Abstract
Background: Marfan Syndrome (MFS) is one of the most common connective tissue disorders. The aim of this study was to characterize an adult population with MFS and evaluate its long-term prognosis. Methods: A retrospective analysis of adult patients with MFS followed up during the past 40 years in a tertiary congenital heart disease outpatient clinic was performed. Survival analysis was performed according to different parameters, and survival curves were compared using the log-rank test. Results: A total of 62 MFS patients were followed up for a mean period of 12 years (47% male; mean age, 39 years). The baseline mean aortic root diameter (ARD) at the Valsalva sinus was 42.4 ± 10.3 mm, with 15% of patients having moderate-to-severe aortic regurgitation and seven patients with acute aortic syndrome. The Bentall procedure was the most commonly performed surgical technique, and five patients required re-operation. Of the 17 pregnancies, 29% developed fetal complications; however, there was no maternal morbidity or mortality. A total of ten deaths occurred at a mean age of 52 years. Patients with an ARD ≤ 45 mm had a significantly lower all-cause mortality rate than patients with 45 < ARD ≤ 50 mm or with ARD > 50 mm (P = 0.004 and P < 0.001, respectively). Heart failure symptoms were associated with a worse outcome (P = 0.041), while the presence of extracardiac involvement had a protective effect (P < 0.001). Conclusion: MFS-related aortopathy is associated with high morbidity rates. In the overall population, an ARD > 45 mm at the time of diagnosis was associated with higher mortality during follow-up.
Keywords: Marfan Syndrome, acute aortic syndrome, congenital heart disease, follow-up, aortic root surgery
Introduction
Marfan Syndrome (MFS) is a connective tissue disorder with autosomal dominant inheritance, high penetrance but variable expression, and an estimated incidence of 1:3000 to 1:5000 people, mostly caused by mutations in the gene encoding fibrillin-1 (FBN1) [1].
Approximately 25% of patients do not have a family history and represent new cases due to sporadic mutations. It is a multisystem disease with cardinal features associated with the cardiovascular, ocular, and musculoskeletal systems; however, the condition displays a high clinical variability and a wide spectrum of clinical severity, making its diagnosis markedly challenging [2,3]. The diagnosis of MFS relies on defined clinical criteria (Ghent nosology), which were revised in 2010, focusing on cardiovascular and ocular manifestations, namely aortic root (AR) aneurysm and ectopia lentis, respectively [3]. Approximately 60%-80% of adult patients have dilatation of the AR, often accompanied by aortic regurgitation; dilatation may involve other segments of the thoracic aorta, abdominal aorta, or even the supra-aortic trunks and cerebral arteries [4,5]. Prognosis is mainly determined by progressive AR dilatation, especially at the sinus of Valsalva, potentially leading to aortic dissection or rupture, which may affect 9.7% of individuals with an average mortality of approximately 10.6% [4,6,7]. Mitral valve prolapse (MVP) is the second most common cardiac abnormality associated with MFS, with 1 in 8 cases with this condition developing moderate to severe mitral regurgitation, the main cause of morbidity and mortality in children with this disease [6,8-10]. For many years, this population had a limited life expectancy that was 20 to 30 years below the average population, but the prognosis has improved due to the use of medical therapy, routine and serial monitoring of aortic dimensions, restriction of vigorous physical exercise, and timely elective repair of the AR, so that the life expectancy has risen to over 70 years according to recent series [6,11].
There is scarce data in literature regarding the long-term morbidity and mortality of patients with Marfan Syndrome. In this sense, the authors found pertinent to share their center’s decades long experience concerning the management of this population, particularly regarding the risk for long-term complications, namely infective endocarditis, need for reoperation and cerebrovascular events. Also, in a cohort of young adults with a genetic disease, it is also imperative to analyze maternal and fetal complications and their appropriate management. The approach to the management of patients with Marfan Syndrome is influenced by the patient’s age, clinical penetrance and presentation, namely the degree of cardiac involvement. Thus, our aim was to assess the real-world long-term morbidity and mortality of an adult population of MFS patients followed up in a tertiary center [12-15].
Materials and methods
Patient population and study protocol
This was a single-center retrospective observational registry that included all adult patients with Marfan Syndrome followed up in the adult CHD outpatient clinic of our tertiary center between January 1978 and May 2020. We evaluated demographic, clinical, echocardiographic, imaging, and genetic testing data from clinical files.
Diagnosis was considered to have been made at a pediatric age if made when the patient was younger than 18 years. The clinical presentation that led to the diagnosis was divided into cardiac symptoms/acute aortic syndrome (AAS), family screening of affected individuals, extra-cardiac manifestations, and routine echocardiographic examination. The New York Heart Association (NYHA) functional classification was used to evaluate the severity of heart failure (HF) symptoms. Data from the first transthoracic echocardiogram (TTE) performed in our center were analyzed, and the ejection fraction was calculated from end-diastolic and end-systolic volumes indexed to body surface area. AR diameter (ARD) was measured at the sinus of Valsalva using the leading-edge-to-leading-edge method. Most patients also underwent cross-sectional aortic imaging with computed tomography (CT) or magnetic resonance imaging (MRI) to confirm AR, and ascending aorta size as measured by TTE (using the inner edge-to-inner edge method), to evaluate more distal segments of the aorta/supra-aortic trunks or during the follow-up of patients who underwent AR surgery.
All cases were retrospectively analyzed, and the diagnosis of Marfan Syndrome was established if the patients met the revised Ghent nosology for Marfan Syndrome [3].
Study endpoints
During follow-up, all-cause mortality data, as well as other morbidity endpoints, such as cardioembolic stroke (CS), infective endocarditis (IE), and the need for aortic reoperation were assessed. The diagnosis of IE was established based on the modified Duke criteria. CS was defined by the documentation of a cortical infarct by head CT/MRI in the presence of a potential intracardiac source of embolism and after exclusion of alternative causes for the cerebrovascular event.
Pregnancy outcomes were assessed from clinical file data, namely maternal (acute heart failure or acute aortic syndrome) or fetal complications (spontaneous abortion, premature birth, stillbirth, or congenital heart defect).
Ethics
The investigation conformed to the principles outlined in the Declaration of Helsinki. The study protocol was approved by the Institutional Ethics Committee and was assigned the approval number 1196/2022.
Statistical analysis
Baseline characteristics and follow-up workup data were summarized as frequencies (percentages) for categorical variables, as means and standard deviations for continuous variables when normality was verified, and as median and interquartile range when normality was not verified by the Kolmogorov-Smirnov test. The Student’s t-test for independent samples or the Mann-Whitney test (when normality was not confirmed) was used for all comparisons. The chi-square test or Fisher’s exact test was used to compare categorical variables. Survival curves were determined using the Kaplan-Meier method and compared using the log-rank test. A two-tailed probability value of < 0.05, was considered statistically significant.
Results
Population baseline profile
A total of 62 patients were enrolled in the registry (Table 1). The mean age of the study cohort was 39.0 ± 13.3 years with a mean follow-up time of 12.4 ± 8.7 years and 47% of the patients were male. There was a low prevalence of medical comorbidities, and the mean baseline ARD at the time of diagnosis was 45.0 ± 10.8 mm, with 28 patients presenting with MVP. Approximately 45% of the population had a family history of MS, and 15% had sudden cardiac death or AAS. There was a high prevalence of extracardiac involvement, namely, musculoskeletal manifestations. The diagnosis was made at a pediatric age in 45% of patients, mainly elicited by extracardiac manifestations (in 39% of patients) and in the setting of familial screening (25.8%).
Table 1.
Clinical profile of Marfan Syndrome patients
| Total Population (n = 62) | |
|---|---|
| Male gender, n (%) | 29 (46.7%) |
| Mean age, years | 39.0 ± 13.3 |
| Mean follow-up time, years | 12.4 ± 8.7 |
| Hypertension, n (%) | 19 (30.6%) |
| Dyslipidemia, n (%) | 15 (24.2%) |
| Diabetes, n (%) | 7 (11.3%) |
| Coronary artery disease (%) | 4 (6.5%) |
| Chronic kidney disease, n (%) | 6 (9.7%) |
| Pediatric diagnosis, n (%) | 28 (45.2%) |
| Genetic testing, n (%) | 25 (40.3%) |
| Family history of MS, n (%) | 28 (45.2%) |
| Family history of SCD or aortic dissection, n (%) | 9 (14.5%) |
| Extra-cardiac manifestations, n (%) | 55 (88.7%) |
| Ocular | 24 (38.7%) |
| Musculoskeletal | 32 (51.6%) |
| Pulmonary | 2 (3.2%) |
| Central nervous system | 3 (4.8%) |
| Marfanoid habitus | 32 (51.6%) |
| Mean aortic root diameter, mm | 42.4 ± 10.3 |
| Moderate to severe AR, n (%) | 9 (14.5%) |
| Mitral valve prolapse, n (%) | 28 (45.2%) |
| Moderate to severe MR, n (%) | 12 (19.4%) |
| Mean left ventricular ejection fraction, % | 54.4 ± 9.3 |
| Overt HF | 18 (29.0%) |
| NYHA II | 12 (19.4%) |
| NYHA III | 6 (9.7%) |
| NYHA IV | 0 |
| Aortic root surgery, n (%) | 18 (29%) |
| Acute aortic syndrome, n (%) | 7 (11.3%) |
| Mitral valve surgery, n (%) | 5 (8.1%) |
| Under beta-blocker, n (%) | 36 (58.1%) |
| Under ARB, n (%) | 25 (40.3%) |
| Associated congenital heart defects, n (%) | 3 (4.8%) |
| Infective endocarditis, n (%) | 3 (4.8%) |
| Stroke, n (%) | 8 (12.9%) |
| Death, n (%) | 10 (16.1%) |
Values are mean ± SD, n (%), or median (interquartile range). Abbreviations: BNP, Brain Natriuretic Peptide; CABG, Coronary Artery Bypass Grafting; CAD, Coronary Artery Disease; CCS, Canadian Cardiovascular Society; CIED, Cardiac Implantable Electronic Device; GLS, Global Longitudinal Strain; LVEF, Left Ventricular Ejection Fraction; MI, Myocardial Infarction; NYHA, New York Heart Association; PCI, Percutaneous Coronary Intervention.
Twenty-five patients underwent genetic testing, with almost half revealing a pathogenic FBN1 mutation (Table 2).
Table 2.
Genetic testing results
| Total Tests (n = 25) | |
|---|---|
| Positive (FBN1 mutation) | 12 (48.0%) |
| Negative | 9 (36.0%) |
| Inconclusive | 4 (16.0%) |
Values are mean ± SD, n (%), or median (interquartile range).
Surgical management
Eighteen patients underwent AR surgery, mostly a Bentall procedure (61% of cases), which was mainly performed in a non-urgent setting, as only seven patients suffered an AAS. The majority of patients with AAS did not have a previous diagnosis of MFS. Five patients underwent reoperation, with a mean time to reoperation of 11 years (Table 3). Five patients underwent mitral valve surgery for symptomatic severe mitral regurgitation.
Table 3.
Characteristics of aortic root and mitral surgery
| Aortic Root Surgery | Total Procedures (n = 18) |
|
| |
| Type of Surgery | |
| Bentall | 11 (61.1%) |
| David | 7 (38.9%) |
| Setting | |
| Urgent | 7 (38.9%) |
| Prophylactic | 11 (61.1%) |
| Reoperation | 5 (27.8%) |
| Reoperation Type | |
| Chronic dissection distal to aortic graft | 2 (40%) |
| Descending thoracic aortic aneurysm | 3 (60%) |
| Mean time to reoperation (years) | 10.9 ± 6.5 |
|
| |
| Mitral Valve Surgery | Total Procedures (n = 5) |
|
| |
| Type of Surgery | |
| Mitral valve replacement | 3 (60%) |
| Mitral valve repair | 2 (40%) |
Patients who underwent AR surgery had a significantly higher prevalence of arterial hypertension (66.7% vs. 15.9%, P < 0.001), dyslipidemia (55.6% vs. 11.4%, P = 0.001), and chronic kidney disease (27.8% vs. 2.3%, P = 0.006), were more frequently diagnosed in adulthood (83.3% vs. 43.2%, P = 0.004), and had a higher baseline ARD (47.5 ± 10.8 vs. 34.4 ± 8.8, P = 0.039). These patients also presented more frequently with HF symptoms (66.7% vs. 15.9%, P < 0.001) and were less likely to be under medical therapy (11.1% vs. 54.5%, P = 0.002); however, this did not translate into a worse long-term prognosis, as the groups had similar all-cause mortality rates (Table 4).
Table 4.
Clinical profile of patients that underwent aortic root surgery
| Aortic Root Surgery (N - 18) | No Aortic Root Surgery (N - 44) | p value | |
|---|---|---|---|
| Male gender, n (%) | 13 (72.2%) | 26 (59.1%) | 0.331 |
| Hypertension, n (%) | 12 (66.7%) | 7 (15.9%) | < 0.001 |
| Dyslipidemia, n (%) | 10 (55.6%) | 5 (11.4%) | 0.001 |
| Diabetes, n (%) | 3 (16.7%) | 4 (9.1%) | 0.404 |
| Coronary artery disease (%) | 3 (16.7%) | 1 (2.3%) | 0.070 |
| Chronic kidney disease, n (%) | 5 (27.8%) | 1 (2.3%) | 0.006 |
| Pediatric diagnosis, n (%) | 3 (16.7%) | 25 (56.8%) | 0.004 |
| Positive genetic test, n (%) | 3 (16.7%) | 9 (20.5%) | 0.910 |
| Family history of MS, n (%) | 10 (55.6%) | 18 (40.9%) | 0.293 |
| Family history of SCD or aortic dissection, n (%) | 3 (16.7%) | 6 (13.6%) | 0.711 |
| Extra-cardiac manifestations, n (%) | 15 (83.3%) | 40 (90.9%) | 0.404 |
| Mean aortic root diameter, mm | 47.5 ± 10.8 | 34.4 ± 8.8 | 0.039 |
| Moderate to severe AR, n (%) | 3 (16.7%) | 6 (13.6%) | 0.711 |
| Mitral valve prolapse, n (%) | 8 (44.4%) | 20 (45.4%) | 0.883 |
| Moderate to severe MR, n (%) | 5 (27.8%) | 7 (15.9%) | 0.305 |
| Mean left ventricular ejection fraction, % | 51.3 ± 10.1% | 55.8 ± 8.9% | 0.098 |
| Overt HF | 12 (66.7%) | 6 (13.6%) | < 0.001 |
| Mitral valve surgery, n (%) | 3 (16.7%) | 2 (4.5%) | 0.141 |
| Under beta-blocker, n (%) | 2 (11.1%) | 24 (54.5%) | 0.002 |
| Under ARB, n (%) | 9 (50.0%) | 16 (36.4%) | 0.320 |
| Infective endocarditis, n (%) | 2 (11.1%) | 1 (2.3%) | 0.200 |
| Stroke, n (%) | 6 (33.3%) | 2 (4.5%) | 0.006 |
| Pregnancy, n (%) | 2 (11.1%) | 11 (25.0%) | 0.312 |
| Death, n (%) | 5 (27.8%) | 5 (11.4%) | 0.137 |
Values are mean ± SD, n (%), or median (interquartile range). Abbreviations: BNP, brain natriuretic peptide; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCS, Canadian Cardiovascular Society; CIED, cardiac implantable electronic device; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Pregnancy outcomes
Thirteen women became pregnant (39% of the female population), with a total of 17 pregnancies (Table 5). The mean AR diameter at the time of pregnancy was 36.2 mm and only 2 patients underwent AR surgery. There were no maternal deaths or complications, such as preeclampsia, eclampsia, acute HF, or aortic syndrome. There were four fetal complications, namely two premature births and two stillbirths, but no fetal mortality. All offspring were referred to the pediatric cardiology department and genetic consultation for evaluation. Two children were born with mild congenital heart disease and an atrial septal defect.
Table 5.
Characteristics of pregnant patients with Marfan Syndrome during follow-up
| Patient | Aortic Root Diameter (mm) | Aortic Regurgitation | Mitral Valve Prolapse | Previous Aortic Root Surgery | Previous Mitral Surgery | Nr. of pregnancies | Fetal/maternal complications | Offspring born with CHD |
|---|---|---|---|---|---|---|---|---|
| 1 | 45 | Moderate | No | No | No | 1 | None | No |
| 2 | 33 | Not significant | No | No | No | 1 | None | Yes |
| 3 | 39 | Not significant | Yes | No | No | 2 | SA (Spontaneous Abortion) | Yes |
| 4 | 52 | Mild | Yes | Yes | No | 3 | None | No |
| 5 | 39 | Not significant | No | No | No | 1 | SA (Spontaneous Abortion) | No |
| 6 | Unknown | Severe | Yes | No | No | 1 | None | No |
| 7 | 34 | Mild | No | No | No | 1 | None | No |
| 8 | 41 | Mild | Yes | No | No | 1 | PB (Premature birth) | No |
| 9 | 31 | Not significant | No | No | No | 1 | None | No |
| 10 | 29 | Not significant | Yes | Yes | No | 1 | None | No |
| 11 | Unknown | Not significant | No | No | No | 1 | PB (Premature birth) | No |
| 12 | 25 | Not significant | Yes | No | No | 1 | None | No |
| 13 | 30 | Mild | No | No | No | 2 | None | Yes |
Abbreviations: CHD, congenital heart disease; Nr, Number.
Survival and follow-up
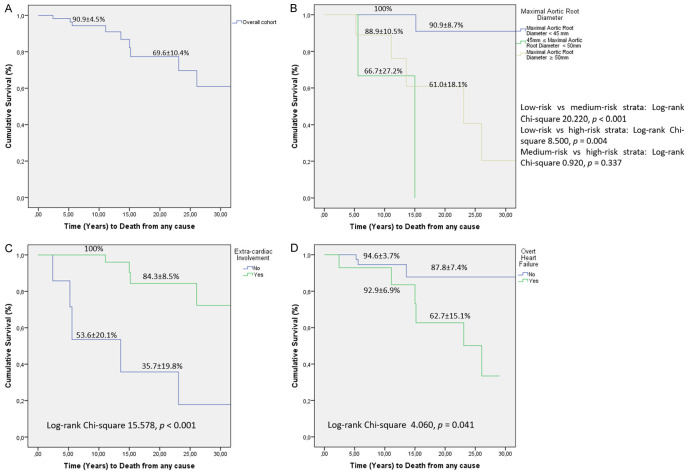
During a median follow-up of 12 years, 10 patients (16.1%) died, with a survival rate of 91% and 70% at the 10- and 20-year follow-up, respectively (Figure 1A). The profiles of the deceased patients are detailed in Table 6. The mean age of these patients at the time of death was 52 ± 16 years, with half of these patients having undergone a previous AR surgery and three of them requiring reoperation. Only one patient had been submitted to mitral valve surgery, and the majority was treated with beta-blocker (BB) and/or angiotensin receptor blocker (ARB) therapy. Three patients (4.8%) were diagnosed with IE during follow-up, and 12.9% suffered a CS.
Figure 1.
A. Survival rates of adult patients with Marfan Syndrome during follow-up. B. Survival curves according to different baseline aortic root diameter cut-offs (45 and 50 mm). C. Survival analysis according to the presence of extra-cardiac manifestations. D. Survival analysis according to the presence of heart failure symptoms.
Table 6.
Clinical profile of deceased patients with Marfan Syndrome
| Gender | Age at death | Follow-up Time (Years) | Aortic Root Diameter (mm)1 | Moderate to Severe AR2 | MVP | Moderate to Severe MR3 | Previous Aortic Root Surgery | Previous Mitral Surgery | Need for reoperation | Medication | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | 59 | 11 | 56 | No | No | No | Yes | No | Yes | BB | Perioperative ischemic stroke following aortic reoperation |
| Male | 67 | 23 | 55 | No | No | No | Yes | No | Yes | BB | Perioperative ventilator-associated pneumonia |
| Female | 74 | 3 | 54 | Yes | Yes | Yes | No | No | No | None | End-stage HF |
| Female | 33 | 15 | 41 | No | Yes | Yes | No | No | No | BB and ARB | Sudden Death |
| Male | 67 | 36 | 56 | Yes | Yes | Yes | No | Yes | No | BB and ARB | End-stage HF |
| Male | 61 | 5 | 53 | No | No | No | Yes | No | Yes | BB | Sudden Death |
| Male | 44 | 6 | 49 | Yes | No | No | Yes | No | No | BB and ARB | Sudden Death while on heart transplant waiting list |
| Female | 31 | 14 | 55 | Yes | Yes | Yes | No | No | No | BB | Unknown |
| Male | 46 | 26 | 59 | No | No | No | Yes | No | No | BB and ARB | Hemorrhagic Stroke |
| Female | 37 | 15 | 46 | No | Yes | Yes | No | No | No | BB and ARB | Sudden Death - while awaiting mitral valve annuloplasty |
1,2,3Previous to Surgery. Abbreviations: AR, aortic regurgitation; ARB, angiotensin receptor blocker; BB, beta-blocker; HF, heart failure; MR, mitral regurgitation; MVP, mitral valve prolapse.
A survival analysis was performed comparing three risk strata divided according to two AR diameter cut-offs: 45 and 50 mm (Figure 1B). Patients with an AR diameter ≥ 45 mm at the time of diagnosis had a significantly lower survival during follow-up than the remainder risk strata; there was no difference in the prognosis of patients with an AR diameter between 45 and 50 mm and those with an AR diameter ≥ 50 mm. Patients with extracardiac manifestations had a significantly higher 20-year follow-up survival (84% vs. 36%, P < 0.001), as did patients without HF symptoms, with a 20-year follow-up survival of 88% vs. 63%, P = 0.041 (Figure 1C and 1D, respectively). In the female cohort, pregnancy was not associated with worse long-term prognosis.
Discussion
The main cardiovascular manifestation of MFS is dilatation of the AR and the proximal ascending aorta, which can lead to aortic dissection and premature death [6]. MFS is found in 50% of patients with aortic dissection aged under 40 years [5]. According to current guidelines, surgery is recommended when the maximal ARD is ≥ 50 mm or ≥ 45 mm when the following additional risk factors are present [16,17]: uncontrolled hypertension, family history of dissection at a low diameter, size increase > 3 mm/year, severe aortic or mitral regurgitation, or desire for pregnancy. In our analysis, the follow-up mortality rate was not negligible, with 20% mortality at the 20-year follow-up. Survival analysis revealed that an ARD < 45 mm at the time of diagnosis was associated with a better prognosis than those between 45 and 50 mm and above 50 mm, while prognosis was similar between the strata with ARD between 45 and 50 mm and the strata with ARD > 50 mm. This should raise awareness of the importance of a timely diagnosis, strict serial aortic dimension monitoring, and the presence of additional risk factors that may warrant an earlier elective surgery. The mortality risk of prophylactic aortic surgery is only 1%-2% [6]. In our population, only two patients died after prophylactic aortic surgery; however, both were reoperations, and the cause of death was post-operative complications (ischemic stroke and ventilator-associated pneumonia). There was no difference in mortality between patients who underwent AR surgery and those who did not, which is in concordance with what is reported in the literature.
MVP is a frequently neglected cardiovascular manifestation in patients with MFS, despite affecting almost 80% of this population. Although commonly asymptomatic, it can present as severe acute mitral regurgitation secondary to chordae tendineae rupture, and in 1 in 8 patients, it can progress to moderate-to-severe mitral regurgitation, making it the most common cause of mortality and morbidity in the pediatric Marfan population [8-10]. Only 20% of patients who undergo elective AR replacement have concomitant MV procedures, despite it being reasonable to combine both procedures in patients with significant mitral regurgitation [10]. Several studies have shown better survival with valve repair than with replacement [8-10]. In our population, only 45% of patients had MVP and nine eventually progressed to moderate-to-severe mitral regurgitation, with five of them undergoing MV surgery. In our experience, neither MVP nor the presence of moderate-to-severe MR was associated with higher follow-up mortality.
Patients who developed overt HF symptoms during follow-up (NYHA functional classes II, III, and IV) had significantly worse survival. These were mostly patients who developed ventricular dysfunction due to severe aortic/mitral regurgitation or post-cardiotomy shock after AR surgery. Of note, three patients died due to end-stage HF, and one of them was on the heart transplant waiting list. In the literature, there is a poor correlation between the severity of the cardiovascular, ocular, and skeletal manifestations [18]; however, in our analysis, patients with extracardiac manifestations displayed better survival, which may be explained by an earlier age of diagnosis and management elicited by the ocular or musculoskeletal manifestations.
Prophylactic treatment with BB has been considered the mainstay of care for adult patients with MFS [11,17] since an open-label randomized trial showed that propranolol use was associated with a significantly slower rate of aortic dilatation and better mid-term survival [19]. Mullen et al. (2019) reported that irbesartan was associated with a reduction in the rate of aortic dilatation in young adults with MFS [20]. In our experience, neither BB use nor angiotensin II receptor blockade was associated with lower mortality during follow-up.
Not only are pregnancy and the postpartum period high-risk periods for aortic dissection and rupture in women with MFS, but they may also increase the long-term rate of aortic dilatation [16,21]. These women should receive counseling and require specialized management, especially during delivery and the postpartum period. Women with an AR diameter > 45 mm are strongly discouraged from becoming pregnant without prior elective AR repair, and when the AR diameter is between 40 and 45 mm, aortic growth rate and family history of dissection should be taken into account in the decision [16]. These patients were followed up by a multidisciplinary team comprising a dedicated specialized cardiologist, obstetricians, and an anesthesiologist. Despite the fact that some patients had undergone pregnancy without the recommended prior aortic root repair, there were no maternal complications. There were two spontaneous abortions (11.8%), both from patients with an AR diameter < 40 mm and two premature births.
Thus, our study characterizes the long-term outcomes of adults with Marfan Syndrome followed in a tertiary centre. To the best of our knowledge, this is the largest Portuguese report on this subject. As we notice in our study, Marfan Syndrome is still a high-risk population demanding close and multidisciplinary clinical follow-up, multimodality imaging techniques and a specialized surgical care.
Study limitations
First, the study’s unicentre design and relatively small cohort size may limit some of our conclusions. Second, its retrospective nature also imposes some limitations, since there is no informative data regarding the first follow-up years of the first patients followed up in our center. As our analysis included patients who were followed up for four decades, it may include outdated clinical practices regarding diagnosis, management, and surgical techniques, which would impact patient prognosis. Furthermore, only a limited proportion of our population underwent genetic testing, so it is possible that some patients with other thoracic aortic syndromes may have been misdiagnosed with MFS. In addition, being a retrospective study with some patients having been diagnosed with MFS after an AAS may have biased our analysis, thereby explaining that the use of BB/ARB or a previous AAS was not associated with a lower mortality. Finally, our analysis may also have a selection bias, since all the included MFS patients had some degree of cardiac involvement and, therefore, may not be representative of an MFS population.
Conclusions
Our work refers to the largest Portuguese cohort of patients with MFS with one of the longest follow-up periods reported in the literature. It sheds light on specific aspects of these patients’ management, such as the need for continued aortic root dimension monitoring after surgery and the need for multidisciplinary pregnancy management. Patients with an AR diameter > 45 mm at the time of diagnosis had significantly worse survival during follow-up.
Disclosure of conflict of interest
None.
References
- 1.Ho NC, Tran JR, Bektas A. Marfan’s syndrome. Lancet. 2005;366:1978–81. doi: 10.1016/S0140-6736(05)66995-4. [DOI] [PubMed] [Google Scholar]
- 2.De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan Syndrome. Am J Med Genet. 1996;62:417–26. doi: 10.1002/(SICI)1096-8628(19960424)62:4<417::AID-AJMG15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 3.Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, Hilhorst-Hofstee Y, Jondeau G, Faivre L, Milewicz DM, Pyeritz RE, Sponseller PD, Wordsworth P, De Paepe AM. The revised Ghent nosology for the Marfan Syndrome. J Med Genet. 2010;47:476–85. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 4.Isekame Y, Gati S, Aragon-Martin JA, Bastiaenen R, Kondapally Seshasai SR, Child A. Cardiovascular management of adults with Marfan Syndrome. Eur Cardiol. 2016;11:102–110. doi: 10.15420/ecr/2016:19:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ammash NM, Sundt TM, Connolly HM. Marfan Syndrome-diagnosis and management. Curr Probl Cardiol. 2008;33:7–39. doi: 10.1016/j.cpcardiol.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Coelho SG, Almeida AG. Revista Portuguesa de Cardiologia. 2020. Marfan Syndrome revisited: from genetics to clinical practice; pp. 215–226. [DOI] [PubMed] [Google Scholar]
- 7.Wright MJ, Connolly HM. In: Genetics, clinical features, and diagnosis of Marfan Syndrome and related disorders. UpToDate, Post TW, editor. Waltham, MA: UpToDate; 2020. [Google Scholar]
- 8.Thacoor A. Mitral valve prolapse and Marfan Syndrome. Congenit Heart Dis. 2017;12:430–434. doi: 10.1111/chd.12467. [DOI] [PubMed] [Google Scholar]
- 9.Kunkala MR, Schaff HV, Li Z, Volguina I, Dietz HC, LeMaire SA, Coselli JS, Connolly H. Mitral valve disease in patients with Marfan Syndrome undergoing aortic root replacement. Circulation. 2013;128(Suppl 1):S243–7. doi: 10.1161/CIRCULATIONAHA.112.000113. [DOI] [PubMed] [Google Scholar]
- 10.Bhudia SK, Troughton R, Lam BK, Rajeswaran J, Mills WR, Gillinov AM, Griffin BP, Blackstone EH, Lytle BW, Svensson LG. Mitral valve surgery in the adult Marfan Syndrome patient. Ann Thorac Surg. 2006;81:843–8. doi: 10.1016/j.athoracsur.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 11.Wright MJ, Connolly HM. In: Management of Marfan Syndrome and related disorders. UpToDate, Post TW, editor. Waltham, MA: UpToDate; 2020. [Google Scholar]
- 12.Marsalese DL, Moodie DS, Vacante M, Lytle BW, Gill CC, Sterba R, Cosgrove DM, Passalacqua M, Goormastic M, Kovacs A. Marfan’s syndrome: natural history and long-term follow-up of cardiovascular involvement. J Am Coll Cardiol. 1989;14:422–8. doi: 10.1016/0735-1097(89)90197-6. [DOI] [PubMed] [Google Scholar]
- 13.Gallotti R, Ross DN. The Marfan Syndrome: surgical technique and follow-up in 50 patients. Ann Thorac Surg. 1980;29:428–33. doi: 10.1016/s0003-4975(10)61673-6. [DOI] [PubMed] [Google Scholar]
- 14.Nicolo F, Romeo F, Lio A, Bovio E, Scafuri A, Bassano C, Polisca P, Pellegrino A, Nardi P, Chiariello L, Ruvolo G. Long-term results of aortic root surgery in Marfan Syndrome patients: a single-center experience. J Heart Valve Dis. 2017;26:397–404. [PubMed] [Google Scholar]
- 15.Nardi P, Saitto G, Ruvolo G. Long-term follow-up experience on the aortic root surgery in patients affected by Marfan Syndrome. J Vasc Endovasc Surg. 2016;1:4. [Google Scholar]
- 16.Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke-Baerwolf C, Kaemmerer H, Kilner P, Meijboom F, Mulder BJ, Oechslin E, Oliver JM, Serraf A, Szatmari A, Thaulow E, Vouhe PR, Walma E Task Force on the Management of Grown-up Congenital Heart Disease of the European Society of Cardiology (ESC); Association for European Paediatric Cardiology (AEPC); ESC Committee for Practice Guidelines (CPG) ESC Guidelines for the management of grown-up congenital heart disease (new version 2010) Eur Heart J. 2010;31:2915–57. doi: 10.1093/eurheartj/ehq249. [DOI] [PubMed] [Google Scholar]
- 17.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchoukos NT, Lytle BW, Milewicz DM, Reich DL, Sen S, Shinn JA, Svensson LG, Williams DM American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American College of Radiology; American Stroke Association; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of Thoracic Surgeons; Society for Vascular Medicine. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 18.Bruno L, Tredici S, Mangiavacchi M, Colombo V, Mazzotta GF, Sirtori CR. Cardiac, skeletal, and ocular abnormalities in patients with Marfan’s syndrome and in their relatives. Comparison with the cardiac abnormalities in patients with kyphoscoliosis. Br Heart J. 1984;51:220–30. doi: 10.1136/hrt.51.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shores J, Berger KR, Murphy EA, Pyeritz RE. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan’s syndrome. N Engl J Med. 1994;330:1335–41. doi: 10.1056/NEJM199405123301902. [DOI] [PubMed] [Google Scholar]
- 20.Mullen M, Jin XY, Child A, Stuart AG, Dodd M, Aragon-Martin JA AIMS Investigators. Irbesartan in Marfan Syndrome (AIMS): a double-blind, placebo-controlled randomised trial. Lancet. 2019;394:2263–2270. doi: 10.1016/S0140-6736(19)32518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright MJ, Connolly HM. In: Pregnancy and Marfan Syndrome. UpToDate, Post TW, editor. Waltham, MA: UpToDate; 2020. [Google Scholar]