Abstract
Aim:
This study aimed to evaluate the effects of factors like demographic items, comorbidities, and drug history on the inadequacy of colonic preparation before colonoscopy.
Background:
Inadequate bowel preparation can lead to lower polyp detection rates, longer procedure times, and lower cecal intubation rates.
Methods:
This population-based study was conducted on 2476 Iranian adults who were referred to two tertiary centers for elective colonoscopy between 2017 and 2018. Bowel preparation quality was scored by the Boston bowel preparation scale (BBPS). Univariate and multivariate logistic regressions were used to find the independent predictors of bowel preparation inadequacy.
Results
The results showed that 31.8% of patients had inadequate bowel preparation before their colonoscopy. Higher age, BMI>25, abdominal circumference>95 cm, low fruit consumption, and history of smoking were independently correlated with bowel preparation inadequacy. Additionally, using NSAIDs and SSRIs were correlated with bowel preparation adequacy in multivariate regression analysis. Finally, age, gender, ethnicity, BMI, abdominal circumference, fruit consumption, smoking, NSAIDs, SSRIs, education, constipation, physical activity, and diabetes entered the predictive model of this study. The area under the curve (AUC) reached 0.70 in the final step.
Conclusion:
The independent risk factors associated with colonic preparation inadequacy were identified, and herein, a predictive model is suggested for identifying patients with a high risk of bowel preparation inadequacy before a colonoscopy so that alternative preparation techniques can be employed among high-risk groups to yield optimal preparation quality.
Key Words: Colonoscopy, Colon cleaning, Bowel preparation, Risk factors of bowel cleansing, Quality of colonoscopy
Introduction
Colonoscopy has long been considered the gold standard for colorectal cancer (CRC) screening because of its capabilities in exploring the colon and removing colorectal polyps that could turn cancerous in the future (1). It is critical to have an appropriate bowel preparation before performing a colonoscopy examination to achieve a high quality, effective, and safe procedure (2). In addition, suitable colonoscopy preparation must effectively clean the lumen, while having no histological adverse effects on colonic mucosa (3-5).
Inadequate bowel preparation can result in failure to detect polyps equal to or larger than 5 mm in size and increase the risk of procedural adverse events, such as bleeding or perforation (3). Moreover, insufficient bowel preparation can lead to lower detection of polyps and adenomas of various sizes (6, 7), longer overall procedure time, more frequent repetitions of colonoscopies, higher risk of perforation, longer CIT, prolonged hospitalization, and higher costs of procedures (3, 8, 9). Cecal intubation time (CIT) is defined as the time between insertion of the colonoscope into the anus and when the colonoscope tip passes to a point proximal to the ileocecal valve in which the base of the cecum is visible. Actually, in clinical practice, longer CITs can be seen in patients with inadequate preparation (10). Therefore, inadequate preparation reduces the quality of colonoscopy and increases the likelihood of lesions and evidence of disease (such as mucosal changes suggesting ulcerative colitis) not being identified; this delay in diagnosis and, consequently, lack of timely treatment will lead to the progression of the disease and its complications.
A lack of proper preparation can also increase the risk of complications from the procedure, morbidity and mortality, length of hospital stay, and the cost of treatment.
Previous studies have reported that 17.2% to 44.2% of colonoscopies were performed under inadequate preparation conditions (11-16). Furthermore, some authors have declared that various demographic factors, socioeconomic factors, previous comorbidities, and drug history may affect the quality of colonoscopy preparation (17, 18). Accordingly, several studies have evaluated the factors associated with bowel preparation quality (16, 18-20); however, only a small fraction of them has proposed a predictive model to identify those patients with a high risk of bowel preparation inadequacy before a colonoscopy (13, 21, 22). Therefore, the current study evaluated the effects of some factors, i.e. demographic items, comorbidities, and drug history, on the inadequacy of colonic preparation before colonoscopy. Accordingly, a predictive model is suggested for identifying patients with a high risk of bowel preparation inadequacy before colonoscopy so that alternative preparation techniques can be employed and the cleansing regimen intensified so as to yield optimal preparation quality.
Methods
Participants
This study was conducted on Iranian adults who referred to Taleghani Hospital, which is a gastroenterology university center in Tehran, Iran, for elective colonoscopy during 2017 and 2018. All patients shared similar socio-economic statuses (SES). This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Research Ethics Committee of the Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences. Informed written consent was obtained from each subject.
Data collection
Information on the demographic, anthropometric and lifestyle features, socioeconomic status, and medical and family histories was collected for each of the patients in a visit before a colonoscopy. Educational level was categorized as being illiterate or literate (primary school or higher), low fruit consumption from low (never use to weekly use) to high (daily use), vegetable consumption from low (not eating to weekly use) to high (daily use), smoking as never and ever (smoking now or was a smoker and quit), physical activity as never (never or less than 30 minutes in a day, weekly) and ever (regular exercise or more than 30 minutes per day/weekly), and ethnicity as Fars or other Iranian ethnic group.
Colonoscopy preparation method
The preparation regimen of the patients scheduled for a morning colonoscopy was comprised of six liters of polyethylene glycol (PEG) solution and three bisacodyl tablets (FDA-approved regimen, i.e. PEG-containing regimen) one day before the colonoscopy. Patients undergoing an evening colonoscopy consumed five liters of PEG and three bisacodyl tablets the day before the procedure, and another one liter in the morning of the day of the procedure. A liquid-based regimen was started the day before the procedure, and having proper physical activity in the preparation period was recommended. All patients received preparation instructions at their appointment in the clinic. The preparation regimen, the importance of bowel preparation, and the effect of diet modification were described. A brochure containing instructions was given to all patients.
Evaluation of bowel preparation
Colonoscopies were performed by three expert colonoscopists, who performed an annual minimum of 400 colonoscopies each year. Fentanyl and midazolam were used for moderate conscious sedation during the procedure. Reaching the cecum and ileocecal valve by colonoscope tip was considered the end of the examination. Colonic preparation was evaluated in the whole colon and in its three anatomic parts separately, as follows: ascending, descending, and transverse. Bowel preparation quality was scored by the Boston bowel preparation scale (BBPS), a valid and reliable measure of bowel preparation that reflects the colon’s cleanliness during the inspection phase of the procedure (23). Each segment of the colon is scored between 0 (unprepared colon) to 3 (fully prepared colon), 0-1 is considered as inadequate and 2-3 as adequate preparation. Finally, the sum of each three segments score created the BBPS score, in which BBPS score 0-5 was considered as an inadequate bowel preparation and 6-9 (certainly preparation of any segment should not be less than 2) as an adequate preparation.
Statistical analysis
Continuous and categorical variables are described as mean (SD) and number (%), respectively. The distribution of variables that were significantly different between adequately and inadequately prepared individuals was forced into the regression models. Thereafter, the candidate variables were examined based on their univariable association with the outcome, and then, seven significant preparation indicators, i.e. BMI, abdominal circumference, education, fruit consumption, vegetable consumption, smoking, and physical activity, were combined to generate a variable with all possible configurations. Moreover, multivariable logistic regression models were used to estimate the odds of having inadequate colon preparation, given the “combined variable” as the risk indicator of interest. Multivariable linear regression that treats variables as independent variables was done to evaluate the roles of demographic variables, diseases, and drug consumption on colon preparation. While the effect of one demographic variable in these models was of interest, other demographic variables were considered as potential confounders and remained in the model. The full model was built with the covariate effects using the stepwise inclusion method. Then, the significance of covariates was tested in the full model using the backward elimination method. The Akaike information criterion (AIC) and Bayesian information criterion (BIC) were computed each time a variable was included/excluded, and the model with the lower AIC or BIC value was preferred. Additionally, the interactions of age, gender, ethnicity, and other predictors in the model were tested and model performances determined by examining measures of calibration and discrimination. Calibration refers to how closely the predicted probability of having inadequate colon preparation agrees with the observed inadequacy status (24), assessed by the Hosmer-Lemeshow test. Discrimination refers to the ability of the clinical decision rule to differentiate between individuals with and without adequate colon preparation, as measured by the area under the receiver operating characteristic (ROC) curve (AUC) statistic. In this study, an AUC value of 0.5 is described as no discrimination and a value of one is perfect discrimination. Stata software (version 14) was used for analyses, and the results were considered as statistically significant at a p=0.05 levels.
Results
Baseline characteristics:
A total of 2476 participants aged between 18 and 80 years were enrolled in the current study. The mean age of the study participants was 51.32 14.80 years. Out of all participants, 1407 (56.82%) were female, and the ethnicity of 1314 (53.2%) of them were Fars. The analyses showed that 68.2% of participants had adequate bowel preparation measured by BBPS score, and 85.7% of procedures were conducted in the evening. No significant difference was observed in the amount of PEG consumption between the patients with adequate and those with inadequate bowel preparation or in the prevalence of preparation inadequacy between all three parts of the colon (Table 1). CIT was registered in a subgroup of patients (n=228), in which it was calculated as 263.5 95.5 s in patients with adequate bowel preparation and 349.8 203.7 s in patients with inadequate bowel preparation, indicating a higher CIT in the inadequate bowel preparation group compared to those with adequate preparation (p<0.001) (Table1).
Table 1.
Baseline characteristics of patients enrolled in this study
| Studied Patients n=2476 (%) | |
|---|---|
| BMI (Mean SD)1 | 26.40 5.22 |
| Median/ Rang (Min, Max) | 26.03/ 59.59 (13.06, 72.65) |
| Abdominal circumference (Mean SD)2 | 90.52 12.34 |
| Median/ Rang (Min, Max) | 90.00/ 78.00 (62.00, 140.00) |
| Indication of colonoscopy | |
| FIT positive | 288 (11.6) |
| Family history of polyps | 887 (35.8) |
| Abdominal pain | 719 (29.0) |
| Constipation | 261 (10.5) |
| Other reasons | 321 (13.1) |
| Fruit consumption | |
| High | 1590 (65.1) |
| Never or Low | 852 (34.9) |
| Vegetable consumption | |
| High | 1065 (44.1) |
| Never or Low | 1350 (55.9) |
| Smoking | |
| Never | 2064 (84.4) |
| Ever | 382 (15.6) |
| Physical Activity | |
| Never | 531 (21.9) |
| Ever | 1894 (78.1) |
| PEG consumption (Mean SD)3 | |
| Adequate | 5.15 1.25 |
| Inadequate | 5.07 1.51 |
| Cecum Intubation time (Mean SD)4 | 299.5 155.8 |
| Median/ Rang (Min, Max) Adequate inadequate |
240.0/ 969.0 (120.0, 1089.0) |
| 263.5 95.5 | |
| 349.8 203.7 | |
| Preparation quality by BBPS | |
| Adequate | 1688 (68.2) |
| Inadequate | 788 (31.8) |
| Preparation quality of ascending colon | |
| Adequate | 1820 (73.5) |
| Inadequate | 656 (26.5) |
| Preparation quality of transverse colon | |
| Adequate | 1817 (73.4) |
| Inadequate | 659 (26.6) |
| Preparation quality of descending colon | |
| Adequate | 1782 (72.0) |
| Inadequate | 694 (28.0) |
Univariate analysis of risk factors
The univariate analysis of demographic, anthropometric, and clinical characteristics of participants in the current study is summarized in Table 2. The results showed that bowel preparation inadequacy measured by BBPS was significantly correlated with higher age, higher BMI, larger abdominal circumference, illiteracy, lower fruit consumption, lower vegetable consumption, lower physical activity, and smoking (p<0.05). Moreover, diabetes and constipation were other conditions that significantly affected the colonic preparation and were correlated with the inadequacy of bowel preparation by BBPS (p<0.05). Other variables, including gender, ethnicity, IBD, and coronary heart disease, were not associated with colon preparation quality. Furthermore, it was found that ASA, NSAIDs, SSRI, PPI, gliclazide, insulin, metformin, vitamin D3, ACEI or ARB, GnRH, and calcium were significantly correlated with the improvement of bowel preparation adequacy by BBPS score (p<0.05). The association between use of various drugs and colonic preparation is presented in Table 3.
Table 2.
Demographic, anthropometric, and clinical features and their relationship to colonic preparation in the studied Population
| BBPS score | |||||
|---|---|---|---|---|---|
| Adequate 1688 (68.2) |
Inadequate 788 (31.8) |
OR (95% CI)* | P-value | ||
| Age | |||||
| Mean SD | 50.44 14.57 | 53.22 15.12 | 1.01 (1.01- 1.02) | <0.0001 | |
| Gender | |||||
| Female | 979 (58.0) | 428 (54.3) | |||
| Male | 709 (42.0) | 360 (45.7) | 1.16 (0.98- 1.38) | 0.085 | |
| Ethnicity | |||||
| Fars | 904 (53.6) | 410 (52.2) | 0.510 | ||
| Other ethnic groups | 781 (46.4) | 375 (47.8) | 1.06 (0.89- 1.25) | ||
| BMI | |||||
| Mean SD | 25.51 4.10 | 28.16 6.57 | 1.11 (1.09- 1.13) | <0.0001 | |
| Abdominal circumference | |||||
| Mean SD | 88.68 11.65 | 94.17 12.84 | 1.04 (1.03- 1.04) | <0.0001 | |
| Education | |||||
| Educated | 1507 (89.3) | 664 (84.6) | 1.53 (1.19- 1.95) | 0.001 | |
| Illiterate | 180 (10.7) | 121 (15.4) | |||
| Fruit consumption | |||||
| High | 1195 (71.3) | 394 (51.6) | 2.33 (1.95- 2.78) | <0.0001 | |
| Never/Low | 482 (28.7) | 370 (48.4) | |||
| Vegetable consumption | |||||
| High | 788 (47.0) | 276 (37.4) | 1.49 (1.24- 1.78) | <0.0001 | |
| Never/Low | 887 (53.0) | 462 (62.6) | |||
| Smoking | |||||
| Never | 1431 (85.8) | 633 (81.4) | 1.39 (1.11- 1.74) | 0.005 | |
| Ever | 236 (14.2) | 145 (18.6) | |||
| Physical activity | |||||
| Never | 383 (23.2) | 149 (19.3) | 1.26 (1.02- 1.56) | 0.030 | |
| Ever | 1268 (76.8) | 624 (80.7) | |||
| IBD | |||||
| No | 1527 (92.4) | 719 (94.4) | 0.72 (0.51- 1.04) | 0.077 | |
| UC1 | 111 (6.7) | 33 (4.3) | |||
| Yes CD2 | 12 (0.7) | 9 (1.2) | |||
| IC3 | 3 (0.2) | 1 (0.1) | |||
| Diabetes | |||||
| No | 1561 (94.2) | 691 (91.3) | 1.55 (1.12- 2.15) | 0.008 | |
| Yes | 96 (5.8) | 66 (8.7) | |||
| Coronary heart disease | |||||
| No | 1554 (93.8) | 694 (92.3) | 1.26 (0.90- 1.76) | 0.174 | |
| Yes | 103 (6.2) | 58 (7.7) | |||
| Constipation | |||||
| No | 1031 (60.8) | 442 (57.1) | 1.20 (1.00-1.43) | 0.045 | |
| Yes | 652 (39.2) | 332 (42.9) | |||
* OR is reported for inadequate groups compared with adequate groups as reference group. 1 UC, Ulcerative colitis. 2 CD, Crohn’s disease. 3 IC, indeterminate colitis. Data adjusted for age, gender, and ethnicity
Table 3.
Drug consumption features and their relationship to colonic preparation in the studied population
| BBPS score | |||||
|---|---|---|---|---|---|
| Adequate 1688 (68.2) | Inadequate 788 (31.8) | OR (95% CI)* | P-value | ||
| ASA | No | 1415 (85.3) | 626 (81.4) | ||
| Yes | 243 (14.7) | 143 (18.6) | 1.33 (1.06- 1.67) | 0.014 | |
| NSAID | No | 1565 (94.6) | 744 (97.5) | ||
| Yes | 89 (5.4) | 19 (2.5) | 0.45 (0.27- 0.74) | 0.002 | |
| Anti-TNF | No | 1638 (99.0) | 755 (98.8) | ||
| Yes | 16 (1.0) | 9 (1.2) | 1.22 (0.54- 2.77) | 0.635 | |
| Prednisolone | No | 1637 (99.0) | 756 (99.0) | ||
| Yes | 17 (1.0) | 8 (1.0) | 1.02 (0.44- 2.37) | 0.965 | |
| Azathioprine | No | 1628 (98.4) | 756 (99.0) | ||
| Yes | 26 (1.6) | 8 (1.0) | 0.66 (0.30- 1.47) | 0.312 | |
| TCA | No | 1646 (99.5) | 760 (99.5) | ||
| Yes | 8 (0.5) | 4 (0.5) | 1.08 (0.32- 3.61) | 0.897 | |
| SSRI | No | 1568 (94.8) | 746 (97.5) | ||
| Yes | 86 (5.2) | 19 (2.5) | 0.46 (0.28- 0.77) | 0.003 | |
| PPI | No | 1405 (84.7) | 675 (88.5) | ||
| Yes | 254 (15.3) | 88 (11.5) | 0.72 (0.56- 0.93) | 0.013 | |
| Gliclazide | No | 1645 (99.5) | 750 (98.0) | ||
| Yes | 9 (0.5) | 15 (2.0) | 3.66 (1.59- 8.39) | 0.002 | |
| Insulin | No | 1629 (98.4) | 738 (96.5) | ||
| Yes | 26 (1.6) | 27 (3.5) | 2.29 (1.33- 3.95) | 0.003 | |
| Glibenclamide | No | 1628 (98.4) | 748 (97.8) | ||
| Yes | 26 (1.6) | 17 (2.2) | 1.42 (0.77- 2.64) | 0.263 | |
| Metformin | No | 1541 (93.2) | 687 (89.9) | ||
| Yes | 113 (6.8) | 77 (10.1) | 1.53 (1.13- 2.07) | 0.006 | |
| Ferrous Sulfate | No | 1627 (98.2) | 753 (98.6) | ||
| Yes | 30 (1.8) | 11 (1.4) | 0.79 (0.39- 1.59) | 0.512 | |
| Statin | No | 1422 (85.9) | 674 (87.3) | ||
| Yes | 234 (14.1) | 98 (12.7) | 0.85 (0.66- 1.10) | 0.222 | |
| ACE-I Or ARB | No | 1389 (83.8) | 601 (79.0) | ||
| Yes | 269 (16.2) | 160 (21.0) | 1.37 (1.11- 1.71) | 0.004 | |
| GnRH | No | 1643 (99.3) | 749 (97.9) | ||
| Yes | 11 (0.7) | 16 (2.1) | 3.19 (1.47- 6.91) | 0.003 | |
| Vitamin D3 | No | 1316 (79.4) | 645 (84.8) | ||
| Yes | 341 (20.6) | 116 (15.2) | 0.69 (0.55- 0.87) | 0.002 | |
| Calcium | No | 1361 (82.1) | 654 (85.9) | ||
| Yes | 296 (17.9) | 107 (14.1) | 0.72 (0.59- 0.96) | 0.020 | |
* OR is reported for inadequate groups compared with adequate groups as reference group
Predictive model based on demographic factors
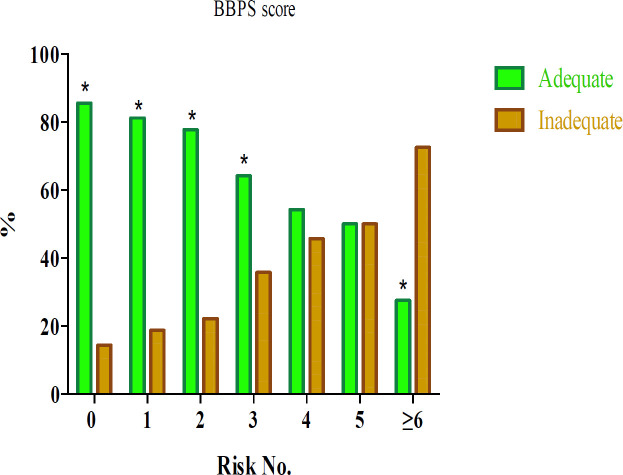
In order to suggest a predictive model for identifying patients with a high risk of bowel preparation inadequacy, the joint assessment of the seven demographic risk factors of bowel preparation inadequacy, i.e. BMI, abdominal circumference, education, fruit consumption, vegetable consumption, smoking, and physical activity, was performed. Stepwise escalation of risk was sequentially observed for single up to over six positive subjects and yielded adjusted odds ratios ranging from 1.32 to 14.35 as compared with the sixth negative reference subjects. In the group of the patients with ≥6 risk factors, the likelihood of inadequacy was approximately 14 times greater than in patients with no risk factor, and the number of patients with inadequate bowel preparation was significantly higher than the number of patients with adequate preparation (p<0.001) (Figure 1). Also evaluated was the stepwise inclusion of these seven demographic risk factors in a logistic model (model A). Additionally, stepwise inclusion of each parameter to
Figure 1.
The effect of the combination of demographic factors on inadequacy of bowel preparation
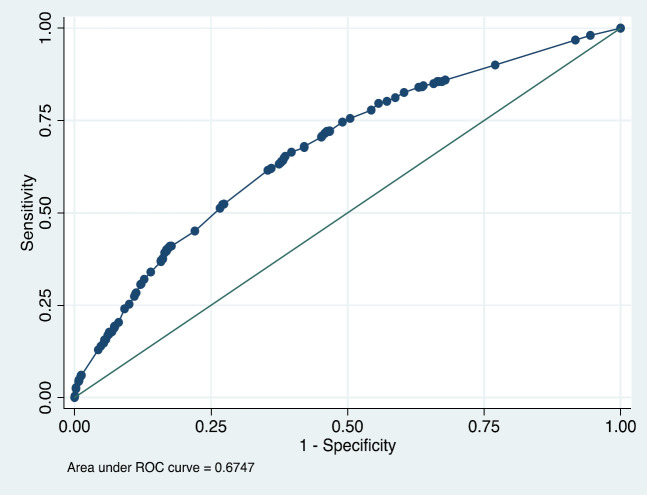
the model decreased the AIC and BIC and increased AUC (except for vegetable consumption) to 0.67 in the final step (Figure 2).
Figure 2.
ROC curve of combined logistic model of demographic factors (including BMI, abdominal circumference, education, fruit consumption, vegetable consumption, smoking, and physical activity) (Model A)
Multivariate analysis of risk factors
Demographic, anthropometric, and drug consumption data was entered into the multivariate logistic analysis and is shown in Table 4 as a crude model. The effect of age, gender, and ethnicity on colon preparation changed when these variables were simultaneously included in the model; however, the association between gender, ethnicity and other variables was found to be non-significant (Model B, Table 4). Age (OR=1.01, 95% CI 1.01-1.02, p<0.0001), BMI>25 (OR=1.59, 95% CI 1.26-2.01, p<0.0001), abdominal circumference >95 cm (OR=1.48, 95% CI 1.17-1.87, p=0.001), low fruit consumption (OR=2.57, 95% CI 2.05-3.23, p<0.0001), and history of smoking (OR=1.34, 95% CI 1.02-1.75, p=0.035) were significantly correlated with bowel preparation inadequacy in the multivariate regression analysis. Furthermore, NSAIDs (OR=0.42, 95% CI 0.24-0.74, p=0.003) and SSRI (OR=0.40, 95% CI 0.22-0.71, p=0.002) were significantly correlated with bowel preparation adequacy in the multivariate regression analysis.
Table 4.
Logistic model
| Characteristic | Crude model | Model B* | Model C** | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | ||||||||
| General characteristics | |||||||||||||
| Age (Years old) | 1.01 (1.00- 1.02) | <0.0001 | 1.01 (1.01- 1.02) | <0.0001 | 1.01 (1.01- 1.02) | <0.0001*** | |||||||
| Gender | |||||||||||||
| Female | 1 | 1 | 1 | ||||||||||
| Male | 1.16 (0.98- 1.38) | 0.085 | 1.16 (0.98- 1.38) | 0.085 | 1.08 (0.87- 1.34) | 0.465 | |||||||
| Ethnicity | |||||||||||||
| Fars | 1 | 1 | 1 | ||||||||||
| Non-Fars | 2.7 (2.1- 3.6) | <0.0001 | 1.08 (0.91- 1.28) | 0.371 | 1.07 (0.88- 1.31) | 0.469 | |||||||
| BMI | |||||||||||||
| 25 | 1 | 1 | |||||||||||
| >25 | 2.1 (1.8- 2.5) | <0.0001 | 1.59 (1.26- 2.01) | <0.0001*** | |||||||||
| Abdominal circumference | |||||||||||||
| 95 | 1 | 1 | |||||||||||
| >95 | 2.1 (1.7- 2.5) | <0.0001 | 1.48 (1.17- 1.87) | 0.001*** | |||||||||
| Education | |||||||||||||
| Educated | 1 | 1 | |||||||||||
| Illiterate | 1.53 (1.19- 1.95) | 0.001 | 1.31 (0.96- 1.78) | 0.085 | |||||||||
| Fruit consumption | |||||||||||||
| High | 1 | 1 | |||||||||||
| Never/Low | 2.33 (1.95- 2.78) | <0.0001 | 2.57 (2.05- 3.23) | <0.0001*** | |||||||||
| Vegetable consumption | |||||||||||||
| High | 1 | 1 | |||||||||||
| Never/Low | 1.48 (1.24- 1.78) | <0.0001 | 1.11 (0.89- 1.39) | 0.343 | |||||||||
| Smoking | |||||||||||||
| Never | 1 | 1 | |||||||||||
| Ever | 1.39 (1.11- 1.74) | 0.005 | 1.34 (1.02- 1.75) | 0.035*** | |||||||||
| Physical activity | |||||||||||||
| Never | 1 | 1 | |||||||||||
| Ever | 1.26 (1.02- 1.56) | 0.030 | 1.14 (0.89- 1.45) | 0.299 | |||||||||
| Diseases | |||||||||||||
| History of Diabetes | |||||||||||||
| No | 1 | 1.52 (0.92- 2.50) | 0.101 | ||||||||||
| Yes | 1.55 (1.12- 2.15) | 0.008 | |||||||||||
| Constipation | |||||||||||||
| No | 1 | ||||||||||||
| Yes | 1.19 (1.00- 1.43) | 0.045 | 0.87 (0.70- 1.07) | 0.188 | |||||||||
| Drug Usage | |||||||||||||
| ASA | |||||||||||||
| No | 1 | 1 | |||||||||||
| Yes | 1.33 (1.06- 1.67) | 0.014 | 0.96 (0.71- 1.29) | 0.774 | |||||||||
| NSAID | |||||||||||||
| No | 1 | 1 | |||||||||||
| Yes | 0.45 (0.27- 0.74) | 0.002 | 0.42 (0.24- 0.74) | 0.003*** | |||||||||
| SSRI | |||||||||||||
| No | 1 | 1 | |||||||||||
| Yes | 0.46 (0.28- 0.77) | 0.003 | 0.40 (0.22- 0.71) | 0.002*** | |||||||||
| PPI | |||||||||||||
| No | 1 | 1 | |||||||||||
| Yes | 0.72 (0.56- 0.93) | 0.013 | 0.83 (0.62- 1.10) | 0.199 | |||||||||
| Gliclazide | |||||||||||||
| No | 1 | 1 | |||||||||||
| Yes | 3.65 (1.59- 8.39) | 0.002 | 1.24 (0.38- 3.99) | 0.718 | |||||||||
| Insulin | |||||||||||||
| No | 1 | 1 | |||||||||||
| Yes | 2.29 (1.33 3.95) | 0.003 | 1.30 (0.61- 2.75) | 0.492 | |||||||||
| Metformin | |||||||||||||
| No | 1 | 1 | |||||||||||
| Yes | 1.53 (1.13- 2.07) | 0.006 | 1.11 (0.72- 1.70) | 0.636 | |||||||||
| ACE-I Or ARB | |||||||||||||
| No | 1 | 1 | |||||||||||
| Yes | 1.37 (1.11- 1.71) | 0.004 | 1.19 (0.91- 1.56) | 0.194 | |||||||||
| GnRH | |||||||||||||
| No | 1 | 1 | |||||||||||
| Yes | 3.19 (1.47- 6.91) | 0.003 | 2.07 (0.86- 5.02) | 0.105 | |||||||||
| Vitamin D3 | |||||||||||||
| No | 1 | 1 | |||||||||||
| Yes | 0.69 (0.55- 0.87) | 0.002 | 0.82 (0.61- 1.12) | 0.209 | |||||||||
| Calcium | |||||||||||||
| No | 1 | 1 | |||||||||||
| Yes | 0.72 (0.59- 0.96) | 0.020 | 0.84 (0.61- 1.16) | 0.295 | |||||||||
*Adjusted for Age, gender, and ethnicity; ** Adjusted for all variables within the table. ***Selected statistically significant predictors for the final model. Clinically significant predictors
Final predictive model
The results of univariable and multivariable logistic regression analyses along with the AIC and BIC values corresponding to the inclusion/exclusion of each predictor were used to select the predictors of the full
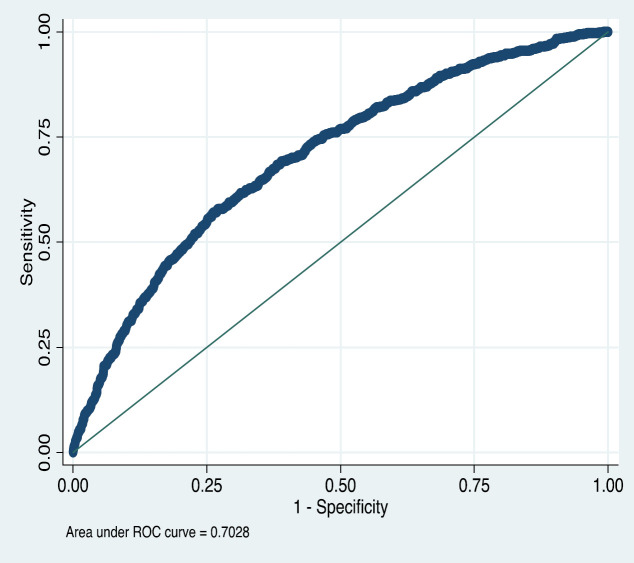
predictive (logistic) model (Model C, Table 4). The optimum model was selected by both methods corresponding with the model consisting of 13 predictors, as shown in Table 5. Additionally, the stepwise inclusion of each parameter to the model decreased AIC and BIC and increased AUC to 0.7 in the final step. Although the association of gender, ethnicity, physical activity, and education with colon preparation was not statistically significant in either univariable or multivariable analyses, they were forced into the final predictive model due to their clinical relevance. The Hosmer-Lemeshow statistic suggested that the fit of the model was acceptable for the development dataset (p = 0.811). Figure 3 shows the ROC curves for the diagnosis of colon preparation adequacy, where the sensitivity and specificity for several risk thresholds are plotted.
Table 5.
Full diagnostic (logistic) model for colon preparation adequacy, including the intercept (Model C)
| Intercept and Predictors | Coefficient | SE | OR | 95% CI | P-value | |
|---|---|---|---|---|---|---|
| General characteristics | ||||||
| Intercept | -2.27 | 0.23 | - | - | ||
| Age (Years old) | 0.01 | 0.00 | 1.01 | 1.00- 1.02 | < 0.0001 | |
| Gender | Female | |||||
| Male | 0.13 | 0.11 | 1.14 | 0.93- 1.41 | 0.205 | |
| Ethnicity | Fars | |||||
| Non-Fars | 0.06 | 0.10 | 1.06 | 0.87- 1.29 | 0.551 | |
| BMI | 25 | |||||
| >25 | 0.51 | 0.11 | 1.67 | 1.33- 2.09 | < 0.0001 | |
| Abdominal circumference | 95 | |||||
| >95 | 0.39 | 0.12 | 1.48 | 1.17- 1.86 | 0.001 | |
| Education | Educated | |||||
| Illiterate | 0.32 | 0.15 | 1.37 | 1.02- 1.86 | 0.039 | |
| Fruit consumption | High | |||||
| Never/Low | 1.07 | 0.10 | 2.90 | 2.38- 3.54 | < 0.0001 | |
| Smoking | Never | |||||
| Ever | 0.28 | 0.14 | 1.32 | 1.01- 1.72 | 0.041 | |
| Physical activity | Never | |||||
| Ever | 0.12 | 0.12 | 1.13 | 0.89- 1.43 | 0.315 | |
| History of Diabetes | No | |||||
| Yes | 0.50 | 0.19 | 1.65 | 1.14- 2.41 | 0.008 | |
| Constipation | No | |||||
| Yes | -0.19 | 0.10 | 0.83 | 0.68- 1.01 | 0.060 | |
| NSAID | No | |||||
| Yes | -0.92 | 0.28 | 0.40 | 0.23- 0.69 | 0.001 | |
| SSRI | No | |||||
| Yes | -0.97 | 0.28 | 0.38 | 0.21- 0.66 | 0.001 | |
| LR chi2 | 252.19 | |||||
| Hosmer-Lemshow GOF | X2 = 4.48, P value= 0.811 | |||||
| ROC area (95% CI) | 0.70 (0.68, 0.72) | |||||
Predictor value that is one when it is present and zero when it is absent. SE: Standard error. OR: Odds Ratio. X2: Chi square statistic. GOF: Goodness of fit. ROC = Receiver-operating characteristic.
Figure 3.
ROC curve of full diagnostic (logistic) model (Model C)
Discussion
Having appropriate bowel preparation before a colonoscopy is necessary for performing a high quality, safe, and effective procedure. In the current study, the association between bowel preparation inadequacy and demographic factors, comorbidities, and drug history was evaluated, and a predictive model is suggested for identifying those patients with a high risk of bowel preparation inadequacy before a colonoscopy to employ alternative preparation techniques for yielding optimal preparation quality.
The current results showed that 31.8% of the enrolled patients had inadequate bowel preparation before a colonoscopy, which was in concordance with previous studies reporting that bowel preparation inadequacy occurred in 17.2% to 44.2% of colonoscopies (11-16). Contrary to prior studies, however, there were no significant differences in the prevalence of preparation inadequacy between ascending, descending, and transverse colon in this study, even though several authors have described that preparation inadequacy is more frequent in ascending colon (21, 25). These controversies could be due to the difference in preparation regimens between studies, as a higher amount of PEG was used in the current study than in earlier ones.
The demographic, clinical, and drug-related factors were investigated, and it was found that age, BMI>25, and abdominal circumference >95 cm are independent risk factors for inadequate bowel preparation. In addition, patient age is considered a predictor of inadequate bowel preparation (13, 18, 19). Accordingly, impaired gastrointestinal motility, higher constipation rate (26), and a higher prevalence of comorbidities in older ages (27, 28) can be the reasons. Similar to the current findings, some previous studies confirmed that overweight and obesity are associated with bowel preparation inadequacy (13, 29). Borg et al. declared that BMI>25 is an independent risk factor of bowel preparation inadequacy, and each unit of increase in BMI could enhance the likelihood of inadequate bowel preparation by 2.1% (29). Several studies have reported that obesity (i.e. BMI>30), but not overweight (i.e. 30>BMI≥25), is associated with bowel preparation inadequacy (15, 30). This effect can be attributed to lower colonic motility and higher rates of constipation among obese patients compared to individuals with normal weight (31). The association between abdominal circumference and preparation inadequacy has not been studied previously; however, waist circumference has been reported to be positively correlated with BMI, and patients with higher adnominal circumference tend to have more BMI (32). Accordingly, this can be the reason for the association between abdominal circumference >95 cm and preparation inadequacy in the current study. Moreover, the current study found history of smoking and low fruit consumption to be independent risk factors of inadequate bowel preparation. Several studies have reported that smoking is independently associated with lower quality of bowel preparation (15, 29). Importantly, smoking can also increase colon transit time, which may explain the greater amount of residue in the colon, leading to inappropriate bowel preparation (33, 34). Although daily fruit consumption is associated with better colonic motility and lower constipation rates (35), there is no report evaluating the effect of routine dietary fibers on preparation quality. Hence, it is recommended that physicians also consider the patient’s diet.
The impact of diabetes and constipation remains controversial in previous studies. Similar to the current study, several investigations have reported that diabetes (16, 22, 36) and constipation (13, 14, 22, 36) are not independently associated with bowel preparation inadequacy. Conversely, a considerable number of studies and two meta-analyses indicated that constipation and diabetes can significantly affect the bowel preparation quality (14, 18, 19, 21, 37, 38). The 5-HT3 receptor located in both the enteric nervous system and the central nervous system (CNS) is related to colonic peristaltic reflex, colonic motility, colonic transit, GI secretion, and sensation (39-41). Previous studies have found that serotoninergic agents can activate and increase the colonic peristaltic reflex, resulting in increased colon transit and reduced constipation (42-44). This may explain the association between these drugs and colonic preparation adequacy. No data is available on the effect of NSAIDs on colonic preparation quality; however, some authors have declared that COX-2 inhibition affects colonic smooth muscles, resulting in decreased colon transit time (45-47), which could explain the effect of NSAIDs on the enhancement of bowel preparation. Conversely, numerable studies have indicated that NSAIDs usage can cause constipation (37, 48), and therefore, decrease the bowel preparation quality. This dilemma needs further study to be accurately evaluated.
In the predictive model herein, patients with ≥6 risk factors had a chance of inadequacy approximately 14 times greater than those with no risk factor. Yaldapati et al. evaluated the effect of the number of demographic, socioeconomic, and clinical risk factors on bowel preparation inadequacy among inpatients and indicated that as the number of risk factors increased, patients were more likely to have inadequate preparation (36). In the joint assessment model for demographic risk factors in the current study, the chance of having inadequate bowel preparation was significantly higher in patients with ≥3 risk factors compared to those without any risk factors, and thus, employing alternative preparation techniques should be considered for high risk patients. The stepwise inclusion of these seven demographic risk factors in a logistic model (model A) was also evaluated, and it finally reached an AUC of 0.67 in the final step.
To enhance the discriminatory power of this model, we suggest the final logistic predictive model (model C) comprising age, BMI>25, abdominal circumference>95 cm, low fruit consumption, and smoking by the use of NSAID and SSRI, which were independent risk factors of inadequate bowel preparation in the current study. Additionally, illiteracy (19) and diabetes (13, 18, 19, 22) have previously been described as associated risk factors with preparation inadequacy, which is in accordance with the univariate analysis performed herein. Male gender (13, 18, 20), ethnicity (non-Fars in the current study) (49), low physical activity (50-52), and constipation (18, 21, 22) were not significantly associated with preparation inadequacy in univariate and multivariate analyses; however, previous studies have indicated their relevance, and adding them to the final model increased AUC and decreased AIC and BIC.
An AUC of 0.70 was achieved in our final model, which included thirteen factors. Gimeno-García et al. evaluated 1076 patients from a tertiary referral hospital in a development and validation cohort. The preparation regimen in their study was distinct from the current one; their regimen contained three bowel agents, sodium picosulphate plus magnesium citrate plus citric acid, PEG plus ascorbic acid, and PEG. Four independent factors that significantly affect the bowel preparation adequacy were entered into the model. The final predictive model reached an AUC of 0.70 in the validation cohort. Moreover, a scoring system was suggested to identify patients with a high risk of inadequate bowel preparation with a negative predictive value of 89.1% (21). Hassan et al. conducted a prospective multi-center study on 2811 patients and finally suggested a predictive model including eight risk factors of bowel preparation inadequacy. Their preparation method consisted of six different regimens, and the AUC of the model was 0.63 (13). Dik et al. performed a multi-center prospective study on 1996 Dutch participants who were randomly assigned into validation and development cohorts. All patients used a split-dose preparation regimen, but four different medications were used. Eventually, a logistic model comprised of eight items was proposed for predicting inadequacy in bowel preparation with an AUC of 0.77 in the validation cohort [21]. Importantly, it should be noted that preparation protocols used differed among all of these previous studies and the current one. In the present study, the patients who underwent a morning colonoscopy using a single-dose preparation regimen, and those undergoing an evening colonoscopies used a split-dose preparation regimen. A higher volume of PEG was used compared to the mentioned studies (6 liters of PEG+3 tablets of bisacodyl).
The proposed model has a fundamental difference from those in previous studies; in addition to independent risk factors of bowel preparation inadequacy, risk factors that were significantly associated with bowel preparation inadequacy in previous studies or were associated only with preparation inadequacy in univariate analysis were entered into the model. However, increased AUC in the stepwise inclusion enhanced the discriminative ability of the final model. The AUC of the proposed model was similar to that of previous studies [9, 20, 21], which showed fair discriminative ability.
The strengths of the current study are: (1) large population from a gastroenterology university center; (2) complete evaluation of drug associations with bowel preparation inadequacy (which has not been completely studied); and (3) entering the factors that were significantly associated with bowel preparation inadequacy in previous studies in addition to independent predictors of preparation inadequacy in the current study to improve the generalizability and discriminative abilities of the predictive model.
However, some limitations of this study need to be addressed. First, this study suggests a predictive model for bowel preparation inadequacy, but no validation study was done for the predictive rule. Second, the discriminative ability of the proposed model is moderate. Finally, no validated score was suggested to predict the preparation inadequacy in the current study.
The current study identified some independent risk factors that are associated with bowel preparation inadequacy, and accordingly, a predictive model for identifying patients with a high risk of inadequate bowel preparation before colonoscopy is suggested. Identifying and eliminating reversible causes of inadequate preparation, alternative cleansing techniques, and intensified preparation protocols are necessary for these patients to reach acceptable preparation adequacy before colonoscopy. Further studies are needed to validate the model, or to suggest a model with higher discriminative ability, and to invent a predictive score for identifying patients with a higher risk of bowel preparation inadequacy.
Conflict of interests
The authors declare that they have no conflict of interest.
Acknowledgment
The authors wish to thank the member of the Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- 1.Bénard F, Barkun AN, Martel M, von Renteln D. Systematic review of colorectal cancer screening guidelines for average-risk adults: Summarizing the current global recommendations. World J Gastroenterol. 2018;24:124–38. doi: 10.3748/wjg.v24.i1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jover R, Herraiz M, Alarcon O, Brullet E, Bujanda L, Bustamante M, et al. Clinical practice guidelines: quality of colonoscopy in colorectal cancer screening. Endoscopy. 2012;44:444–51. doi: 10.1055/s-0032-1306690. [DOI] [PubMed] [Google Scholar]
- 3.Johnson DA, Barkun AN, Cohen LB, Dominitz JA, Kaltenbach T, Martel M, et al. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the US multi-society task force on colorectal cancer. Gastroenterology. 2014;147:903–24. doi: 10.1053/j.gastro.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Mamula P, Adler DG, Conway JD, Diehl DL, Farraye FA, Kantsevoy SV, et al. Colonoscopy preparation. Gastrointest Endosc. 2009;69:1201–9. doi: 10.1016/j.gie.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 5.Connor A, Tolan D, Hughes S, Carr N, Tomson C. Consensus guidelines for the safe prescription and administration of oral bowel-cleansing agents. Gut. 2012;61:1525–32. doi: 10.1136/gutjnl-2011-300861. [DOI] [PubMed] [Google Scholar]
- 6.Adler A, Wegscheider K, Lieberman D, Aminalai A, Aschenbeck J, Drossel R, et al. Factors determining the quality of screening colonoscopy: a prospective study on adenoma detection rates, from 12,134 examinations (Berlin colonoscopy project 3, BECOP-3) Gut. 2013;62:236–41. doi: 10.1136/gutjnl-2011-300167. [DOI] [PubMed] [Google Scholar]
- 7.Sulz MC, Kroger A, Prakash M, Manser CN, Heinrich H, Misselwitz B. Meta-Analysis of the Effect of Bowel Preparation on Adenoma Detection: Early Adenomas Affected Stronger than Advanced Adenomas. PLoS One. 2016;11:e0154149. doi: 10.1371/journal.pone.0154149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saltzman JR, Cash BD, Pasha SF, Early DS, Muthusamy VR, Khashab MA, et al. Bowel preparation before colonoscopy. Gastrointest Endosc. 2015;81:781–94. doi: 10.1016/j.gie.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 9.Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol. 2002;97:1696–700. doi: 10.1111/j.1572-0241.2002.05827.x. [DOI] [PubMed] [Google Scholar]
- 10.Jaruvongvanich V, Sempokuya T, Laoveeravat P, Ungprasert P. Risk factors associated with longer cecal intubation time: a systematic review and meta-analysis. Int J Colorectal Dis. 2018;33:359–65. doi: 10.1007/s00384-018-3014-x. [DOI] [PubMed] [Google Scholar]
- 11.Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;73:1207–14. doi: 10.1016/j.gie.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lebwohl B, Wang TC, Neugut AI. Socioeconomic and other predictors of colonoscopy preparation quality. Dig Dis Sci. 2010;55:2014–20. doi: 10.1007/s10620-009-1079-7. [DOI] [PubMed] [Google Scholar]
- 13.Hassan C, Fuccio L, Bruno M, Pagano N, Spada C, Carrara S, et al. A predictive model identifies patients most likely to have inadequate bowel preparation for colonoscopy. Clin Gastroenterol Hepatol. 2012;10:501–6. doi: 10.1016/j.cgh.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 14.Chung YW, Han DS, Park KH, Kim KO, Park CH, Hahn T, et al. Patient factors predictive of inadequate bowel preparation using polyethylene glycol: a prospective study in Korea. J Clin Gastroenterol. 2009;43:448–52. doi: 10.1097/MCG.0b013e3181662442. [DOI] [PubMed] [Google Scholar]
- 15.Fayad NF, Kahi CJ, Abd El-Jawad KH, Shin AS, Shah S, Lane KA, et al. Association between body mass index and quality of split bowel preparation. Clin Gastroenterol Hepatol. 2013;11:1478–85. doi: 10.1016/j.cgh.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng CL, Liu NJ, Tang JH, Kuo YL, Hung HL, Tsui YN, et al. Predictors of Suboptimal Bowel Preparation Using 3-l of Polyethylene Glycol for an Outpatient Colonoscopy: A Prospective Observational Study. Dig Dis Sci. 2017;62:345–51. doi: 10.1007/s10620-016-4343-7. [DOI] [PubMed] [Google Scholar]
- 17.Anderson JC, Butterly LF, Robinson CM, Goodrich M, Weiss JE. Impact of fair bowel preparation quality on adenoma and serrated polyp detection: data from the New Hampshire colonoscopy registry by using a standardized preparation-quality rating. Gastrointest Endosc. 2014;80:463–70. doi: 10.1016/j.gie.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:819–26. doi: 10.1097/MEG.0000000000001175. [DOI] [PubMed] [Google Scholar]
- 19.Gandhi K, Tofani C, Sokach C, Patel D, Kastenberg D, Daskalakis C. Patient Characteristics Associated With Quality of Colonoscopy Preparation: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:357–69. doi: 10.1016/j.cgh.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Yee R, Manoharan S, Hall C, Hayashi A. Optimizing bowel preparation for colonoscopy: what are the predictors of an inadequate preparation? Am J Surg. 2015;209:787–92. doi: 10.1016/j.amjsurg.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Gimeno-Garcia AZ, Baute JL, Hernandez G, Morales D, Gonzalez-Perez CD, Nicolas-Perez D, et al. Risk factors for inadequate bowel preparation: a validated predictive score. Endoscopy. 2017;49:536–43. doi: 10.1055/s-0043-101683. [DOI] [PubMed] [Google Scholar]
- 22.Dik VK, Moons LM, Huyuk M, van der Schaar P, de Vos Tot Nederveen Cappel WH, Ter Borg PC, et al. Predicting inadequate bowel preparation for colonoscopy in participants receiving split-dose bowel preparation: development and validation of a prediction score. Gastrointest Endosc. 2015;81:665–72. doi: 10.1016/j.gie.2014.09.066. [DOI] [PubMed] [Google Scholar]
- 23.Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620–25. doi: 10.1016/j.gie.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaha MJ. The Critical Importance of Risk Score Calibration: Time for Transformative Approach to Risk Score Validation? J Am Coll Cardiol. 2016;67:2131–4. doi: 10.1016/j.jacc.2016.03.479. [DOI] [PubMed] [Google Scholar]
- 25.Parra-Blanco A, Nicolas-Perez D, Gimeno-Garcia A, Grosso B, Jimenez A, Ortega J, et al. The timing of bowel preparation before colonoscopy determines the quality of cleansing, and is a significant factor contributing to the detection of flat lesions: a randomized study. World J Gastroenterol. 2006;12:6161–6. doi: 10.3748/wjg.v12.i38.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emmanuel A, Mattace-Raso F, Neri MC, Petersen KU, Rey E, Rogers J. Constipation in older people: A consensus statement. Int J Clin Pract. 2017:71. doi: 10.1111/ijcp.12920. [DOI] [PubMed] [Google Scholar]
- 27.Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004;33:351–75. doi: 10.1016/j.ecl.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Romero RV, Mahadeva S. Factors influencing quality of bowel preparation for colonoscopy. World J Gastrointest Endosc. 2013;5:39–46. doi: 10.4253/wjge.v5.i2.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borg BB, Gupta NK, Zuckerman GR, Banerjee B, Gyawali CP. Impact of obesity on bowel preparation for colonoscopy. Clin Gastroenterol Hepatol. 2009;7:670–5. doi: 10.1016/j.cgh.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharara AI, Harb AH, Sarkis FS, Chalhoub JM, Habib RH. Body mass index and quality of bowel preparation: Real life vs. clinical trials. Arab J Gastroenterol. 2016;17:11–6. doi: 10.1016/j.ajg.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Mushref MA, Srinivasan S. Effect of high fat-diet and obesity on gastrointestinal motility. Ann Transl Med. 2013;1:14. doi: 10.3978/j.issn.2305-5839.2012.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Booth ML, Hunter C, Gore CJ, Bauman A, Owen N. The relationship between body mass index and waist circumference: implications for estimates of the population prevalence of overweight. Int J Obes Relat Metab Disord. 2000;24:1058–61. doi: 10.1038/sj.ijo.0801359. [DOI] [PubMed] [Google Scholar]
- 33.Meier R, Beglinger C, Dederding JP, Meyer-Wyss B, Fumagalli M, Rowedder A, et al. Influence of age, gender, hormonal status and smoking habits on colonic transit time. Neurogastroenterol Motil. 1995;7:235–8. doi: 10.1111/j.1365-2982.1995.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 34.Bohlin J, Dahlin E, Dreja J, Roth B, Ekberg O, Ohlsson B. Longer colonic transit time is associated with laxative and drug use, lifestyle factors, and symptoms of constipation. Acta Radiol Open. 2018;7:2058460118807232. doi: 10.1177/2058460118807232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dreher M. Whole fruits and fruit fiber emerging health effects. Nutrients. 2018;10:1833. doi: 10.3390/nu10121833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yadlapati R, Johnston ER, Gregory DL, Ciolino JD, Cooper A, Keswani RN. Predictors of Inadequate Inpatient Colonoscopy Preparation and Its Association with Hospital Length of Stay and Costs. Dig Dis Sci. 2015;60:3482–90. doi: 10.1007/s10620-015-3761-2. [DOI] [PubMed] [Google Scholar]
- 37.Chang JY, Locke GR, Schleck CD, Zinsmeister AR, Talley NJ. Risk factors for chronic constipation and a possible role of analgesics. Neurogastroenterol Motil. 2007;19:905–11. doi: 10.1111/j.1365-2982.2007.00974.x. [DOI] [PubMed] [Google Scholar]
- 38.Rotondano G, Rispo A, Bottiglieri ME, De Luca L, Lamanda R, Orsini L, et al. Quality of bowel cleansing in hospitalized patients undergoing colonoscopy: A multicentre prospective regional study. Dig Liver Dis. 2015;47:669–74. doi: 10.1016/j.dld.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 39.Bjornsson ES, Chey WD, Hooper F, Woods ML, Owyang C, Hasler WL. Impaired gastrocolonic response and peristaltic reflex in slow-transit constipation: role of 5-HT(3) pathways. Am J Physiol Gastrointest Liver Physiol. 2002;283:G400–7. doi: 10.1152/ajpgi.00082.2001. [DOI] [PubMed] [Google Scholar]
- 40.Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–86. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crowell MD. The role of serotonin in the pathophysiology of irritable bowel syndrome. Am J Manag Care. 2001;7:S252–60. [PubMed] [Google Scholar]
- 42.Grover M, Camilleri M. Effects on gastrointestinal functions and symptoms of serotonergic psychoactive agents used in functional gastrointestinal diseases. J Gastroenterol. 2013;48:177–81. doi: 10.1007/s00535-012-0726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terry N, Margolis KG. Serotonergic Mechanisms Regulating the GI Tract: Experimental Evidence and Therapeutic Relevance. Handb Exp Pharmacol. 2017;239:319–42. doi: 10.1007/164_2016_103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cash BD, Chey WD. Review article: The role of serotonergic agents in the treatment of patients with primary chronic constipation. Aliment Pharmacol Ther. 2005;22:1047–60. doi: 10.1111/j.1365-2036.2005.02696.x. [DOI] [PubMed] [Google Scholar]
- 45.Lin YM, Sarna SK, Shi XZ. Prophylactic and therapeutic benefits of COX-2 inhibitor on motility dysfunction in bowel obstruction: roles of PGE(2) and EP receptors. Am J Physiol Gastrointest Liver Physiol. 2012;302:G267–75. doi: 10.1152/ajpgi.00326.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smid SD, Svensson KM. Inhibition of cyclooxygenase-2 and EP1 receptor antagonism reduces human colonic longitudinal muscle contractility in vitro. Prostaglandins Other Lipid Mediat. 2009;88:117–21. doi: 10.1016/j.prostaglandins.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Cong P, Pricolo V, Biancani P, Behar J. Abnormalities of prostaglandins and cyclooxygenase enzymes in female patients with slow-transit constipation. Gastroenterology. 2007;133:445–53. doi: 10.1053/j.gastro.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 48.Jones RH, Tait CL. Gastrointestinal side-effects of NSAIDs in the community. Br J Clin Pract. 1995;49:67–70. [PubMed] [Google Scholar]
- 49.Anderson JM, Stemboroski L , Aldridge P, Shuja A, Malespin M, de Melo Jr SW. Patients Come Clean: How Patient Factors and the Bowel Preparation Experience Influence Bowel Preparation Quality. Biomedical Journal of Scientific and Technical Research. 2018;7:5647–53. [Google Scholar]
- 50.Peters HP, De Vries WR, Vanberge-Henegouwen GP, Akkermans LM. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. 2001;48:435–9. doi: 10.1136/gut.48.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dukas L, Willett WC, Giovannucci EL. Association between physical activity, fiber intake, and other lifestyle variables and constipation in a study of women. Am J Gastroenterol. 2003;98:1790–6. doi: 10.1111/j.1572-0241.2003.07591.x. [DOI] [PubMed] [Google Scholar]
- 52.De Schryver AM, Keulemans YC, Peters HP, Akkermans LM, Smout AJ, De Vries WR, et al. Effects of regular physical activity on defecation pattern in middle-aged patients complaining of chronic constipation. Scand J Gastroenterol. 2005;40:422–9. doi: 10.1080/00365520510011641. [DOI] [PubMed] [Google Scholar]