This cross-sectional study examines whether new formulations of brand-name novel drugs were associated with novel drugs’ sales and/or therapeutic value as well as characterizes new formulations’ approval timing relative to the novel drug’s generic approval.
Key Points
Questions
Are sales or therapeutic value associated with approval of new formulations of brand-name novel drugs, and does timing coincide with generic competition?
Findings
In this cross-sectional study of 206 brand-name drugs approved in tablet or capsule form by the US Food and Drug Administration between 1995 and 2010, approval of new formulations was 4 times more likely among blockbuster drugs and 5.5 times more likely among drugs granted accelerated approval. First new formulation approval was statistically significantly lower after approval of generic competition.
Meaning
Manufacturers pursue new formulations of best-selling brand-name drugs and those granted accelerated approval but less frequently once generic competition begins.
Abstract
Importance
New formulations of prescription drugs can improve convenience and tolerability for patients, but they also constitute manufacturer strategies to extend brand-name drug market exclusivity periods.
Objective
To examine whether new formulations of brand-name novel drugs were associated with novel drugs’ sales and/or therapeutic value, as well as characterize first new formulations’ approval timing relative to the novel drug’s generic approval.
Design, Setting, and Participants
This cross-sectional study used the Drugs@FDA database to identify all novel tablet and capsule drugs approved by the US Food and Drug Administration (FDA) between 1995 and 2010 and followed through December 31, 2021.
Exposures
Novel drugs’ blockbuster status, defined as annual sales of $1 billion or greater, and therapeutic value, measured by (1) accelerated approval status, (2) World Health Organization Model Lists of Essential Medicines inclusion, (3) innovativeness, and (4) clinical usefulness.
Main Outcomes and Measures
Approval of a new formulation and timing relative to a novel drug’s first generic’s approval.
Results
Among the 206 novel drugs in tablet or capsule form approved by the FDA from 1995 to 2010, 81 (39.3%) were followed by an FDA-approved new formulation, and 167 (81.1%) had a generic version as of December 31, 2021. In multivariable analyses, new formulations were statistically significantly more likely among blockbuster drugs vs not (58.2% vs 27.6%; adjusted odds ratio [AOR], 4.72; 95% CI, 2.26-9.87; P < .001) and those granted accelerated approval vs not (50.0% vs 37.6%; AOR, 5.48; 95% CI, 1.52-19.67; P = .009), and less likely among orphan products vs not (11.8% vs 44.8%; AOR, 0.13; 95% CI, 0.03-0.52; P = .004). Essential medicine listing vs no listing (47.8% vs 36.9%; AOR, 1.32; 95% CI, 0.52-3.34; P = .56), first-in-class or advance-in-class status vs addition-to-class status (37.8% vs 40.5%; AOR, 0.71; 95% CI, 0.32-1.58; P = .40), and categorization as clinically useful vs not useful (40.9% vs 44.8%; AOR, 0.81; 95% CI, 0.34-1.92; P = .64) were not associated with increased likelihood of a new formulation. First new formulations were statistically significantly less likely to be approved after the novel drug’s first generic approval (84.6% vs 15.4%; P < .001).
Conclusions and Relevance
In this cross-sectional study of novel drugs in tablet or capsule form approved by the FDA between 1995 and 2010, manufacturers pursued new formulations of best-selling brand-name drugs and those granted accelerated approval but did so less frequently once generic competitors entered the market. Other measures of therapeutic value were not associated with new formulations.
Introduction
Brand-name drug manufacturers can modify existing drugs through various means, including changes in delivery mechanisms to decrease dosing frequency, slight chemical alterations, and combinations of multiple active ingredients.1 Such new formulations can be useful for patients by increasing convenience, improving adherence through dosing frequency reductions or better tolerability, and offering additional treatments for diseases.2,3,4,5 However, in some cases new formulations, particularly tablets and capsules, may not be clinically superior to the novel drug,6,7,8 and their potential convenience benefits may be outweighed by their cost. Moreover, whether new formulations are more common for drugs that are therapeutically valuable remains unclear.
Another concern is that new formulations constitute one of numerous well-described strategies—called “evergreening” or “life-cycle management”—used by brand-name drug manufacturers to extend periods of market exclusivity protection and thus revenue associated with drugs,1,9,10,11,12 particularly as they face generic competition.13 One report found that new formulation approvals are considerably higher around the first generic’s entry and that manufacturers decide when to pursue new formulations based on the extent to which their sales would cut into those of the novel drug.14 Strategically delaying new formulation applications until the time of generic entry complicates the ability of generic versions to achieve widespread use and lower prescription drug spending. In a strategy known as “product hopping,”9,15 for example, brand-name manufacturers may simultaneously heavily promote the new formulation over the novel drug and rely on laws that prevent pharmacies from automatically substituting the new formulation with generic versions of the novel drug. Product-hopping strategies lead to substantial excess spending owing to the delayed availability of potentially useful formulations16,17,18 and the introduction of extended-release formulations8,19 and fixed-dose combination drugs20,21 of uncertain marginal value.
Previous studies have characterized specific examples of product hopping,16,17,22,23 assessed new formulations over narrow time frames24 or formulation types,19,25 or sought to determine the therapeutic value of new formulations.6,26,27,28 To our knowledge, no prior studies, however, have examined additional characteristics, including therapeutic value, of novel drugs for which manufacturers pursued new formulations. Studying the therapeutic value of novel drugs that are formulated again holds particular salience for prescription drug pricing reform and incentives for pharmaceutical innovation. In this cross-sectional study, we assessed the association between the presence of a new formulation and the novel drug’s sales and therapeutic value using 4 different, objective measures. We examined the timing of the first new formulation’s approval since the novel drug’s approval and before and after the first generic version’s approval.
Methods
Data and Study Sample
We used the Drugs@FDA database to identify all new drug applications for new molecular entities (novel drugs) in tablet and capsule form approved by the US Food and Drug Administration (FDA) between January 1, 1995, and December 31, 2010, excluding tentative approvals, biological treatments, over-the-counter drugs, and duplicate listings (eFigure 1 in the Supplement). We excluded novel drugs withdrawn from the market due to safety concerns. For the secondary outcome of new formulation approval timing, we excluded drugs with active patent protections, other market exclusivity, or ongoing litigation as of December 2021, identified from the FDA’s Orange Book drug database, public sources, company press releases, and US Securities and Exchange Commission documents, because these drugs were not yet likely to face generic competition.
For each novel drug in the final cohort, new formulations were identified as of December 31, 2021, using Drugs@FDA. New formulations were included if they were19 (1) approved through a new drug application with at least 1 active ingredient matching the novel drug’s, (2) approved as a tablet or capsule, (3) approved more than 6 months after the novel drug (to account for possible regulatory delays in simultaneous application filings), and (4) included in an application filed by the same manufacturer as the novel drug or a manufacturer that had acquired or merged with the original manufacturer or a different manufacturer but with the drug sharing the source brand name. We excluded new formulations that were new active ingredients, marketed but unapproved, or only divisible dosage changes (eg, change to 40-mg dose from an existing 20-mg dose). For fixed-dose combination drugs, these criteria were applied to each active ingredient of the combination individually to be included as a new formulation, if the active ingredient met the inclusion criteria for novel drugs. For example, amlodipine besylate/atorvastatin calcium (Caduet) was included as a new combination formulation for atorvastatin calcium (Lipitor) but not amlodipine besylate (Norvasc) because amlodipine besylate was first approved in 1992, before the start of the study period.
For each novel drug, we then used Drugs@FDA to identify the first FDA-approved bioequivalent generic drug by matching active ingredient and formulation as of December 31, 2021. We disregarded multiple dosage strengths approved on the same date and counted the generic drug only once. We excluded tentative approvals and authorized generics.29
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cross-sectional studies. Institutional review board approval was not necessary because this study did not include human participant data.
Sales
Drugs with blockbuster status have annual global sales of $1 billion or greater, indicating large profits.30 Using annual sales extracted from public sources, company press releases, and US Securities and Exchange Commission documents, each novel drug in the present cohort was classified by whether it was a blockbuster drug based on whether it had, during any year since its approval, annual global sales of $1 billion or greater.
Therapeutic Value
The therapeutic value of a drug to patients is dependent on a number of factors, including whether the drug is a promising treatment beyond existing therapeutic alternatives, clinically important and indicated for treatment of a widely prevalent disease, an innovative advance, and clinically useful. We estimated the therapeutic value of each novel drug in the sample using these 4 proxy measures.31
The promise of a novel drug was assessed by whether it was granted accelerated approval by the FDA. Drugs that received accelerated approval were identified using Drugs@FDA, including FDA approval letters. Therapeutics that address a serious unmet medical need may be eligible for accelerated approval status, allowing the FDA to approve the drug on the basis of surrogate markers of disease as clinical trial end points.32
Clinical importance of novel drugs was assessed through a proxy measure of whether the drug was included in the World Health Organization’s (WHO) Model Lists of Essential Medicines,33 which list drugs that address a population’s priority health care needs. These lists offer a straightforward metric of both the drug’s utility and the relative prevalence of the disease(s) for which it is indicated.
To assess whether each novel drug was considered innovative, we followed a schematic established by FDA research,34 which categorized drugs as first in class, advance in class, or addition to class. Using this schematic, innovative drugs that were the first approved within their respective drug class were considered first in class because they represent a new pathway for treating a disease. Drugs that were not first in class but received priority review status were categorized as advance in class. All other drugs were categorized as addition to class. In the present analysis, we grouped first in class and advance in class together compared with addition to class.
We assessed clinical usefulness of each novel drug by using an established source31,35 of ratings by the French drug industry watchdog Prescrire International,36 an independent, nonprofit organization that reviews new treatments. Drugs that were categorized by Prescrire as “possibly helpful,” “offers an advantage,” “a real advance,” or “bravo” were grouped together as “clinically useful.” Drugs that were categorized as “nothing new” and “not acceptable” were grouped together as “clinically not useful.” The remaining drugs were categorized as “judgment reserved.” For drugs for which Prescrire ratings were not available, we reviewed Prescrire materials and guidelines to extrapolate assessments. If no statements could be found, drugs were grouped together with judgment reserved.
Other Drug Characteristics
Drugs are granted orphan status by the FDA if they treat a rare disease, defined as affecting fewer than 200 000 individuals in the US annually. The Orphan Drug Product designation database was used to determine whether novel drugs had received designation as orphan products for the first approval indication.37,38
Using the WHO’s Anatomical Therapeutic Classification system,39 we categorized each novel drug into 1 of 9 treatment classes based on the indication for which the drug was first approved: autoimmune or musculoskeletal; cancer; cardiovascular, diabetes, or hyperlipidemia; gastrointestinal; genitourinary/sex hormones; infectious disease; neurology; psychiatry; and other (eTable 1 in the Supplement). Finally, we categorized the approval year for each novel drug into 3-year increments from 1995 to 2010.
Presence and Timing of New Formulations
The primary outcome was whether each novel drug in the sample was followed by at least 1 new FDA-approved formulation of the same drug. The secondary outcome was the timing of the first new formulation approval, categorized into before and after the first generic approval. The timing of the first new formulation approval, particularly relative to the novel drug’s first generic competitor’s approval, is an important indicator of whether the new formulation is a potential tool for evergreening.
Statistical Analysis
We used descriptive statistics to characterize novel drugs and their first new formulations and first generics. We conducted bivariate and multivariable logistic regression analyses with presence of at least 1 new formulation as the outcome, and we report unadjusted odds ratios (ORs) and adjusted ORs (AORs), respectively, with 95% CIs for 8 novel drug characteristics: (1) blockbuster status, (2) accelerated approval status, (3) WHO Model Lists of Essential Medicines inclusion, (4) orphan product, (5) innovation status, (6) clinical usefulness (based on Prescrire ratings), (7) therapeutic area, and (8) approval year.
For novel drugs with at least 1 new formulation and 1 generic, we produced Kaplan-Meier estimates to plot the presence of a new formulation as a function of time since the novel drug’s approval, with 2-sided log-rank tests to assess for differences in events over time among all 8 novel drug characteristics. We then performed a χ2 goodness-of-fit test to examine whether the observed proportion of new formulations before and after the first generic approval was statistically significantly different from the expected equal proportions. An additional timing analysis was specified to account for regulatory approval delays by examining the likelihood of a new formulation in different windows relative to the novel drug’s first generic approval.
All statistical tests were 2-tailed and used P = .05 as a threshold for statistical significance. We used Stata, version 16 (StataCorp), and R, version 4.0.4 (R Foundation for Statistical Computing), to conduct all analyses.
Additional Analyses
Given the possibility of blockbuster drugs also being most therapeutically valuable, we conducted bivariate and multivariable logistic regression analyses with blockbuster status as the outcome and each measure of therapeutic value and other drug characteristics as covariates. In addition, because of likely correlation between different measures of therapeutic value, we performed bivariate analyses using χ2 tests to examine associations between measures.
Results
Characteristics of Novel Drugs
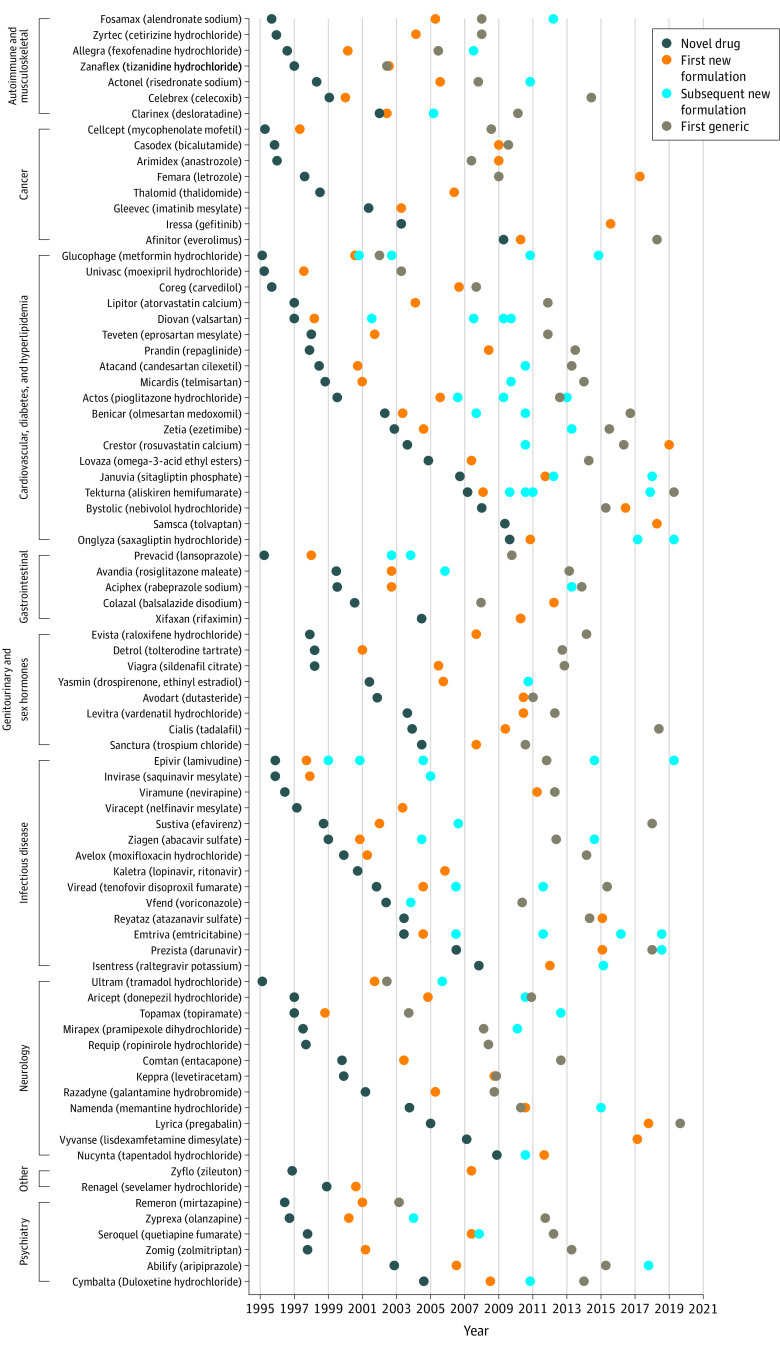
A total of 206 novel drugs in tablet or capsule formulation were approved by the FDA between 1995 and 2010 (Table 1). Eighty-one (39.3%) were followed by at least 1 new FDA-approved formulation (Figure 1 and eFigure 2 in the Supplement), and 167 (81.1%) had at least 1 generic version approved by the FDA as of December 31, 2021 (Table 2). Among the 206 novel drugs, nearly one-third (n = 65 [31.6%]) were followed by at least 1 new formulation and a generic.
Table 1. Characteristics of Novel Drugs in Tablet or Capsule Formulation Approved by the US Food and Drug Administration, 1995-2010 (N = 206).
| Characteristic | No. (%) |
|---|---|
| Blockbuster statusa | |
| Yes | 79 (38.4) |
| No | 127 (61.7) |
| Accelerated approval | |
| Yes | 28 (13.6) |
| No | 178 (86.4) |
| WHO essential medicine | |
| Yes | 46 (22.3) |
| No | 160 (77.7) |
| Innovation status | |
| First in class or advance in class | 90 (43.7) |
| Addition to class | 116 (56.3) |
| Clinical usefulnessb | |
| Judgment reserved | 44 (21.4) |
| Clinically not useful | 96 (46.6) |
| Clinically useful | 66 (32.0) |
| Orphan status | |
| Yes | 34 (16.5) |
| No | 172 (83.5) |
| Therapeutic area | |
| Autoimmune or musculoskeletal | 14 (6.8) |
| Cancer | 25 (12.1) |
| Cardiovascular, diabetes, or hyperlipidemia | 39 (18.9) |
| Gastrointestinal | 15 (7.3) |
| Genitourinary/sex hormones | 18 (8.7) |
| Infectious disease | 36 (17.5) |
| Neurology | 33 (16.0) |
| Psychiatry | 17 (8.3) |
| Other | 9 (4.4) |
| Approval year | |
| 1995-1997 | 58 (28.2) |
| 1998-2000 | 48 (23.3) |
| 2001-2003 | 31 (15.1) |
| 2004-2006 | 31 (15.1) |
| 2007-2010 | 38 (18.5) |
Abbreviation: WHO, World Health Organization.
Drugs were categorized as blockbuster if they had annual global sales of $1 billion during any year after the novel drug’s approval.
Clinical usefulness was determined through review of ratings from Prescrire International, the French drug industry watchdog. If drugs were not evaluated by Prescrire, they were categorized as “judgment reserved.” If the drugs were categorized by Prescrire as “possibly helpful,” “offers an advantage,” “a real advance,” or “bravo,” they were grouped together as “clinically useful.” If the drugs were categorized by Prescrire as “nothing new” or “not acceptable,” they were grouped together as “clinically not useful.”
Figure 1. Timeline of Novel Tablets and Capsules Approved by the US Food and Drug Administration, 1995-2010, Followed by New Formulation and Generic Version Approvals Grouped by Therapeutic Area (n = 81).
Table 2. Characteristics and Approval Timing of New Formulations of Novel Tablet or Capsule Drugs Approved by the US Food and Drug Administration, 1995-2010 (N = 206).
| Characteristics and timing of new formulations and generics | No. (%) |
|---|---|
| No. of new formulations per novel drug | |
| 0 | 125 (60.7) |
| 1 | 45 (21.8) |
| 2 | 23 (11.2) |
| ≥3 | 13 (6.3) |
| New formulations and generics of novel drugs | |
| With ≥1 new formulation | 81 (39.3) |
| With ≥1 generic | 167 (81.1) |
| With 0 new formulations and 0 generics | 23 (11.2) |
| With ≥1 new formulation and 1 generic | 65 (31.6) |
| First new formulation type (n = 81) | |
| Dosage forma | 34 (41.9) |
| Combination | 29 (35.8) |
| Indication | 13 (16.0) |
| Other | 5 (6.2) |
| Approval timing of novel drug, new formulation, and generic, median (IQR), y | |
| Novel drug and first new formulation approval | 4.6 (2.3-8.6) |
| Novel drug and first generic approval | 11.4 (8.1-14.5) |
| First new formulation approval and first generic approval | 5.9 (1.5-10.9) |
Carvedilol had a new formulation that was both a switch from tablet to extended-release capsule (ie, a new dosage form) and approved as carvedilol phosphate (ie, a new active ingredient). This drug was included in the sample as a new dosage form.
Among these 206 drugs, the most common clinical indications were for cardiovascular disease, diabetes, or hyperlipidemia (n = 39 [18.9%]), followed by infectious disease (n = 36 [17.5%]) and neurologic conditions (n = 33 [16.0%]). One-sixth of novel drugs were designated as orphan products (n = 34 [16.5%]). More than one-third were blockbuster drugs (n = 79 [38.4%]). Overall, 121 (58.7%) drugs qualified for at least 1 proxy of therapeutic value (eTable 2 in the Supplement). Less than one-fifth were granted accelerated approval by the FDA (n = 28 [13.6%]). One-fifth were considered essential medicines by WHO (n = 46 [22.3%]). Nearly half were considered innovative, categorized as first-in-class or advance-in-class drugs (n = 90 [43.7%]). Approximately one-third were considered clinically useful based on Prescrire ratings (n = 66 [32.0%]), whereas nearly half were considered clinically not useful (n = 96 [46.6%]).
Presence and Timing of New Formulations and Generics
Approximately one-fifth of novel drugs had 1 new FDA-approved formulation (n = 45 [21.8%]), 23 (11.2%) had 2, and 13 (6.3%) had 3 or more (Table 2). Among the 81 drugs with at least 1 new formulation, nearly half of the first new formulations were new dosage forms (eg, extended-release versions) (n = 34 [41.9%]), more than one-third were combination drugs (n = 29 [35.8%]), and nearly one-sixth were approved for new indications (n = 13 [16.0%]). Among drugs with at least 1 new formulation, the median (IQR) time from novel drug to first new formulation approval was 4.6 (2.3-8.6) years. Among drugs with at least 1 generic, the median (IQR) time from novel drug to first generic approval was 11.4 (8.1-14.5) years. For novel drugs that had both a new formulation and a generic, the first new formulation was approved a median (IQR) of 5.9 (1.5-10.9) years prior to the first generic’s approval.
Factors Associated With Presence of a New Formulation
In multivariable analyses, new formulations were statistically significantly more likely among blockbuster drugs (AOR, 4.72; 95% CI, 2.26-9.87; P < .001) and novel drugs granted accelerated approval (AOR, 5.48; 95% CI, 1.52-19.67; P = .009), and less likely among orphan products (AOR, 0.13; 95% CI, 0.03-0.52; P = .004) (Table 3). Essential medicine list inclusion (AOR, 1.32; 95% CI, 0.52-3.34; P = .56), first-in-class or advance-in-class status (AOR, 0.71; 95% CI, 0.32-1.58; P = .40), and categorization as clinically useful based on Prescrire ratings (AOR, 0.81; 95% CI, 0.34-1.92; P = .64) were not associated with increased likelihood of a new formulation. There were no statistically significant differences in the likelihood of new formulations by therapeutic area.
Table 3. New Formulation Approval and Associations Between Approval and Characteristics of Novel Tablets and Capsules Approved by the US Food and Drug Administration, 1995-2010 (N = 206).
| Novel drug characteristic | Proportion with a new formulation, % (95% CI) | Bivariate analysis | Multivariable analysis | ||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Adjusted odds ratio (95% CI) | P value | ||
| Blockbuster status | |||||
| No | 27.6 (20.5-36.0) | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 58.2 (47.1-68.6) | 3.66 (2.03-6.63) | <.001 | 4.72 (2.26-9.87) | <.001 |
| Accelerated approval | |||||
| No | 37.6 (30.8-45.0) | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 50.0 (32.2-67.8) | 1.66 (0.74-3.69) | .22 | 5.48 (1.52-19.67) | .009 |
| WHO essential medicine | |||||
| No | 36.9 (29.7-44.7) | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 47.8 (33.9-62.1) | 1.57 (0.81-3.04) | .18 | 1.32 (0.52-3.34) | .56 |
| Orphan status | |||||
| No | 44.8 (37.5-52.3) | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 11.8 (4.5-27.6) | 0.16 (0.06-0.49) | .001 | 0.13 (0.03-0.52) | .004 |
| Innovation status | |||||
| Addition to class | 40.5 (31.9-49.7) | 1 [Reference] | NA | 1 [Reference] | NA |
| First in class or advance in class | 37.8 (28.3-48.2) | 0.89 (0.51-1.57) | .69 | 0.71 (0.32-1.58) | .40 |
| Clinical usefulnessa | |||||
| Clinically not useful | 44.8 (35.1-54.9) | 1 [Reference] | NA | 1 [Reference] | |
| Judgment reserved | 25.0 (14.4-39.8) | 0.41 (0.19-0.91) | .03 | 0.56 (0.21-1.51) | .25 |
| Clinically useful | 40.9 (29.7-53.1) | 0.85 (0.45-1.61) | .62 | 0.81 (0.34-1.92) | .64 |
| Therapeutic area | |||||
| Autoimmune or musculoskeletal | 50.0 (25.8-74.2) | 1 [Reference] | NA | 1 [Reference] | NA |
| Cancer | 32.0 (16.8-52.3) | 0.47 (0.12-1.80) | .27 | 0.78 (0.14-4.55) | .79 |
| Cardiovascular, diabetes, or hyperlipidemia | 48.7 (33.6-64.1) | 0.95 (0.28-3.22) | .93 | 1.46 (0.36-5.90) | .59 |
| Gastrointestinal | 33.3 (14.5-59.5) | 0.50 (0.11-2.24) | .37 | 1.56 (0.26-9.20) | .63 |
| Genitourinary/sex hormones | 44.4 (23.9-67.1) | 0.80 (0.20-3.25) | .76 | 1.68 (0.33-8.48) | .53 |
| Infectious disease | 38.9 (24.5-55.5) | 0.64 (0.18-2.21) | .48 | 0.72 (0.15-3.54) | .68 |
| Neurology | 36.4 (21.9-53.8) | 0.57 (0.16-2.02) | .39 | 1.52 (0.36-6.42) | .57 |
| Psychiatry | 35.3 (16.7-59.7) | 0.55 (0.13-2.31) | .41 | 0.81 (0.15-4.30) | .80 |
| Other | 22.2 (5.6-58.1) | 0.29 (0.04-1.89) | .19 | 1.55 (0.18-13.33) | .69 |
| Approval year | |||||
| 1995-1997 | 53.4 (40.6-65.9) | 1 [Reference] | NA | 1 [Reference] | NA |
| 1998-2000 | 37.5 (25.0-51.9) | 0.52 (0.24-1.14) | .10 | 0.37 (0.14-0.94) | .04 |
| 2001-2003 | 54.8 (37.3-71.2) | 1.06 (0.44-2.54) | .90 | 0.98 (0.35-2.76) | .98 |
| 2004-2006 | 22.6 (11.1-40.5) | 0.25 (0.09-0.68) | .007 | 0.19 (0.06-0.59) | .004 |
| 2007-2010 | 21.1 (10.8-36.9) | 0.23 (0.09-0.59) | .002 | 0.23 (0.08-0.68) | .008 |
Abbreviations: NA, not applicable; WHO, World Health Organization.
Clinical usefulness was determined through review of ratings from Prescrire International, the French drug industry watchdog. If drugs were not evaluated by Prescrire, they were categorized as “judgment reserved.” If the drugs were categorized by Prescrire as “possibly helpful,” “offers an advantage,” “a real advance,” or “bravo,” they were grouped together as “clinically useful.” If the drugs were categorized by Prescrire as “nothing new” or “not acceptable,” they were grouped together as “clinically not useful.”
Timing of New Formulations Relative to Generics
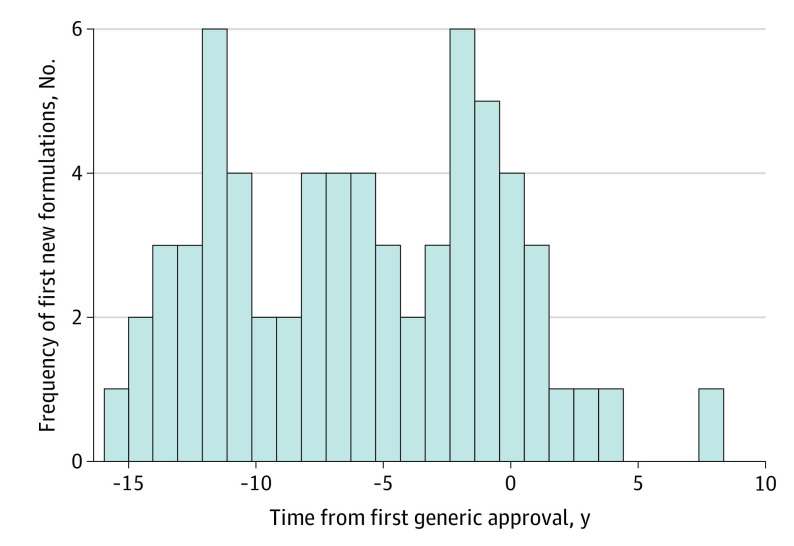
Among the 65 novel drugs with at least 1 new formulation and a generic, 55 (84.6%) new formulations were approved before the novel drug’s first generic approval and 10 (15.4%) were approved after generic approval (Figure 2). First new formulations were less likely to be approved after the novel drug’s first generic approval compared with before generic approval (χ2 = 31.2; P < .001). Results from the additional timing analysis in different time windows relative to the first generic approval are reported in eMethods in the Supplement.
Figure 2. Timing of Novel Tablet and Capsule First New Formulation Approved by the FDA Relative to Generic Drug Approval (n = 65).
FDA indicates US Food and Drug Administration.
Additional Analyses
In multivariable analysis, drugs considered essential medicines were more likely to be blockbuster drugs (AOR, 4.94; 95% CI, 1.91-12.76; P = .001). Blockbuster status was not associated with accelerated approval status (AOR, 0.82; 95% CI, 0.24-2.80; P = .76), first-in-class or advance-in-class status (AOR, 1.37; 95% CI, 0.67-2.82; P = .39), or categorization as clinically useful based on Prescrire ratings (AOR, 0.93; 95% CI, 0.42-2.06; P = .86). Results from bivariate analyses were similar (eTable 3 in the Supplement). Measures of therapeutic value were statistically significantly correlated with each other (eTable 4 in the Supplement).
Discussion
In this analysis of 206 novel drugs approved in tablet or capsule form by the FDA from 1995 to 2010, one-third had at least 1 new formulation approved through December 2021, with blockbuster drugs 4 times more likely and drugs granted accelerated approval 5.5 times more likely to have a new formulation. Other measures of therapeutic value, however, were not associated with an increased likelihood of a new formulation. Additionally, there was a sharp decrease in new formulation approvals after the first generic’s approval. Taken together, these results suggest that revenue is a substantial driver of whether and when a manufacturer secures FDA approval of the first new formulation of existing drugs, reinforcing concerns that manufacturers are using evergreening strategies to maintain revenue and avoid generic competition.
These findings are supported by a recent report, which found that top-selling novel drugs were twice as likely to be followed by a new formulation.14 The present study advances this literature by also examining measures of novel drugs’ therapeutic value to determine whether products of greatest importance to patients are being formulated again to improve convenience or tolerability. With the exception of accelerated approval status, no measure of therapeutic value, including innovativeness and Prescrire rating, was associated with approval of a new formulation. This may, in part, reflect the considerable number of novel drugs in this study that are similar to existing drugs within a therapeutic class. Furthermore, because drugs that are granted accelerated approval are most commonly indicated to treat cancer,40 it is perhaps unsurprising that these drugs were more likely to be formulated again to improve patient convenience or tolerability.
The analyses of new formulations’ approval timing suggest manufacturers time them strategically, particularly relative to the first generic approval. Once specific drug markets face generic competition, new formulations are far less common, indicating a possible diminishment of manufacturers’ ability or interest to continue incremental innovation. Another study examining new formulations based on new indications found that 32% of drugs had a new indication approved prior to generic entry, but such approvals declined afterward.41 The present findings of a concentration of new formulation approvals in the period leading up to first generic approval are consistent with previous research on the variety of evergreening strategies used by manufacturers to impede generic competition.15,23,42
New formulations have important consequences for patient access, clinical care, and health care finances. For patients, convenience or clinical benefit from a new formulation may be diminished by its expense compared with the novel drug’s generic version, which has implications for cost-related nonadherence.43,44 In the case of the antiepileptic drug levetiracetam, for example, the brand-name manufacturer received FDA approval for an extended-release version on September 12, 2008, before the first levetiracetam immediate-release generic version was FDA approved on November 4, 2008. A recent study19 found that Medicaid spent nearly $130 million between 2008 and 2016 on extended-release levetiracetam, despite therapeutic equivalence with the immediate-release version. In 2017 alone, Medicare Part D and Medicaid could have saved up to $2.6 billion by switching patients from extended-release formulations to therapeutically comparable immediate-release generics.8 Such examples of new formulations may have unclear patient adherence benefits45 and yet place financial strain on patients and the health care system.46 This strain may be compounded if the novel drug itself is not particularly therapeutically valuable.
This study has implications for prescription drug pricing reform, patent law, and other incentives for pharmaceutical innovation. Much attention, including in a recent letter from the FDA to the US Patent and Trademark Office47 and a US Department of Health and Human Services report,48 has been focused on the issue of strategically timed new formulations and their effect on generic competition and drug prices. The present findings highlight the importance of reforming incentives to encourage more meaningful pharmaceutical innovation. In particular, to minimize new formulations of novel drugs that do not offer therapeutic value, one approach is to align the duration of patent and regulatory exclusivities for both the novel drug and all of its new formulations with their value to patients and effect on public health.49 Greater duration of market exclusivity can potentially motivate subsequent innovation, but much of this innovation may be “me too” drugs that are not meaningful clinical advancements.50 Although we only examined therapeutic value of the novel drug in this study, the findings also underscore the need for reform of incentives for incremental innovation. For example, small changes to pharmaceutical formulations could be deemed legally insufficient to warrant new patents, or these patents could be subjected to heightened antitrust scrutiny.51
Limitations
This study has limitations. First, we did not assess the potential value of new formulations, including differences in value between novel drugs and their new formulations, an important direction for future research. The research design, in which the presence of a new formulation is an outcome and not all novel drugs were followed by a new formulation, precludes an analysis of the difference between novel drugs and their new formulations.
Second, we are unable to account for the potential deterrence effect of a new formulation’s approval on the novel drug’s first generic’s approval. After a new formulation’s approval, generic manufacturers may shift their efforts to the new formulation.
Third, examining novel drugs approved through 2010 in this study meant that the minimum follow-up period of 11 years may have underestimated the number of new formulations and generics ultimately approved. However, only 6 of 206 (2.9%) novel drugs in the sample were approved in 2010. Thus, most drugs were followed for 12 or more years, which is the average duration of market exclusivity periods.52,53
Fourth, we only examined novel drugs’ blockbuster status, and we were unable to estimate associations with more granular degrees of drug sales. Finally, the analysis used data on regulatory drug approvals, including for generic versions, which does not necessarily indicate that the generic drug was marketed. However, this likely applies to few, if any, drugs in the sample because we excluded novel drugs with ongoing patents and exclusivity or litigation in the timing analysis.
Conclusions
In this cross-sectional study of novel drugs approved by the FDA between 1995 and 2010, blockbuster drugs and those granted accelerated approval were more likely to be formulated again, but other measures of therapeutic value of the novel drug were not associated with new formulation approvals. Subsequent approval of a first new formulation was statistically significantly lower after the novel drug’s first generic approval. This study reinforces concerns that manufacturers are using evergreening strategies to maintain revenue and avoid generic competition. It suggests that policy makers should consider the role of new formulations more carefully to incentivize therapeutically valuable innovation and minimize strategies to avoid generic competition.
eFigure 1. Drug Sample Selection
eTable 1. Novel Drug Therapeutic Area Classification
eFigure 2. Proportion of Novel Tablets and Capsules Approved by the US FDA From 1995 to 2010 with New Formulation Approvals, Overall and According to Blockbuster Status, Accelerated Approval, WHO Essential Medicine, Orphan Drug Status, Innovation Status, Clinical Usefulness, Therapeutic Area, and Approval Year
eTable 2. Measures of Therapeutic Value of Novel Tablets and Capsules Approved by the US FDA From 1995 to 2010, Including Accelerated Approval, WHO Essential Medicine, Innovation Status, and Clinical Usefulness
eMethods. Additional New Formulation Approval Timing Analysis With Time Windows Relative to Novel Drug’s First Generic Approval
eTable 3. Correlation Between Blockbuster Status and Measures of Therapeutic Value of Novel Tablets and Capsules Approved by the US FDA From 1995 to 2010, Including Accelerated Approval, WHO Essential Medicine, Innovation Status, and Clinical Usefulness
eTable 4. Correlation Between Measures of Therapeutic Value of Novel Tablets and Capsules Approved by the US FDA From 1995 to 2010, Including Accelerated Approval, WHO Essential Medicine, Innovation Status, and Clinical Usefulness
References
- 1.Hitchings AW, Baker EH, Khong TK. Making medicines evergreen. BMJ. 2012;345:e7941. doi: 10.1136/bmj.e7941 [DOI] [PubMed] [Google Scholar]
- 2.Riedel M, Schmitz M, Østergaard PK, et al. Comparison of the effects of quetiapine extended-release and quetiapine immediate-release on cognitive performance, sedation and patient satisfaction in patients with schizophrenia: a randomised, double-blind, crossover study (eXtRa). Schizophr Res. 2015;162(1-3):162-168. doi: 10.1016/j.schres.2014.12.027 [DOI] [PubMed] [Google Scholar]
- 3.Gottwald-Hostalek U, Sun N, Barho C, Hildemann S. Management of hypertension with a fixed-dose (single-pill) combination of bisoprolol and amlodipine. Clin Pharmacol Drug Dev. 2017;6(1):9-18. doi: 10.1002/cpdd.309 [DOI] [PubMed] [Google Scholar]
- 4.Pan F, Chernew ME, Fendrick AM. Impact of fixed-dose combination drugs on adherence to prescription medications. J Gen Intern Med. 2008;23(5):611-614. doi: 10.1007/s11606-008-0544-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15(6):e22-e33. [PubMed] [Google Scholar]
- 6.Tarry-Adkins JL, Grant ID, Ozanne SE, Reynolds RM, Aiken CE. Efficacy and side effect profile of different formulations of metformin: a systematic review and meta-analysis. Diabetes Ther. 2021;12(7):1901-1914. doi: 10.1007/s13300-021-01058-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gellad WF, Choi P, Mizah M, Good CB, Kesselheim AS. Assessing the chiral switch: approval and use of single-enantiomer drugs, 2001 to 2011. Am J Manag Care. 2014;20(3):e90-e97. [PubMed] [Google Scholar]
- 8.Sumarsono A, Sumarsono N, Das SR, Vaduganathan M, Agrawal D, Pandey A. Economic burden associated with extended-release vs immediate-release drug formulations among Medicare Part D and Medicaid beneficiaries. JAMA Netw Open. 2020;3(2):e200181. doi: 10.1001/jamanetworkopen.2020.0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta R, Shah ND, Ross JS. Generic drugs in the United States: policies to address pricing and competition. Clin Pharmacol Ther. 2019;105(2):329-337. doi: 10.1002/cpt.1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemphill CS, Sampat BN. Evergreening, patent challenges, and effective market life in pharmaceuticals. J Health Econ. 2012;31(2):327-339. doi: 10.1016/j.jhealeco.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 11.Feldman R. May your drug price be evergreen. J Law Biosci. 2018;5(3):590-647. doi: 10.1093/jlb/lsy022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapczynski A, Park C, Sampat B. Polymorphs and prodrugs and salts (oh my!): an empirical analysis of “secondary” pharmaceutical patents. PLoS One. 2012;7(12):e49470. doi: 10.1371/journal.pone.0049470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobo-Rubio R. Essays on the US pharmaceutical industry (1984-2013). University of Georgia . 2014. Accessed April 20, 2022. https://getd.libs.uga.edu/pdfs/jacobo-rubio_ruben_201408_phd.pdf
- 14.Fowler AC. Hurry up or wait? strategic delay in the introduction of pharmaceutical line extensions. Harvard University . December 23, 2019. Accessed April 20, 2022. https://scholar.harvard.edu/files/afowler/files/Fowler_JMP.pdf
- 15.Carrier MA, Shadowen S. Pharmaceutical product hopping: a proposed framework for antitrust analysis. HealthAffairs . June 1, 2017. Accessed April 20, 2022. https://www.healthaffairs.org/do/10.1377/hblog20170601.060360/full/
- 16.Rome BN, Tessema FA, Kesselheim AS. US spending associated with transition from daily to 3-times-weekly glatiramer acetate. JAMA Intern Med. 2020;180(9):1165-1172. doi: 10.1001/jamainternmed.2020.2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Downing NS, Ross JS, Jackevicius CA, Krumholz HM. Avoidance of generic competition by Abbott Laboratories’ fenofibrate franchise. Arch Intern Med. 2012;172(9):724-730. doi: 10.1001/archinternmed.2012.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro BT. Estimating the cost of strategic entry delay in pharmaceuticals: the case of Ambien CR. Quant Marketing Econ. 2016;14:201-231. doi: 10.1007/s11129-016-9170-9 [DOI] [Google Scholar]
- 19.Dickson S. Effect of evergreened reformulations on Medicaid expenditures and patient access from 2008 to 2016. J Manag Care Spec Pharm. 2019;25(7):780-792. doi: 10.18553/jmcp.2019.18366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacks CA, Lee CC, Kesselheim AS, Avorn J. Medicare spending on brand-name combination medications vs their generic constituents. JAMA. 2018;320(7):650-656. doi: 10.1001/jama.2018.11439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakim A, Ross JS. High prices for drugs with generic alternatives: the curious case of duexis. JAMA Intern Med. 2017;177(3):305-306. doi: 10.1001/jamainternmed.2016.8423 [DOI] [PubMed] [Google Scholar]
- 22.Schwartz LM, Woloshin S. How the FDA forgot the evidence: the case of donepezil 23 mg. BMJ. 2012;344:e1086. doi: 10.1136/bmj.e1086 [DOI] [PubMed] [Google Scholar]
- 23.Carrier MA, Shadowen SD. Product hopping: a new framework. Notre Dame Law Rev. 2016;92(1):167-230. [Google Scholar]
- 24.Beall RF, Kesselheim AS, Sarpatwari A. New drug formulations and their respective generic entry dates. J Manag Care Spec Pharm. 2019;25(2):218-224. doi: 10.18553/jmcp.2019.25.2.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao J, Rodriguez-Monguio R, Seoane-Vazquez E. Fixed-dose combination drug approvals, patents and market exclusivities compared to single active ingredient pharmaceuticals. PLoS One. 2015;10(10):e0140708. doi: 10.1371/journal.pone.0140708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Udelson JE, Pressler SJ, Sackner-Bernstein J, et al. Adherence with once daily versus twice daily carvedilol in patients with heart failure: the Compliance And Quality of Life Study Comparing Once-Daily Controlled-Release Carvedilol CR and Twice-Daily Immediate-Release Carvedilol IR in Patients with Heart Failure (CASPER) Trial. J Card Fail. 2009;15(5):385-393. doi: 10.1016/j.cardfail.2008.12.010 [DOI] [PubMed] [Google Scholar]
- 27.Beckebaum S, Iacob S, Sweid D, et al. Efficacy, safety, and immunosuppressant adherence in stable liver transplant patients converted from a twice-daily tacrolimus-based regimen to once-daily tacrolimus extended-release formulation. Transpl Int. 2011;24(7):666-675. doi: 10.1111/j.1432-2277.2011.01254.x [DOI] [PubMed] [Google Scholar]
- 28.Strohbehn GW, Kacew AJ, Goldstein DA, Feldman RC, Ratain MJ. Combination therapy patents: a new front in evergreening. Nat Biotechnol. 2021;39(12):1504-1510. doi: 10.1038/s41587-021-01137-6 [DOI] [PubMed] [Google Scholar]
- 29.FDA listing of authorized generics. US Food and Drug Administration . Accessed April 20, 2022. https://www.fda.gov/media/77725/download
- 30.Berghauser Pont L, Keirsse J, Moss R, Poda P, Robke L, Wurzer S. Developing blockbuster drugs: both nature and nurture. Nat Rev Drug Discov. 2021;20(6):421-422. doi: 10.1038/d41573-020-00061-9 [DOI] [PubMed] [Google Scholar]
- 31.Greenway T, Ross JS. US drug marketing: how does promotion correspond with health value? BMJ. 2017;357:j1855. doi: 10.1136/bmj.j1855 [DOI] [PubMed] [Google Scholar]
- 32.Accelerated approval program. US Food and Drug Administration . Updated October 26, 2020. Accessed April 20, 2022. https://www.fda.gov/drugs/information-health-care-professionals-drugs/accelerated-approval-program/
- 33.WHO model lists of essential medicines. World Health Organization . Accessed April 20, 2022. https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists/
- 34.Lanthier M, Miller KL, Nardinelli C, Woodcock J. An improved approach to measuring drug innovation finds steady rates of first-in-class pharmaceuticals, 1987-2011. Health Aff (Millwood). 2013;32(8):1433-1439. doi: 10.1377/hlthaff.2012.0541 [DOI] [PubMed] [Google Scholar]
- 35.Hwang TJ, Ross JS, Vokinger KN, Kesselheim AS. Association between FDA and EMA expedited approval programs and therapeutic value of new medicines: retrospective cohort study. BMJ. 2020;371:m3434. doi: 10.1136/bmj.m3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prescrire International. Accessed April 20, 2022. https://english.prescrire.org/en/Summary.aspx/
- 37.Designating an orphan product: drugs and biological products. US Food and Drug Administration . Updated September 7, 2021. Accessed April 20, 2022. https://www.fda.gov/industry/developing-products-rare-diseases-conditions/designating-orphan-product-drugs-and-biological-products/
- 38.Search orphan drug designations and approvals. US Food and Drug Administration . Accessed April 20, 2022. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/
- 39.Anatomical therapeutic chemical (ATC) classification. World Health Organization . Accessed April 20, 2022. https://www.who.int/tools/atc-ddd-toolkit/atc-classification/
- 40.Hwang TJ, Franklin JM, Chen CT, et al. Efficacy, safety, and regulatory approval of Food and Drug Administration–designated breakthrough and nonbreakthrough cancer medicines. J Clin Oncol. 2018;36(18):1805-1812. doi: 10.1200/JCO.2017.77.1592 [DOI] [PubMed] [Google Scholar]
- 41.Sahragardjoonegani B, Beall RF, Kesselheim AS, Hollis A. Repurposing existing drugs for new uses: a cohort study of the frequency of FDA-granted new indication exclusivities since 1997. J Pharm Policy Pract. 2021;14(1):3. doi: 10.1186/s40545-020-00282-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellison G, Ellison SF. Strategic entry deterrence and the behavior of pharmaceutical incumbents prior to patent expiration. National Bureau of Economic Research working paper 13069. April 2007. Accessed April 20, 2022. http://papers.nber.org/papers/w13069
- 43.Gaffney A, Bor DH, Himmelstein DU, Woolhandler S, McCormick D. The effect of Veterans Health Administration coverage on cost-related medication nonadherence. Health Aff (Millwood). 2020;39(1):33-40. doi: 10.1377/hlthaff.2019.00481 [DOI] [PubMed] [Google Scholar]
- 44.Madden JM, Graves AJ, Zhang F, et al. Cost-related medication nonadherence and spending on basic needs following implementation of Medicare Part D. JAMA. 2008;299(16):1922-1928. doi: 10.1001/jama.299.16.1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterson AM, Takiya L, Finley R. Meta-analysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm. 2003;60(7):657-665. doi: 10.1093/ajhp/60.7.657 [DOI] [PubMed] [Google Scholar]
- 46.Hwang TJ, Feng J, Maini L, Kesselheim AS. Medicaid expenditures and estimated rebates on line extension drugs, 2010-2018. J Gen Intern Med. 2022. doi: 10.1007/s11606-022-07435-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodcock J. Performing the functions and duties of the Under Secretary of Commerce for Intellectual Property and Director of the United States Patent and Trademark Office. US Food and Drug Administration . September 10, 2021. Accessed April 20, 2022. https://www.fda.gov/media/152086/download
- 48.Becerra X. Comprehensive plan for addressing high drug prices: a report in response to the Executive Order on competition in the American economy. US Department of Health and Human Services . September 9, 2021. Accessed April 20, 2022. https://aspe.hhs.gov/sites/default/files/2021-09/Competition%20EO%2045-Day%20Drug%20Pricing%20Report%209-8-2021.pdf
- 49.Beall RF, Hollis A, Kesselheim AS, Spackman E. Reimagining pharmaceutical market exclusivities: should the duration of guaranteed monopoly periods be value based? Value Health. 2021;24(9):1328-1334. doi: 10.1016/j.jval.2021.04.1277 [DOI] [PubMed] [Google Scholar]
- 50.Gilchrist DS. Patents as a spur to subsequent innovation? evidence from pharmaceuticals. Am Econ J Appl Econ. 2016;8(4):189-221. doi: 10.1257/app.20150373 [DOI] [Google Scholar]
- 51.Affordable Prescriptions for Patients Act of 2021, S 1435, 117th Cong, 1st Sess (2021). Accessed April 20, 2022. https://www.congress.gov/bill/117th-congress/senate-bill/1435/text/
- 52.Wang B, Liu J, Kesselheim AS. Variations in time of market exclusivity among top-selling prescription drugs in the United States. JAMA Intern Med. 2015;175(4):635-637. doi: 10.1001/jamainternmed.2014.7968 [DOI] [PubMed] [Google Scholar]
- 53.Grabowski H, Long G, Mortimer R, Boyo A. Updated trends in US brand-name and generic drug competition. J Med Econ. 2016;19(9):836-844. doi: 10.1080/13696998.2016.1176578 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Drug Sample Selection
eTable 1. Novel Drug Therapeutic Area Classification
eFigure 2. Proportion of Novel Tablets and Capsules Approved by the US FDA From 1995 to 2010 with New Formulation Approvals, Overall and According to Blockbuster Status, Accelerated Approval, WHO Essential Medicine, Orphan Drug Status, Innovation Status, Clinical Usefulness, Therapeutic Area, and Approval Year
eTable 2. Measures of Therapeutic Value of Novel Tablets and Capsules Approved by the US FDA From 1995 to 2010, Including Accelerated Approval, WHO Essential Medicine, Innovation Status, and Clinical Usefulness
eMethods. Additional New Formulation Approval Timing Analysis With Time Windows Relative to Novel Drug’s First Generic Approval
eTable 3. Correlation Between Blockbuster Status and Measures of Therapeutic Value of Novel Tablets and Capsules Approved by the US FDA From 1995 to 2010, Including Accelerated Approval, WHO Essential Medicine, Innovation Status, and Clinical Usefulness
eTable 4. Correlation Between Measures of Therapeutic Value of Novel Tablets and Capsules Approved by the US FDA From 1995 to 2010, Including Accelerated Approval, WHO Essential Medicine, Innovation Status, and Clinical Usefulness