Abstract
Background
Transient thyrotoxicosis has been documented in the setting of hyperemesis gravidarum (HG) with elevated human chorionic gonadotropin (hCG) levels. Thyroid storm in pregnancy is rarer and typically associated with autoimmune hyperthyroidism. We described thyroid storm in a primigravid 18-year-old patient due to hCG level elevation secondary to HG, which resolved in the second trimester of pregnancy.
Case Report
Our patient presented with vomiting, hyperthyroidism, and cardiac and renal dysfunction at 16 weeks’ gestation. She was clinically found to have a thyroid storm, with undetectable thyroid-stimulating hormone (TSH) and a free thyroxine level of >6.99 ng/dL. The hCG level was elevated at 246 030 mIU/L (9040-56 451 mIU/L). She was treated with methimazole, saturated solution potassium iodide, and propranolol. Because thyroid autoantibodies were absent, thyroid ultrasound yielded normal results, and thyroid function testing results rapidly improved as the hCG level decreased, the medications were tapered and ultimately discontinued by day 10 of hospitalization. The thyroid function remained normal after discharge.
Discussion
Because hCG and TSH have identical alfa subunits and similar beta subunits, hCG can bind to the TSH receptor and stimulate thyroxine production. The hCG level peaks at around 8-14 weeks of gestation, correlating with decreased TSH levels in this same time frame. This case emphasizes the relevant physiology and importance of timely and thorough evaluation to determine the appropriate management, prognosis, and follow-up for patients with thyroid storm in the setting of HG.
Conclusion
Although transient thyrotoxicosis is documented in patients with HG, thyroid storm is rare, and our case illustrates a severe example of these comorbidities.
Key words: thyroid storm, hyperemesis gravidarum, pregnancy, hCG
Abbreviations: AKI, acute kidney injury; GTT, gestational transient thyrotoxicosis; HG, hyperemesis gravidarum; IV, intravenous; TSH, thyroid-stimulating hormone; PTU, propylthiouracil; SSKI, saturated solution potassium iodide
Introduction
Hyperemesis gravidarum (HG) affects 0.3% to 3% of pregnancies and is a common cause of hospitalization during early pregnancy.1 Although the etiology remains unconfirmed, there is a strong positive association between HG incidence and hCG levels. The condition manifests at 6 to 8 weeks of gestation and spontaneously resolves by 20 weeks, following the natural curve of hCG production over time.2,3 However, there have been reports of HG persisting throughout pregnancy.1 The most common complications include dehydration, electrolyte imbalances, nutritional deficiencies, and weight loss.1,4 The severe risk factors for morbidity include Wernicke encephalopathy, renal impairment, and extreme weight loss.4 Patients may also present with orthostatic hypotension, signs and symptoms of dehydration, acute kidney injury (AKI), transaminitis, hyperbilirubinemia, electrolyte abnormalities, and hyperthyroidism.5
Hyperthyroidism affects 0.1% to 0.4% of pregnancies, with a majority occurring because of pre-existing autoimmune hyperthyroidism (Graves disease) and the remainder occurring because of gestational transient thyrotoxicosis (GTT).6 In 1982, Bouillon et al7 described an association between hyperthyroidism and HG. GTT affects up to two thirds of pregnancies complicated by HG.2,8 Both the entities are believed to result from elevated hCG levels; the symptoms and laboratory abnormalities correlate directly with hCG trends, and the degree of hCG elevation is associated with the severity of illness.2,9,10 hCG shares an alfa subunit with thyroid-stimulating hormone (TSH) and stimulates the thyrotropin receptor via molecular mimicry. This leads to elevated free thyroxine (T4) and triiodothyronine (T3) levels to an extent greater than those expected because of physiologic increases in the thyroxine-binding globulin level during pregnancy.9,11,12
Here, we described a thyroid storm in a primigravid patient resulting from hCG level elevation secondary to HG, which spontaneously resolved in the second trimester of pregnancy.
Case Report
Our patient was an 18-year-old primigravid woman with no significant medical history at 16 weeks’ gestation with a singleton pregnancy. She presented to her local hospital’s emergency department with a 1-month history of worsening nausea, vomiting, generalized weakness, fatigue, tremor, and a 15-pound weight loss as well as a 1-day history of dark urine, lower extremity edema, blurred vision, difficulty walking, and an altered mental status. She was afebrile, with a temperature of 36.6 °C, and was taking no medications or supplements. Her personal and family histories were negative for autoimmune disease.
A physical examination was notable for tachycardia (125/min), with a regular rhythm, hand tremor, and mild thyromegaly and without thyroid bruit. Laboratory evaluation revealed hyponatremia, hypokalemia, hypochloremia, metabolic alkalosis, and leukocytosis. Hepatic and renal dysfunction was evidenced by an aspartate aminotransferase level of 128 U/L (13-39 U/L), alanine aminotransferase level of 212 U/L (7-52 U/L), total bilirubin level of 4.1 mg/dL (0.3-1.0 mg/dL), and creatinine level of 4.2 mg/dL (0.4-1.4 mg/dL). TSH was undetectable, the free T4 level was elevated to >6.99 ng/dL (0.6-1.20 ng/dL), and the T3 level was elevated to 238 ng/dL (87-178 ng/dL). To our knowledge, thyroid function testing had not been previously performed in this patient for comparison. The Burch-Wartofsky point scale score was 50, determined based on neurologic status, gastrointestinal-hepatic dysfunction, degree of cardiac dysfunction, and underlying pregnancy, consistent with a diagnosis of thyroid storm.13 Treatment with a 1-L normal saline bolus, 1 mg of intravenous (IV) propranolol, 500 mg of oral propylthiouracil (PTU), and 300 mg of IV hydrocortisone was initiated at the outside facility.
Upon transfer to our facility’s intensive care unit, electrolyte disturbances and AKI were addressed using slow fluid resuscitation and electrolyte replacement after a consultation with the pediatric nephrology team. The pediatric endocrinology department was consulted, and treatment was continued with 15 mg of oral methimazole twice daily instead of PTU in the setting of the hepatic injury, with 250 mg of oral saturated solution potassium iodide (SSKI) every 6 hours, 60 mg of oral propranolol every 4 hours, and 100 mg of IV hydrocortisone every 8 hours, while evaluating the underlying etiology. When thyroid-stimulating immunoglobulin testing yielded a negative result— making autoimmune thyroid disease unlikely—thyroid ultrasound was performed, which revealed a normal thyroid size and no increased vascularity or concerning nodules. Then, the hCG level was measured and found to be elevated 5 times the upper reference range for gestational age, at 246 030 mIU/mL (normal range for gestational age 9040-56 451 mIU/L). The obstetrics department was consulted, and obstetric ultrasound was performed, which ruled out gestational trophoblastic disease. A thyroid storm was then presumed to be secondary to the elevated hCG level in the setting of HG.
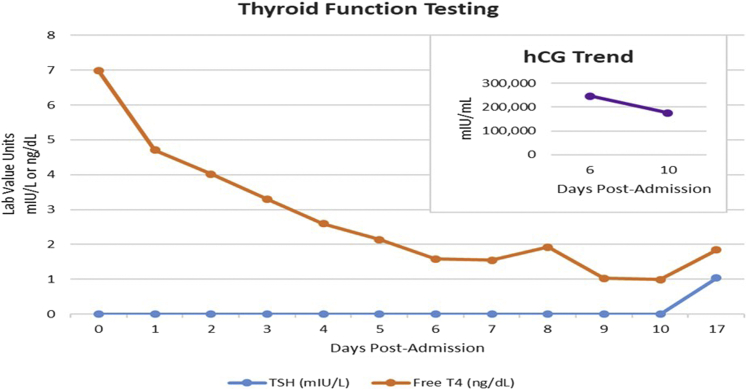
As symptoms and the laboratory findings improved, hydrocortisone was discontinued on day 3 of hospitalization. Methimazole was discontinued on day 5 of hospitalization because of recurrence of elevated liver enzyme levels. The transaminase levels and thyroid function testing results continued to improve, and SSKI was discontinued on day 7 of hospitalization. The free T4 and T3 levels normalized by day 9 of hospitalization. Propranolol was tapered and ultimately discontinued on day 10. HG was treated during admission with IV fluids, IV thiamine, and ondansetron, with resolution of symptoms. The laboratory trends are shown in the Figure.
Fig.
Trends in thyroid function testing and hCG levels correlating throughout the patient’s hospitalization and after discharge. hCG = human chorionic gonadotropin; T4 = thyroxine; TSH = thyroid-stimulating hormone.
Our patient was discharged on day 11 of hospitalization. Repeated outpatient thyroid function testing 1 week after discharge yielded a normal result, and she had no recurrence of symptoms.
Discussion
Because hCG and TSH have identical alfa subunits and similar beta subunits, hCG can bind to the TSH receptor and stimulate thyroxine production.14 The hCG level peaks at around 8 to 14 weeks of gestation, correlating with increased T4 and T3 levels and decreased TSH levels.10 However, higher levels of hCG are required to induce overt hyperthyroidism because this hormone has less thyrotrophic activity.14
Nausea and vomiting during pregnancy are common but can also indicate HG or, less frequently, be associated with hyperthyroidism. There are many overlapping symptoms of HG and hyperthyroidism, including vomiting, weight loss, dehydration, and organ dysfunction, and it is critical to establish the correct diagnosis to determine the appropriate treatment, monitoring, and prognosis. Hyperthyroidism can directly lead to vomiting and can also be a secondary effect of excess hCG, confounding its role in HG.10 Our patient’s presentation with metabolic alkalosis, electrolyte disturbances, and AKI during the second trimester of pregnancy gives credence to a diagnosis of HG with hypovolemia secondary to vomiting. Mortality associated with HG has been linked to the development of Wernicke encephalopathy, which is characterized by the triad of ataxia, confusion, and nystagmus, making thiamine replacement an important component of treatment.4
Hyperthyroidism occurs in approximately 1 to 2 per 1000 pregnancies.8 The differential for hyperthyroidism in pregnancy includes autoimmune hyperthyroidism (Graves disease), functional thyroid nodules, ingestion of exogenous thyroxine, GTT (occurring with physiologic increases in the hCG level or more severe elevations during HG), and gestational trophoblastic disease, also secondary to significant hCG level elevation.10,15 Of these, Graves disease is the most common underlying etiology, occurring in 0.5% to 1.3% of pregnant women.8 GTT occurs in 1% to 3% of pregnancies16; however, the incidence of thyroid storm in this setting has not been reported. In contrast, goiter, exophthalmos, and the common signs and symptoms of hyperthyroidism, including heat intolerance, muscle weakness, or tremor, are frequently seen in patients with Graves disease; these are not commonly seen in patients with GTT associated with hyperemesis.6 Our patient did have some of these symptoms, complicating her presentation. Muscle weakness and tremor might be explained by the electrolyte abnormalities and thyroid enlargement due to thyroid gland stimulation caused by her significantly elevated hCG level. Maternal Graves disease carries the risk of associated neonatal Graves disease in infants because of transplacental passage of maternal thyroid-stimulating immunoglobulin. Intensive monitoring and treatment might be necessary in this instance because there is a high risk of morbidity and mortality.10,15 GTT associated with HG is self-limiting, typically does not require treatment, aside from supportive care, and does not carry the same risks for the neonate after delivery.10 Thyrotoxicosis should spontaneously resolve by 20 weeks if thyroid stimulation is secondary to hCG level elevation, whereas thyroid dysfunction is expected to be more prolonged with other etiologies.14
The progression of hyperthyroidism to thyroid storm is associated with stressors such as trauma, surgery, infection, pregnancy, and delivery.17 The Burch-Wartofsky point scale is a clinical scoring system developed to predict the likelihood of thyroid storm in adult patients with thyrotoxicosis.13 A score of >45 points indicates a thyroid storm, a score between 25 and 44 points indicates concern for an impending storm, and a score of <25 points is reassuring.13 The diagnostic criteria include dysfunction in thermoregulation and symptoms common to the cardiovascular, hepatic, and central nervous systems, including fever, tachycardia, nausea, vomiting, and neurologic disturbances, in the setting of overt hyperthyroid symptoms.18
The treatment of thyroid storm is critical to avoid high-output cardiac failure and multiorgan failure and entails supportive care and initiation of thionamide therapy to decrease thyroid hormone synthesis by inhibiting thyroid peroxidase and blocking organification of iodine.19 Because of the risk of fetal esophageal or choanal atresia and aplasia cutis with methimazole, PTU is used early in pregnancy but has a higher risk of hepatotoxicity.15 Methimazole was chosen for our patient because she already had transaminitis and was in the second trimester of pregnancy. Potassium iodine (Lugol’s solution or SSKI) induces the Wolff-Chaikoff effect, a transient decrease in iodine organification and, therefore, thyroid hormone release.19 It is imperative to administer thionamide at least an hour before potassium iodine is administered to avoid the Jod-Basedow effect (increased thyroxine formation and release).19,20 Beta blockade prevents negative adrenergic effects of excess thyroid hormone. Excessive beta blockade should be avoided in patients with significant cardiovascular dysfunction because this can be associated with circulatory collapse.19 Although most beta blockers provide similar benefits and minimally decrease the peripheral conversion of T4 to T3, propranolol has been the most widely studied in pregnant patients, can be given intravenously, and has a short half-life.18 Lastly, glucocorticoid administration can potentially decrease the peripheral conversion of T4 to T3, improve cardiovascular symptoms, and potentially suppress the autoimmune process, if present.20 Adrenal dysfunction has been documented in the setting of thyroid storm and is also treated with glucocorticoids.19
Fortunately, because our patient’s thyrotoxicosis was not autoimmune in nature and resolved as the hCG levels decreased, she did not require long-term therapy or specialized monitoring for her infant.
Conclusion
This case highlights the importance of maintaining hyperemesis in the differential for causes of hyperthyroidism of pregnancy, particularly in younger patients who may present during early pregnancy.4 Although transient thyrotoxicosis is well documented in the setting of HG, our case illustrates a severe example presenting with thyroid storm and highlights the importance of determining the etiology to allow for appropriate treatment and monitoring.
Disclosure
The authors have no multiplicity of interest to disclose.
References
- 1.McParlin C., O'Donnell A., Robson S.C., et al. Treatments for hyperemesis gravidarum and nausea and vomiting in pregnancy: a systematic review. JAMA. 2016;316(13):1392–1401. doi: 10.1001/jama.2016.14337. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin T.M., Montoro M., Mestman J.H. Transient hyperthyroidism and hyperemesis gravidarum: clinical aspects. Am J Obstet Gynecol. 1992;167(3):648–652. doi: 10.1016/s0002-9378(11)91565-8. [DOI] [PubMed] [Google Scholar]
- 3.Tan J.Y., Loh K.C., Yeo G.S., Chee Y.C. Transient hyperthyroidism of hyperemesis gravidarum. BJOG. 2002;109(6):683–688. doi: 10.1111/j.1471-0528.2002.01223.x. [DOI] [PubMed] [Google Scholar]
- 4.Ismail S.K., Kenny L. Review on hyperemesis gravidarum. Best Pract Res Clin Gastroenterol. 2007;21(5):755–769. doi: 10.1016/j.bpg.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Sun S., Qiu X., Zhou J. Clinical analysis of 65 cases of hyperemesis gravidarum with gestational transient thyrotoxicosis. J Obstet Gynaecol Res. 2014;40(6):1567–1572. doi: 10.1111/jog.12372. [DOI] [PubMed] [Google Scholar]
- 6.Cuff R.D. Hyperthyroidism during pregnancy: a clinical approach. Clin Obstet Gynecol. 2019;62(2):320–329. doi: 10.1097/GRF.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 7.Bouillon R., Naesens M., Van Assche F.A., et al. Thyroid function in patients with hyperemesis gravidarum. Am J Obstet Gynecol. 1982;143(8):922–926. doi: 10.1016/0002-9378(82)90475-6. [DOI] [PubMed] [Google Scholar]
- 8.Cooper D.S., Laurberg P. Hyperthyroidism in pregnancy. Lancet Diabetes Endocrinol. 2013;1(3):238–249. doi: 10.1016/S2213-8587(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 9.Niemeijer M.N., Grooten I.J., Vos N., et al. Diagnostic markers for hyperemesis gravidarum: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211(2):150.e1. doi: 10.1016/j.ajog.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Moleti M., Di Mauro M., Sturniolo G., Russo M., Vermiglio F. Hyperthyroidism in the pregnant woman: maternal and fetal aspects. J Clin Transl Endocrinol. 2019;16:100190. doi: 10.1016/j.jcte.2019.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwin T.M., Montoro M., Mestman J.H., Pekary A.E., Hershman J.M. The role of chorionic gonadotropin in transient hyperthyroidism of hyperemesis gravidarum. J Clin Endocrinol Metab. 1992;75(5):1333–1337. doi: 10.1210/jcem.75.5.1430095. [DOI] [PubMed] [Google Scholar]
- 12.Rodien P., Jordan N., Lefèvre A., et al. Abnormal stimulation of the thyrotrophin receptor during gestation. Hum Reprod Update. 2004;10(2):95–105. doi: 10.1093/humupd/dmh008. [DOI] [PubMed] [Google Scholar]
- 13.Burch H.B., Wartofsky L. Life-threatening thyrotoxicosis: thyroid storm. Endocrinol Metab Clin North Am. 1993;22(2):263–277. [PubMed] [Google Scholar]
- 14.Walkington L., Webster J., Hancock B.W., Everard J., Coleman R.E. Hyperthyroidism and human chorionic gonadotrophin production in gestational trophoblastic disease. Br J Cancer. 2011;104(11):1665–1669. doi: 10.1038/bjc.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American College of Obstetricians and Gynecologists Thyroid disease in pregnancy: ACOG practice bulletin, number 223. Obstet Gynecol. 2020;135(6):e261–e274. doi: 10.1097/AOG.0000000000003893. [DOI] [PubMed] [Google Scholar]
- 16.Krassas G., Karras S.N., Pontikides N. Thyroid diseases during pregnancy: a number of important issues. Hormones (Athens) 2015;14(1):59–69. doi: 10.1007/BF03401381. [DOI] [PubMed] [Google Scholar]
- 17.Ma Y., Li H., Liu J., Lin X., Liu H. Impending thyroid storm in a pregnant woman with undiagnosed hyperthyroidism: a case report and literature review. Medicine (Baltimore) 2018;97(3) doi: 10.1097/MD.0000000000009606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akamizu T., Satoh T., Isozaki O., et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012;22(7):661–679. doi: 10.1089/thy.2011.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carroll R., Matfin G. Endocrine and metabolic emergencies: thyroid storm. Ther Adv Endocrinol Metab. 2010;1(3):139–145. doi: 10.1177/2042018810382481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blick C., Schreyer K.E. Gestational trophoblastic disease-induced thyroid storm. Clin Pract Cases Emerg Med. 2019;3(4):409–412. doi: 10.5811/cpcem.2019.9.43656. [DOI] [PMC free article] [PubMed] [Google Scholar]