Abstract
Purpose
The purpose of this study was to explore the therapeutic effect of human umbilical cord mesenchymal stem cell (HUMSC) transplantation alone or assisted with ultrasound targeted microbubble destruction (UTMD) on optic neuropathy in a novel and practical model of experimental glaucoma in rabbits.
Methods
Eight New Zealand white healthy rabbits were used as the control group (group A). Twenty-four experimental glaucomatous rabbits were established as described previously and randomly divided into three groups: (1) received no treatment (group B); (2) received intravitreal transplantation of HUMSCs (group C); and (3) received UTMD-assisted intravitreal transplantation of HUMSCs (group D). After 4 weeks of treatment, the distribution of HUMSCs, retinal thickness, layer structure, retinal ganglion cells (RGCs), and their axons were examined.
Results
After 4 weeks of treatment, HUMSCs were successfully scattered under the retina. HUMSC transplantation significantly increased the regeneration of RGCs and their axons, and restored the retinal structure in glaucomatous rabbits. Moreover, the application of UTMD enhances HUMSC distribution and achieved more significant therapeutic effect.
Conclusions
Intravitreal transplantation of HUMSCs effectively repaired glaucomatous optic nerve injury, and UTMD enhanced the successful delivery of HUMSCs into injured retina, promoting its therapeutic effects remarkably.
Translational Relevance
This study demonstrated that HUMSC transplantation repaired the glaucoma-caused nerve injury significantly and the combination of UTMD can augment the therapeutic effect further, which has important clinical guiding significance for the development of therapeutic strategies of glaucoma.
Keywords: human umbilical cord mesenchymal stem cell (HUMSC), mesenchymal stem cells (MSCs), retinal ganglion cells (RGCs), ultrasound targeted microbubble destruction (UTMD)
Introduction
Glaucoma is a group of neurodegenerative diseases mainly caused by pathologic elevation of intraocular pressure.1-3 which is accompanied with the progressive loss of retinal ganglion cells (RGCs) and their axons, eventually resulting in optic atrophy and even blindness without proper and timely therapy.4 Glaucomatous optic nerve injury cannot be completely prevented through reducing intraocular pressure (IOP) and there are no effective therapeutic strategies for the injury until the development of stem cell-based cellular therapy in regenerative medicine, which provides new hope for the treatment of glaucoma.
Mesenchymal stem cells have the potential of multidirectional differentiation, among which human umbilical cord mesenchymal stem cells (HUMSCs) can be obtained directly from the fetal umbilical cord blood, which guarantees the sufficient number of cells. In addition, the low immunogenicity of mesenchymal stem cells effectively alleviated the formidable graft rejection problem of transplantation, which makes it a promising candidate for cell therapy.5,6 Moreover, mesenchymal stem cells (MSCs) and their secretome have evolved as new therapeutic agents for glaucoma treatment.7 MSC-derived exosomes can remarkably promote survival and regeneration of damaged RGCs caused by glaucoma and attenuate retinal inflammation through releasing trophic and immunomodulatory factors.8 MSCs can also repopulate RGCs through generating RGC-like cells and through facilitating proliferation and differentiation of residential retinal stem cells.9,10 Rui Liu et al. showed that vitreous injection of human umbilical cord MSCs have provided a protection for RGCs in an acute ocular hypertension mouse model.11 However, considering the less permeability of cells compared to other therapeutic agents, such as small molecules and proteins, how to enhance the transplantation efficiency of MSCs remains to be explored. In addition, an appropriate and practical glaucoma model is also critical for the evaluation of the therapeutic efficiency of treatments.
Ultrasound targeted microbubble destruction (UTMD) can increase the permeability of cell membranes localized to the site of microbubble ultrasound interaction, resulting in the deposition of microbubble shell components.12 In the past decade, UTMD has evolved as a promising strategy to enhance target-specific gene delivery, thus improving the effect of therapy, and it has been successfully evaluated for the treatment of many diseases.13 Meanwhile, it also provides a novel approach for gene therapy of ophthalmic diseases.14 However, it remains unclear whether this technique can also be applied to enhance the efficiency of MSC transplantation and improve its repair effect on glaucoma-induced optic nerve injury.
In the present study, a new and practical glaucomatous rabbit model without observable ocular damage or inflammatory response was established through intracameral injections of fluorescent microsphere, as described previously.15 Then, PKH-26 labeled HUMSCs were transplanted into the vitreous cavity of glaucomatous rabbits alone or assisted with UTMD in order to investigate the repair effect of HUMSC transplantation on glaucomatous optic nerve injury and the influence of UTMD on therapeutic efficacy. The results demonstrated that HUMSC transplantation repaired the nerve injury caused by glaucoma significantly, and moreover the combination of UTMD can augment the therapeutic effect further. This study indicates the repair effect of HUMSC transplantation on glaucoma-caused nerve injury with a practical rabbit model and provides a strategy to further enhance the therapeutic effect, which has important clinical guiding significance.
Methods
Culture and Labeling of HUMSCs
HUMSCs were purchased from American Type Culture Collection (ATCC) and cultured with Dulbecco's modified Eagle's medium (DMEM) medium with 10% fetal bovine serum (FBS). After digested, 2 × 107 cells were collected and washed with serum-free medium. Then, the cells were resuspended with 1 mL diluent C to ensure complete dispersion of cells. PKH-26 dye solution was placed in a new centrifuge tube, and HUMSCs were added to the dye solution and immediately mixed evenly and quickly. After incubation for 2 to 5 minutes, 2 mL serum was added and incubated with HUMSCs for 1 minute to stop the staining reaction. Cells were washed three times with culture medium and appropriate volumes of phosphate-buffered saline (PBS) were added to resuspend the cells to the concentration of 1 × 105 cells /mL.
Animal Study
Forty-eight male New Zealand white rabbits were included in this study and the experimental rabbit model of glaucoma was successfully established, as described previously,15 through 2 intracameral injection of 50 µL microbead (5 × 106 beads/mL) at day 0 and day 21 to induce IOP elevation. Eight healthy rabbits were used as control group (group A). Twenty-four experimental glaucomatous rabbits were randomly divided into 3 groups with 8 rabbits in each group and received following 3 treatments: (1) no treatment; (2) HUMSC transplantation through intravitreal delivery; and (3) UTMD-assisted HUMSC transplantation. After 4 weeks of treatment, the distribution of PKH-26-labeled HUMSCs in the retina was tracked through immunofluorescence analysis, and the repair of the retina was evaluated. All animal experiments in this study complied with the Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of Shenzhen Eye Hospital Affiliated to Jinan University.
Intravitreal Injection of HUMSCs in Rabbits
Rabbits were anesthetized generally through intramuscular injection of 0.2 mL/ kg xylazine hydrochloride, supplemented with tetracaine hydrochloride eye drops for local topical anesthesia. Compound tropicamide eye drops were administered to fully dilate the pupils. Eyes were disinfected first and covered with a sterile sheet before the surgery. After opening the eyelid, the conjunctival sac was rinsed with 1% iodine volt. Under the eye surgery microscope, a microsyringe (30 G, 50 µL) was inserted vertically into the sclera at the 12 o'clock position, 3 mm behind the angular sclera margin, and then punctured into the vitreous cavity. After the needle tip was clearly placed in the vitreous cavity through the dilated pupil, 50 µL HUMSC suspension and 50 µL ultrasonic microbubbles were injected slowly and continuously. The coupling agent was evenly coated after the rabbit eye was closed. The ultrasonic probe was lightly placed over the rabbit eye, and the eyeball was immediately irradiated with a frequency of 1 MHz and a sound intensity of 0.5 W /cm2 for 60 seconds, as described previously.16
HUMSC Tracking Through Immunofluorescence Assay
Rabbits were euthanized 4 weeks after the transplantation of HUMSCs into the ocular cavity. After the eyeball was extracted, the retinal tissue was obtained quickly and embodied with optimal cutting temperature compound (OCT). The frozen sections with a thickness of 10 µm were prepared, fixed with 75% alcohol, and stained with 4′,6-diamidino-2-phenylindole (DAPI). The distribution of HUMSCs in the retina was observed under the fluorescence microscope.
Detection and Counting of RGCs and the Optic Nerve Through Light Microscope
Rabbits were euthanized 4 weeks after the vitreous cavity transplantation of HUMSCs. The retina and optic nerve were immediately obtained and fixed in 4% paraformaldehyde for 24 hours. The optic nerve was excised 1.5 mm posterior to the eyeball and cross-section slices were constructed. The optic papilla and retina were excised at the 6 and 12 o'clock sectors relative to the optic disc. After paraffin embedding and hematoxylin and eosin (H&E) staining, the tissues were observed and images were acquired through the microscope with the 40 × magnification. The area of the transverse section of the optic nerve and the number of RGCs in the range of 5 mm and 10 mm on both sides of the optic papilla were measured with the image measuring system (Image Pro-Plus 6.0). Although we used samples in the same location of the eyeballs in different groups, the present analysis is limited. Ideally, RGC numbers should be done using wholemounts, as the death can be patchy, and non-uniform.
Detection and Counting of RGC Axons and Optic Nerve Through Transmission Electron Microscope
Rabbits were euthanized 4 weeks after the vitreous cavity transplantation of HUMSCs. The eyeball was isolated and the eye wall was cut open along the edge of the cornea. The optic nerve was obtained immediately after removing the cornea, crystal, and vitreous body. After fixed in 2.5% glutaraldehyde phosphate buffer for 2 hours, the optic nerve was then fixed with 1% osmic acid, dehydrated with acetone step by step, and embedded with EPon812 epoxy resin. Ultrathin slices were made after positioning semithin sections with the thickness of 1 µm by the light microscope. After stained with uranium acetate and lead citrate, ultrathin slices of the optic nerve were observed with the transmission electron microscopy. Twelve standard rectangular regions (246 µm2) were randomly selected from each image with the magnification of 5800 ×. All axons in the images were counted and the average density of the axons in the 12 selected regions was calculated with the Image-Pro Plus 6.0. The percentage of RGC axon loss was obtained through calculating the ratio of the number of RGC axons in each group to that in the contralateral eye.
Statistical Analysis
SPSS version 20.0 statistical software was used for statistical analysis. The data of the measured indicators in this study were normally distributed by Shapiro-Wilk test and represented as mean ± SD. The experimental design of rabbit grouping was completely randomized. One-way ANOVA was used to compare the overall differences in RGC count, retinal thickness change, RGC axon count, and GFAP expression in the retinal tissue of each group. Pair comparison between groups was tested by the Student-Newman-Keuls (SNK) method, and P ≤ 0.05 was considered statistically significant.
Results
HUMSCs were Successfully Delivered to Retina and Ultrasound-Driven Microbubbles Enhanced the Delivery Efficiency of HUMSCs
The experimental rabbit model of glaucoma was successfully established, as described previously,15 after 2 intracameral injections of 50 µL microbeads. The peak IOP was 23.80 ± 1.14 mm Hg in the glaucomatous group, which was significantly higher than that in the control group (13.60 ± 0.90 mm Hg) and maintained for at least 8 weeks.
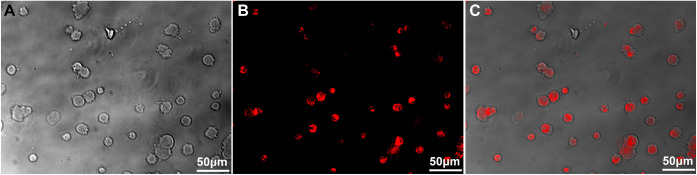
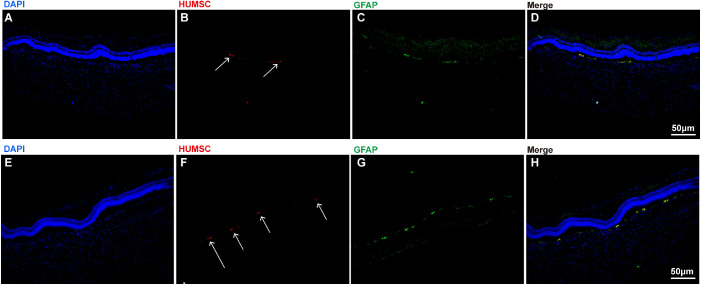
HUMSCs were cultured with DMEM medium with 10% FBS. Then, the 2 × 107 cells were digested and resuspended to be stained with PKH-26 dye solution. As observed under fluorescence microscope, the digested HUMSCs were round and the majority of the cells were stained with orange red uniformly and stably (Figs. 1A–C). Then PKH-26 labeled HUMSCs were injected intravitreally into glaucomatous rabbits alone or assisted with UTMD. Four weeks later, the HUMSC distribution and GFAP expression were assessed with the fluorescence microscope. It was observed that transplanted HUMSCs were clustered in the retina and showed GFAP positive expression. No inflammatory cell invasion or tumor formation was observed after HUMSC transplantation (Figs. 2A–D). Moreover, compared with group C, where HUMSCs were injected alone, significantly more HUMSCs were integrated into the retinal tissue when HUMSCs were combined with UTMD in group D (Figs. 2E–H). The quantification result indicating the number of GFAP+ PKH26+ cells in the images was shown in Figure 3, which suggests that the combination of UTMD technology can significantly enhance the transplantation efficiency of HUMSCs.
Figure 1.
Cell morphology and staining efficiency of PKH-26 labeled HUMSCs. (A) Cell morphology of suspension HUMSCs after digestion. (B) The staining results of PKH-26 labeled HUMSCs. (C) The merged image of A and B. Scale bar, 50 µm.
Figure 2.
The retinal distribution and GFAP expression of transplanted HUMSCs in glaucomatous rabbits treated with HUMSC transplantation alone (A, B, C, D) or assisted with UTMD (E, F, G, H). A The structure of retinal tissue stained with DAPI (blue) in rabbits. B HUMSCs labeled with PKH-26 (orange red, directed by arrows). C The expression of GFAP in HUMSCs transplanted into the retina (green, directed by arrows). D The merged image of A to C. E The structure of retinal tissue stained with DAPI (blue) in rabbits. F HUMSCs labeled with PKH-26 (orange red, directed by arrows). G The expression of GFAP in HUMSCs transplanted into the retina (green, directed by arrows). H The merged image of E to G. HUMSCs were directed with arrows. Scale bar, 50 µm.
Figure 3.
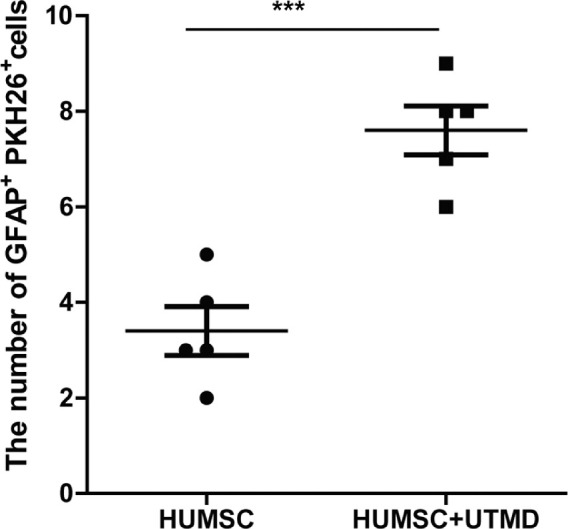
The quantification result showing the numbers of GFAP+ PKH26+ cells successfully transplanted into the retina in glaucomatous rabbits treated with HUMSC transplantation alone or assisted with UTMD.
HUMSC Transplantation Repaired the Retinal Structure Injured by Glaucoma
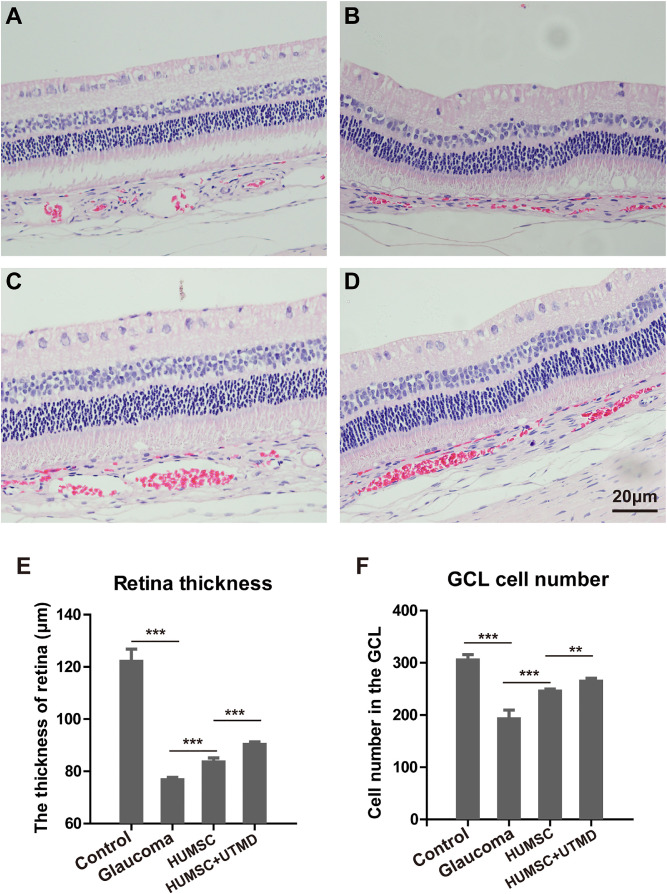
To investigate the effect of HUMSCs on retinal structure, retinas tissues were extracted from rabbits and H&E staining was performed. Under light microscope, it was observed that compared with the control group, the retinal layers of glaucomatous rabbits were disorganized, and the RGCs were sparsely arranged. The inner layer cells were thinner and less clear, and the outer layer cells and photoreceptor cells were disorganized (Figs. 4A, 4B). However, after the therapy of HUMSC transplantation, the histology of retinal layers (in groups C and D) appeared to be normal and exhibited relatively dense and orderly RGCs in the ganglion cell layer (GCL). The inner plexus layer was thicker and showed obvious reticular structure with larger nuclei and darker staining. The outer nuclear layer and photoreceptor cells were closely and neatly arranged (Figs. 4C, 4D). The above observations demonstrate that HUMSC transplantation repairs the retinal structure injured by glaucoma.
Figure 4.
The structure of retinal tissue with H&E staining in every group of rabbits. (A) The structure of retinal tissue in the control healthy rabbits. (B) The structure of retinal tissue in glaucomatous rabbits without treatment. (C) The structure of retinal tissue in glaucomatous rabbits with HUMSC transplantation. (D) The structure of retinal tissue in glaucomatous rabbits with UTMD-assisted HUMSC transplantation. Scale bar, 20 µm. The thickness of retina (E) and cell number in the GCL (F) in different groups of rabbits. Data were presented as mean values ±SD (error bars). *P < 0.05; **P < 0.01; ***P < 0.001.
HUMSCs Alleviated Glaucoma-Caused RGC Loss and Restored Retinal Thickness
RGCs are pivotal nervous retinal elements which connect the visual receptors to the brain forming the nervous visual system. Glaucoma is characterized by progressive loss of RGCs and their axons. Considering the RGCs are mostly located in the GCL, to evaluate the influence of HUMSCs on RGC regeneration, the cell number in the GCL and retinal thickness were examined and analyzed. As the results showed, the cell numbers in the GCL in the 4 groups were 302.50 ± 13.26, 189.67 ± 19.72, 245.25 ± 7.65, and 262.25 ± 8.18 /mm, respectively, and the corresponding retinal thickness were 121.63 ± 5.21, 76.23 ± 1.49, 83.13 ± 2.04, and 89.80 ± 1.51 µm, respectively (Figs. 4E, 4F). These results demonstrated that glaucoma led to a dramatic RGC loss and significant reduction of retinal thickness, whereas intravitreal injection of HUMSCs in rabbits could alleviate glaucoma-caused RGC loss and decrease of retinal thickness significantly, and moreover the combination of UTMD achieved a more efficient therapeutic effect.
HUMSCs Repaired the Optic Nerve Injury Caused by Glaucoma
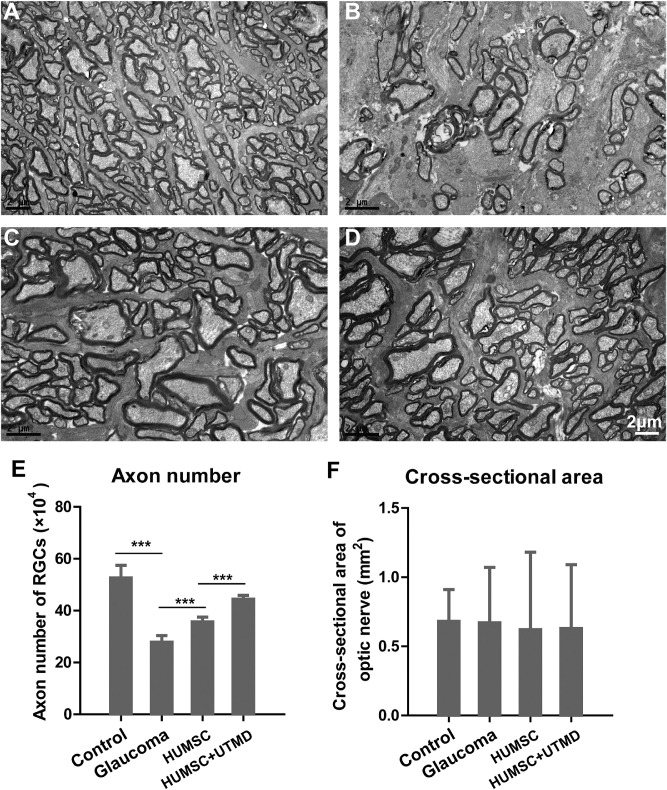
Next, we further evaluated the impact of HUMSCs on the optic nerve through the transmission electron microscope. It was observed that the axons of the optic nerve in healthy rabbits were regular, with intact myelin sheath, and organelles, such as the microtubules and microfilaments, can be clearly observed in the axoplasm (Fig. 5A). In glaucomatous rabbits (group B), the myelin of the optic nerve was dissolved and loose, accompanied with disordered axon structure. Microtubules and microfilaments disappeared, and mitochondria swelled significantly (Fig. 5B). However, after therapy of HUMSC transplantation, the myelin of the optic nerve in group C and group D restored integrity, and was closely arranged, but not orderly. The microtubules and microfilaments in axons can be observed in Figures 5C and 5D.
Figure 5.
The structure of optic nerves observed with transmission electron microscope in every group of rabbits. (A) The tissue structure of optic nerve in the control healthy rabbits. (B) The tissue structure of optic nerve in glaucomatous rabbits without treatment. (C) The tissue structure of optic nerve in glaucomatous rabbits with HUMSC transplantation. (D) The tissue structure of optic nerve in glaucomatous rabbits with UTMD-assisted HUMSC transplantation. Scale bar, 2 µm. The axon numbers of RGCs (E) and cross-sectional areas of the optic nerve (F) in different groups of rabbits. Data were presented as mean values ±SD (error bars). *P < 0.05; **P < 0.01; ***P < 0.001.
The axons of the RGC constitute the major element of the optic nerve and play prominent roles in conveying visual information from the retina to the brain. Therefore, we also evaluated the influence of HUMSC transplantation on RGC axon regeneration. RGC axons were counted through a transmission electron microscope. The axon numbers in groups A, B, C, and D were 52.65 ± 4.83 × 104, 27.82 ± 2.54 × 104, 35.68 ± 1.80 × 104, and 44.45 ± 1.46 × 104, respectively, and the differences between every 2 groups were all significant (Fig. 5E). These results showed that the occurrence of glaucoma led to remark the loss of axons, whereas HUMSC transplantation could alleviate the axon loss effectively. Furthermore, UTMD-assisted HUMSC injection could improve this therapeutic effect. However, the cross-sectional areas of the optic nerve in groups A, B, C, and D were 0.68 ± 0.23, 0.67 ± 0.40, 0.62 ± 0.56 and 0.63 ± 0.46, respectively, with no significant difference among groups (Fig. 5F).
Discussion
In the present study, UTMD-assisted intravitreal injection of HUMSCs was applied to the treatment of glaucoma-caused optic nerve injury in rabbits and significant therapeutic efficiency was achieved. Previous studies have shown that ultrasonic targeted microbubble was relatively safe in the treatment of fundus oculi disease without obvious damage to the retinal choroid tissue.17,18 which paved a new avenue for the experimental research and clinical practice of UTMD-assisted optic nerve protection in glaucoma.
HUMSCs are a type of adult stem cells with the potential of self-renewal, high proliferation, and multidirectional differentiation.19 In the past decade, breakthroughs have been made in stem cell research, making stem cell replacement and regenerative therapy among the most intensively studied areas.5 Glaucoma is a degenerative disease characterized by the progressive apoptosis of RGCs, so, theoretically, it can be treated through cell replacement therapy, especially stem cell therapy. Recently, increasing successful treatments of optic nerve injury by stem cell transplantation have been reported.20–22 These results pave the way for stem cell treatment of optic nerve damage in glaucoma.
In this study, we found that after 4 weeks of UTMD-assisted intravitreal injection of HUMSCs in the glaucomatous rabbits, PKH-26 labeled HUMSCs were scattered in the entire retinal tissue. Moreover, GFAP expression was observed in HUMSCs after migration, indicating that HUMSCs have certain retinal cell-like functions after transplantation. In addition, no obvious inflammatory cell infiltration or tumor formation was observed after HUMSC transplantation, demonstrating the safety of this therapy.
The loss of RGCs is the major pathological characteristics of glaucoma. The present study showed that RGCs in glaucomatous rabbits were significantly lost and the retinal thickness was significantly reduced compared with healthy rabbits, whereas intravitreal injection of HUMSCs increased the regeneration of RGCs and restored the retinal thickness, suggesting that HUMSC transplantation elicits repair effect for the high IOP induced optic nerve damage. Moreover, the combination of UTMD and HUMSC injection can further promote the RGC regeneration and increase the retinal thickness, indicating that the assistance of UTMD can remarkably enhance the therapeutic effect of HUMSCs cells for glaucomatous optic nerve injury.
Previous studies have reported that the elevation of IOP could interfere with the physiological function of normal cells in the axon of the optic nerve, resulting in the accumulation of intracellular material and the swelling and degeneration of axons.4 The present study showed that in the condition of high IOP-induced glaucoma, optic nerve axons were disorganized, myelin sheaths were dissolved and loosed to varying degrees, and microtubules and microfilaments disappeared. After the HUMSC transplantation, the myelin arrangement of the optic nerve became close and tidy, accompanied with the appearance of microtubules and microfilaments. Meanwhile, the number of RGC axons in glaucomatous rabbits was significantly lower than that in healthy rabbits, whereas HUMSC transplantation remarkably increased the number of axons. Furthermore, the combination of UTMD and HUMSC injection led to a much greater increase of RGC axons. The above results demonstrate that in addition to repairing RGCs, UTMD-assisted HUMSC transplantation can also exhibit repair effect on RGC axons damaged by high IOP.
Another observation in this study is the high expression of GFAP in transplanted HUMSCs. GFAP is a filamentous protein residing in the glial cells of the central nervous system.23 which is abundantly expressed in the condition of retinal ischemia, hypoxia, and other environmental stress.24,25 and its expression changes in neurodegenerative diseases have attracted more and more attention in recent years.26 This study showed that GFAP was obviously expressed in HUMSCs transplanted in the retinal tissue, indicating that HUMSCs have the capability of differentiating into retinoid cells and have remarkable repair effect on glaucoma-induced optic nerve damage in rabbits.
Compared with intravenous injection, the intravitreal administration allows HUMSCs to reach the eyes more directly and avoid immune rejection, so as to maximize the therapeutic effect. The present study combined UTMD technology and allowed HUMSCs to be successfully delivered into the vitreous cavity and migrate to the retina of the glaucomatous rabbits, which laid an experimental foundation for the future research on nerve regeneration and further treatment of optic nerve diseases. However, whether the HUMSCs could establish nerve conduction with the original retina and optic nerve after transplantation remains to be determined, which still needs further research and exploration.
Acknowledgments
Supported by the Science, Technology, and Innovation Commission of Shenzhen Municipality Grants (GJHZ 20180420180937076 and JCYJ20180228164400218), and the Sanming Project of Medicine in Shenzhen Grant (SZSM201812090).
Disclosure: T. Zhu, None; X. Huang, None; S. Peng, None; Y. Ye, None; J. Zhao, None
References
- 1. Quigley HA,Broman AT.. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90(3): 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kass MA, Heuer DK, Higginbotham EJ, et al.. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120(6): 701–713; discussion 829-830. [DOI] [PubMed] [Google Scholar]
- 3. Roh M, Zhang Y, Murakami Y, et al.. Etanercept, a widely used inhibitor of tumor necrosis factor-alpha (TNF-alpha), prevents retinal ganglion cell loss in a rat model of glaucoma. PLoS One . 2012; 7(7): e40065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fan BJ,Wiggs JL.. Glaucoma: genes, phenotypes, and new directions for therapy. J Clin Invest. 2010; 120(9): 3064–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ding DC, Chang YH, Shyu WC, et al.. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant . 2015; 24(3): 339–347. [DOI] [PubMed] [Google Scholar]
- 6. Kong L, Xu X, Zhang H, et al.. Human umbilical cord-derived mesenchymal stem cells improve chronic pancreatitis in rats via the AKT-mTOR-S6K1 signaling pathway. Bioengineered. 2021; 12(1): 1986–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harrell CR, Fellabaum C, Arsenijevic A, et al.. Therapeutic Potential of Mesenchymal Stem Cells and Their Secretome in the Treatment of Glaucoma. Stem Cells Int . 2019; 2019: 7869130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Osborne A, Sanderson J,Martin KR.. Neuroprotective Effects of Human Mesenchymal Stem Cells and Platelet-Derived Growth Factor on Human Retinal Ganglion Cells. Stem Cells . 2018; 36(1): 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roozafzoon R, Lashay A, Vasei M, et al.. Dental pulp stem cells differentiation into retinal ganglion-like cells in a three dimensional network. Biochem Biophys Res Commun. 2015; 457(2): 154–160. [DOI] [PubMed] [Google Scholar]
- 10. Li Y, He X, Li J, et al.. Proliferation and differentiation of direct coculture of bone marrow mesenchymal stem cells and pigmented cells from the ciliary margin. Mol Med Rep . 2017; 15(6): 3529–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu R, Shi Q, Yang H, et al.. Protective effects of human umbilical cord mesenchymal stem cells on retinal ganglion cells in mice with acute ocular hypertension. Int J Ophthalmol . 2021; 14(2): 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carson AR, McTiernan CF, Lavery L, et al.. Ultrasound-targeted microbubble destruction to deliver siRNA cancer therapy. Cancer Res . 2012; 72(23): 6191–6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu J, Li RK.. Ultrasound-targeted microbubble destruction in gene therapy: A new tool to cure human diseases. Genes Dis . 2017; 4(2): 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wan C, Li F, Li H.. Gene therapy for ocular diseases meditated by ultrasound and microbubbles (Review). Mol Med Rep . 2015; 12(4): 4803–4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao J, Zhu TH, Chen WC, et al.. Optic neuropathy and increased retinal glial fibrillary acidic protein due to microbead-induced ocular hypertension in the rabbit. Int J Ophthalmol . 2016; 9(12): 1732–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shen X, Huang L, Ma D, et al.. Ultrasound Microbubbles Enhance the Neuroprotective Effect of Mouse Nerve Growth Factor on Intraocular Hypertension-Induced Neuroretina Damage in Rabbits. J Ophthalmol . 2016; 2016: 4235923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li H, Qian J, Yao C, et al.. Combined ultrasound-targeted microbubble destruction and polyethylenimine-mediated plasmid DNA delivery to the rat retina: enhanced efficiency and accelerated expression. J Gene Med . 2016; 18(4–6): 47–56. [DOI] [PubMed] [Google Scholar]
- 18. Xie W, Liu S, Su H, et al.. Ultrasound microbubbles enhance recombinant adeno-associated virus vector delivery to retinal ganglion cells in vivo. Acad Radiol. 2010; 17(10): 1242–1248. [DOI] [PubMed] [Google Scholar]
- 19. Mei Q, Mou H, Liu X, et al.. Therapeutic Potential of HUMSCs in Female Reproductive Aging. Front Cell Dev Biol . 2021; 9: 650003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cen LP, Ng TK, Liang JJ, et al.. Human Periodontal Ligament-Derived Stem Cells Promote Retinal Ganglion Cell Survival and Axon Regeneration After Optic Nerve Injury. Stem Cells . 2018; 36(6): 844–855. [DOI] [PubMed] [Google Scholar]
- 21. Zhao T, Li Y, Tang L, et al.. Protective effects of human umbilical cord blood stem cell intravitreal transplantation against optic nerve injury in rats. Graefes Arch Clin Exp Ophthalmol. 2011; 249(7): 1021–1028. [DOI] [PubMed] [Google Scholar]
- 22. da Silva-Junior AJ, Mesentier-Louro LA, Nascimento-Dos-Santos G, et al.. Human mesenchymal stem cell therapy promotes retinal ganglion cell survival and target reconnection after optic nerve crush in adult rats. Stem Cell Res Ther . 2021; 12(1): 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sukhorukova EG, Kruzhevskii DE,Alekseeva OS. [Glial fibrillary acidic protein: the component of intermediate filaments in the vertebrate brain astrocytes]. Zh Evol Biokhim Fiziol. 2015; 51(1): 3–10. [PubMed] [Google Scholar]
- 24. Kim YH, Oh TW, Park E, et al.. Neuroprotective effects of Acer palmatum thumb. leaf extract (KIOM-2015E) against ischemia/reperfusion-induced injury in the rat retina. Mol Vis. 2020; 26: 691–704. [PMC free article] [PubMed] [Google Scholar]
- 25. Becerra-Gonzalez M, Varman Durairaj R, Ostos Valverde A, et al.. Response to Hypoxic Preconditioning of Glial Cells from the Roof of the Fourth Ventricle. Neuroscience . 2020; 439: 211–229. [DOI] [PubMed] [Google Scholar]
- 26. Hol EM,Pekny M.. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015; 32: 121–130. [DOI] [PubMed] [Google Scholar]