Abstract
Background: Diabetes mellitus causes ischemic heart disease (IHD) through macrovascular or microvascular involvement. Diabetes-associated hypertension, dyslipidemia, and obesity further increase coronary artery disease risk and can cause left ventricular hypertrophy leading to heart failure with preserved ejection fraction independent of IHD. This study was undertaken to evaluate the differences in demographics, clinical characteristics, Echocardiographic parameters, management, and outcomes between non-ischemic cardiomyopathy (NICM) and ischemic cardiomyopathy (ICM) patients in cohort of diabetes patients. Methods: This retrospective study included diabetes patients with reduced ejection fraction (≤40) who were hospitalized with heart failure between January 2014 and February 2020. Patients were divided into two groups: group 1; ICM and group 2; NICM. Data obtained on above mentioned features including mortality and heart failure readmissions were compared between the two groups. Results: A total of 612 diabetes patients admitted with acute heart failure were screened of which 442 were included. Group 1 (ICM) had 361 patients (81.7%) and group 2 (NICM) had 81 patients (18.3%). Patients in group 1 were older, predominantly males and with higher prevalence of hypertension, smoking and insulin dependent Diabetes while group 2 patients had higher BMI and higher prevalence of cardiac rhythm problems. No significant difference was detected in 5-year-mortality between the two groups (P=0.165). However, heart failure associated hospitalizations were higher in group 2 though it was not statistically significant (P=0.062). Conclusion: There was no difference in 5-years mortality between ICM and NICM in diabetes patients. However, NICM patients had higher prevalence of obesity and rhythm problems.
Keywords: Heart failure, diabetes mellitus, ischemic cardiomyopathy, non-ischemic cardiomyopathy
Introduction
Diabetes mellitus (DM) can affect the cardiovascular system in different aspects. It can cause ischemic heart disease (IHD) through macrovascular or microvascular involvement. It is also associated with other cardiac risk factors like hypertension (HTN), dyslipidemia and obesity, which increase the risk for coronary artery disease (CAD). Diabetes can also cause left ventricular hypertrophy, which leads to heart failure with preserved ejection fraction (HFpEF) independent of IHD [1]. Presence of DM in heart failure (HF) patients has prognostic significance that may be influenced by the underlying etiology. Patients with ischemic cardiomyopathy (ICM) have worse survival rates than those with non-ischemic cardiomyopathy (NICM) [1,2]. However, the impact of DM on NICM is not clearly defined. While some studies showed that there was no association between DM and mortality risk in patients with NICM [3], other studies found it resulting in increased mortality rate only in this subgroup of patients [4-6]. Additionally, some studies supported the hypothesis of a possible direct detrimental effect of DM on the myocardium [7,8]. On the other hand, a study in Denmark showed that DM has similar adverse impact on long term prognosis of both ICM and NICM [9].
Several mechanisms have been described in pathogenesis of NICM in diabetics. Myocardial inflammation is a possible pathophysiologic process contributing to cardiac hypertrophy, fibrosis, and dysfunction in this subgroup of heart failure (HF) patients [10-12].
Therefore, the objective of this study was to evaluate the differences in clinical characteristics, echocardiographic parameters, management, and outcomes of NICM and ICM in diabetes patients who are admitted to the hospital with acute heart failure (AHF).
Methods
Definition and classification of cardiomyopathy
Patients with a history of coronary artery disease (CAD) documented by coronary angiography, myocardial infarction (MI), or coronary revascularization were classified as ischemic cardiomyopathy (ICM). For NICM, we have used the 2008 European Society of Cardiology (ESC) working group classification of cardiomyopathy: “A myocardial structural or functional disorder in the absence of common causes like coronary artery disease, hypertension, valve heart disease and congenital heart disease” [13].
AHF and HF with reduced EF were determined based on the 2021 European Society of Cardiology (ESC) HF guidelines [14]. Patients admitted with AHF were considered to have acute onset of symptoms (dyspnea at rest or on exercise, fatigue, tiredness, ankle swelling) and signs (tachycardia, tachypnea, elevated jugular venous pressure, pulmonary rales, pleural effusion, hepatomegaly, peripheral edema) secondary to abnormal cardiac function (with objective evidence of structural or functional abnormality of the heart at rest such as third heart sound, murmurs, cardiomegaly, abnormal echocardiogram, raised natriuretic peptide concentration).
Inclusion criteria
The study included patients (1) aged older than 18 years, (2) diagnosed with diabetes and on anti-diabetic medications, (3) had EF≤40% by visual echocardiographic assessment, and (4) were admitted with AHF (whether acute de novo or acute on chronic HF).
Exclusion criteria
Patients with any of the following conditions were excluded: valvular or rheumatic heart disease, congenital heart disease, alcohol cardiomyopathy, peripartum cardiomyopathy, endocrine induced cardiomyopathy, iron overload related cardiomyopathy, hypertrophic cardiomyopathy, pulmonary heart disease, and/or pericardial diseases.
Study design and study population
In this retrospective, observational, single-center study, 612 consecutive patients with diabetes who were hospitalized due to AHF from January 2014 to February 2020 were screened. Of them, 160 patients were excluded due to HF with preserved ejection fraction, and 10 others because they met one of the exclusion criteria. Hence, a total of 442 patients were included in the study, out of whom 361 patients had ICM and 81 patients had NICM.
Data collection
Baseline data at presentation including patients’ demographics, comorbidities, clinical history, physical examination findings and medications were collected from electronic medical records. In this study, we included only diabetes patients with confirmed diagnosis of DM and on anti-diabetic medications. Chronic kidney disease (CKD) was defined as individuals who had an eGFR below 60 mL/min per 1.73 m2 for three months or more, irrespective of the cause [15,16]. Obesity was defined according to Center of Disease Control and Prevention (CDC) as body mass index (BMI) of 30 kg/m2 or higher.
Laboratory investigations and diagnostics reports including N-terminal pro-B type natriuretic peptide (NT-pro BNP) were retrieved from the hospital central lab data base. Electrocardiograms (ECG) and Echocardiography data were collected from the cardiac non-invasive laboratory. Also, data on cardiac procedures like percutaneous interventions (PCI), Coronary Artery Bypass Grafting (CABG), implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy (CRT) were collected from the catheterization laboratory and operating room records.
Follow-up endpoints
Follow up data were collected on recurrent JF hospitalizations and/or emergency visits as well as mortality over 5 years. Hospitalization was considered as HF hospitalization if the definition of AHF in the European Society of Cardiology guidelines (no new ischemic changes and negative troponin) was met [14].
There were some patients who could not be classified and hence, are not reported in the present paper. These patients included those in whom a specific etiology could not either be ascertained, even after thorough screening of patient files, or in whom the etiology was a mix between different etiology groups.
The echocardiographic methods
Echocardiographic studies of AHF patients were done and analyzed on admission in the non-invasive core laboratory of the hospital. The echocardiograms were obtained with patients in the left lateral decubitus position with the head of the examining table elevated to about 30°, using the available echocardiographic equipment-(Philips iE33, Probe-S5-1). Standard images were obtained from multiple tomographic planes. Imaging and Doppler echocardiograms were performed. Studies were performed using a standardized protocol and phased-array echocardiographs with M-mode, 2-dimensional, pulsed, continuous-wave, and color-flow Doppler capabilities. Recordings were sent and stored in the non-invasive laboratory data base.
Echocardiographic measurements
The main echo data comprised of ejection fraction (EF), Grade of left ventricular hypertrophy (LVH), left ventricular (LV) size, left atrial (LA) size, right ventricular systolic pressure (RVSP), Grade of LV diastolic dysfunction and right ventricle (RV) systolic function.
LV internal dimension and interventricular septal and posterior wall thicknesses were measured at end diastole and end systole as recommended by the American Society of Echocardiography (ASE) [17]. When optimal orientation of the M-mode line could not be obtained, correctly oriented leading-edge linear dimension measurements were made from 2-dimensional images as per ASE recommendations.
Using the criteria from the American Society of Echocardiography, February, 2009 [18], diastolic dysfunction was divided into: grade I diastolic dysfunction with impaired relaxation but normal LV filling pressures at rest, grade IA LV diastolic dysfunction with impaired relaxation and likely elevated LV end-diastolic pressure, grade II LV diastolic dysfunction with increased LV filling pressures (pseudo normal pattern), and grade III LV diastolic dysfunction with high LV filling pressures (restrictive pattern).
Quantification of Mitral regurgitation and Tricuspid regurgitation severity were based mainly on color Doppler, pulse and continuous Doppler signals from multiple windows of 2-D echo. RVSP was calculated from the tricuspid regurgitation velocity and estimated RA pressure. RA pressure was estimated from the inferior vena cava size and collapsibility. Right ventricle systolic function was assessed mainly by tricuspid annular plane systolic excursion (TAPSE; normal ≥1.7 cm) and visual estimation.
Statistical analysis
Categorized data were summarized as percentages whereas continuous data were summarized as means and standard Deviations (SD) or Medians and Inter-quartile ranges (IQR). Comparisons between different groups were performed using Chi-square test or Fisher’s exact test for categorical variables, where Student t-test or Mann-Whitney u test for continuous data. All statistical analysis were performed using SAS version 9.2 (SAS Institute, Inc. Cary, NC) and (R foundation for Statistical Computing, Vienna, Austria).
Results
A total of 442 patients were included in the study and were divided into two groups: the ICM group (361 patients, 81.7%) and NICM group (81 patients, 18.3%).
Patients’ demographics and clinical characteristics
The overall cohort had a mean age of 67.47±11.3 years in which 74.6% were male. Half of this cohort population had history of CKD with median GFR of 50.44±30.87, 24.2% had anemia, mean HbA1C level was 8.5±2.06 and mean NT pro BNP level was 7149±8210 with no differences in these demographics between the two groups (Table 1).
Table 1.
Baseline clinical demographics and laboratory investigation of ICM vs. NICM
| ICM N=361 (81.67%) | NICM N=81 (18.33%) | Total N=442 | P-value | |
|---|---|---|---|---|
| Age (mean ± SD) | 68.23±10.97 | 64.09±12.22 | 67.47±11.31 | 0.003 |
| Male, n (%) | 278 (77.01%) | 52 (64.20%) | 330 (74.66%) | 0.017 |
| Nationality | ||||
| Saudi, n (%) | 270 (75.00%) | 70 (86.42%) | 340 (77.10%) | 0.027 |
| Non-Saudi, n (%) | 90 (25.00%) | 11 (13.58%) | 101 (22.90%) | |
| BMI kg/m2, mean (SD) | 28.19±5.90 | 30.83±7.28 | 28.68±6.26 | <0.001 |
| SBP mmHg median (IQR) | 119.9±21.51 | 122.1±22.35 | 120.4±21.66 | 0.415 |
| DBP mmHg median (IQR) | 67.80±12.38 | 69.23±13.59 | 68.07±12.61 | 0.356 |
| Resting HR B/min median (IQR) | 78.13±14.12 | 77.56±16.12 | 78.03±14.50 | 0.751 |
| JVP | ||||
| Normal n (%) | 179 (66.30%) | 27 (52.94%) | 206 (64.17%) | 0.068 |
| Raised n (%) | 91 (33.70%) | 24 (47.06%) | 115 (35.83%) | |
| NYHA CLASS | ||||
| I n (%) | 92 (31.51%) | 14 (24.14%) | 106 (30.29%) | 0.535 |
| II n (%) | 134 (45.89%) | 29 (50.00%) | 163 (46.57%) | |
| III n (%) | 50 (17.12%) | 13 (22.41%) | 63 (18.00%) | |
| IV n (%) | 16 (5.48%) | 2 (3.45%) | 18 (5.14%) | |
| EF% mean (SD) | 29.19±8.68 | 28.00±7.91 | 28.98±8.55 | 0.259 |
| HTN n (%) | 308 (93.05%) | 70 (86.42%) | 378 (91.75%) | 0.052 |
| Dyslipidaemia n (%) | 93 (31.85%) | 19 (23.75%) | 112 (30.11%) | 0.162 |
| Smoking n (%) | 90 (27.86%) | 7 (8.75%) | 97 (24.07%) | <0.001 |
| Atrial fibrillation history n (%) | 66 (19.02%) | 25 (31.25%) | 91 (21.31%) | 0.016 |
| H/O MI | 228 (63%) | 0 (0%) | 228 (63%) | 0.001 |
| H/O PCI | 120 (33%) | 0 (0%) | 120 (33%) | 0.001 |
| H/O CABG | 100 (28.6%) | 0 (0%) | 100 (28.6%) | 0.001 |
| ICD/CRTD n (%) | 77 (22.32%) | 28 (34.57%) | 105 (24.65%) | 0.021 |
| Anaemia n (%) | 84 (23.27%) | 23 (28.40%) | 107 (24.21%) | 0.330 |
| CKD n (%) | 190 (52.63%) | 37 (45.68%) | 227 (51.36%) | 0.258 |
| eGFR ml/minmedian (IQR) | 49.98±30.61 | 52.50±32.15 | 50.44±30.87 | 0.509 |
| Obesity n (%) | 199 (56.53%) | 53 (65.43%) | 252 (58.20%) | 0.143 |
| Thyroid disease n (%) | 37 (10.69%) | 10 (12.35%) | 47 (11.01%) | 0.669 |
| ECG | ||||
| None from those 4 findings n (%) | 171 (58.97%) | 19 (26.03%) | 190 (52.34%) | <0.001 |
| LVH n (%) | 22 (7.59%) | 8 (10.96%) | 30 (8.26%) | |
| LBBB n (%) | 31 (10.69%) | 14 (19.18%) | 45 (12.40%) | |
| Paced n (%) | 29 (10.00%) | 14 (19.18%) | 43 (11.85%) | |
| AF in ECG n (%) | 37 (12.76%) | 18 (24.66%) | 55 (15.15%) | |
| HgB gm/dl, median (IQR) | 119.5±20.56 | 117.1±20.15 | 119.1±20.48 | 0.324 |
| Serum Cr μmol /L median (IQR) | 168.5±106.8 | 170.1±129.4 | 168.8±111.2 | 0.904 |
| K, mmol/L median (IQR) | 4.41±0.61 | 4.26±0.63 | 4.38±0.62 | 0.054 |
| Iron, µmol/L median (IQR) | 9.28±5.38 | 9.97±5.76 | 9.40±5.44 | 0.406 |
| Ferritin, mcg/L median (IQR) | 202.4±218.5 | 259.6±659.8 | 212.4±338.0 | 0.278 |
| HbA1C% median (IQR) | 8.58±2.05 | 8.11±2.13 | 8.50±2.06 | 0.094 |
| NT pro BNP pg/mL, median (IQR) | 7214±8238 | 6850±8128 | 7149±8210 | 0.728 |
Values are the number of patients (%), unless indicated otherwise. BMI: Body mass index; HTN: hypertension; HR: Heart Rate; SBP: systolic blood pressure; NT-proBNP: N-terminal Pro B-type natriuretic peptide; EF: ejection fraction; ICD: implantable cardioverter defibrillator; CRT: cardiac resynchronization therapy; NYHA: New York heart association; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; bpm: beats per minute; LV: left ventricular; IQR: interquartile range; AF: atrial fibrillation; GFR: glomerular filtration rate.
The ICM group included older patients (68.23±10.97 vs. 64.09±12.22, P=0.003), more males (77% vs. 64.2%, P=0.017) and higher prevalence of hypertension (93% vs. 86.42%, P=0.05), smoking (27.86% vs. 8.75%, P=0.001), and insulin dependent Diabetes (58.12% vs. 40%, P=0.004) than the NICM group. Additionally, patients in this group had the following medical history: 63% myocardial infarction (63%), PCI (one-third), and CABG (one-third).
The NICM patients had higher BMI (30.83±7.28 vs. 28.19±5.90, P<0.001) and higher prevalence of rhythm problems (LBBB, paced rhythm and atrial fibrillation) (63% vs. 34.87%, P<0.001) compared to ICM patients. Additionally, more patients in the NICM group received ICD/CRTD therapy (34.6% vs. 22.3%, P=0.021). Other demographic and clinical Characteristics are described in Table 1.
Echocardiographic data
Mean EF was 28.98±8.55. 26% had moderate to severe LV dilatation, 28.5% had moderate to severe pulmonary hypertension and 28% had moderate to severe RV dysfunction with no significant differences between both groups. Also, we have looked at the grade of left ventricular diastolic dysfunction (LVDD) in these two groups and found that 42.8% of patients had Grade III LVDD (Restrictive pattern), 29% had Grade II LVDD (Pseudo normal pattern) and 27% have grade I LVDD (impaired LV relaxation) with no significant differences between both groups. Echocardiographic characteristics are shown in Table 2.
Table 2.
Echocardiographic parameters of ICM vs. NICM
| ICM N=361 (81.7%) | NICM N=81 (18.3%) | Total N=442 | P-value | |
|---|---|---|---|---|
| LVH grade | ||||
| None to mild | 343 (95.01%) | 76 (93.82%) | 419 (94.8%) | 0.417 |
| Moderate to severe | 18 (4.99%) | 5 (6.17%) | 23 (5.2%) | |
| LV size | ||||
| None to mild | 271 (75.06%) | 56 (69.13%) | 327 (74%) | 0.168 |
| Moderate to severe | 90 (24.93%) | 25 (30.86%) | 115 (26%) | |
| LA size | ||||
| None to mild | 202 (55.95%) | 36 (44.44%) | 238 (53.8%) | 0.040 |
| Moderate to severe | 159 (44.04%) | 45 (55.55%) | 204 (46.2%) | |
| Grade of diastolic dysfunction | ||||
| Impaired relaxation (Grade I) n (%) | 59 (25.43%) | 12 (37.50%) | 71 (26.89%) | 0.437 |
| Impaired relaxation with high filling pressure (Grade IA) n (%) | 4 (1.72%) | 0 (0.00%) | 4 (1.52%) | |
| Pseudo normal (Grade II) n (%) | 69 (29.74%) | 7 (21.88%) | 76 (28.79%) | |
| Restrictive (Grade III) n (%) | 100 (43.10%) | 13 (40.63%) | 113 (42.80%) | |
| RVSP | ||||
| None to mild | 260 (72.02%) | 56 (69.13%) | 316 (71.5%) | 0.347 |
| Moderate to severe | 101 (27.97%) | 25 (30.86%) | 126 (28.5%) | |
| RV function | ||||
| None to mild | 263 (72.85%) | 55 (67.90%) | 318 (71.9%) | 0.222 |
| Moderate to severe | 98 (27.14%) | 26 (32.09%) | 124 (28.1%) | |
| Mitral regurgitation | ||||
| None to mild | 266 (73.68%) | 55 (67.90%) | 321 (72.6%) | 0.179 |
| Moderate to severe | 95 (26.31%) | 26 (32.09%) | 121 (27.4%) | |
| Tricuspid regurgitation | ||||
| None to mild | 289 (80.05%) | 61 (75.30%) | 350 (79.2%) | 0.210 |
| Moderate to severe | 72 (19.94%) | 20 (24.69%) | 92 (20.8%) |
LVH, left ventricle hypertrophy; LV, left ventricle; LA, left atrium; RVSP, Right ventricle systolic pressure; RV, right ventricle.
In-hospital management
In terms of HF medications, it was found that Lasix was used in 93.3%, B-blockers in 96.4%, ACEI in 39%, ARB in 17.3% and ARNI in 12% of patients in the whole cohort with no differences between the two groups.
The use of Aspirin (ASA), Isosorbide Dinitrate and statins was more in ICM compared to NICM (78.33% vs. 33.8%, 42% vs. 22.5% and 93% vs. 74% respectively, P<0.001). The use of anticoagulation and Spironolactone was more in NICM compared to ICM (35.4% vs. 26%, P=0.091) and (46% VS. 30%, P=0.011) respectively (Figure 1).
Figure 1.
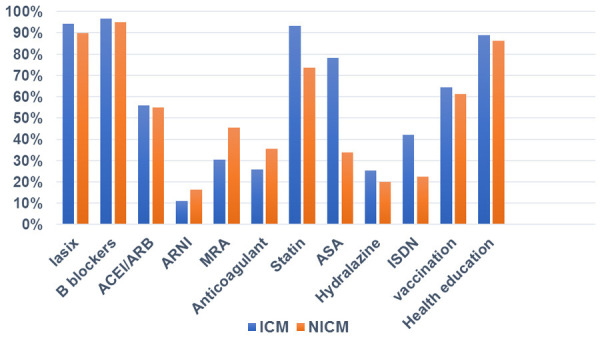
In-hospital heart failure medications, vaccination and health education in ICM vs. NICM. B blockers, beta blockers; ACEI, Angiotensin-converting-enzyme inhibitors; ARB, Angiotensin receptor blockers; ARNi, Angiotensin receptor-neprilysin Inhibitors; MRA, mineralocorticoid receptor antagonist; ASA, acetylsalicylic acid (aspirin); ISDN, isosorbide dinitrate.
About two thirds of the cohort patients (63.75%) received yearly Influenza and Pneumococcal vaccine with no significant difference between the two groups. Health education was provided to 88% of the study population about their disease, side effects of their medications, compliance with diet and other medications. No significant differences were found between the two groups (Figure 1).
A total of 254 patients (57.5%) were on oral hypoglycemic agents only while 240 patients (54.5%) were on insulin. The patients in the ICM group used more insulin than those in the NICM group (58% vs. 40%, P=0.004). One-third of the patients were on DDP4 (26%) and only few (5%) patients were on SGLT2 inhibitors (Figure 2).
Figure 2.
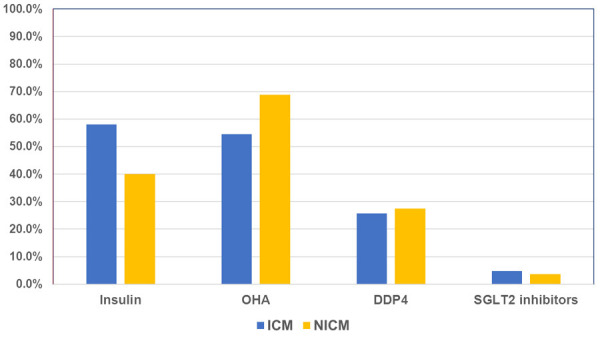
Diabetic medications prescribed in ICM vs. NICM. OHA, oral hypoglycemic agents; DDP4, Dipeptidyl peptidase-4 inhibitor; SGLT2 inhibitors, Sodium-glucose cotransporter-2 Inhibitors.
Long term mortality and heart failure hospitalization
There was no significant difference in 5-year-mortality between ICM and NICM (39.5% vs. 34.1%, P=0.165). However, compared to ICM group, there was a higher rate of HF hospitalizations in NICM group though it was just shy of statistical significance (49.38% vs. 36.11%, P=0.062) (Table 3).
Table 3.
Long term outcomes mortality and Hf hospitalization
| Outcomes | Diabetic ICMP | Diabetic NICMP | Total | P-value |
|---|---|---|---|---|
| status | ||||
| Alive | 128 (35.56%) | 33 (40.74%) | 161 (36.51%) | 0.165 |
| Dead | 123 (34.17%) | 32 (39.51%) | 155 (35.15%) | |
| Unknown | 109 (30.28%) | 16 (19.75%) | 125 (28.34%) | |
| Hospitalization for HF | ||||
| No | 129 (35.83%) | 26 (32.10%) | 155 (35.15%) | 0.062 |
| Yes | 130 (36.11%) | 40 (49.38%) | 170 (38.55%) | |
| Unknown | 101 (28.06%) | 15 (18.52%) | 116 (26.30%) |
Discussion
In the current study we aimed to analyze the differences in clinical characteristics, Echocardiographic parameters, management and outcomes between ICM and NICM in diabetes patients to study the differential impact of diabetes on mortality and recurrent HF hospitalization in the absence of ischemic burden. Diabetes patients with reduced EF who were admitted with acute decompensated HF were included and the cohort was divided into two groups-ICM and NICM. Patients in the ICM were older, with more males and had higher prevalence of other cardiac risk factors. The NICM patients had higher BMI with higher prevalence of rhythm problems (LBBB, paced rhythm and atrial fibrillation).
On analysis, no difference was found in 5-year-mortality between the ICM and NICM patients. However, a trend of increased hospitalization due to recurrent HF was noted in the NICM group compared to ICM though was not statistically significant. The available data on the impact of DM on long term outcomes of HF patients without ischemic heart disease is conflicting [3-9]. Anderson et al [9] demonstrated that HF patients with concomitant diabetes had an approximately 40% increased risk of death, compared to HF patients without diabetes and this increased mortality was similar among patients with ischemic and non-ischemic HF, which corroborates with findings. The fact that the present study found the prognosis to be similar in both groups, and the fact that some of the previous studies have reported no interaction between ischemic HF etiology and diabetes suggest that the mechanism behind the increased mortality risk associated with diabetes is multi-factorial and not an effect of serious ischemic heart disease alone.
A retrospective analysis of 6,797 participants of the Studies of Left Ventricular Dysfunction (SOLVD) Prevention and Treatment trials found that diabetes imparted an increased risk for all-cause mortality in patients with ischemic cardiomyopathy (relative risk [RR] 1.37, p 0.0001), but not in those with nonischemic cardiomyopathy (RR 0.98, p 0.98) [3]. On the contrary, a 12-year prospective survival analysis [4] done on patients hospitalized with AHF, half of whom had a history of ischemic cardiomyopathy, found that DM increases mortality among HF patients without ischemic cardiomyopathy whether they have preserved or reduced LVSF.
Obesity is common in our population as shown in this cohort and it is one of the common comorbidities in female patients with diabetes that also include hypertension, atrial fibrillation and AHF with preserved or reduced ejection fraction. The trend of increased hospitalization in NICM patients compared to ICM points out the difficulty in managing HF in this group of patients that may require special attention to their risk factors including obesity and dysrhythmias.
Although the primary etiology of HF in NICM is non-ischemic, patients with diabetes might still have some ischemic features, such as diffuse vascular or microvascular disease, compared to patients without diabetes. Additionally, diabetes induced cellular and biochemical changes like, altered myocyte metabolism, impaired glucose utilization, increased lipolysis and myocardial fibrosis [19], may contribute to the adverse outcomes in this group.
It is to be noted that HF patients in our population present at a relatively younger age, have a much higher incidence of DM, and predominantly have LV systolic dysfunction, which is mainly ischemic in origin, compared with patients in other ethnic groups [20]. More than 30 years ago, the Framingham Heart Study presented data showing an increased mortality rate of HF in patients with diabetes, compared to those without [21]. However, studies on the differential effect of DM on the outcome of HF patients with presence or absence of ischemia showed conflicting evidence [5-9].
We have reported several findings in this cohort. The average age of our patients was 60-70 years in both ICM and NICM groups. This was 10 years more than what was reported before in our national registries SPACE and HEARTS [22,23], where the average age of HF patients was 57-60 years in both registries. This may imply improvement in the management of HF over the last five to seven years which also reduced mortality rate of these patients. In SPACE HF [22] the prevalence of HF complicating acute coronary syndrome (ACS) increased considerably with age, which matches our findings in this study. It was interesting to note in our findings that, compared to ICM, patients who presented with HF due to NICM were more likely to be women, obese, have higher EF and have atrial fibrillation. This is expected because HF in the presence of LVH is the most common subtype of HF in women, with higher prevalence compared to men and associated with several comorbidities, such as older age, obesity, diabetes mellitus, hypertension, and hyperlipidemia, which define phenotypic profiles associated with HFpEF [24].
History of AF was found in 21% of patients who presented to the hospital with HF. This was comparable to the findings in the atrial fibrillation HF sub study of HEARTS, where out of 2593 patients admitted with HF, 449 (17.8%) had AF at presentation [25]. In our cohort we also noted that AF is more common in NICM compared to ICM patients.
In the present study, 50% of the patients in this cohort had impaired kidney function and this may explain the lower use of ACEI and ARB in both groups. The use of beta-blockers, ASA and isosorbide dinitrate (ISDN) was higher in the ICM group compared to NICM which is expected in patients with coronary artery disease. The use of anticoagulants was much higher in NICM, most likely reason being a higher rate of AF compared to ICM.
On the other hand, devices like ICDs and CRTs were under prescribed. This could be attributed to lack of application of knowledge to clinical practice in our population. It could also be related to improve EF after revascularization in the ICM group which obviates the need for these devices, which we had earlier stated in HEARTS-chronic sub study [26]. Verifying the indication for an ICD-/CRT-D implantation is important as patients with DM and HF are at increased risk of malignant ventricular arrhythmias and sudden cardiac death. This was shown in the CHARM trial that revealed a higher rate of sudden cardiac death in patients with DM compared to non-diabetics irrespective of HF phenotype [27].
Besides the advancement in HF treatment there are new anti-diabetic medications that have shown a significant outcome improvement in terms of both mortality and recurrent hospitalization needs in HF patients. Two large randomized controlled trials investigating the cardiovascular safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors, such as empagliflozin and canagliflozin, have shown a significant reduction in hospitalization need in HF patients with both drugs [28,29]. Recently the DAPA-HF trial showed a significant reduction in deterioration or death from cardiovascular causes among patients with HF and reduced EF by dapagliflozin compared to placebo. Interestingly, this effect was independent of the presence of diabetes [30]. In our study, very few patients were on SGLT2 inhibitors as part of anti-diabetic regimen that might be attributed to non-availability of the drug in our hospital at the time of patients’ recruitment. Additionally, the poor glycemic control, in this cohort (HbA1C level 8.5±2.06), may be responsible for the worse outcomes in our patients independent of the underlying HF etiology. Lawson reported that patients grouped by levels of HbA1c showed a U-shaped relationship with outcomes, with the lowest and highest HbA1c groups associated with the highest risk of first hospitalization and all-cause mortality [31]. Evidence on HbA1c impact on outcomes in HF has been inconsistent. This is an area of potential future investigation in diabetes patients with HF.
Despite the fact that amongst diabetics NICM had similar mortality and HF re-hospitalization rates to ICM patients, we think that NICM in diabetics may have different precipitating risk factors for HF re-hospitalization like dysrhythmias, obesity and poor glycemic control as shown in our study. This should be investigated further in future studies and should be the focus of future efforts in managing these patients.
Study limitations
The study was limited by its relatively small sample size. Additionally, it was limited to diabetes patients admitted with acute decompensated HF, and hence it does not represent the universe of CHF. However, the aim of this study was to describe, for the first time, the clinical characteristics and outcomes of NICM compared to ICM specifically in diabetes patients. Higher numbers of patients with more geographical representation is needed to confirm the findings of our study.
Conclusion
There was no difference in 5-year-mortality between ischemic and non-ischemic cardiomyopathy in diabetes patients. However, diabetes patients with non-ischemic cardiomyopathy had higher prevalence of obesity and dysrhythmias compared to ischemic cardiomyopathy. These areas need to be explored in future studies to help establish better strategies in the management of these patients.
Acknowledgements
This project was supported by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University and King Saud University medical city. We would like to thank the Heart failure educator Mrs. Gihan S. El Behiry for her contribution in collecting some of the patient’s data for this study.
Disclosure of conflict of interest
None.
References
- 1.De Groote P, Lamblin N, Mouquet F, Plichon D, McFadden E, Van Belle E, Bauters C. Impact of diabetes mellitus on long-term survival in patients with congestive heart failure. Eur Heart J. 2004;25:656–662. doi: 10.1016/j.ehj.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Domanski M, Krause-Steinrauf H, Deedwania P, Follmann D, Ghali JK, Gilbert E, Haffner S, Katz R, Lindenfeld J, Lowes BD, Martin W, McGrew F, Bristow MR BEST Investigators. The effect of diabetes on outcomes of patients with advanced heart failure in the BEST trial. J Am Coll Cardiol. 2003;42:914–922. doi: 10.1016/s0735-1097(03)00856-8. [DOI] [PubMed] [Google Scholar]
- 3.Dries DL, Sweitzer NK, Drazner MH, Stevenson LW, Gersh BJ. Prognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunction. J Am Coll Cardiol. 2001;38:421–428. doi: 10.1016/s0735-1097(01)01408-5. [DOI] [PubMed] [Google Scholar]
- 4.Varela-Roman A, Grigorian Shamagian L, Barge Caballero E, Mazon Ramos P, Rigueiro Veloso P, Gonzalez-Juanatey JR. Influence of diabetes on the survival of patients hospitalized with heart failure: a 12-year study. Eur J Heart Fail. 2005;7:859–864. doi: 10.1016/j.ejheart.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 5.From AM, Leibson CL, Bursi F, Redfield MM, Weston SA, Jacobsen SJ, Rodeheffer RJ, Roger VL. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006;119:591–599. doi: 10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 6.Meindl C, Hochadel M, Frankenstein L, Bruder O, Pauschinger M, Hambrecht R, von Scheidt W, Pfister O, Hartmann A, Maier LS, Senges J, Unsöld B. The role of diabetes in cardiomyopathies of different etiologies-characteristics and 1-year follow-up results of the EVITA-HF registry. PLoS One. 2020;15:e0234260. doi: 10.1371/journal.pone.0234260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawson CA, Zaccardi F, McCann GP, Davies MJ, Kadam UT, Khunti K. Trends in cause-specific outcomes among individuals with type 2 diabetes and heart failure in the United Kingdom, 1998-2017. JAMA Netw Open. 2019;2:e1916447. doi: 10.1001/jamanetworkopen.2019.16447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seferovic PM, Paulus WJ. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J. 2015;36:1718–1727. doi: 10.1093/eurheartj/ehv134. [DOI] [PubMed] [Google Scholar]
- 9.Andersson C, Weeke P, Pecini R, Kjaergaard J, Hassager C, Køber L, Torp-Pedersen C. Long-term impact of diabetes in patients hospitalized with ischemic and non-ischemic heart failure. Scand Cardiovasc J. 2010;44:37–44. doi: 10.3109/14017430903312438. [DOI] [PubMed] [Google Scholar]
- 10.Frieler RA, Mortensen RM. Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation. 2015;131:1019–1030. doi: 10.1161/CIRCULATIONAHA.114.008788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116:1254–1268. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 13.Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on myocardial and pericardial diseases. Eur Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 14.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO controversies conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 17.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American society of echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 18.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Marwick TH. Diabetic heart disease. Heart. 2006;92:296–300. doi: 10.1136/hrt.2005.067231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AlHabib KF, Elasfar AA, AlBackr H, AlFaleh H, Hersi A, AlShaer F, Kashour T, AlNemer K, Hussein GA, Mimish L. Design and preliminary results of the heart function assessment registry trial in Saudi Arabia (HEARTS) in patients with acute and chronic heart failure. Eur J Heart Fail. 2011;13:1178–1184. doi: 10.1093/eurjhf/hfr111. [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 22.Albackr HB, Alhabib KF, Ullah A, Alfaleh H, Hersi A, Alshaer F, Alnemer K, Al Saif S, Taraben A, Kashour T. Prevalence and prognosis of congestive heart failure in Saudi patients admitted with acute coronary syndrome (FROM SPACE REGISTRY) Coron Artery Dis. 2013;24:596–601. doi: 10.1097/MCA.0b013e328364d98f. [DOI] [PubMed] [Google Scholar]
- 23.AlHabib KF, Elasfar AA, Alfaleh H, Kashour T, Hersi A, AlBackr H, Alshaer F, AlNemer K, Hussein GA, Mimish L, Almasood A, AlHabeeb W, AlGhamdi S, Alsharari M, Chakra E, Malik A, Soomro R, Ghabashi A, Al-Murayeh M, Abuosa A. Clinical features, management, and short- and long-term outcomes of patients with acute decompensated heart failure: phase I results of the hearts database. Eur J Heart Fail. 2014;16:461–469. doi: 10.1002/ejhf.57. [DOI] [PubMed] [Google Scholar]
- 24.Tibrewala A, Yancy CW. Heart failure with preserved ejection fraction in women. Heart Fail Clin. 2019;15:9–18. doi: 10.1016/j.hfc.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Ajlan M, Almazroa L, AlHabib KF, Elasfar AA, Alfaleh H, Albackr H, Kashour T, Hersi A, Hussein GA, Mimish L, Almasood A, AlHabeeb W, AlGhamdi S, Alsharari M, Chakra E, Malik A, Soomro R, Ghabashi A, Al-Murayeh M, Abuosa A. Atrial fibrillation in patients hospitalized with heart failure: patient characteristics and outcomes from the hearts registry. Angiology. 2018;69:151–157. doi: 10.1177/0003319717711764. [DOI] [PubMed] [Google Scholar]
- 26.Alhabeeb W, Elasfar A, AlBackr H, AlShaer F, Almasood A, Alfaleh H, Kashour T, Hersi A, Asfina KN, Altaradi H, AlShaqhaa W, Alayoubi F, AlHabib KF. Clinical characteristics, management and outcomes of patients with chronic heart failure: results from the heart function assessment registry trial in Saudi Arabia (HEARTS-chronic) Int J Cardiol. 2017;235:94–99. doi: 10.1016/j.ijcard.2017.02.087. [DOI] [PubMed] [Google Scholar]
- 27.MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, Solomon SD, Granger CB, Swedberg K, Yusuf S, Pfeffer MA, McMurray JJ CHARM Investigators. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–1385. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 28.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 29.Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Fabbrini E, Sun T, Li Q, Desai M, Matthews DR CANVAS Program Collaborative Group. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (Canagliflozin cardiovascular assessment study) Circulation. 2018;137:323–334. doi: 10.1161/CIRCULATIONAHA.117.032038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 31.Lawson CA, Jones PW, Teece L, Dunbar SB, Seferovic PM, Khunti K, Mamas M, Kadam UT. Association between type 2 diabetes and all-cause hospitalization and mortality in the UK general heart failure population: stratification by diabetic glycemic control and medication intensification. JACC Heart Fail. 2018;6:18–26. doi: 10.1016/j.jchf.2017.08.020. [DOI] [PubMed] [Google Scholar]


