Abstract
Background: Ostial left anterior descending (LAD) artery lesions are a critical area for coronary stenting, given that the location subtends a large area of the myocardium and can also be more technically challenging. It remains controversial whether crossover stenting of ostial LAD back into the left-main (LM) is advantageous over stenting the ostium alone. Methods: To evaluate the long-term clinical outcomes of stenting ostial LAD lesions, we retrospectively reviewed all ostial LAD lesions cases at QEII Health Science Centre between 2008 and 2018. Specifically, we compared the outcomes in those patients that had left main stent crossover vs. ostial stenting (OS) alone. Results: The total number of patients included in the study was 175, with 25 patients (14%) having a crossover to the LM and 150 (86%) having OS. There were more patients with previous CABG (24%) in the crossover group compared to the OS group (9.2%) (P = 0.042). The one-year MACE was not significantly different between CO vs. OS (13.3% (10.5-16.1) vs. 12% (5.5-18.5)). The five-year MACE was numerically higher, although statistically not significant, in CO vs. OS (19.3 (15.9-22.7) vs. 25.9 (16.6-35.2)). Conclusion: This study shows that percutaneous intervention provides reasonable long-term outcomes and low rates of repeat revascularization for isolated ostial LAD lesions, with no noticeable difference in outcomes with crossover stenting into the LM vs. OS alone. A larger, prospective study may be required to determine the optimal strategy for treating ostial LAD lesions.
Keywords: PCI - percutaneous coronary intervention (PCI), LM - left main coronary disease, stenting technique
Introduction
Ostial coronary lesions are a unique subset of lesions that carry distinctive technical and procedural challenges during the percutaneous coronary intervention (PCI). The shear stress on the main branch provides important mechanics for ostial lesion atherosclerosis [1-4]. The large amount of myocardium in jeopardy is always an area of concern during these interventions. The left anterior descending artery (LAD), specifically with it is connection to the left main (LM) and left circumflex artery (LCX), may provide a particular challenge during PCI. Ostial LAD lesions may often extend from the distal left-main artery [5]. Compared to non-ostial lesions, ostial lesions have high rates of Instent restenosis and long-term adverse events [6-8]. Balloon slippage and extension of dissection into the LM could result in poor patient outcomes. Stent struts protrusion into the left-main vs. incomplete coverage of the ostial lesion are not uncommon in intravascular ultrasound (IVUS) studies [9] and can lead to increased risk of restenosis and stent thrombosis [10]. Moreover, the adjacent LCX artery may be compromised during ostial LAD stenting and may lead to ischemia and unwanted consequences [11].
To improve clinical and angiographic outcomes, the use of cutting balloons, rotational atherectomy [12-14], different wiring techniques [15] and the use of drug-eluting stents (DES) compared to bare-metal stents (BMS) [16-19] have been studied in ostial LAD lesions and may improve long-term outcomes. Beyond this, some have suggested crossover stenting of ostial LAD back into the left main showed favourable outcomes in in-vitro models [2,4] and clinical studies [11,20-23]. Furthermore, intravascular imaging with optical coherence tomography (OCT) and intravascular ultrasound (IVUS) [13,18] may optimize procedural and long-term clinical outcomes in these cases; however, it is unknown whether LM to LAD crossover stenting is superior to stenting the LAD ostial disease alone.
Thus, our goal was to evaluate the outcomes in patients undergoing PCI for ostial LAD lesions while comparing the short- and long-term outcomes between those treated with a stent crossing over to the LM versus ostial stenting (OS) alone.
Methods
Patient cohort and eligibility criteria
We retrospectively reviewed all patients with ostial LAD lesions PCI at the QEII Health Science Centre in Halifax from January 2008 until December 2018. Patients were eligible for the study if they were ≥18 years old and had received a coronary intervention of an ostial LAD lesion. Two expert interventional cardiologists (OE and ST) reviewed all coronary angiogram images of all ostial LAD interventions. We included only ostial LAD lesions interventions, which were defined as >50% stenosis within 5mm of the ostium of the LAD.
We excluded patients with concomitant significant LM disease defined as >40% stenosis or concomitant ostial LCx lesion of >50% and patients with protected LM.
We divided patients into two groups based on their stenting strategy. The first group included patients with left-main stent crossover (CO), and the second group comprised patients with OS alone. All patients had Informed consent obtained before the procedure. The institutional research ethics board approved the study (NSHA REB ROMEO File# 1023428).
Patient details were retrieved from the catheterization laboratory Cardiovascular Information System (CVIS) database. The CVIS database includes patient demographics, procedural complications, devices used, and procedural outcomes. We completed missing data and mortality outcomes by linking the CVIS database to a population-level provincial registry of Cardiovascular Health Nova Scotia (CVHNS) database. A manual chart review was done to complete missing data and review the need for repeat revascularization procedures.
The catheterization laboratory at the QEII health science center in Halifax is the only cath lab in the province of Nova Scotia. To capture all potential repeat catheterizations and revascularizations, we excluded out-of-province patients to minimize the chance of having subsequent catheterizations at another center.
Endpoints and definitions
The primary goal of the study was to compare long-term outcomes of major adverse cardiac events (MACE), all-cause mortality, target lesion revascularization (TLR), target vessel revascularization (TVR) and instent restenosis (ISR) in those patients with CO stenting vs. OS. The MACE was defined as the combination of mortality, TLR and TVR. Mortality in this study is all-cause mortality. TLR is defined as a re-intervention of the target lesion, while TVR is a re-intervention of any segment of the target vessel. ISR is considered when angiographic luminal narrowing of >50% within 5 mm of stented segment. Procedural success is defined as the successful implantation of a stent with TIMI 3 flow in the treated vessel.
Procedural technique
The indications for the cardiac catheterization were stable angina with ongoing symptoms despite medical therapy and acute coronary syndrome (ACS), which included ST-segment elevation myocardial infarction (STEMI), non-ST segment elevation myocardial infarction (NSTEMI) and unstable angina (UA).
Percutaneous coronary interventions and stenting were performed according to standard techniques. All patients were pre-treated with dual antiplatelet therapy, including aspirin and loading dose of either clopidogrel or ticagrelor.
The procedure is performed through either a transradial or transfemoral approach. Unfractionated heparin or bivalirudin was given to all patients during the procedure with a target activated clotting time of >250 seconds. Two main strategies for treating ostial LAD lesions were used and analyzed in this study. The first strategy included crossover stenting (CO) from the LM into the LAD, using standard provisional bifurcation techniques and proximal stent optimization (POT), where post dilatation was typically performed. The second strategy is stenting of the LAD to the ostium (OS) with no crossover back to the LM. The choice of the technique was left to the discretion of the treating interventional cardiologist.
The use of intravascular imaging was left to the discretion of the operator. Two primary modalities were used: Intravascular ultrasound (IVUS) and optical coherence tomography (OCT). IVUS uses an ultrasound probe to generate an image of the vessel wall layers, while OCT uses near-infrared light waves to provide high-definition images of the vessel wall. IVUS was available through the study period, and OCT was available since 2013.
Twelve-month Dual antiplatelet therapy was typically used in most patients with DES and for one month in those with BMS and lifelong aspirin therapy.
Statistical analyses
Baseline patient demographic variables were summarized with descriptive statistics as means (standard deviations), medians (interquartile range) or counts (percentages) for categorical variables. Chi-square tests, fishers exact test, student’s t-test and Wilcoxon rank-sum test were used, where appropriate, to assess differences in baseline characteristics between the two patient groups. Kaplan-Meier survival function was used to characterize the outcomes of MACE and mortality. The Log Rank test was used, and the p-value was calculated. The one-year and five-year survival rates were calculated. All comparisons were based on two-sided tests, and a p-value of less than 0.05 was considered to indicate statistical significance. All analyses were performed using SPSS statistical software (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp).
Results
We identified 283 patients for potential ostial LAD intervention. Figure 1 shows the workflow for patient selection. 38 patients were excluded because of significant LM disease, while another 50 were identified as non-ostial LAD lesions and excluded. Data were missing on 12 patients, seven patients did not have any interventions, and finally, 2 out of province patients were excluded.
Figure 1.
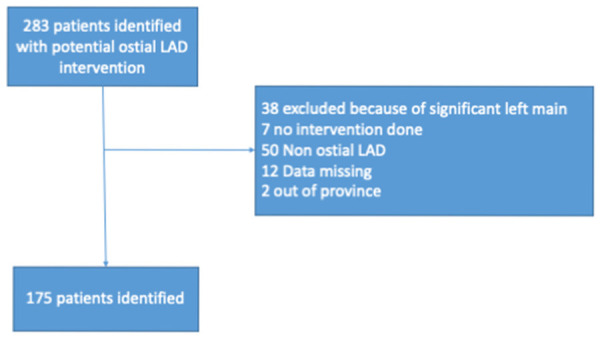
Study flow chart for selected patients. LAD = Left anterior descending artery.
Overall, 175 patients fulfilled the criteria for our study, with 150 patients undergoing ostial LAD stenting (OS) and 25 patients having LM crossover (CO). The mean follow-up was 65.2 months for the OS group and 62.8 months for the CO group. Most of the patients were men at 77.7%, with a ratio of 1 woman to 3 men (Table 1).
Table 1.
Baseline characteristics for patients with ostial LAD versus crossover
| Variables | Overall Total = 175 | Ostial LAD n = 150 | Crossover to LM n = 25 | p-value |
|---|---|---|---|---|
| Age Mean (±SD) | 65 (12) | 64.5 (11.7) | 67.7 (13.7) | 0.213 |
| Male % | 136 (77.7) | 118 (78.7) | 18 (72) | 0.445 |
| Female % | 39 (22.3) | 32 (21.3) | 7 (28) | |
| Indication % | ||||
| NSTEMI | 37 (21.1) | 35 (23.3) | 2 (8) | 0.368 |
| STEMI | 43 (24.6) | 37 (24.7) | 6 (24) | |
| Unstable Angina | 48 (27.4) | 39 (26.0) | 9 (36) | |
| Stable Angina | 35 (20) | 30 (20) | 5 (20) | |
| Other | 12 (6.9) | 9 (6) | 3 (12) | |
| Cardiogenic Shock % | 3 (1.7) | 3 (1.7) | 0 (0) | 0.476 |
| Previous PCI % | 28 (16) | 23 (15.3) | 5 (20) | 0.559 |
| Myocardial Infarction % | 49 (28.2) | 40 (26.7) | 9 (37) | 0.329 |
| Hypertension % | 111 (64.5) | 97 (66.0) | 14 (56) | 0.370 |
| Diabetes % | 54 (31.4) | 46 (31.3) | 8 (32) | 1 |
| Dyslipidemia % | 125 (74) | 101 (70.1) | 24 (96) | 0.005 |
| Smoker % | 108 (61.7) | 87 (58) | 21 (84) | 0.014 |
LAD = Left anterior descending; LM = Left Main; NSTEMI = Non-ST Elevation Myocardial Infarction; STEMI = ST Elevation Myocardial Infarction; PCI = Percutaneous coronary intervention.
Table 1 shows the baseline characteristics, primarily similar for both groups, with a mean age of 64.5 for OS versus 67.7 for the CO group. The CO group had more patients with reported dyslipidemia (96% vs. 70.1%; P = 0.005) and more significant history of smoking (84% versus 58%; P = 0.014) than the OS group.
Table 2 shows procedural characteristics for OS versus CO group. Angiographically, non-significant LM disease was present in 64% of the CO group compared to 12.7% of the OS group. The use of imaging such as IVUS and OCT was significantly higher in the CO group compared to the OS group (64% vs. 20%; P≤0.001). Procedural complications were not significantly different between the groups (2.7% in the OS group v.s 4% in the CO group; P = 0.7).
Table 2.
Procedural characteristics for patients with ostial LAD versus crossover
| Variables | Overall Total = 175 | Ostial LAD N = 150 | Crossover to LM n = 25 | p-value |
|---|---|---|---|---|
| Stent Diameter Mean (±SD) | 3.2 (0.38) | 3.2 (0.39) | 3.2 (0.31) | 0.91 |
| Stent Length Mean (±SD) | 19.1 (7.93) | 18.66 (7.86) | 21.7 (7.9) | 0.07 |
| Lesion Length Mean (±SD) | 20.16 (11.32) | 20.07 (11.38) | 20.7 (11) | 0.8 |
| Distal Reference Mean (±SD) | 3.29 (0.47) | 3.3170 (0.48) | 3.1 (0.3) | 0.1 |
| Number of stents Mean (±SD) | 1.54 (0.78) | 1.51 (0.74) | 1.76 (0.97) | 0.13 |
| Number of Vessels PCI % | ||||
| (2 vessel) | 30 (17) | 22 (14.7) | 8 (32) | 0.06 |
| (3 Vessel) | 8 (4.6) | 6 (4) | 2 (8) | |
| Stent % | ||||
| DES | 154 (88) | 130 (86.7) | 24 (96) | 0.409 |
| BMS | 20 (11.4) | 19 (12.7) | 1 (4) | |
| POBA | 1 (0.6) | 1 (0.7) | 0 | |
| Cutting Balloon % | 6 (3.4) | 6 (3.4) | 0 | 0.596 |
| Thrombectomy % | 10 (5.7) | 10 (5.7) | 0 (0) | 0.361 |
| IABP % | 8 (4.6) | 7 (4.7) | 1 (4) | 1 |
| Rotablation % | 4 (2.3) | 4 (2.7) | 0 (0) | 1 |
| Left main disease % | 35 (20) | 19 (12.7) | 16 (64) | ≤0.001 |
| Instent restenosis % | 10 (5.7) | 8 (5.3) | 2 (8) | 0.637 |
| IVUS % | 33 (18.9) | 19 (12.7) | 14 (56) | ≤0.001 |
| OCT % | 13 (7.4) | 11 (7.3) | 2 (8) | |
| Procedural Success % | 173 (98.9) | 149 (99.3) | 24 (96) | 0.266 |
| Procedural complications % | 5 (2.9) | 4 (2.7) | 1 (4) | 0.7 |
LAD = Left anterior descending; LM = Left Main; PCI = Percutaneous coronary intervention; DES = Drug eluting stent; BMS = Bare metal stent; POBA = plain old balloon angioplasty; IABP = intra-aortic balloon angioplasty; IVUS = intravascular ultrasound; OCT = optical coherence tomography.
The mean stent length in the OS group was 18.66 mm vs. 21.7 mm in the CO group (P = 0.07). Rotational atherectomy use was low and was not used in the CO patients.
Intervention on the LCX or Ramus branch was required in 5 patients of the CO group. Two patients required bifurcation stenting, and three were treated with balloon angioplasty only.
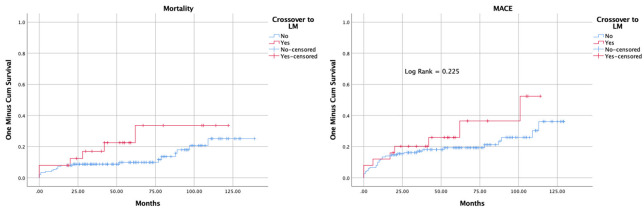
All-cause mortality tended to be higher in the CO group during follow-up, but this was not significantly different from the OS group (Kaplan-Meier Log Rank = 0.115) (Figure 2). Table 3 shows clinical outcomes. The one-year mortality following the procedure was similar in both groups (7.3% in the CO group vs. 8% in the OS group). TLR and TVR in the CO group were 4% and 12% vs. 7.3% and 8%, respectively, in the OS group, and these were not statistically significant (P = 0.5).
Figure 2.
Kaplan-Meier curve for Mortality and MACE, including TLR, TVR and all-cause mortality. MACE = major adverse cardiac events, LM = Left-Main, TLR = target lesion revascularization, TVR = target vessel revascularization.
Table 3.
Clinical outcomes for patients with ostial LAD versus crossover
| Variables | Overall Total = 175 | Ostial LAD n = 150 | Crossover to LM n = 25 |
|---|---|---|---|
| Mortality (1 year) % (CI) | 7.43 (5.45-9.41) | 7.3 (5.2-9.4) | 8 (2.6-13.4) |
| Mortality (5 year) % (CI) | 11.71 (9.13-14.29) | 9.9 (7.3-12.5) | 22.5 (13.5-31.5) |
| MACE (1 Year) % (CI) | 13.1 (10.5-15.7) | 13.3 (10.5-16.1) | 12 (5.5-18.5) |
| MACE (5 year) % (CI) | 20.2 (17-23.4) | 19.3 (15.9-22.7) | 25.9 (16.6-35.2) |
| TLR (Total) n (%) | 12 (6.9) | 11 (7.3) | 1 (4) |
| TVR (Total) n (%) | 15 (8.6) | 12 (8) | 3 (12) |
| Restenosis >50% (Total) n (%) | 16 (9.1) | 15 (10) | 1 (4) |
LAD = Left anterior descending; LM = Left Main; MACE = major adverse cardiac events; TLR = target lesion revascularization; TVR = target vessel revacularization.
The one-year MACE was not significantly different between CO vs. OS (13.3% (10.5-16.1) vs. 12% (5.5-18.5)). The five-year MACE was numerically higher, although statistically not significant, in CO vs. OS (19.3 (15.9-22.7) vs. 25.9 (16.6-35.2)) (Table 3). The Kaplan-Meier curve for overall MACE is shown in Figure 2. There was no significant survival difference between the groups with a log-rank test of 0.225.
Discussion
This study is one of the largest retrospective studies for ostial LAD lesion interventions with 175 patients and long-term outcomes extending beyond an average of 63 months. Our study showed that ostial LAD lesion PCI generally had favourable outcomes. The long-term outcomes were comparable to non-ostial LAD studies [24].
Furthermore, it appeared as though a strategy crossover to left-main versus ostial LAD stenting showed no significant difference in outcomes, although the numbers of CO were relatively small in our cohort.
The overall 1-year MACE rate (TLR, TVR and mortality) was 13.1%, with a five-year MACE of 20.2%. Our study’s overall 1-year and 5-year mortality was 7.43% and 11.71%, respectively. These results are comparable to non-ostial LAD outcomes. In the PROTECT trial [24], there was no significant difference between proximal LAD and non-proximal LAD long-term outcomes. The one-year proximal LAD rates of TLR and TVR were 3.5% 5.3%, respectively. The long-term 4-year MACE was also similar to our study at 15%. Also, four-year mortality was 5.8%, comparable to ostial LAD outcomes in our study. These results confirm that although ostial LAD lesion location involves more myocardium at risk and stenting could jeopardize the left-main, outcomes are comparable to proximal LAD and non-proximal LAD stenting.
Although ostial LAD lesion intervention has a high success rate and acceptable outcomes, the outcomes with BMS are inferior. Some studies reported a 32% risk of angiographic restenosis with a TLR rate of 12% [13]. Debulking techniques showed mixed results regarding improved outcomes in ostial LAD lesion interventions [7,12]. In our study, the use of BMS was limited and insufficient to allow for direct comparison to DES. Debulking techniques were rarely used in our study, and thus we cannot determine if they impacted the outcome.
It has been shown previously that DES significantly improved outcomes [16] compared to BMS. Seung et al. [18] showed significant reductions in angiographic restenosis, with 5.1% in DES compared to 32% in BMS. The TLR rate was 0% in DES compared to 17% in BMS. Another study [8] compared DES in ostial lesions to non-ostial lesions found that the restenosis rate was 7% with a MACE of 13% with no difference between the groups. The study concluded that ostial LAD lesions are no longer a risk factor for restenosis with DES. In a large study with 162 ostial LAD lesions, the use of DES showed good outcomes with overall TLR rates of 8.3% [16]. These results are similar to our study, as most of our patients were treated with DES.
It remains unclear whether crossover stenting back into the LM may improve outcomes over ostial stenting alone. Rigatelli et al. [23] retrospectively evaluated 74 patients with ostial LAD lesions. Of those, 36 (49%) patients had crossover stenting to the LM. The study outcomes showed a numerical decrease in MACE in the crossover group vs. ostial stent (10 vs. 21%; P = 0.20). However, TVR was significantly lower in the crossover technique (5.6% versus 10.1%; P = 0.04). Another study showed that crossover is a safe and effective strategy and potentially has favourable outcomes than ostial stenting [16]. In our study, only 25 (14.3%) had a crossover to left-main, and there was no significant difference between a crossover and ostial stenting strategy in terms of long-term MACE and Mortality. These results suggest the crossover technique may be a reasonable and safe strategy, especially when there is not enough landing zone or disease in the left-main or if there is a disease in the distal left-main.
Crossover stenting from the LM into the LAD is safe even without opening the LCX struts [21]. Yamamoto [21] confirmed these results in 76 patients with ostial LAD who had crossover stenting versus ostial LAD. Although a Kissing balloon was required in 30% of their crossover group, no cases needed LCx intervention, and MACE was comparable. Proximal optimization techniques (POT) have been recommended in crossover stenting.Rigatelli et al. [23] reported the use of POT in all patients with crossover stenting and with the need for ballooning of the LCx in 6 out of 36 patients only. These techniques are recommended to optimize the crossover technique and may contribute to favourable outcomes, with only a minimal risk for subsequent intervention to the circumflex. In our study, the stenting strategy was left to the treating physician’s discretion, including the use of POT. Bifurcation stenting was required in two patients, and in another three patients, only balloon angioplasty was required to the circumflex.
It is well recognized that ostial LAD disease may often co-exist with distal left-main disease [25]. Intravascular imaging is a valuable tool in exploring the extent of the disease back into the left main vessel. IVUS guidance showed favourable outcomes in ostial LAD lesions, especially with distal left-main involvement [18]. The complete lesion coverage guided by IVUS could potentially help in reducing adverse cardiac outcomes. Although it did not affect restenosis rate, IVUS guided PCI of ostial LAD lesion showed strut protrusion in 53% of cases and incomplete coverage in 33% of cases [9]. The stent cross-sectional area is a significant predictor of outcomes. Kim et al. Showed that IVUS guided larger stent area is associated with more favourable outcomes, regardless of the technique used [13]. Intravascular imaging use was 26% in our cohort, and it was used more commonly in the crossover group. In our experience, intravascular imaging for stent optimization could improve outcomes. However, our study cannot determine whether the use of imaging contributed to an improved outcome due to the small numbers.
The limitation of the study includes the inherent drawback of retrospective studies, which include missing data and selection bias. Complete follow-up data is potentially missing, given the study’s retrospective nature, but the data on repeat revascularization and vital status is almost complete by excluding out-of-province patients. Selection bias for the technique used is another shortcoming of the study and limits the direct comparison between both groups. On the other hand, the relatively large number of patients included in this study with the long-term outcomes are the strengths of this analysis.
Prospective long-term data is missing on this subset of patients with ostial LAD lesions. Future research should focus on prospective randomized clinical trials for patients with ostial LAD lesions with a direct comparison of the two techniques.
Conclusions
In conclusion, ostial LAD lesion PCI showed reasonable outcomes in this large retrospective study. Left-main crossover techniques compared to ostial stenting showed comparable and favourable outcomes regardless of the technique used. Either technique appears to be safe and should be considered on a patient-by-patient basis. Left-main crossover technique should be considered when appropriate but may occasionally result in further intervention to the adjacent circumflex. Further prospective studies are needed to confirm these results and guide stent optimization techniques.
Disclosure of conflict of interest
None.
References
- 1.Texon M. The hemodynamic basis of atherosclerosis. Further observtions: the ostial lesion. Bull N Y Acad Med. 1972;48:733–740. [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng X, Peng H, Zhao D, Ma Q, Fu K, Chen G, Fan Q, Liu J. Optimal revascularization strategy on medina 0,1,0 left main bifurcation lesions in type 2 diabetes. J Diabetes Res. 2016;2016:1702454. doi: 10.1155/2016/1702454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konishi T, Yamamoto T, Funayama N, Nishihara H, Hotta D. Relationship between left coronary artery bifurcation angle and restenosis after stenting of the proximal left anterior descending artery. Coron Artery Dis. 2016;27:449–459. doi: 10.1097/MCA.0000000000000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Liu J, Zheng X, Rong X, Peng H, Silber-Li Z, Li M, Liu L. Three-dimensional virtual surgery models for percutaneous coronary intervention (PCI) optimization strategies. Sci Rep. 2015;5:10945. doi: 10.1038/srep10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L, Dash D, Gai LY, Cao YS, Zhao Q, Wang YR, Zhang YJ, Zhang JX. Intravascular ultrasound classification of plaque in angiographic true bifurcation lesions of the left main coronary artery. Chin Med J (Engl) 2016;129:1538–1543. doi: 10.4103/0366-6999.184456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park SW, Park HK, Hong MK, Lee SG, Lee IS, Kim JW, Lee CW, Kim JJ, Park SJ. Comparison of slotted tube versus coil stent implantation for ostial left anterior descending coronary artery stenosis: initial and late clinical outcomes. J Korean Med Sci. 1998;13:483–487. doi: 10.3346/jkms.1998.13.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SJ, Lee CW, Hong MK, Kim JJ, Park SW. Stent placement for ostial left anterior descending coronary artery stenosis: acute and long-term (2-year) results. Catheter Cardiovasc Interv. 2000;49:267–271. doi: 10.1002/(sici)1522-726x(200003)49:3<267::aid-ccd9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh IC, Chen CC, Chang SH, Hsieh MJ, Wang CY, Lee CH, Lin FC. Acute and long-term outcomes of drug-eluting stent implantations in aorto-ostial, left anterior descending artery-ostial, and nonostial lesions. Catheter Cardiovasc Interv. 2013;82:727–734. doi: 10.1002/ccd.24943. [DOI] [PubMed] [Google Scholar]
- 9.Kang SJ, Ahn JM, Kim WJ, Lee JY, Park DW, Lee SW, Kim YH, Lee CW, Mintz GS, Park SW, Park SJ. Intravascular ultrasound assessment of drug-eluting stent coverage of the coronary ostium and effect on outcomes. Am J Cardiol. 2013;111:1401–1407. doi: 10.1016/j.amjcard.2013.01.291. [DOI] [PubMed] [Google Scholar]
- 10.Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56:1897–1907. doi: 10.1016/j.jacc.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 11.Nam CW, Hur SH, Koo BK, Doh JH, Cho YK, Park HS, Yoon HJ, Kim H, Chung IS, Kim YN, Fearon WF, Tahk SJ, Kim KB. Fractional flow reserve versus angiography in left circumflex ostial intervention after left main crossover stenting. Korean Circ J. 2011;41:304–307. doi: 10.4070/kcj.2011.41.6.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bramucci E, Repetto A, Ferrario M, Canosi U, Boschetti E, Brambilla N, Gnecchi M, Merlini PA, Ardissino D, Angoli L, Tavazzi L. Effectiveness of adjunctive stent implantation following directional coronary atherectomy for treatment of left anterior descending ostial stenosis. Am J Cardiol. 2002;90:1074–1078. doi: 10.1016/s0002-9149(02)02772-8. [DOI] [PubMed] [Google Scholar]
- 13.Kim YH, Hong MK, Lee SW, Lee CW, Han KH, Kim JJ, Park SW, Mintz GS, Park SJ. Randomized comparison of debulking followed by stenting versus stenting alone for ostial left anterior descending artery stenosis: intravascular ultrasound guidance. Am Heart J. 2004;148:663–669. doi: 10.1016/j.ahj.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Dahm JB, Ruppert J, Hartmann S, Vogelgesang D, Hummel A, Felix SB. Directional atherectomy facilitates the interventional procedure and leads to a low rate of recurrent stenosis in left anterior descending and left circumflex artery ostium stenoses: subgroup analysis of the FLEXI-CUT study. Heart. 2006;92:1285–1289. doi: 10.1136/hrt.2005.081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu K, Hundal H, Zynda T, Seto A. Three-dimensional optical coherence tomography reconstruction of bifurcation stenting using the Szabo anchor-wire technique. World J Cardiol. 2017;9:384–390. doi: 10.4330/wjc.v9.i4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capranzano P, Sanfilippo A, Tagliareni F, Capodanno D, Monaco S, Sardella G, Giordano A, Sangiorgi GM, Tamburino C. Long-term outcomes after drug-eluting stent for the treatment of ostial left anterior descending coronary artery lesions. Am Heart J. 2010;160:973–978. doi: 10.1016/j.ahj.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Kishi K, Kimura T, Morimoto T, Namura M, Muramatsu T, Nishikawa H, Hiasa Y, Isshiki T, Nobuyoshi M, Mitsudo K j-Cypher Registry Investigators. Sirolimus-eluting stent implantation for ostial left anterior descending coronary artery lesions: three-year outcome from the j-Cypher Registry. Circ Cardiovasc Interv. 2011;4:362–370. doi: 10.1161/CIRCINTERVENTIONS.111.961904. [DOI] [PubMed] [Google Scholar]
- 18.Seung KB, Kim YH, Park DW, Lee BK, Lee CW, Hong MK, Kim PJ, Chung WS, Tahk SJ, Park SW, Park SJ. Effectiveness of sirolimus-eluting stent implantation for the treatment of ostial left anterior descending artery stenosis with intravascular ultrasound guidance. J Asm Coll Cardiol. 2005;46:787–792. doi: 10.1016/j.jacc.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Golmohamadi Z, Sokhanvar S, Aslanabadi N, Ghaffari S, Sohrabi B. One-year outcomes after everolimus-eluting stents implantation in ostial lesions of left anterior descending coronary arteries. Cardiol Res. 2013;4:192–198. doi: 10.4021/cr295w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CH, Choi SW, Hwang J, Kim IC, Cho YK, Park HS, Yoon HJ, Kim H, Han S, Kim JY, Lee JM, Doh JH, Shin ES, Koo BK, Hur SH, Nam CW. 5-year outcomes according to ffr of left circumflex coronary artery after left main crossover stenting. JACC Cardiovasc Interv. 2019;12:847–855. doi: 10.1016/j.jcin.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 21.Lee HM, Nam CW, Cho YK, Yoon HJ, Park HS, Kim H, Chung IS, Heo YS, Hur SH, Kim YN, Kim KB. Long-term outcomes of simple crossover stenting from the left main to the left anterior descending coronary artery. Korean J Intern Med. 2014;29:597–602. doi: 10.3904/kjim.2014.29.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto K, Sakakura K, Akashi N, Watanabe Y, Noguchi M, Taniguchi Y, Wada H, Momomura SI, Fujita H. Clinical outcomes of left main crossover stenting for ostial left anterior descending artery acute myocardial infarction. Heart Vessels. 2018;33:33–40. doi: 10.1007/s00380-017-1033-0. [DOI] [PubMed] [Google Scholar]
- 23.Rigatelli G, Zuin M, Baracca E, Galasso P, Carraro M, Mazza A, Lanza D, Roncon L, Daggubati R. Long-term clinical outcomes of isolated ostial left anterior descending disease treatment: Ostial stenting versus left main crossover stenting. Cardiovasc Revasc Med. 2019;20:1058–1062. doi: 10.1016/j.carrev.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 24.Roguin A, Camenzind E, Kerner A, Beyar R, Boersma E, Mauri L, Steg PG, Wijns W. Long-term outcomes of stenting the proximal left anterior descending artery in the PROTECT trial. JACC Cardiovasc Interv. 2017;10:548–556. doi: 10.1016/j.jcin.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 25.Oviedo C, Maehara A, Mintz GS, Araki H, Choi SY, Tsujita K, Kubo T, Doi H, Templin B, Lansky AJ, Dangas G, Leon MB, Mehran R, Tahk SJ, Stone GW, Ochiai M, Moses JW. Intravascular ultrasound classification of plaque distribution in left main coronary artery bifurcations: where is the plaque really located? Circ Cardiovasc Interv. 2010;3:105–112. doi: 10.1161/CIRCINTERVENTIONS.109.906016. [DOI] [PubMed] [Google Scholar]