Abstract
Background
: The rhythm-monitoring strategy after catheter ablation (CA) for atrial fibrillation (AF) impacts the detection of atrial arrhythmia recurrence and is not well characterized. We performed a systematic review and meta-regression analysis to determine whether the duration and mode of rhythm monitoring after CA affects detection of atrial arrhythmia recurrence.
Methods
Databases were systematically searched for randomized controlled trials of adult patients undergoing first CA for AF from 2007 to 2021. Duration and strategy of rhythm monitoring were extracted. Meta-regression was used to identify any association between duration of monitoring and detection of atrial arrhythmia recurrence. The primary measure of outcome was single-procedure recurrence of atrial arrhythmia.
Results
The search strategy yielded 57 trial arms from 56 randomized controlled trials comprising 5322 patients: 36 arms of patients with paroxysmal AF (PAF), and 21 arms of patients with persistent AF (PeAF) or both PAF/PeAF. Intermittent monitoring was associated with detection of significantly less atrial arrhythmia recurrence than continuous monitoring in PAF arms (31.2% vs 46.9%, P = 0.001), but not in PeAF/PAF-PeAF combined arms (43.3% vs 63.6%, P = 0.12). No significant relationship was seen between the duration of intermittent rhythm monitoring and atrial arrhythmia recurrence detection in either the PAF (P = 0.93) or PeAF/PAF-PeAF combined arms (P = 0.20).
Conclusions
Continuous rhythm monitoring detected higher atrial arrhythmia recurrence rates, compared to intermittent rhythm monitoring, in patients with PAF. The duration of intermittent monitoring did not show a statistically significant relationship to the yield of arrhythmia detection, in near identical cohorts of trial subjects undergoing similar interventions, with clinical and research implications.
Résumé
Contexte
La stratégie qui consiste à surveiller le rythme cardiaque après une ablation par cathéter dans le traitement de la fibrillation auriculaire (FA) a un effet sur la détection de récidive de l’arythmie auriculaire, mais elle n’est pas bien définie. Nous avons mené une revue systématique et une méta-régression pour déterminer si le mode employé pour surveiller le rythme après une ablation par cathéter et la durée de cette surveillance ont un effet sur la détection de récidive de l’arythmie auriculaire.
Méthodologie
Des bases de données ont été systématiquement épluchées à la recherche d’essais contrôlés randomisés menés auprès d’adultes subissant leur première ablation par cathéter pour une FA entre 2007 et 2021. La durée et la stratégie utilisées dans la surveillance du rythme ont été recensées. La méta-régression a été utilisée pour déceler tout lien entre la durée de la surveillance et la détection d’une récidive de l’arythmie auriculaire. Le paramètre d’évaluation principal était la récidive de l’arythmie auriculaire avec une seule intervention.
Résultats
La stratégie de recherche a fait ressortir 57 groupes de 56 essais contrôlés randomisés comprenant 5 322 patients : 36 groupes de patients présentant une FA paroxystique et 21 groupes de patients présentant une FA persistante ou ces deux types de FA (paroxystique et persistante). La surveillance intermittente a été associée à une moins grande détection de cas d’arythmie auriculaire récidivante, comparativement à la surveillance constante (31,2 % vs 46,9 %, p = 0,001), ce qui n’a pas été le cas dans les groupes où les types de FA (persistante ou paroxystique et persistante) étaient combinés (43,3 % vs 63,6 %, p = 0,12). Aucun lien notable n’a été observé entre la durée de la surveillance intermittente du rythme et la détection de l’arythmie auriculaire récidivante dans le groupe FA paroxystique (p = 0,93) ou dans le groupe des types de FA combinés (p = 0,20).
Conclusions
Le taux de détection de l’arythmie auriculaire récidivante était plus élevé avec la surveillance constante qu’avec la surveillance intermittente chez les patients atteints de FA paroxystique. La durée de la surveillance intermittente n’a pas eu de lien statistiquement significatif avec le rendement de détection de l’arythmie, dans des cohortes presque identiques de participants aux essais subissant des interventions similaires, comportant des implications cliniques ou expérimentales.
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia worldwide and is associated with impaired quality of life (QoL), heart failure, thromboembolic events, and mortality.1, 2, 3, 4 AF-related morbidity has led to increased healthcare expenditure and is burdening healthcare systems around the world.5, 6, 7, 8, 9, 10 Multiple randomized studies have shown that catheter ablation for AF improves QoL, reduces cardiovascular hospitalization and mortality, and is superior to antiarrhythmic therapy for maintaining sinus rhythm in subjects with paroxysmal AF (PAF).11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 More recently, the Early Treatment of Atrial Fibrillation for Stroke Prevention Trial 4 (EAST-AFNET 4) trial has shown that early rhythm control therapy is associated with a lower risk of adverse cardiovascular outcomes compared to usual care among patients with AF, suggesting that rhythm control should be the preferred strategy in most individuals with AF.23
Post-ablation symptoms correlate poorly with arrhythmia recurrence, irrespective of pre-ablation symptoms.24,25 Accordingly, postprocedural follow-up strategies consist of regular intermittent or continuous ambulatory rhythm monitoring in addition to symptom-driven rhythm assessments. Intermittent rhythm monitoring includes standard 12-lead electrocardiograms, transtelephonic monitoring systems, patient-activated event recorders, Holter monitors, and newer handheld recording devices.26,27 Continuous rhythm monitoring includes implantable loop recorders (ILRs), pacemakers, or implantable cardiac defibrillators (ICDs). Although international consensus guidelines for studies assessing ablation in PAF recommend a minimum of one 24-hour Holter in addition to regular event recording throughout the follow-up period, the optimal frequency and duration of monitoring are not known.5
Observational and randomized prospective studies have suggested that continuous rhythm monitoring detects more recurrent arrhythmia than intermittent monitoring, and that a longer total duration of intermittent rhythm monitoring results in more arrhythmia detection.28, 29, 30, 31, 32 Continuous rhythm-monitoring devices are more expensive, require implantation, and may not be available in all healthcare settings. Conversely, prolonged-duration intermittent rhythm monitoring can be burdensome for patients and can result in reduced compliance.33
In this systematic review with meta-analyses, we assessed the postprocedural rhythm-monitoring strategies in randomized controlled trials (RCTs) of AF ablation, to establish the impact of duration and frequency of rhythm monitoring on AF recurrence rates. We hypothesized that studies with a higher frequency and longer duration of rhythm monitoring would report increased rates of arrhythmia recurrence.
Methods
Research ethics board approval for this type of research is not required at our institution. This study has been reported per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplemental Appendix S2). The search and study protocol were registered on the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42020161817). This systematic reveiw represents a protocol deviation in that we directed our analysis to trial arms of patients undergoing solely pulmonary vein isolation (PVI).
Search strategy
The search strategy was designed with the help of an experienced cardiology research librarian and can be found in the Supplemental Appendix S1. MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched for the date range of January 31, 2007 to September 22, 2021. The search dates were chosen after consensus discussion among the authors, as ablation technology prior to 2007 was less well established. All references of eligible studies and the ClinicalTrials.gov registry were searched to identify other eligible studies. The grey literature was not searched. Abstracts were screened independently and in duplicate by 4 authors (R.U., L.P., W.A., and A.Z.), with any positive screen subject to full-text review. Full-text review was performed in duplicate by 3 reviewers (R.U., J.Z., R.O.), with discrepancies resolved through consensus discussion among authors. A senior author was consulted for any unresolved disagreements (G.N., P.N.).
Study eligibility
Studies were included if they were RCTs assessing first-time catheter ablation for PAF or persistent AF (PeAF) in adult patients (age ≥ 18 years) and reported an endpoint of single-procedure freedom (or recurrence) of atrial arrhythmia (AF, atrial flutter [AFL], or atrial tachyarrhythmia). In addition, one of the trial arms had to have used PVI or pulmonary vein antral isolation (both referred to here as PVI), with no other left-sided interventions (eg, mitral isthmus line), and with no other intervention drugs. Only studies published in English were included.
Studies were excluded if they assessed surgical or hybrid ablation, atrioventricular nodal ablation, atrial flutter ablation, or repeat ablation procedures. They were also excluded if they reported less than 12 months of follow-up or did not provide sufficient detail surrounding monitoring to calculate total duration of rhythm monitoring. Long-term follow-up reports of already included studies were excluded.
Data extraction
All data extraction was done independently and in duplicate (by R.U. and one of J.Z., R.O., N.F.H., and R.P.), with all discrepancies resolved through consensus discussion. Data extraction was performed using DistillerSR (Evidence Partners, Ottawa, ON) and stored in Microsoft Excel (Redmond, WA). Patient demographics, comorbidities, and echocardiographic features (left atrial diameter and left ventricular ejection fraction) were extracted. Procedural data were extracted, including the type of ablation lesion sets, energy source, and antiarrhythmic drug use. Postprocedural rhythm monitoring data were extracted, including the number of monitoring instances in the follow-up period and the duration of each monitoring instance (eg, 72-hour Holter monitoring performed every 3 months). Assessment of bias was conducted through a validated quality-assessment tool used for RCTs (maximum, 13 points; poor, ≤ 5 of 13; intermediate, 6-9 of 13; and high, ≥ 10 of 13) derived from validated quality-assessment tools.34,35 The results of the quality assessment showed that all the trials were of high quality (one of the RCTs scored 13 of 13, and all others scored 12 of 13 on the quality score). The results are summarized in Supplemental Table S1.
Duration of monitoring
To determine the duration of monitoring for a patient in the first 12 months, the number of monitoring instances outside of the blanking period was multiplied by the duration of each monitoring instance (eg, 4 occurrences × 7-day Holter monitoring = 28 days). Blanking periods were respected in calculations. For continuous monitors, the duration of monitoring in the first 12 months outside of the blanking period was assigned as 274 days. Regular 12-lead electrocardiograms and transtelephonic monitoring were assumed to have a negligible duration of monitoring when combined with another modality; however, if transtelephonic monitoring was the sole rhythm-monitoring strategy, it was assigned a total duration of 1 day. For studies with a mean follow-up greater than 1 year, the number of months after 1 year was multiplied by the frequency of monitoring instances after 1 year, to yield the total duration of monitoring after 1 year.
Handling of multiple definitions for atrial arrhythmia recurrence
As multiple definitions for atrial arrhythmia were used in the various studies, the following definitions were used hierarchically, per expert consensus recommendations: (i) single-procedure recurrence of AF, AFL, or atrial tachyarrhythmia of at least 30 seconds duration, off antiarrhythmics; (ii) single-procedure recurrence of AF, AFL, or atrial tachyarrhythmia of any duration, off antiarrhythmics; (iii) single-procedure AF of at least 30 seconds duration off antiarrhythmic therapy; (iv) single-procedure AF or AFL of any duration; and (v) single-procedure AF of any duration.5
Data analysis
The primary outcome was single-procedure recurrence rate of atrial arrythmia. Studies were analyzed separately, based on type of AF, to reduce heterogeneity across studies, given known differences in recurrence between PAF and PeAF. For studies including patients with both PeAF and PAF, analysis was done with the PeAF (referred to as PeAF/Combined).
Descriptive statistics are provided as medians with interquartile ranges, and 95% confidence intervals (CIs) where appropriate. Meta-analysis of recurrence rates was performed using inverse variance random-effect models in each subgroup. To compare the difference of arrhythmia recurrence between subgroups, χ2 tests were used. The heterogeneity was assessed by examining the forest plots and results of the I2 statistics. If the effects observed across studies were inconsistent and varied to a large extent (eg, I2 > 50%), the results were explored to assess whether the difference could be explained by clinical or methodological features. The meta-regression method was used to assess the effect of duration of monitoring on arrhythmia recurrence using mixed-effects logistic regression models. All meta-analysis was performed using Review Manager 5.3 (Cochrane Collaboration, Copenhagen, Denmark) and Comprehensive Meta-analysis V3 (Biostat, Englewood, NJ). The significance threshold was set at P < 0.05. All data analyses and details of statistical analyses are available via the corresponding author.
Results
The study flow diagram is provided in the Supplemental Figure S1. Our search strategy identified 15,603 unique studies, with 2583 records undergoing full-text screening. A total of 57 arms comprising 5312 patients, from 56 RCTs in which radiofrequency PVI or pulmonary vein antrum isolation was the only left-sided ablation that took place, and in which no medication or imaging intervention was received. One RCT36 compared medical therapy to a treatment arm of ablation with radiofrequency PVI only; therefore, the treatment arm was included. One study37 included 2 different intervention protocols stratified by type of AF (PAF vs non-PAF), for a total of 4 arms. We included 2 eligible arms in our PAF and PAF/PeAF combined analyses, respectively (Supplemental Table S2).
Demographics and study endpoints
Of the PVI arms, 36 were arms of patients with PAF (2944 patients), and 21 were arms of patients with either PeAF or both PAF/PeAF (2368 patients; Table 1). Overall, the prevalences of major comorbidities, including diabetes, hypertension, and stroke/transient ischemic attack, were similar. The means and standard deviations for left ventricular ejection fraction, left atrial diameter, as well as postprocedure antiarrhythmic drug use were also similar within arms.
Table 1.
Trial and patient characteristics of randomized controlled trials of AF ablation from 2007 to 2021, with a study arm of solely PVI or pulmonary vein antrum isolation
| Characteristic | Paroxysmal AF | Persistent AF | Mixed paroxysmal and persistent AF |
|---|---|---|---|
| Number of arms | 36 | 9 | 12 |
| Number of patients in PVI arms (%) | 2944 (55.4) | 744 (14.0) | 1624 (30.6) |
| Male | 1988 (67.5) | 523 (70.2) | 1169 (72.0) |
| Female | 956 (32.5) | 221 (29.8) | 455 (28.0) |
| Age, y | 59.4 (4.1) | 60.5 (3.6) | 59.9 (4.8) |
| LAD, mm | 40.5 (2.5) | 44.4 (1.44) | 43.2 (3.1) |
| LVEF, % | 60.9 (3.7) | 57.4 (3.3) | 62.3 (2.3) |
| Diabetes, % | 8.3 (5.5–1.7) | 8.9 (3.4–16.8) | 12.0 (3.7–14.7) |
| Hypertension, % | 40.5 (35.7–52.8) | 49.5 (47.8–57.1) | 56.3 (43.1–61.6) |
| Stroke, % | 6.0 (3.0–7.7) | 8.1 (7.9–9.0) | 10.8 (4.0–20.7) |
| Heart failure, % | 3.6 (1.4–10.5) | 11.3 (3.8–19.2) | 8.9 (3.0–16.9) |
Values are median (interquartile range) or mean (standard deviation), unless otherwise specified. LAD was reported in 51 study arms; LVEF was reported in 41 arms; hypertension was reported in 54 arms; stroke was reported in 33 arms; diabetes was reported in 43 arms; and heart failure was reported in 22 arms.
AF, atrial fibrillation; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; PVI, pulmonary vein isolation.
Quality of RCTs and bias assessment
Recurrence endpoints
For the definition of atrial arrhythmia recurrence, the majority of arms (32; 56%) reported the recurrence endpoint of AF or atrial tachyarrhythmia lasting greater than 30 seconds. Two studies (4%) utilized AF or antiarrhythmia > 60 seconds; 6 studies (11%) used AF and atrial tachycardia > 30 seconds; 7 studies (12%) utilized AF > 30 seconds; 3 studies (5%) utilized AF > 60 seconds; and 2 studies (4%) used 0% AF burden/any AF. One study (2%) (for each of the following) utilized: arrhythmia (no other qualifiers); palpitations/AF/AFl > 30 seconds; AF or atypical AFl ≥ 2 minutes; AF ≥ 2 minutes; AF (no other qualifiers); and “AF or atrial arrhythmia > 30 seconds or antiarrhythmic drug prescription or repeat ablation.”
Duration of rhythm monitoring and PVI-arm rhythm-monitoring strategies
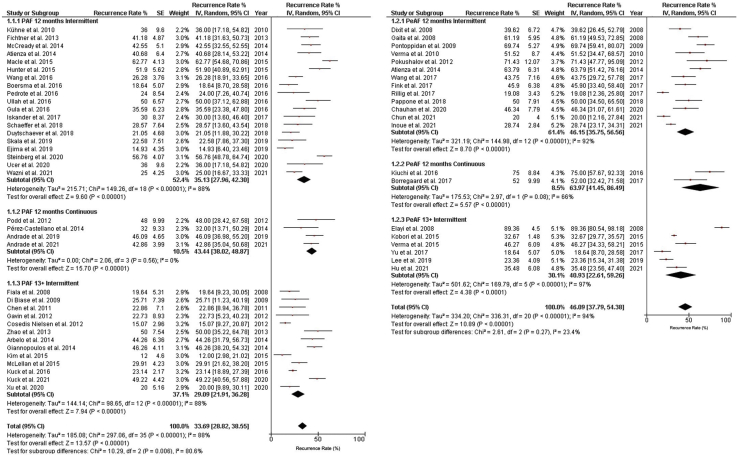
The pooled recurrence rate among the PVI-PAF arm was 33.7% (95% CI, 28.82% to 38.55%; I2 = 88% for overall group; I2 = 80.1% for subgroup differences). Among the PeAF/Combined arms, the pooled recurrence rate was 46.1% (95% CI, 37.8% to 54.4%; I2 = 94% for overall group; I2 = 23.4% for subgroup differences). The heterogeneity index is high in the RCTs included in our systematic review, as the intervention arms are different in each of the RCTs. This difference is the reason that we selected subjects exclusively from the arms of the RCTs undergoing PVI (a well-established and standardized intervention) and performed meta-regression analysis and not meta-analysis (between-group comparison) in order to reduce heterogeneity. In addition, the monitoring strategies used in these RCTs were varied in duration and type of monitoring, and this also contributed to the observed heterogeneity (Fig. 1). Most of the included arms utilized intermittent rhythm-monitoring strategies for AF recurrence detection, and used single-device strategies to do so (Table 2). Fifty study arms (88%) utilized Holter monitors, and 5 (11%) used ILRs or non-ILR cardiac implantable electronic devices. Of the studies that employed intermittent rhythm monitoring, the median duration of rhythm monitoring, excluding measurements made during a 3-month blanking period, was 10 days in PAF arms and 5 days in the PeAF/Combined arms. Only one study (Iskandar et al. 2017; all references for trials in the systematic review are listed in the Supplemental Appendix S1) utilized solely patient-activated rhythm monitoring with a 30-day event monitor 3 times within a mean 12-month follow-up period.
Figure 1.
Rates of atrial arrhythmia recurrence as detected in pulmonary vein isolation arms of randomized controlled trials of atrial fibrillation (AF) ablation from 2007 to 2021. Arms are grouped by type of AF and duration of study follow-up. Square markers indicate the point estimate of AF recurrence. The size of each square is proportional to the corresponding weight of the given study. Horizontal lines indicate 95% confidence interval (CI). Solid diamonds represent the estimated 95% CI for the recurrence rate of all pooled trial arms. IV, intra venous; PAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation (including studies with combined PeAF and PAF); SE, standard error. A complete list of trial references can be found in the Supplemental References.
Table 2.
Post-procedural rhythm-monitoring devices and duration in randomized controlled trials of AF ablation between 2007 and 2021, with a study arm of solely pulmonary vein isolation or pulmonary vein antrum isolation
| Intermittent rhythm-monitoring device and intensity, n∗ | All arms | PAF arms | PeAF/combined arms |
|---|---|---|---|
| Holter | 51 | 32 | 19 |
| 24-h | 25 | 15 | 10 |
| 48-h | 10 | 6 | 4 |
| 72-h | 5 | 2 | 3 |
| 168-h | 10 | 9 | 1 |
| Event monitor | |||
| 72-h | 1 | 1 | — |
| 168-h | 1 | 1 | — |
| 30-d | 1 | 1 | — |
| 5-mo | 1 | 1 | — |
| 6-mo | 1 | 1 | — |
| 2-wk loop recorder | 1 | 1 | — |
| Transtelephonic monitor | 4 | 3 | 1 |
| Continuous rhythm-monitoring devices | 6 | 4 | 2 |
| Implantable loop monitor (continuous) | 5 | 4 | 1 |
| Permanent pacemaker or ICD | 1 | — | 1 |
| Study arms using 1 device in addition to regular ECGs | 47 (82.5) | 29 (80.6) | 18 (85.7) |
| Study arms using 2 devices in addition to regular ECGs | 10 (17.5) | 7 (19.4) | 3 (14.3) |
| Duration of rhythm monitoring, median (IQR), d | 9 (4, 21) | 10 (4, 22) | 5 (3, 9) |
| Duration of rhythm monitoring in intermittent rhythm-monitoring strategies, median (IQR), d | 8 (4, 12) | 9.5 (4, 20) | 4.1 (3, 9) |
| Shortest measured duration of rhythm monitoring† d | 2 | 2 | 2 |
| Longest measured duration of intermittent rhythm monitoring, d | 90 | 90 | 21 |
Values are n or n (%), unless otherwise specified. All studies collected standard 12-lead electrograms at each follow-up clinic visit and symptoms-based ambulatory ECG monitoring.
AF, atrial fibrillation; ECG, electrocardiogram; ICD, implantable cardiac defibrillator; IQR, interquartile range; PAF, paroxysmal AF; PeAF, persistent AF.
The number of devices is greater than the number of arms included, as some included studies used multiple rhythm-monitoring devices.
Minimum duration excludes transtelephonic monitoring, which is assigned a duration of < 1 day.
Intermittent vs continuous monitoring and effect of trial follow-up length
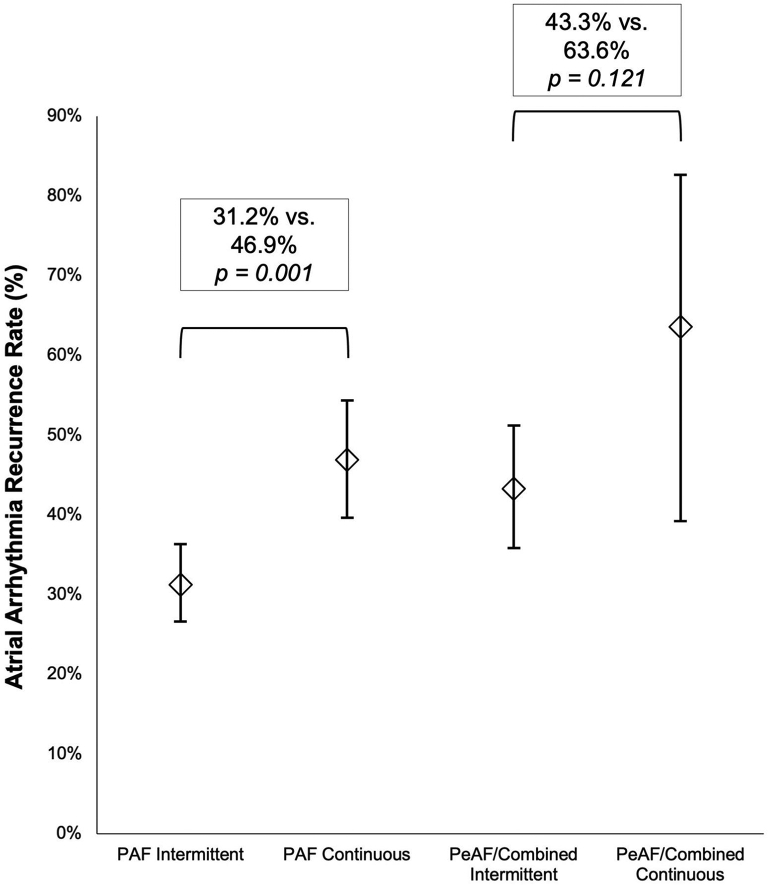
Among the PVI-PAF arms, there was less atrial arrhythmia recurrence detected in arms that underwent intermittent monitoring vs continuous monitoring (31.2% vs 46.9%, P < 0.01; Fig. 2). Less atrial arrhythmia recurrence was detected with intermittent monitoring among the PVI-PeAF/Combined arms, but the difference was not significant (43.3% vs 63.6%, P = 0.12).
Figure 2.
Pulmonary vein isolation arms and type of monitoring. Pooled atrial arrhythmia recurrence rates of pulmonary vein isolation arms of randomized controlled trials of atrial fibrillation ablation from 2007 to 2021, by type of atrial fibrillation and monitoring. The diamonds represent the mixed-effects analysis of pooled recurrence rates for each group of trial arms. The short horizontal lines represent the upper and lower bounds of 95% confidence intervals. PAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation.
Among the PVI-PAF arms with intermittent rhythm monitoring, atrial arrhythmia recurrence was not significantly different in arms with a follow-up of 12 months, compared to arms that were followed for longer than 12 months (35.1% vs 29.1%, P = 0.24). Similarly, among the PVI-PeAF/Combined arms with intermittent monitoring, no difference in atrial arrythmia recurrence rates was detected between arms followed for 12 months, compared to patient arms followed for longer than 12 months (46.2% vs 40.1%, P = 0.63). There were no arms of PVI-PAF or PVI-PeAF/Combined with continuous monitoring followed for greater than 12 months.
Duration of intermittent rhythm monitoring on detection of atrial arrhythmia recurrence
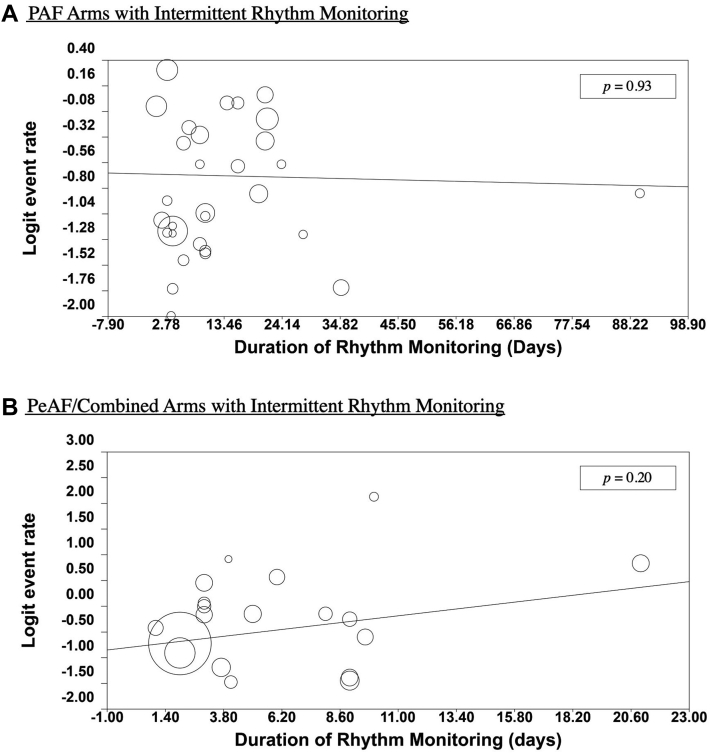
Among the PVI-PAF arms that performed intermittent rhythm monitoring, there was no significant correlation between atrial arrhythmia recurrence and duration of rhythm monitoring (P = 0.93; Fig. 3A). Excluding the outlier study by Iskandar et al. (all references for trials in the systematic review are listed in the Supplemental Appendix S1), there was still no significant increase in atrial arrhythmia recurrence (P = 0.76; Supplemental Fig. S4). Similarly, among the PVI-PeAF/Combined arms that underwent intermittent rhythm monitoring, no significant increase in atrial arrhythmia recurrence was found with increased duration of rhythm monitoring (P = 0.20; Fig. 3B). Fixed and random-effects cumulative meta-analyses were also performed for both PVI-PAF and PVI-PeAF arms, which also did not reveal significant trends in recurrence rate with increasing duration of rhythm monitoring (Supplemental Figs. S2 and S5).
Figure 3.
Duration of intermittent rhythm monitoring and atrial arrhythmia recurrence rate in pulmonary vein isolation arms of randomized controlled trials of atrial fibrillation ablation from 2007 to 2021. Circles represent arms of patients and logit recurrence rates of atrial arrhythmia. The solid lines show mixed-effects logistic regressions of duration of rhythm monitoring and logit atrial arrhythmia recurrence for (A) paroxysmal atrial fibrillation (PAF) arms and (B) persistent AF (PeAF)/Combined PeAF and PAF arms.
Discussion
In this systematic review, we examined postprocedural rhythm-monitoring strategies used in RCTs of catheter ablation for AF. There was a significant increase in detection with continuous-monitoring devices, compared to that with intermittent monitoring in studies of PAF, but not in those of PeAF or combined PAF/PeAF. We found no significant increase in the detection of atrial arrhythmia with increasing total duration of intermittent rhythm monitoring in studies of both PAF and PeAF. We also found no significant difference in arrhythmia recurrence in trials with a total duration of 12 months (or less), compared to those greater than 12 months.
Intermittent vs continuous monitoring
Our finding of a significant difference in recurrence rates between intermittent and continuous monitoring in patients with PAF is reflected in the results from recent large RCTs. The Cryoballoon vs Irrigated Radiofrequency Catheter Ablation: Double Short vs Standard Exposure Duration (CIRCA-DOSE) and Early Aggressive Invasive Intervention for Atrial Fibrillation (EARLY-AF) clinical trials of catheter ablation for AF both used continuous monitoring for postprocedure follow-up and demonstrated low AF-free survival, due to the continuous-monitoring strategy used after catheter ablation.18,32 Two similar clinical trials using intermittent/symptom-guided monitoring strategies showed much higher AF-free survival.17,38 Both the EARLY-AF18 and the Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP AF)17 studies compared drug therapy vs cryoablation for early PAF. The EARLY-AF study used ILR/continuous monitoring with 57.1% freedom from recurrence outcome in the ablation arm, whereas the (STOP-AF) study used Holter/intermittent monitoring with a 74.6% freedom from recurrence outcome in the ablation arm. These differences likely reflect failure to detect asymptomatic AF episodes occurring during unmonitored epochs in the follow-up period.
Our systematic review suggests that when intermittent rhythm-monitoring strategies were used for detection of atrial arrhythmia recurrence, the duration of intermittent monitoring (median duration, 7 days) did not show a statistically significant relationship to the yield of arrhythmia detection, in near identical cohorts of trial subjects undergoing similar interventions. This finding is most probably related to the relatively short duration of intermittent monitoring (24 hours to 7 days, 1 to 3 times in the year following catheter ablation) in these clinical trials. Very few trials had longer durations of intermittent monitoring (eg, 14 days, 3 times in a year). Although the short duration of intermittent monitoring limits the ability to identify the true incidence of recurrent arrhythmias after catheter ablation and arrhythmia burden, it can suffice to evaluate the comparative efficacy of 2 different catheter ablation strategies in clinical trials.
The absence of a significant difference between intermittent and continuous monitoring in the PeAF/Combined arms may be due to a limited number of studies in the combined arms (only 2 studies). However, it may also reflect that the recurrence in patients with PeAF is more frequent and persistent by nature, reducing the potential benefit gained from continuous monitoring. In PAF, transient episodic recurrences, regardless of their symptomology, may be missed by intermittent rhythm monitoring, thus leading to a greater relative increase in arrhythmia detection with continuous monitoring.
From a clinical perspective, continuous-monitoring strategies in AF ablation clinical trials offer the following advantages: the true incidence of recurrent arrhythmias and burden of AF recurrence may offer novel endpoints compared to the binary outcome of 30 seconds of sustained atrial arrhythmias that is currently used as a primary outcome in most AF catheter ablation trials.
Reduction of AF burden may be a more meaningful endpoint than 30 seconds of asymptomatic AF from the patient’s perspective. Continuous monitoring can rule out recurrent atrial arrhythmias with greater certainty compared to intermittent-monitoring strategies and assist the clinician and patient with the decision regarding discontinuation of systemic oral anticoagulation in subjects without symptomatic AF, but with a risk factor that necessitates ongoing systemic oral anticoagulation. The higher incidence of recurrent arrhythmia detected using ILR-based strategies has implications for sample size calculations in clinical trials evaluating the efficacy of treatment strategies for AF. From a trial design perspective, continuous monitoring not only allows accurate comparisons of AF burden with different ablation strategies, but also has a higher arrhythmia detection rate, which may result in smaller sample size requirements to evaluate interventions, thereby reducing the cost of RCTs.
Duration of intermittent monitoring
In 2006, Ziegler et al. examined pacemaker records of 576 patients and simulated intermittent rhythm-monitoring strategies of different durations by randomly selecting a number of days to see if AF was detected.39 A lower monitoring duration was found to have significantly less sensitivity for detection of arrhythmia recurrence compared to higher total monitoring duration and continuous rhythm-monitoring strategies. Similar simulation and observational studies examining this topic have suggested similar conclusions.28,29,40 Our findings challenge those of these earlier studies and suggest that small differences in monitoring duration (for example, 3 days vs 12 days in 1 year) are likely irrelevant with respect to the sensitivity of arrhythmia recurrence detection (Supplemental Table S3 and Supplemental Fig. S3).
One explanation for these findings may be that the true effect of total duration of monitoring on detection of recurrence may be quite small compared to other predictors of success (clinical profile, operator success, and technique). Among the PVI-PAF arms, we found a 13.3% difference in recurrence between all intermittent monitoring and continuous monitoring. This relatively small difference suggests even smaller incremental gains in arrhythmia detection when intermittent monitoring duration is increased from 3 days to 21 days, for example—a fraction of the days monitored with a continuous-monitoring strategy. Also important to recall is that a significant proportion of post-ablation arrhythmia recurrence remains symptomatic. The Discerning Symptomatic and Asymptomatic Episodes Pre and Post Radiofrequency Ablation of Atrial Fibrillation (DISCERN-AF) study found that although 56% of all episodes of AF/AFL were asymptomatic, only 12% of patients had asymptomatic episodes only.24 As symptomatic recurrences prompt additional workup, such as emergency department visits and additional ambulatory electrocardiographic monitoring, the relative effect of more intensive rhythm monitoring may be quite small.
From a clinical perspective, our findings suggest that clinicians using an intermittent rhythm-monitoring strategy need not burden patients with several long and intensive protocols, which may promote dropout from follow-up. Our findings similarly suggest that trialists utilizing intermittent rhythm monitoring can avoid costly follow-up protocols and use a shorter duration of rhythm monitoring.
Over the past few years, consensus has been growing that freedom from atrial arrhythmia may not be the only, or indeed optimal, metric of success for catheter ablation.41 The CIRCA-DOSE trial showed that despite a relatively low single-procedure rate of freedom of arrhythmia, catheter ablation reduced the total time in AF (burden of AF) by over 99% compared to medical therapy.32 Although AF ablation may not consistently cure AF, the reduction in AF burden may be correlated to stroke risk, although this has not been conclusively demonstrated.32,41 Our findings of relatively small differences in single-procedure success rates despite large differences in monitoring underline how existing studies of AF ablation using this endpoint may not truly represent the efficacy of ablation.
We also found no significant increase in detection of atrial arrhythmia in studies with 12 months of follow-up vs longer than 12 months. This finding is supported by studies that indicate the majority of AF recurrence occurs in the first 6 months post-ablation.42,43 Although these findings suggest that trialists wishing to study ablation in specific scenarios may not need to follow up patients for greater than 12 months, further study is required.
Limitations
Our study has several limitations. First, several arms of patients captured in our search strategy were excluded due to additional left-sided ablations performed, or failure to sufficiently detail the rhythm-monitoring strategy for total duration of rhythm monitoring to be calculated. Our meta-regression analysis of arms of patients from different RCTs relies on the similarity of patients within each arm and similarity of technique. Although hypertension, the prevalence of diabetes and stroke, and left atrial diameter were all largely similar within both the PAF and PeAF/Combined groups of study arms, confounders not reported in each study, such as operator/centre experience and variations in ablation technique, may weaken the analysis. The limited number of studies with patients undergoing continuous monitoring, and the slightly different endpoints used for atrial arrhythmia recurrence, may also affect analysis.
Additionally, studies using transtelephonic monitoring were assigned a total duration of 1 day of rhythm monitoring because this modality records very short electrocardiograms. Other measures of intensity of monitoring (for example, “number of days in which monitoring took place,” in which case the relative intensity with transtelephonic monitoring would be much higher than that in our study) were not analyzed and are potential topics for additional analyses.
Conclusion
In conclusion, among patients undergoing PVI for AF, continuous rhythm monitoring detected higher rates of atrial arrhythmia recurrence compared to RCTs utilizing an intermittent rhythm-monitoring strategy. However, among arms undergoing intermittent rhythm monitoring, the total duration did not influence reported atrial arrhythmia recurrence. These findings have important implications in the design of RCTs evaluating treatments for AF, due to the impact of outcome detection and event rates on estimation of sample size, effectiveness of trial interventions, and the trial budget.
Acknowledgments
Funding Sources
The authors have no funding sources to declare.
Disclosures
Dr Girish M. Nair reports honoraria, speaking fees, and grant support from Bisosense Webster Canada, not related to this work (modest). Dr Pablo B. Nery reports honoraria, speaking fees, and grant support from Bisosense Webster Canada, not related to this work (modest). Dr David H. Birnie reports grants from Boehringer Ingelheim, Germany, and from Pfizer and Bristol-Myers Squibb, New York (modest). The other authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: Research ethics board approval for this type of research is not required at our institution.
Registration: PROSPERO: CRD42020161817.
See page 495 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2022.02.001.
Supplementary Material
References
- 1.January C.T., Wann L.S., Alpert J.S., et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 2.European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery. Camm A.J., et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D.M., Wang T.J., Leip E.P., et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 4.Wolf P.A., Mitchell J.B., Baker C.S., Kannel W.B., D'Agostino R.B. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med. 1998;158:229–234. doi: 10.1001/archinte.158.3.229. [DOI] [PubMed] [Google Scholar]
- 5.Calkins H., Hindricks G., Cappato R., et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1–160. doi: 10.1093/europace/eux274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ringborg A., Nieuwlaat R., Lindgren P., et al. Costs of atrial fibrillation in five European countries: results from the Euro Heart Survey on atrial fibrillation. Europace. 2008;10:403–411. doi: 10.1093/europace/eun048. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin E.J., Wolf P.A., D'Agostino R.B., et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 8.Wolf P.A., Abbott R.D., Kannel W.B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 9.Stewart S., Hart C.L., Hole D.J., McMurray J.J. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 10.Andrade J.G., Verma A., Mitchell L.B., et al. 2018 focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol. 2018;34:1371–1392. doi: 10.1016/j.cjca.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Nair G.M., Nery P.B., Diwakaramenon S., et al. A systematic review of randomized trials comparing radiofrequency ablation with antiarrhythmic medications in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:138–144. doi: 10.1111/j.1540-8167.2008.01285.x. [DOI] [PubMed] [Google Scholar]
- 12.Morillo C.A., Verma A., Connolly S.J., et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014;311:692–700. doi: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 13.Verma A., Macle L., Cox J., Skanes A.C. CCS Atrial Fibrillation Guidelines Committee. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: catheter ablation for atrial fibrillation/atrial flutter. Can J Cardiol. 2011;27:60–66. doi: 10.1016/j.cjca.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Proietti R., Santangeli P., Di Biase L., et al. Comparative effectiveness of wide antral versus ostial pulmonary vein isolation: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2014;7:39–45. doi: 10.1161/CIRCEP.113.000922. [DOI] [PubMed] [Google Scholar]
- 15.Conti S., Weerasooriya R., Novak P., et al. Contact force sensing for ablation of persistent atrial fibrillation: a randomized, multicenter trial. Heart Rhythm. 2018;15:201–208. doi: 10.1016/j.hrthm.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Fink T., Schluter M., Heeger C.H., et al. Stand-alone pulmonary vein isolation versus pulmonary vein isolation with additional substrate modification as index ablation procedures in patients with persistent and long-standing persistent atrial fibrillation: the Randomized Alster-Lost-AF Trial (Ablation at St. Georg Hospital for Long-Standing Persistent Atrial Fibrillation) Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.117.005114. [DOI] [PubMed] [Google Scholar]
- 17.Wazni O.M., Dandamudi G., Sood N., et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384:316–324. doi: 10.1056/NEJMoa2029554. [DOI] [PubMed] [Google Scholar]
- 18.Andrade J.G., Wells G.A., Deyell M.W., et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384:305–315. doi: 10.1056/NEJMoa2029980. [DOI] [PubMed] [Google Scholar]
- 19.Marrouche N.F., Brachmann J., Andresen D., et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 20.Jones D.G., Haldar S.K., Hussain W., et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. 2013;61:1894–1903. doi: 10.1016/j.jacc.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 21.De Potter T., Van Herendael H., Balasubramaniam R., et al. Safety and long-term effectiveness of paroxysmal atrial fibrillation ablation with a contact force-sensing catheter: real-world experience from a prospective, multicentre observational cohort registry. Europace. 2018;20:f410–f418. doi: 10.1093/europace/eux290. [DOI] [PubMed] [Google Scholar]
- 22.Wilber D.J., Pappone C., Neuzil P., et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–340. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 23.Kirchhof P., Camm A.J., Goette A., et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422. [DOI] [PubMed] [Google Scholar]
- 24.Verma A., Champagne J., Sapp J., et al. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): a prospective, multicenter study. JAMA Intern Med. 2013;173:149–156. doi: 10.1001/jamainternmed.2013.1561. [DOI] [PubMed] [Google Scholar]
- 25.Arya A., Piorkowski C., Sommer P., Kottkamp H., Hindricks G. Clinical implications of various follow up strategies after catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2007;30:458–462. doi: 10.1111/j.1540-8159.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- 26.Goldenthal I.L., Sciacca R.R., Riga T., et al. Recurrent atrial fibrillation/flutter detection after ablation or cardioversion using the AliveCor KardiaMobile device: iHEART results. J Cardiovasc Electrophysiol. 2019;30:2220–2228. doi: 10.1111/jce.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarakji K.G., Wazni O.M., Callahan T., et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm. 2015;12:554–559. doi: 10.1016/j.hrthm.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Dagres N., Kottkamp H., Piorkowski C., et al. Influence of the duration of Holter monitoring on the detection of arrhythmia recurrences after catheter ablation of atrial fibrillation: implications for patient follow-up. Int J Cardiol. 2010;139:305–306. doi: 10.1016/j.ijcard.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Mulder A.A., Wijffels M.C., Wever E.F., Kelder J.C., Boersma L.V. Arrhythmia detection after atrial fibrillation ablation: value of incremental monitoring time. Pacing Clin Electrophysiol. 2012;35:164–169. doi: 10.1111/j.1540-8159.2011.03202.x. [DOI] [PubMed] [Google Scholar]
- 30.Kottkamp H., Tanner H., Kobza R., et al. Time courses and quantitative analysis of atrial fibrillation episode number and duration after circular plus linear left atrial lesions: trigger elimination or substrate modification: early or delayed cure? J Am Coll Cardiol. 2004;44:869–877. doi: 10.1016/j.jacc.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 31.Senatore G., Stabile G., Bertaglia E., et al. Role of transtelephonic electrocardiographic monitoring in detecting short-term arrhythmia recurrences after radiofrequency ablation in patients with atrial fibrillation. J Am Coll Cardiol. 2005;45:873–876. doi: 10.1016/j.jacc.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 32.Andrade J.G., Champagne J., Dubuc M., et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140:1779–1788. doi: 10.1161/CIRCULATIONAHA.119.042622. [DOI] [PubMed] [Google Scholar]
- 33.Vasamreddy C.R., Dalal D., Dong J., et al. Symptomatic and asymptomatic atrial fibrillation in patients undergoing radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2006;17:134–139. doi: 10.1111/j.1540-8167.2006.00359.x. [DOI] [PubMed] [Google Scholar]
- 34.Shea B.J., Grimshaw J.M., Wells G.A., et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nair G.M., Raut R., Bami K., et al. Efficacy of adjunctive measures used to assist pulmonary vein isolation for atrial fibrillation: a systematic review. Curr Opin Cardiol. 2017;32:58–68. doi: 10.1097/HCO.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 36.Cosedis Nielsen J., Johannessen A., Raatikainen P., et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 37.Atienza F., Almendral J., Ormaetxe J.M., et al. Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: a noninferiority randomized multicenter RADAR-AF trial. J Am Coll Cardiol. 2014;64:2455–2467. doi: 10.1016/j.jacc.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 38.Masuda M., Asai M., Iida O., et al. Additional low-voltage-area ablation in patients with paroxysmal atrial fibrillation: results of the randomized controlled VOLCANO trial. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.015927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziegler P.D., Koehler J.L., Mehra R. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm. 2006;3:1445–1452. doi: 10.1016/j.hrthm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 40.Charitos E.I., Stierle U., Ziegler P.D., et al. A comprehensive evaluation of rhythm monitoring strategies for the detection of atrial fibrillation recurrence. Circulation. 2012;126:806–814. doi: 10.1161/CIRCULATIONAHA.112.098079. [DOI] [PubMed] [Google Scholar]
- 41.Chen L.Y., Chung M.K., Allen L.A., et al. Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: a scientific statement from the American Heart Association. Circulation. 2018;137:e623–e644. doi: 10.1161/CIR.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oral H., Knight B.P., Ozaydin M., et al. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol. 2002;40:100–104. doi: 10.1016/s0735-1097(02)01939-3. [DOI] [PubMed] [Google Scholar]
- 43.Hussein A.A., Saliba W.I., Martin D.O., et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:271–278. doi: 10.1161/CIRCEP.111.962100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.