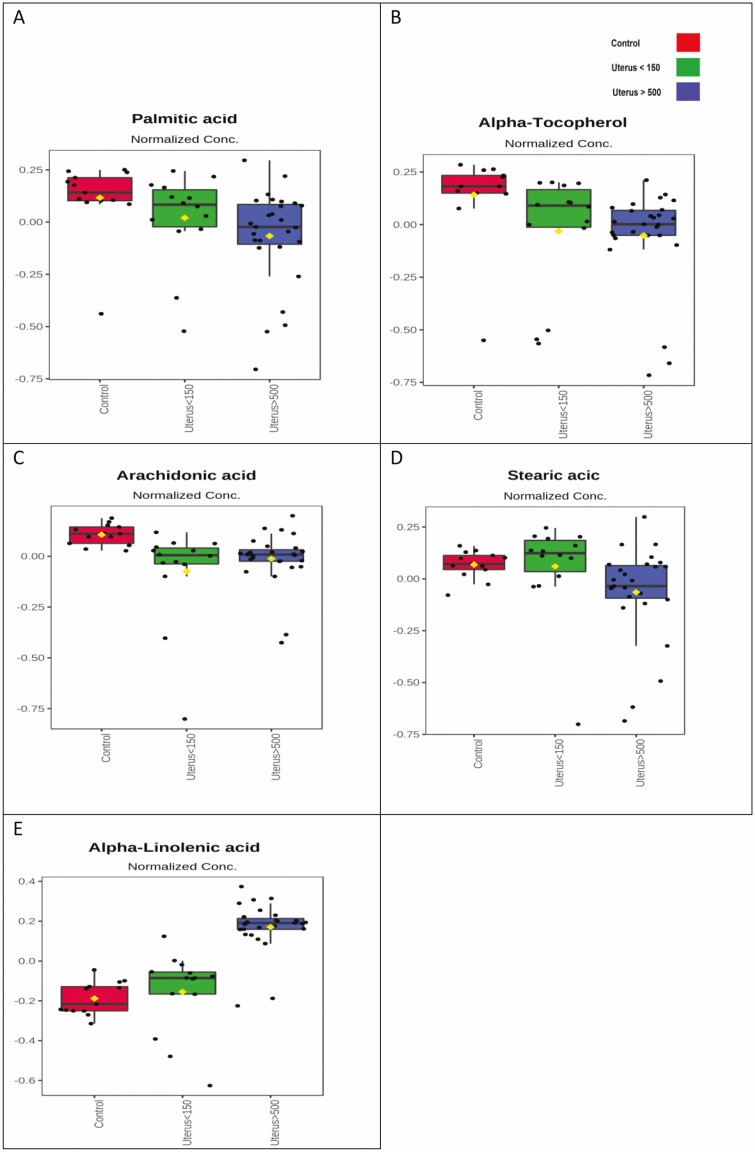
Figure 5 shows significant variation in fatty acid between the 3 groups. Palmitic acid and alpha-tocopherol showed a progressive drop between the C group and the U150 and U500 groups. Reduction in arachidonic acid in both leiomyoma groups compared to the C group is also observed. The level of stearic acid practically did not change in the C and U150 groups, with a drop only for the U500 group. Alpha-linolenic acid, however, showed a progressive increase in the U150 group and a more significant increase in the U500 group.
Figure 5.
Qualitative comparison between plasma levels of each fatty acid by experimental group with significant differences. The mean abundance for each metabolite and the variance are shown. Control group (red), U150 (green), and U500 (blue). C, control group (normal uterus); U150, uterus < 150 cm3; U500, uterus > 500 cm3.
In Figure 6, carbohydrate compounds show significant variation between the 3 groups. The carbohydrates myo-inositol and D-threitol showed a similar trend of progressive decrease when comparing the C group with the U150 and U500 groups, respectively; however, the decrease in D-threitol was visually subtler. Decreasing levels for D-ribose were seen in the U150 group; however, when comparing the C and U500 groups, there was practically no variation for this carbohydrate.
Figure 6.
Qualitative comparison between plasma carbohydrate levels by experimental group showing significant differences. The mean abundances for each metabolite and the variance are shown. Control group (red), U150 (green), and U500 (blue). C, control group (normal uterus); U150, uterus < 150 cm3; U500, uterus > 500 cm3.
Organic acids, nucleosides, and other metabolites showing significant variation between groups are represented in Figure 7. Uric acid and hydrogen phosphate level differences were visually subtle between all 3 groups.
Discussion
Our study identified 46 metabolites in the patients’ plasma. In the MVA, although PCA showed good robustness and reproducibility of the analysis, the predictive value of Q2 quality in PLS showed weak separation between groups and was not strong enough to highlight significant compounds for classification. On the other hand, in the univariate analysis, 14 metabolites had statistically significant differences between the 3 experimental groups, evidencing a metabolic distinction between them. Most metabolites were significantly less abundant in patients with leiomyomas compared to patients in the C group. In addition, some of these metabolites were even less abundant in patients with larger uterus (U500) compared to patients with smaller uterus (U150).
The metabolome of human biological samples is highly dynamic and influenced by numerous factors such as nutritional status, dietary supplements, hydration, circadian rhythm, lifestyle, and individual pathological conditions [21-23]. When studying plasma samples, a wide range of conditions can affect the metabolome and act as confounding factors that should always be considered and minimized [24]. Our study used 3 experimental groups with demographic, anthropometric, and laboratory characteristics as homogeneous as possible to minimize potential biases and focus on the possible metabolic differences due to the presence of leiomyoma and its variations. Age, race, body mass index, kidney function, liver function, and hormonal status did not differ between the 3 groups. Factors with statistical differences identified in the initial sample analysis, such as parity, number of pregnancies, and family history, are epidemiological factors already known to be related to an increased prevalence of leiomyomas [4].
A higher or lower plasma concentration of a specific metabolite is usually related to 2 main reasons: first, due to an eventual larger or smaller supply of that metabolite in the diet or any specific individual condition and, second, due to a lower or higher use of that metabolite [23] in a particular condition, for example, when a leiomyoma is present.
As previously mentioned, Heinonen et al [13] observed metabolomic differences between 3 different genetic subtypes of leiomyomas and between tumor and healthy myometrium samples. The FH leiomyoma subtype had a metabolomic profile showing increased levels of metabolites in the chromatographic analysis. In contrast, fibroids of the MED12 subtype showed a reduction in the levels of metabolites [13]. The authors also observed that MED12 subtype fibroids were phenotypically smaller in volume. In contrast, the FH subtype was larger [13]. Such clinical findings are compatible with our study. The study of plasma metabolome samples, as seen here, shows a scenario of free circulating metabolites [24], and a lower concentration of most metabolites was observed in the plasma of patients with large leiomyomas in our study. These findings suggest that some metabolites could be consumed or accumulated in tumor tissues, as observed in patients with phenotypically larger myomas of the FH subtype [13]. The same reasoning applies to smaller leiomyomas whose metabolic plasma levels varied in magnitude in our study. Our results are compatible with a moderate metabolomic profile found in tumor tissues of MED12 fibroids and HMGA2 [13], which are usually smaller [13].
Amino Acids
We observed reduced levels of amino acids, except glutamine, in the plasma of patients with leiomyomas, especially those with large leiomyomas (U500). Isoleucine, valine, and pyroglutamic acid were significantly reduced in the plasma of U500 patients with large leiomyomas (U500). Interestingly, most of these amino acids (valine and isoleucine) are present in large quantities in ECM proteins, particularly in collagen composition [25]. Our results suggest that leiomyomas, especially those with larger volumes, use up these types of amino acids, probably accumulating them as unstructured collagen-like proteins of the ECM.
Large leiomyomas generally present enormous amounts of disorganized collagen in the extracellular matrix (ECM), compared to normal myometrium [26, 27]. The structure of ECM in leiomyomas has significant amounts of collagen in its composition, disorganized by metalloproteases, which break its compounds and structure [28]. Thus, the excessive, degraded, and disorganized deposition of ECM components can lead to tumor growth [26, 27, 29, 30]. Furthermore, Islam et al [31] showed that the main composition of leiomyomas is based not only on the deposition of structural components in the ECM, such as collagen proteins, laminins, and proteoglycans, but on the constant transformation of such components by a molecular mechanism called mechanotransduction [26, 27, 31]. This phenomenon involves inflammation and fibrosis, requiring adhesion, migration, and consumption of molecules (among them, amino acids), which are constantly forming large new protein molecules [26, 27].
It is interesting to observe the trend for glutamine, which, unlike the other amino acids, was significantly increased in patients presenting small (U150) and large leiomyomas (U500). Glutamine is a nonessential amino acid and can be supplied from the diet. It is the precursor of glutamate and plays a central role in cellular metabolism. Interestingly, the glutaminolysis process and the consequent formation of glutamate, promoted by the loss of ammonia from glutamine, significantly contribute to cell proliferation and growth via the synthesis of fatty acids, amino acids, and nucleic acids [32-35]. Its role in tumorigenesis and tumor growth is very complex and has been the subject of many studies [33-35].
Targeted therapies involving glutaminase inhibition are a possible strategy for tumorigenesis inhibition [33]. One mechanism of carcinogenesis revolves around glutamine catabolism and glutamate production, both responsible for cell proliferation and growth [33]. Correlating with our study, glutamine is not a component of the ECM and, therefore, would not be used up in the leiomyoma ECM. A possible explanation for the progressive increase in plasma levels of glutamine observed in the U150 and U500 groups may involve an increased dietary availability of such metabolites among these women. On the other hand, higher glutamine concentration could be followed by increased glutaminolysis and cell proliferation and growth. However, in our study, we did not observe higher abundances of glutamic acid (or glutamate) in these patients, resulting from supposedly higher glutaminolysis, and plasma levels of glutamic acid were not statistically significant. It is critical to highlight at this point that the literature correlates glutaminolysis with an increase in malignant tumor proliferation [32-34], and the cellular mechanism of action in benign tumors could be different.
Fatty Acids
Regarding fatty acid profiles, such amino acids, lower plasma levels were observed in patients with leiomyomas. The only exception was an increase in α-linolenic acid. Palmitic acid, α-tocopherol, and arachidonic acid levels were reduced in the plasma of patients with leiomyomas. Reduction was progressive for the first 2 fatty acids, whose levels were even lower in women with larger leiomyomas (U500).
Lipid homeostasis is quite complex and fundamental for cell function, acting in cell signaling and energy accumulation, in addition to membrane structuring and cell remodeling [36]. Lipidomics have been studied and related to pathophysiological processes for various diseases [37, 38]. For leiomyomas, however, evidence on the role of lipids is still scarce in the literature.
Palmitic acid and α-tocopherol are essential fatty acids obtained from external sources. They participate in endogenous biochemical processes of paramount importance in our body, as precursors to long-chain fatty acids (mainly palmitic acid) or antioxidants (in the case of α-tocopherol) [39, 40]. Possible reasoning for lower plasma levels of palmitic acid and α-tocopherol observed in patients with leiomyomas could be related to the previously decreased offer of such fatty acids. Consequently, a decrease in these patients’ antioxidant profile and anti-inflammatory process could increase an inflammatory response and tumor growth. Arachidonic acid is an essential fatty acid obtained through diet and has fundamental role in cell membrane structuring. Its precursor is linoleic acid (fatty acid of the ω6 family), and its oxidative enzymatic routes are the lipoxygenase and the cyclooxygenase pathways. The latter is involved in the formation of prostaglandins and thromboxanes, acting directly on inflammatory processes [41].
In 2018, Islam et al observed that arachidonic acid was the most abundant fatty acid in both myoma and myometrium tissue samples, along with oleic, palmitic, and stearic acids. As reported before, the most abundant fatty acid in myoma samples was linoleic acid, a precursor of arachidonic acid [13]. In their 2017 global metabolomic profile, Heinonen et al [13]. identified higher levels of lipids and sphingolipids in myomas compared to healthy myometrium and even higher levels in clinically larger myomas (FH genomic subtype) compared to smaller ones (subtype MED12). Therefore, such findings could explain the observed reduced levels of circulating fatty acids in the plasma of patients with leiomyomas in our study (arachidonic, palmitic, and stearic acids). These fatty acids could be accumulating in the leiomyomas’ tissue and adjacent myometrium, as seen in the previously discussed studies.
However, the abundance of α-linolenic acid was noteworthy, especially in the plasma of patients with large leiomyomas. α-Linolenic acid belongs to the group of essential fatty acids called ω3. It has numerous functions metabolically, involving the structural components of cell membranes and modulation of gene expression, fatty acid metabolism, and inflammation [42-44]. ω3 fatty acids active components, eicosapentaenoic acid and docosahexaenoic acid, may reduce the expression of the CYP11A1 enzyme in myometrial cells [45]. This enzyme catalyzes the conversion of cholesterol to pregnenolone, a precursor of sex steroids. These data suggest that ω3 fatty acids alter the regulatory mechanism of steroidogenesis, acting directly on the pathogenesis of leiomyoma. Moreover, it is known that the pathophysiology of many diseases is related to the imbalance between 2 families of polyunsaturated fatty acids (ω3 and ω6), which are constantly competing for the same desaturation enzyme. However, predicting the ultimate phenotypic results of this imbalance is difficult and complex.
Our study showed progressive increase in plasma levels of α-linolenic acid in patients with small and large leiomyomas, contradicting the expected inhibitory effect of this fatty acid on gene expression and steroidogenesis [45]. We did not observe any statistical difference in the plasma levels of linoleic acid in our patients. However, in patients with leiomyomas, we observed significantly lower plasma levels of arachidonic acid, its active component. As we inferred earlier, arachidonic acid could be used up at the tissue level, promoting higher cell growth. Another possible explanation for such results [46] is that arachidonic acid in these patients’ leiomyomas could overlap the active components of α-linolenic acid, hindering its inhibitory effect.
Another line of reasoning is that our population study has a significantly larger plasma abundance of α-linolenic acid, a fatty acid known for its carcinogenic inhibitory properties [47, 48]. Leiomyoma is a benign disease. We could clinically encounter malignant tumors if the lipid plasma scenario were different for such fatty acids. Such inference is precisely in line with the concept of metabolomics, which translates the phenotypic metabolic expression of a specific disease.
In sum, there is no doubt that ω3 and ω6 fatty acids have central roles in cell communication, balance, and homeostasis and a critical metabolic function in fibroids’ specific growth.
Carbohydrates
Progressively lower and statistically significant levels of mayo-inositol and D-threitol were observed in the plasma of patients with leiomyomas. These carbohydrates can be found in both exogenous and endogenous forms and have different functions in human metabolism, including as precursors of phosphoinositol synthesis (myo-inositol) and nucleic acid synthesis (D-ribose). There are no studies in the literature on carbohydrate metabolomics and their relationship with leiomyomas. However, the metabolic profile of sugars is well studied in oncology. It is noteworthy that our results are consistent with such effects, and it is possible to infer some analogies for uterine fibroids.
It is known that tumors, in general, exhibit a higher concentration of glucose. Understanding the metabolism of other sugars and how the tumor responds is critical for understanding tumors’ pathophysiology [49, 50]. Myo-inositol, or simply inositol, and its compound inositol-hexaphosphate are polyphosphorylated carbohydrates found in plants, fibers, cereals, and vegetables. Inositol-hexaphosphate has a significant antioxidant effect, acting directly on signal transduction and cell proliferation inhibition, in addition to increasing immunity and reducing malignant cell differentiation [51]. Progressively lower plasma myo-inositol levels in our patients may suggest increased cell proliferation and consequent tumor enlargement. D-threitol is a carbohydrate containing 4 carbons and has a weak inhibitory effect on carbonic anhydrase, an enzyme related to CO2 transport, blood pH control, and cell proliferation [52]. Perhaps the lower concentration of D-threitol in plasma observed in patients with leiomyomas reduces the inhibitory effect on myoma cells proliferation.
Organic Acids, Nucleosides, and Other Classes
Plasma levels of uric and acid hydrogen phosphate, despite being significantly different, showed a very subtle visual difference in the 3 experimental groups.
General Considerations
Our study provided 3 well-defined experimental groups, with significantly different leiomyomas’ sizes and plasma levels involving amino acids, fatty acids, and carbohydrates. These concentration differences may be essential for understanding molecular processes of the growth of leiomyomas. Moreover, our study adds to previous metabolomics studies involving leiomyoma tissues by examining the plasma of 2 phenotypically distinct groups of patients with leiomyomas, as well as a C group [13]. Therefore, we generated an unprecedented metabolic panel that represents an initial step forward for the definition of serum biomarkers. This panel brings new reflections on the complex pathophysiology of leiomyomas and gets a little closer to the promising path of noninvasive serum diagnosis and early target therapies [13].
Differences in plasma metabolites levels, especially in patients with large leiomyomas, leads to some inferences and comparisons with oncological conditions. There is a current and persistent search for an early diagnostic method for uterine sarcoma [53]. Yet, to date, there are no studies in the literature correlating metabolomic and uterine sarcomas. However, the molecular action of amino acids in the ECM, the antitumor effects of some carbohydrates, and, especially, the complexity of lipid homeostasis and its controversial pro- and antioncogenic effect regarding ω3 and ω6 fatty acids families are noteworthy and thus reinforce the need for additional studies directed to these specific metabolites in patients with leiomyomas and uterine sarcoma.
Strengths and Limitations
Our study has limitations and reflects an initial and nontargeted assessment of the metabolomics of uterine leiomyomas. As previously discussed, we present data on plasma metabolite concentrations, and although we attempt to establish homogeneous groups, we are aware that plasma metabolites may be subject to other metabolic confounding factors (eg, dietary supplements, physical activity, environmental variables, stage of menstrual cycle) that could affect the interpretation of metabolomic profiles. Therefore, we are aware that our findings represent preliminary data that warrant further study in a randomized fashion after controlling for these variables. However, nontargeted studies are excellent methods for initiating biomarkers discovery, and this novel plasma-profiling study inevitably motivates the search for leiomyoma signatures and raises an interesting pathophysiology discussion. However, it does not validate such metabolites as biomarkers. To do so, a targeted search for significant candidate metabolites using the same samples should be the next step [54]. Another limitation is that we used only GC-MS in our metabolomic analysis. Further analysis by liquid chromatography coupled with mass spectrometry and other analysis platforms could improve accuracy, advancing the scenario for a global fingerprinting of metabolites [13].
Conclusions
The quantitative and nontargeted metabolomic profile obtained from the plasma of patients with leiomyomas shows distinct metabolic profiles compared to leiomyoma-free patients. In addition, women with large leiomyomas have a plasma concentration of metabolites different from those with smaller leiomyomas. Such metabolic differences are mainly related to free amino acids, fatty acids, and carbohydrates, most of which are in low concentrations in the plasma of these patients. This novel metabolic profile constitutes an initial and preliminary step toward defining easily accessible and low-cost biomarkers and metabolic signatures of this prevalent disease. An important next step is a targeted sequential analysis that highlights the amino acids most frequently found in the ECM and fatty acids from the ω3 and ω6 families, whose homeostasis seems to have essential involvement in tumor growth.
Acknowledgments
We have to begin our honorable acknowledgments by expressing our deepest gratitude to Prof M.J.B.C. Girão (in memoriam) for the idealization, support and viabilization of our study. We also sincerely thank the Department of Gynecology of EPM/UNIFESP; M.A. Izidoro and the staff of the Beneficent Association for Blood Collection (Colsan) for all the support and for conducting the metabolomic sample analysis; and P. D’Amora and the staff of the Laboratory of Molecular and Metabolomic Gynecology at EPM / UNIFESP, who also made this study possible.
Contributor Information
Gustavo Anderman Silva Barison, Email: gu.barisa@gmail.com, Department of Gynecology, Federal University of Sao Paulo, Sao Paulo, Brazil.
Paulo D’Amora, Department of Gynecology, Federal University of Sao Paulo, Sao Paulo, Brazil.
Mário Augusto Izidoro, Spectrometry Laboratory; Beneficent Association for Blood Collection (COLSAN), Sao Paulo, Brazil.
Mariana Corinti, Department of Gynecology, Federal University of Sao Paulo, Sao Paulo, Brazil.
Luísa Marcella Martins, Department of Gynecology, Federal University of Sao Paulo, Sao Paulo, Brazil.
Claudio Emílio Bonduki, Department of Gynecology, Federal University of Sao Paulo, Sao Paulo, Brazil.
Rodrigo de Aquino Castro, Department of Gynecology, Federal University of Sao Paulo, Sao Paulo, Brazil.
Manoel João Batista Castello Girão, Department of Gynecology, Federal University of Sao Paulo, Sao Paulo, Brazil.
Mariano Tamura Vieira Gomes, Department of Gynecology, Federal University of Sao Paulo, Sao Paulo, Brazil.
Funding
The authors received no specific funding for this work
Authors’ Contributions
G.A.S.B. was the main author, designed experiments and wrote the manuscript. P.D. helped in the sample initial analysis and project initial structure. M.A.I. helped in the metabolomics experiments and the manuscript writing. M.C.S. and L.M.M. helped in the sample collection. R.A.C. and C.E.B. helped in the project’s structure and manuscript revision. M.J.B.C.G. helped in the revision of the project and the manuscript. M.T.V.G. was the main supervisor and helped in the project structure and revision of the manuscript.
Disclosures
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Data Availability
The data set used during the current study are available from the corresponding author on reasonable request. Supplementary data to this article can be found online at https://doi.org/10.5061/dryad.gb5mkkwrw.
References
- 1. Lemgruber M, Costa O, Lemgruber I.. Mioma uterino. Tratado de Ginecologia. Revinter;2000. [Google Scholar]
- 2. Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nat Rev Dis Primers. 2016;2:1-18. [DOI] [PubMed] [Google Scholar]
- 3. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100-107. [DOI] [PubMed] [Google Scholar]
- 4. Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94(4 suppl 1):435-438. [DOI] [PubMed] [Google Scholar]
- 5. Borgfeldt C, Andolf E. Transvaginal ultrasonographic findings in the uterus and the endometrium: low prevalence of leiomyoma in a random sample of women age 25-40 years. Acta Obstet Gynecol Scandinavia. 2000;79(3):202-207. [PubMed] [Google Scholar]
- 6. Ezzedine D, Norwitz ER. Are women with uterine fibroids at increased risk for adverse pregnancy outcome? Clin Obstet Gynecol. 2016;59(1):119-127. [DOI] [PubMed] [Google Scholar]
- 7.National Cancer Institute. Cancer trends progress report national cancer institute, NIH, HHS, Bethesda, MD. Accessed March 2021. https://progressreport.cancer.gov
- 8. Vikhlyaeva EM, Khodzhaeva ZS, Fantschenko ND. Familial predisposition to uterine leiomyomas. Int J Gynecol Obstet 1995;51(2):127-131. [DOI] [PubMed] [Google Scholar]
- 9. Brosens I, Deprest J, Cin PD, van den Berghe H. Clinical significance of cytogenetic abnormalities in uterine myomas. Fertil Steril. 1998;69(2):232-235. [DOI] [PubMed] [Google Scholar]
- 10. Kjerulff KH, Langenberg P, Seidman JD, Stolley PD, Guzinski GM. Uterine leiomyomas: racial differences in severity, symptoms and age at diagnosis. J Reprod Med Obstetr Gynecol. 1996;41(7):483-490. [PubMed] [Google Scholar]
- 11. Psychogios N, Hau DD, Peng J, et al. The human serum metabolome. PLoS One. 2011;6(2):e16957e16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Griffiths WJ, Koal T, Wang Y, Kohl M, Enot DP, Deigner HP. Targeted metabolomics for biomarker discovery. Angew Chem Int Ed. 2010;49(32):5426-5445. [DOI] [PubMed] [Google Scholar]
- 13. Heinonen HR, Mehine M, Mäkinen N, et al. Global metabolomic profiling of uterine leiomyomas. Br J Cancer. 2017;117(12):1855-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Torell F, Bennett K, Rännar S, Lundstedt-Enkel K, Lundstedt T, Trygg J. The effects of thawing on the plasma metabolome: evaluating differences between thawed plasma and multi-organ samples. Metabolomics. 2017;13(6):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jørgenrud B, Jäntti S, Mattila I, et al. The influence of sample collection methodology and sample preprocessing on the blood metabolic profile. Bioanalysis. 2015;7(8):991-1006. [DOI] [PubMed] [Google Scholar]
- 16. Naz S, García A, Barbas C. Multiplatform analytical methodology for metabolic fingerprinting of lung tissue. Anal Chem. 2013;85(22):10941-10948. [DOI] [PubMed] [Google Scholar]
- 17. Vallejo M, García A, Tuñón J, et al. Plasma fingerprinting with GC-MS in acute coronary syndrome. Anal Bioanal Chem. 2009;394(6):1517-1524. [DOI] [PubMed] [Google Scholar]
- 18. Barison G, Amora P, Izidoro M, et al. Supplemental data for: Leiomyomas metabolomic plasma profiling by GC-MS supplementary data. Dryad. 2022. doi: 10.5061/dryad.gb5mkkwrw [DOI] [Google Scholar]
- 19. Schrimpe-Rutledge A, Codreanu S, Sherrod S, McLean J. Untargeted metabolomics strategies-challenges and emerging directions. J Am Soc Mass Spectrom. 2016;27(12):1897-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gomez E, Nguyen MLT, Fursevich D, Macura K, Gupta A. MRI-based pictorial review of the FIGO classification system for uterine fibroids. Abdom Radiol. 2021;46(5):2146-2155. [DOI] [PubMed] [Google Scholar]
- 21. Tokarz J, Haid M, Cecil A, et al. Endocrinology meets metabolomics: achievements, pitfalls, and challenges. Trends Endocrinol Metab. 2017;28(10):705-721. [DOI] [PubMed] [Google Scholar]
- 22. Lokhov P, Balashova E, Voskresenskaya A, Trifonova O, Maslov D, Archakov A. Mass spectrometric signatures of the blood plasma metabolome for disease diagnostics. Biomed Rep. 2016;4(1):122-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Canuto GAB, da Costa JL, da Cruz PLR, et al. Metabolômica: definições, estado-da-arte e aplicações representativas. Quim Nova. 2018;41(1):75-91. [Google Scholar]
- 24. Rinschen M, Ivanisevic J, Giera M, Siuzdak G. Identification of bioactive metabolites using activity metabolomics. Nat Rev Mol Cell Biol. 2019;20(6):353-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cunningham LW. Structural and contractile proteins. Part A. Extracellular matrix. Methods in Enzymology. 1982;82(Pt A): 1–913. ISSN: 00766879.
- 26. Leppert PC, Jayes FL, Segars JH. The extracellular matrix contributes to mechanotransduction in uterine fibroids. Obstet Gynecol Int. 2014;2014:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seong J, Wang N, Wang Y. Mechanotransduction at focal adhesions: from physiology to cancer development. J Cell Mol Med. 2013;17(5):597-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malik M, Norian J, McCarthy-Keith D, Britten J, Catherino W. Why leiomyomas are called fibroids: the central role of extracellular matrix in symptomatic women. Semin Reprod Med. 2010;28(3):169-179. [DOI] [PubMed] [Google Scholar]
- 29. Gogiel T, Wolańska M, Galewska Z, Kinalski M, Sobolewski K, Romanowicz L. Cathepsin B in human myometrium and in uterine leiomyomas at various stages of tumour growth. Eur J Obstet Gynecol Reprod Biol. 2015;185:140-144. [DOI] [PubMed] [Google Scholar]
- 30. Jayes FL, Liu B, Feng L, Aviles-Espinoza N, Leikin S, Leppert PC. Evidence of biomechanical and collagen heterogeneity in uterine fibroids. PLoS One. 2019;14(4):e02156461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Islam MS, Ciavattini A, Petraglia F, Castellucci M, Ciarmela P. Extracellular matrix in uterine leiomyoma pathogenesis: a potential target for future therapeutics. Hum Reprod Update. 2018;24(1):59-85. [DOI] [PubMed] [Google Scholar]
- 32. Dang CV. Links between metabolism and cancer. Genes Dev. 2012;26:877-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cluntun AA, Lukey MJ, Cerione RA, Locasale JW. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer. 2017;3:169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vettore L, Westbrook RL, Tennant DA. New aspects of amino acid metabolism in cancer. Br J Cancer. 2020;122:150-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoo H, Yu Y, Sung Y, Han J. Glutamine reliance in cell metabolism. Exp Mol Med. 2020;52:1496-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ibarguren M, López D, Escribá P. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim Biophys Acta. 2014;1838:1518-1528. [DOI] [PubMed] [Google Scholar]
- 37. Meikle P, Wong G, Tsorotes D, et al. Plasma lipidomic analysis of stable and unstable coronary artery disease. Arterioscler Thromb Vasc Biol. 2011;31:2723-2732. [DOI] [PubMed] [Google Scholar]
- 38. Han X. Lipid alterations in the earliest clinically recognizable stage of Alzheimer’s disease: implication of the role of lipids in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2005;2:65-77. [DOI] [PubMed] [Google Scholar]
- 39. Olguín-Martínez M, Hernández-Espinosa D, Hernández-Muñoz R. High α-tocopherol dosing increases lipid metabolism by changing redox state in damaged rat gastric mucosa and liver after ethanol treatment. Clin Sci. 2018;132:1281-1296. [DOI] [PubMed] [Google Scholar]
- 40. Lin L, Ding Y, Wang Y, et al. Functional lipidomics: Palmitic acid impairs hepatocellular carcinoma development by modulating membrane fluidity and glucose metabolism. Hepatology. 2017;66(2):432-448. [DOI] [PubMed] [Google Scholar]
- 41. Phinney S. Metabolism of exogenous and endogenous arachidonic acid in cancer. Adv Exp Med Biol. 1996;399:87-94. [DOI] [PubMed] [Google Scholar]
- 42. Saini R, Keum Y. Omega-3 and omega-6 polyunsaturated fatty acids: dietary sources, metabolism, and significance—a review. Life Sci. 2018;203:255-267. [DOI] [PubMed] [Google Scholar]
- 43. Kowalczyk T, Ciborowski M, Kisluk J, Kretowski A, Barbas C. Mass spectrometry based proteomics and metabolomics in personalized oncology. Biochim Biophys Acta Mol Basis Dis. 2020;1866:1-18. [DOI] [PubMed] [Google Scholar]
- 44. Martin C, Almeida V. Ácidos graxos poli-insaturados ômega-3 e ômega-6: importância e ocorrência em alimentos. Rev de Nutr. 2006;19(6). doi:10.1590/S1415-52732006000600011 [Google Scholar]
- 45. Islam MS, Castellucci C, Fiorini R, et al. Omega-3 fatty acids modulate the lipid profile, membrane architecture, and gene expression of leiomyoma cells. J Cell Physiol. 2018;233(9):7143-7156. [DOI] [PubMed] [Google Scholar]
- 46. Fagundes L. Ômega-3 & Ômega-6: O Equilíbrio dos Ácidos Gordurosos Essenciais na Prevenção de Doenças. Fundação de Radioterapia do Rio Grande do Sul; 2002. [Google Scholar]
- 47. Troisi J, Sarno L, Landolfi A, et al. Metabolomic signature of endometrial cancer. J Proteome Res. 2018;17(2):804-812. [DOI] [PubMed] [Google Scholar]
- 48. Maggiora M, Bologna M, Cerù M, et al. An overview of the effect of linoleic and conjugated-linoleic acids on the growth of several human tumor cell lines. Int J Cancer. 2004;112:909-919. [DOI] [PubMed] [Google Scholar]
- 49. Thorens B, Mueckler M. Glucose transporters in the 21st century. Am J Physiol Endocrinol Metab. 2009;298:E141-E145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pavlova N, Thompson C. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vucenik I, Shamsuddin A. Protection against cancer by dietary IP6 and inositol. Nutr Cancer. 2006;55(2):109-125. [DOI] [PubMed] [Google Scholar]
- 52. Innocenti A, Hilvo M, Scozzafava A, et al. Carbonic anhydrase inhibitors: the very weak inhibitors dithiothreitol, β-mercaptoethanol, tris(carboxyethyl)phosphine and threitol interfere with the binding of sulfonamides to isozymes II and IX. Bioorg Med Chem Lett. 2008;18:1898-1903. [DOI] [PubMed] [Google Scholar]
- 53. Suzuki A, Aoki M, Miyagawa C, et al. Differential diagnosis of uterine leiomyoma and uterine sarcoma using magnetic resonance images: a literature review. Healthcare. 2019;7(4):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xia J, Broadhurst D, Wilson M, Wishart D. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics. 2013;9:280-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set used during the current study are available from the corresponding author on reasonable request. Supplementary data to this article can be found online at https://doi.org/10.5061/dryad.gb5mkkwrw.