Abstract
Objective
COVID-19 due to SARS-CoV-2 virus is a new cause of severe acute respiratory syndrome (SARS). Little is known about the short-term cognitive prognosis for these patients. We prospectively evaluated basic cognitive functions shortly after care in the intensive care unit (ICU) and three months later in post-ICU COVID-19 patients.
Material and methods
We performed a prospective single-center study in our institution in Paris. Patients with SARS-CoV-2 SARS were prospectively recruited via our ICU. Patients were evaluated using standardized cognitive tests at baseline and at three months’ follow-up. Our primary endpoint was the evolution of the following five global tests: MMSE, FAB, oral naming test, Dubois five words test and MADRS.
Results
We explored 13 patients at baseline and follow-up. All patients had cognitive impairment at baseline but they all improved at three months, significantly on two of the five global tests after Bonferroni correction for multiple testing: MMSE (median 18 (IQR [15–22]) and 27 (IQR [27–29]) respectively, P = 0.002) and FAB test (median 14 (IQR [14–17]) and 17 (IQR [17,18]) respectively, P = 0.002).
Conclusions
We report here the first longitudinal data on short-term cognitive impairment after intensive care in COVID-19 patients. We found acute and short-term cognitive impairment but significant improvement at three months. This pattern does not seem to differ from other causes of post-intensive care syndrome.
Keywords: Cognitive disorder, COVID-19, Critical care syndrome, Executive function, SARS-CoV-2
1. Introduction
COVID-19 due to SARS-CoV-2 virus is a new cause of severe acute respiratory syndrome (SARS). About 5% of symptomatic patients require extendible intensive care with mechanical ventilation.[1] Severe delirium has been reported in up to 84% of the cases in the intensive care unit (ICU) [2] but little is known about the short-term cognitive prognosis for these patients.
We prospectively evaluated basic cognitive functions shortly after admission to the ICU and three months later in COVID-19 patients.
2. Material and methods
2.1. Patients
We performed a single-center prospective study in our institution in Paris. Patients were prospectively recruited via our ICU.
Inclusion criteria were:
-
•
age ≥ 18 years;
-
•
French speaker;
-
•
admission to the ICU for SARS due to COVID-19 with need for mechanical ventilation with tracheal intubation between April 10th and May 19th;
-
•
discharged to a post-ICU medical unit within our institution.
Exclusion criteria were:
-
•
prolonged stuporous state after ICU discharge;
-
•
focal neurological deficit to avoid any potential associated acute vascular disease.
2.2. Protocol
Patients were evaluated at baseline by both a neurologist (CVDP, AR) and a neuropsychologist (AM, FS) and follow-up examination was performed three months after ICU discharge.
A standardized protocol was used to establish the cognitive evaluation using the five following global tests: Mini Mental State Evaluation(MMSE) for global cognition [3]; Frontal Assessment Battery (FAB) for executive functions [4]; 40 words oral naming test for language [5]; Dubois five words test for episodic memory [6]; and the Montgomery and Asberg Depression Scale (MADRS) [7], [8], [9], [10], [11]. Our main objective was to ascertain the evolution of these five tests. We also used specific executive function tests: forwards or backwards digit spans; similarities test of the Wechsler Adult Intelligence Scale 4th edition (WAIS IV) [7]; Brixton test [8]; Stroop Color-Word Test-Victoria version [9]; categorical and lexical verbal fluencies during two minutes [10]; and finally common bedside praxis. We used published cut-offs for these scores, adjusted for age and educational level as validated. We used the 10-point weighted five words Dubois test meaning that scores below 10 would suggest episodic memory impairment. For the 60-point MADRS, depression is considered mild, medium or severe according to scores between 7 and 19, 20 and 34, and above 34 respectively.
The following clinical data were recorded: demographic data; past medical history and cardiovascular risk factors including: obesity, high blood pressure, diabetes mellitus, smoking, hypercholesterolemia and sleep apnea disorder; oxygen need and level of oxygen; metabolic disorders; infectious diseases and use of psychotropic treatments at baseline. Any neurologic complaint from the patients before or during COVID-19 was recorded.
2.3. Statistics
Statistical analysis was performed using the R language (version 3.2.3). According to the type and distribution of the variables we used the non-parametric paired Wilcoxon test, chi-square test or Fisher's exact test and linear regression. Bonferroni correction has been applied for the five global tests. The threshold p-value corresponded to 0.05/5 = 0.01, which is the number of tests run. Formally, only tests with a p-value lower than 0.01 should be considered statistically significant. P-value under 0.05 was nevertheless considered significant. Graphics were made using ggplot2 R package.
2.4. Protocol approval and patient consent
All patients gave their informed consent. This study was approved and achieved under our institutional review board for clinical studies. This prospective study was in compliance with the tenets of the Helsinki Declaration.
3. Results
Thirty-five patients admitted to the ICU during the study period and fulfilling our inclusion criteria were screened. Among them, 20 patients were excluded (nine could not be evaluated by our neuropsychologists because of lack of availability, seven were directly transferred to another hospital, two did not speak French, and three died). Of the 15 patients finally evaluated at baseline, follow-up was obtained for 13 patients and these constitute our study population (eight women, median age 62, interquartile range, IQR [51–68], 12 right-handed). Education level was secondary school or higher for nine patients and primary school for four. We found at least two cardiovascular risk factors in 62% (8/13) of the patients. Past history of depression was found in 30% (4/13) of the patients. None of the patients had a pre-ICU cognitive complaint.
During the ICU stay, median tracheal intubation duration was 19 days (IQR [15–24]). Neuroleptic drugs were used in 23% (3/13) of the patients. Delayed awakening was noted in two patients and delirium in 46% (6/13) with two cases of transient self-extubation. Two patients underwent brain imaging (one computed tomography and one magnetic resonance imaging) for delirium shortly after ICU discharge with no abnormalities except mild vascular leukopathy.
Median delay between extubation and baseline cognitive assessment was six days (IQR [4–6]). At that time, 23% (3/13) patients were still receiving psychotropic drugs (benzodiazepines only). Evaluation was limited in only one patient due to nervousness. Concerning somatic functions, 46% (6/13) of patients presented various metabolic disorders including three with dialysis for kidney failure. The majority of patients (61%, 8/13) received oxygen at baseline (median level 1.5 l/min, IQR [1–2.25]) but only two patients had oxygen pulse saturation below 95% (93 and 90%). Follow-up evaluation was performed at a median delay of 92 days (IQR [91–95]) after baseline. At that time, none of the patients had returned to work among the five patients who were working before ICU admission.
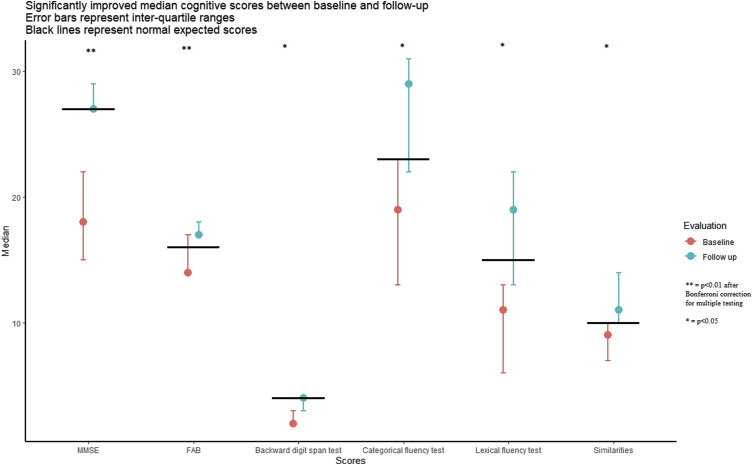
Results of the cognitive tests are reported in Table 1 . None of the patients had normal scores for the whole evaluation at baseline. A total of 92% patients (12/13) exhibited abnormal global cognitive function according to the MMSE score (under 27) and 46% (6/13) had space and temporal disorientation. Significant differences between baseline and follow-up evaluations (Fig. 1 ) were observed for two of the five global tests: MMSE (median 18 (IQR [15–22]) and 27 (IQR [27–29]) respectively, P = 0.002) and FAB test (median 14 (IQR [14–17]) and 17 (IQR [17–18]) respectively, P = 0.002). We also found a significant difference for the following tests: backward digit span test (median scores 2 and 4 respectively (P = 0.005), categorical fluency test (median scores 19 (IQR [13–23]) and 29 respectively (IQR [22–31]), P = 0.006), lexical fluency test (median 11 (IQR [6–13]) and 19 (IQR [13–22]) respectively, P = 0.005), and similarities (median 9 and 10 respectively, P = 0.04). We did not find any language impairment. Using linear regression, we did not find any statistically significant relations between baseline nor follow-up evaluations and: delirium (P = 0.4), severe sepsis (P = 0.4), duration of mechanical ventilation (P = 0.5) or presence of cardiovascular risk factors (P = 0.08), and found that MMSE scores were not linked to:
-
•
delay between extubation and baseline evaluation;
-
•
delay between both evaluations. MADRS scores at baseline were available for 61% (8/13) of the patients and mild-to-moderate depression was reported in 50% of them (respectively 1/8 and 3/8).
Table 1.
Description of cognitive tests results at baseline and follow-up (paired Wilcoxon test, median values and IQR).
| Test | Baseline (IQR) | Follow-up (IQR) | P-value |
|---|---|---|---|
| Global tests | |||
| MMSE | 18 (15–22) | 27 (27–29) | 0.002* |
| FAB | 14 (14–17) | 17 (17–18) | 0.002* |
| Oral naming test | 39 (36–39) | 40 (37–40) | 0.03* |
| Dubois five words test | 9 (6–10) | 10 (9–10) | 0.03* |
| Executive functions | |||
| Forward digit span test | 5 | 5 | 0.30 |
| Backward digit span test | 2 | 4 | 0.005 |
| Categorical fluency test | 19 (13–23) | 29 (22–31) | 0.006 |
| Lexical fluency test | 11 (6–13) | 19 (13–22) | 0.005 |
| Similarities | 9 | 10 | 0.04 |
| Brixton | 15 | 15 | 0.27 |
| Stroop | 1 | 0 | 0.62 |
| Depression | |||
| MADRS | 14 (3·5–23) | 4 (2–10) | 0.55* |
IQR: interquartile range.
For these 5 tests, the threshold p-value taking into account multiplicity of tests (Bonferroni method) is 0.01. Bold font: significant results.
Fig. 1.
Significantly improved median cognitive scores between baseline and follow-up. Error bars represent interquartile ranges. Black lines represent normal expected scores.
Mild-to-moderate depression persisted respectively in two and one patients without statistical difference (P = 0.55) and was not linked to MMSE score at baseline nor at follow-up (P = 0.7, linear regression). One patient with an initially normal MADRS score had mild depression at follow-up, the other eight patients had normal follow-up scores. After their post-ICU stay, 23% (3/13) of the patients returned home and 77% were transferred to rehabilitation units. At follow-up, only one patient was still in a rehabilitation unit and two still had day-basis rehabilitation.
4. Discussion
We performed a prospective single-center study seeking to evaluate cognitive impairment at baseline and three months post-ICU in COVID-19 patients hospitalized for SARS with need for mechanical ventilation via tracheal intubation, without specific neurological complaint. Our results showed severe acute cognitive dysfunction with abnormal scores on the global MMSE test, affecting mainly executive functions (working memory, fluency and inhibition) as mainly shown by the FAB test. We also found episodic memory impairment at baseline for 69% of the patients and for 46% at follow-up (P = 0.03). All patients improved between baseline and follow-up evaluations, with normalization of MMSE score in 83% (11/13). Nevertheless, only two patients (15%) had fully quantitatively normal tests at follow-up, when executive functions remained the main affected fields.
Our results are consistent with the literature on post-intensive care syndrome (PICS), a well-characterized disorder with cognitive impairment reported in 25 to 75% of patients [12]. The main risk factors of PICS are: duration of delirium in ICU; acute brain dysfunction; acute respiratory distress syndrome (ARDS) or cardiac arrest, severe sepsis, use of renal replacement therapy, and prior cognitive impairment [13], [14]. We were unable to identify such risk factors in our study, probably due to the small size of the cohort. According to previous studies [13], cognitive impairment can last from three months (66%) to 12 months (58%) and sometimes many years post recovery [14]. We also found cognitive impairment in 85% of the patients three months after ICU discharge although MMSE scores were normalized in 83% (11/13). Follow-up shows also more involvement of executive functions [13], [15] in the literature and was constituent with our study. Interestingly, 62% (8/13) of our patients were not complaining of these cognitive impediments. Depression in PICS has been reported in 33% of patients two months after ICU discharge [16] which is similar to our results with 38% of depressed patients (5/13).
Few studies, and to our knowledge none using prospective evaluation after ICU, have evaluated short-term cognitive functions after SARS-CoV-2 infections [17], [18], [19]. One study validated the presence of delirium as a known risk factor for cognitive impairment in COVID-19 patients not only after ICU [18]. One evaluated cognitive functions in four patients shortly after ICU discharge, showing initial cognitive impairment but no follow-up was performed and tests were not standardized [17]. Another one evaluated cognitive functions after SARS-CoV-2 infection but not in severe patients and only after four months with no baseline evaluation [20]. No cognitive dysfunctions were found. Lastly, one study compared cognitive assessment in patients shortly after (about 10 days) or later after (25 ± 10 days) SARS-CoV-2 infection and found higher scores (MMSE, MOCA) in the second group [21]. However, the authors did not perform a follow-up evaluation and severe patients were not recruited. The originality of our study was to prospectively evaluate the cognitive functions of post-ICU COVID-19 patients using a wide range of standardized tests.
SARS-CoV-2 has been related to acute encephalopathy without acute respiratory syndrome and one study has shown frontal hypometabolism using positron emission tomography [22]. Nevertheless, the cognitive pattern and prognosis of our patients does not seem to be different than PICS patients after SARS [13], [15], which could argue against a specific SARS-CoV-2 related mechanism to explain cognitive impairment in these patients. Indeed, using MOCA and FAB tests in 13 post-ICU patients, without clear delay from extubation, Beaud and colleagues described a cognitive impairment not different from PICS [23], which is consistent with our longitudinal results. Some authors have explained the need for specific neuropsychological rehabilitation in severe COVID-19 patients [24], [25]. Although we did not find statistical differences between home-discharged and rehabilitation-discharged patients, specific cognitive rehabilitation might be efficient for post-ICU COVID-19 patients as for patients with PICS.
We recognize some limitations for our study and firstly our small cohort. This was due to the organization of the national health care system during the SARS-CoV-2 pandemic first peak when patients were frequently transferred between different ICUs depending on their capacities to receive new patients. We also regret that, mostly because of possible interpatient contagiousness issues and availability of magnetic resonance imaging, when little was known about SARS-CoV-2, we were not able to perform a systematic brain imaging in our patients.
We report here the first longitudinal data on short-term cognitive impairment after intensive care in COVID-19 patients. SARS due to SARS-CoV-2 is responsible for acute and short-term cognitive impairment with major executive dysfunction but significant improvement at three months is the rule. This pattern does not seem to differ from other causes of PICS. Our pragmatic study could not distinguish the part specifically due to SARS-CoV-2 virus from other mechanisms. Studies combining neuropsychological and functional radiological data could help to understand this pathophysiology and more longitudinal long-term studies are needed to evaluate cognitive sequelae.
Funding
No funding was given for this study.
Authors’ contributions
CVDP and AR wrote the protocol. TY and CB recruited the patients. CVDP, AR, AM and FS evaluated the patients. CVDP performed the statistics and wrote the manuscript. CVDP and MZ verified the statement data. MZ and CVDP reviewed the manuscript.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
We want to thank the medical team of the intensive care unit and the pneumology department for their support and help in recruiting the patients, especially Drs Stéphane Jouveshomme and Marie de Torcy.
We want to thank our local clinical research team and especially Emmanuelle Sacco for help in writing the protocol and Mrs Audrey Fels and Pr Gilles Chatellier for help in statistics.
References
- 1.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., et al. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 4.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 5.Azouvi P., Vallat-Azouvi C., Joseph P.-A., Meulemans T., Bertola C., Le Gall D., et al. Executive functions deficits after severe traumatic brain injury: the GREFEX Study. J Head Trauma Rehabil. 2016;31:E10–E20. doi: 10.1097/HTR.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 6.Dubois B., Touchon J., Portet F., Vellas B., Michel B. 2002. « Les 5 mots », épreuve simple et sensible pour le diagnostic de la maladie d’Alzheimer; p. 4. [PubMed] [Google Scholar]
- 7.Wechsler Adult Intelligence Scale | Fourth Edition n.d. https://www.pearsonassessments.com/store/usassessments/en/Store/Professional-Assessments/Cognition-%26-Neuro/Wechsler-Adult-Intelligence-Scale-%7C-Fourth-Edition/p/100000392.html (accessed October 14, 2020).
- 8.Burgess P.W., Shallice T. Bizarre responses, rule detection and frontal lobe lesions. Cortex J Devoted Study Nerv Syst Behav. 1996;32:241–259. doi: 10.1016/s0010-9452(96)80049-9. [DOI] [PubMed] [Google Scholar]
- 9.Spreen O., Spreen P of PO, Strauss E., Spreen P of PE . Oxford University Press; USA: 1998. A compendium of neuropsychological tests: administration, norms, and commentary. [Google Scholar]
- 10.Merck C., Charnallet A., Auriacombe S., Belliard S., Hahn-Barma V., Kremin H., et al. La batterie d’évaluation des connaissances sémantiques du GRECO (BECS-GRECO) : validation et données normatives. Rev Neuropsychol. 2011;3:235–255. [Google Scholar]
- 11.Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry J Ment Sci. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 12.Rawal G., Yadav S., Kumar R. Post-intensive care syndrome: an overview. J Transl Intern Med. 2017;5:90–92. doi: 10.1515/jtim-2016-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandharipande P.P., Girard T.D., Jackson J.C., Morandi A., Thompson J.L., Pun B.T., et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwashyna T.J., Ely E.W., Smith D.M., Langa K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikkelsen M.E., Christie J.D., Lanken P.N., Biester R.C., Thompson B.T., Bellamy S.L., et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davydow D.S., Gifford J.M., Desai S.V., Bienvenu O.J., Needham D.M. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009;35:796–809. doi: 10.1007/s00134-009-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groiss S.J., Balloff C., Elben S., Brandenburger T., Müttel T., Kindgen-Milles D., et al. Prolonged neuropsychological deficits, central nervous system involvement, and brain stem affection after COVID-19 – a case series. Front Neurol. 2020;11:574004. doi: 10.3389/fneur.2020.574004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mcloughlin B.C., Miles A., Webb T.E., Knopp P., Eyres C., Fabbri A., et al. Functional and cognitive outcomes after COVID-19 delirium. Eur Geriatr Med. 2020;11:857–862. doi: 10.1007/s41999-020-00353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowles K.H., McDonald M., Barrón Y., Kennedy E., O’Connor M., Mikkelsen M. Surviving COVID-19 after hospital discharge: symptom, functional, and adverse outcomes of home health recipients. Ann Intern Med. 2021;174:316–325. doi: 10.7326/M20-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Pietro D.A., Comini L., Gazzi L., Luisa A., Vitacca M. Neuropsychological pattern in a series of post-acute COVID-19 patients in a rehabilitation unit: retrospective analysis and correlation with functional outcomes. Int J Environ Res Public Health. 2021;18:5917. doi: 10.3390/ijerph18115917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pistarini C., Fiabane E., Houdayer E., Vassallo C., Manera M.R., Alemanno F. Cognitive and emotional disturbances due to COVID-19: an exploratory study in the rehabilitation setting. Front Neurol. 2021;12:643646. doi: 10.3389/fneur.2021.643646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delorme C., Paccoud O., Kas A., Hesters A., Bombois S., Shambrook P., et al. Covid-19-related encephalopathy: a case series with brain FDG-PET/CT findings. Eur J Neurol. 2020;27:2651–2657. doi: 10.1111/ene.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaud V., Crottaz-Herbette S., Dunet V., Vaucher J., Bernard-Valnet R., Du Pasquier R., et al. Pattern of cognitive deficits in severe COVID-19. J Neurol Neurosurg Psychiatry. 2020;92:567–568. doi: 10.1136/jnnp-2020-325173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker-Davies R.M., O'Sullivan O., Senaratne K.P.P., Baker P., Cranley M., Dharm-Datta S., et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med. 2020;54:949–959. doi: 10.1136/bjsports-2020-102596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabinovitz B., Jaywant A., Fridman C.B. Neuropsychological functioning in severe acute respiratory disorders caused by the coronavirus: implications for the current COVID-19 pandemic. Clin Neuropsychol. 2020;34:1453–1479. doi: 10.1080/13854046.2020.1803408. [DOI] [PubMed] [Google Scholar]