Abstract
Complanadine A and lycodine are representative members of the lycopodium alkaloids with a characteristic pyridine-containing tetracyclic skeleton. Complanadine A has demonstrated promising neurotrophic activity as well as potential for persistent pain management. Herein, we report a pyrrole strategy enabled by one-carbon insertion and polarity inversion for concise total syntheses of complanadine A and lycodine. The use of a pyrrole as the pyridine precursor allowed a rapid construction of their tetracyclic skeleton via a one-pot Staudinger reduction, amine-ketone condensation, and Mannich-type cyclization. The pyrrole group was then converted to the desired pyridine by the Ciamician-Dennstedt rearrangement via a one-carbon insertion process, which also simultaneously introduced a chloride at C3 for the next C-H arylation. Other key steps include a direct anti-Markovnikov hydroazidation, a Mukaiyama-Michael addition, and a Paal-Knorr pyrrole synthesis. Lycodine and complanadine A were prepared in 8 and 11 steps, respectively, from a readily available known compound.
Graphical Abstract
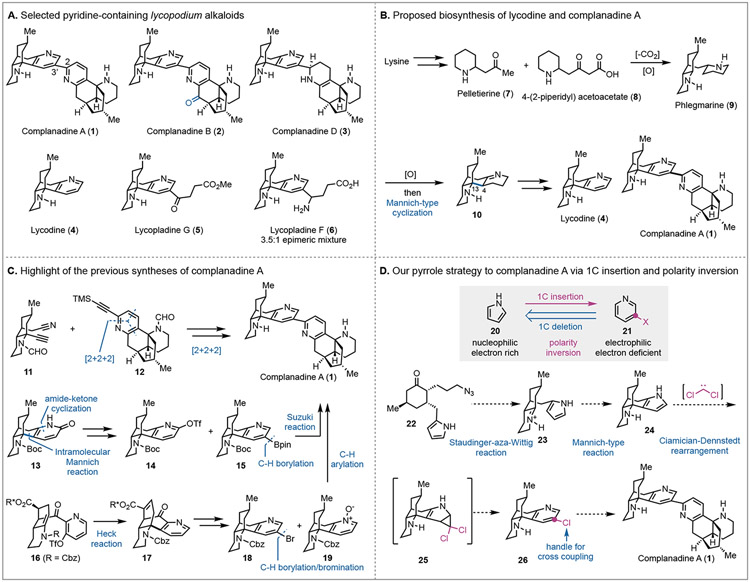
Neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease continue to being insurmountable.1 Recently, exciting progress has been made in amyloid beta-directed monoclonal antibodies for Alzheimer’s disease treatment.2 Meanwhile, naturally-occurring polypeptide neurotrophic factors, such as nerve growth factor (NGF) and brain-derived neurotrophic factor have shown significant therapeutic benefit in treating neurodegenerative disorders.3 However, these natural biologics suffer from poor bioavailability and pharmacokinetics and require unique administration method. Therefore, there are strong interests in small molecules that can promote the production of natural neurotrophic factors or function like them. These efforts have led to the identification of a collection of natural products with neurotrophic activities.4 Among them, the lycopodium alkaloids stood out because they are enriched with molecules having neurotrophic activities.5 For examples, huperzine A, an acetylcholinesterase inhibitor, has entered human clinical trial for treating Alzheimer’s disease;6 lyconadin A7 and complanadine A8 (1, Figure 1A), isolated by Kobayashi and co-workers, demonstrated promising activity in enhancing the mRNA expression for NGF biosynthesis in 1321N1 human astrocytoma cells and the production of NGF in human glial cells. Our interest in the chemical synthesis and biological study of natural products with neurotrophic activities led us to these lycopodium alkaloids. In 2014, we reported the total syntheses of lyconadins A and C.9 Herein, we report our total syntheses of complanadine A (1) and lycodine (4).
Figure 1.
Lycodine and complanadine natural products, proposed biosynthesis, prior total syntheses, and our synthetic plan.
Structurally, complanadine A is an unsymmetrical dimer of lycodine connected via a C2-C3’ linkage. Its mono-oxidized analog complanadine B (2) and partially reduced analogs complanadines D (3) and E were isolated as well.10 Additionally, lycopladine G (5), lycopladine F (6), and others featuring a pyridine C3-linkage with an amino acid or its derivative were identified.11 Biosynthetically, the complanadines and lycodine are proposed to be synthesized from lysine by going through intermediates including pelletierine (7) and phlegmarine (9, Figure 1B).12
Both lycodine and the complanadines have attracted a significant amount of synthetic attention. The groups of Heathcock,13 Hirama/Tsukano,14 and Takayama15 completed elegant total syntheses of lycodine. The reported complanadine A total syntheses are highlighted in Figure 1C. In 2010, the groups of Siegel16 and Sarpong17 reported their total syntheses simultaneously. The Siegel synthesis (18 LLS steps) features two remarkable Co-mediated [2+2+2] cyclizations to form the C2-C3’ bipyridine moiety. Their synthesis also led to lycodine. The Sarpong synthesis (15 LLS steps) employed a biomimetic tandem 1,4-addition/Mannich cyclization/amide-ketone condensation to form key intermediate 13, which was then advanced to triflate 14. After triflate reduction, an Ir(I)-catalyzed C-H borylation was used to produce boronic ester 15 for the next Suzuki cross coupling with 14 and completion of their complanadine A total synthesis. The Tsukano synthesis (20 LLS steps)18 reported in 2013 used an intramolecular Heck reaction to build the tetracyclic core (16→17) and a Pd-catalyzed C-H arylation between 18 and 19 to forge the C2-C3’ linkage. Notably, Siegel et al. identified complanadine A as a selective agonist for the Mas-related G protein-coupled receptor X2, a G protein-coupled receptor that is highly expressed in neurons and functions as a modulator of pain.19 Thus, complanadine A is also a potential lead compound for persistent pain management.
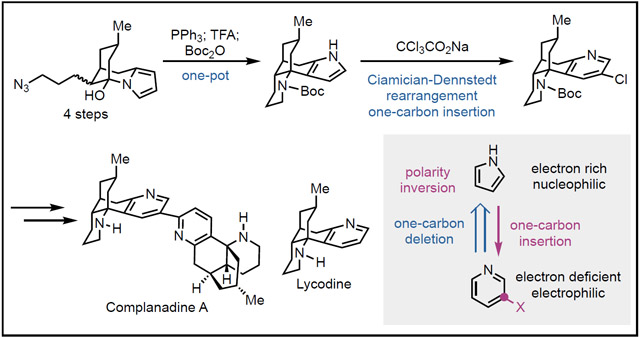
Retrosynthetically, we deleted one carbon (1C) atom from the pyridine group of complanadine A and lycodine and envisioned a pyrrole as its precursor (Figure 1D). This 1C-deletion strategy is critical for our synthesis because it would invert an electron deficient (electrophilic) pyridine to an electron rich (nucleophilic) pyrrole and enable chemistries that are impossible for the pyridine group. In the forward sense, a 1C-insertion tactic would be needed to convert the pyrrole group to a pyridine, ideally functionalized with a handle for the next C-C bond formation step. In this scenario, the Ciamician-Dennstedt rearrangement20 would serve the purpose. It goes through a dihalocarbene cycloaddition on a pyrrole followed by ring expansion to provide a 3-halopyridine. Despite its discovery in 1881, the development of the Ciamician-Dennstedt rearrangement is very limited21 and it has not been used in total synthesis yet. The pyridine to pyrrole retrosynthetic analysis led us to key intermediate 24, which could be converted to chloropyridine 26 via the Ciamician-Dennstedt rearrangement, then to lycodine and complanadine A. While attractive, we were aware of the potential issues associated with the proposed Ciamician-Dennstedt rearrangement. The rearrangement generally has harsh reaction conditions and the dihalocarbene would prefer the more substituted pyrrole double bond instead of the less substituted one. We were hoping that the steric effect would force the [2+1] cycloaddition on the less substituted double bond. Furthermore, the Reimer-Tiemann formylation is always a competing pathway.22 In spite of these challenges, we decided to proceed because this unprecedented strategy once successful would provide a new retrosynthetic analysis for pyridine-containing natural products. More importantly, the use of the pyrrole group would enable a quick synthesis of 24 from 22 via an intramolecular Staudinger-aza-Wittig reaction23 followed by Mannich-type cyclization. The nucleophilicity of the pyrrole group is essential for the proposed Mannich-type cyclization.
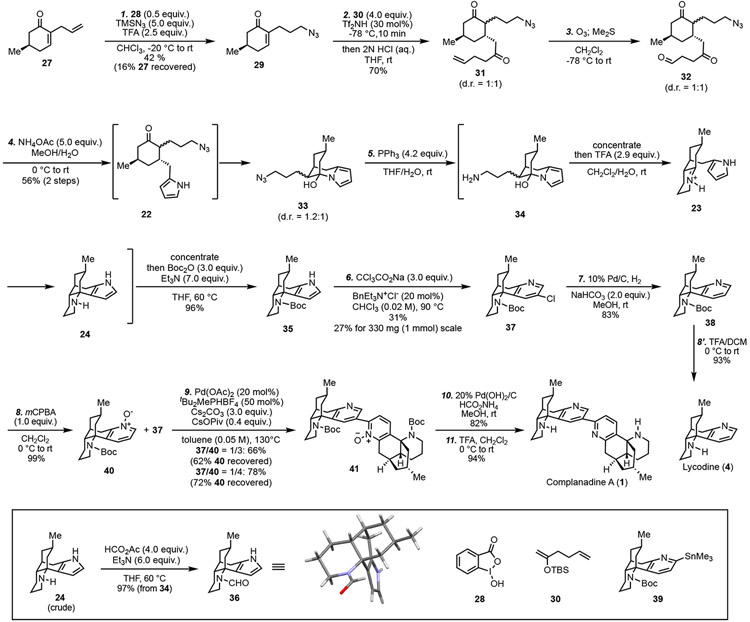
Our synthesis started with known enone 27 (Scheme 1), which can be prepared in large scale from chiral pool molecule (R)-(+)-pulegone (~ 1$/g) in three steps24 or via a one-pot asymmetric organ ocatalytic approach in one step.25 While the terminal olefin can be converted to an alkyl azide via a 3-step sequence (hydroboration-oxidation, activation of the resulting primary alcohol, and nucleophilic substitution with NaN3), we decided to explore the possibility of installing the azide group via a hypervalent iodine-catalyzed direct intermolecular anti-Markovnikov hydroazidation developed by Xu26 and Liu27 independently. After tunings of the reaction conditions developed by Xu et al., 27 was converted to 29 in 42% yield at gram scale with 0.5 equiv. of benziodoxole (28). Our unsuccessful attempts to install the pyrrole group directly prompted us to use 1,4-dicarbonyl 32 as the precursor of pyrrole 22. In this case, an intermolecular Mukaiyama-Michael addition of 30 to 29 was investigated. We were aware that introduction of the azide group at an early stage (cf. 29) could be problematic due to the potential Schmidt-Aube-type rearrangement.28 For example, while TBSOTf was able to promote the conjugate addition on a similar substrate without the azide group, it failed on 29. Eventually, we identified that the azide group could survive the use of triflimide as the promoter at −78 °C. After the reaction was quenched with HCl, product 31 was produced in 70% yield as a 1:1 mixture of epimers at the α position. The 1,4-addition occurred exclusively on the opposite face of the methyl group. This mixture was then subjected to ozonolysis to cleave the terminal olefin for the Paal-Knorr pyrrole synthesis. To our surprise, after 32 was treated with NH4OAc, the isolated product was not 22; instead, 33 was obtained as a 1.2:1 epimeric mixture. It turned out that the newly formed pyrrole further reacted with the ketone to form a stable hemiaminal. We decided to push forward with 33 with the hope that the hemiaminal formation is reversible. Staudinger reduction of azide 33 with PPh3 produced amine 34. After concentration, 34 was treated with trifluoroacetic acid. Under the acidic conditions, the hemiaminal did open to release the ketone, which further underwent condensation with the primary amine to form iminium ion 23. The next Mannich-type cyclization afforded tetracyclic product 24, which was further protected as Boc carbamate 35. Overall, 35 was obtained in 96% yield from 33 in a one-pot procedure. Notably, both epimers of 33 were funneled to the same product 35. The structure of 24 was confirmed by X-ray crystallographic analysis of its derivative 36 (CCDC: 2101755).29
Scheme 1.
Total synthesis of complanadine A and lycodine.
With multiple grams of 35 in hand, we next concentrated on the Ciamician-Dennstedt rearrangement. Chloropyridine 37 could be obtained in 17% yield with a combination of CHCl3 and KOH to generate the dichlorocarbene, but the major identifiable side product (23%) was produced via the Reimer-Tiemann formylation. The use of CHBr3 and KOH is much less effective while bromopyridine is more reactive for the next cross coupling reaction. After further exploration, we identified that the use of CCl3CO2Na to release dichlorocarbene under thermal conditions (70 °C) performed better than the basic conditions and product 37 was obtained in 23%. Further increasing the reaction temperature to 90 °C enhanced the yield to 31%. When the reaction was scaled up to 1 mmol (330 mg) of 35, 37 was obtained in 27% yield.
With sufficient amount of 37 in hand, we proceeded to complete the total synthesis of lycodine and complanadine A. After removal of the chlorine atom and the Boc protecting group, lycodine was prepared in 8 steps from 27. For the synthesis of complanadine A, we first tried to prepare a C2 functionalized intermediate such as 39 via C-H stannation of 38,30 but failed. Thus, 38 was oxidized to pyridine N-oxide 40. In the Tsukano synthesis, a bromopyridine derivative (18, Figure 1C) was used to react with 19, an analog of 40. In our case, chloropyridine 37 is much less reactive than bromopyridine 18. Indeed, when we used the conditions reported by Tsukano et al., C-H arylation of 40 with 37 produced 41 in low yield. Considering the slow oxidative addition with chloropyridine, we switched to more electron rich ligands and identified the conditions developed by Fagnou and co-workers,31 which was recently modified and used by Stoltz and co-workers in their total synthesis of jorunnamycin A and jorumycin.32 Under the Stoltz conditions [Pd(OAc)2, tBu2MePHBF4, Cs2CO3, and CsOPiv in toluene at 130 °C], desired product 41 was produced in 66% yield with a 1/3 ratio of37/40 or 78% yield with a 1/4 ratio of 37/40. The excess amount of 40 can be recovered in almost quantitative yield to avoid loss of material. After pyridine N-oxide reduction and deprotection, the total synthesis of complanadine A was completed in 11 steps from known compound 27.
In summary, a pyrrole strategy was developed to synthesize lycodine and complanadine A. The nucleophilic pyrrole group was used as the precursor of an electrophilic pyridine. This polarity inversion strategy enabled an efficient approach featuring a one-pot Staudinger reduction, amine-ketone condensation, and Mannich-type cyclization to rapidly construct the tetracyclic core skeleton. The Ciamician-Dennstedt one-carbon insertion converted the pyrrole group to a chloropyridine for the next C-H arylation for the complanadine A synthesis. These novel chemistries together with an iodine(III)-mediated direct intermolecular anti-Markovnikov hydroazidation, a triflimide-promoted Mukaiyama-Michael addition, and a Paal-Knorr pyrrole synthesis enabled us to complete the total syntheses of lycodine in 8 steps and complanadine A in 11 steps from readily available known cyclohexenone 27.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by NIH GM128570. The NIH P30 CA023168 is acknowledged for supporting shared NMR resources to Purdue Center for Cancer Research. The XRD data is collected on a new single crystal X-ray diffractometer supported by the NSF CHE 1625543 through the MRI Program.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures and spectra data (PDF file)
Cystallographic data for 36 (cif file)
The authors declare no competing financial interest.
REFERENCES
- (1).Kiaej M New Hopes and Challenges for Treatment of Neurodegenerative Disorders: Great Opportunities for Young Neuroscientists. Basic Clin. Neurosci 2013, 4, 3–4. [PMC free article] [PubMed] [Google Scholar]
- (2).(a) Mintun MA; Lo AC; Evans CD; Wessels AM; Ardayfio PA; Andersen SW; Shcherbinin S; Sparks J; Sims JR; Brys M; Apostolova LG; Salloway SP; Skovronsky DM Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med 2021, 384, 1691–1704. [DOI] [PubMed] [Google Scholar]; (b) FDA’s Decision to Approve New Treatment for Alzheimer’s Disease:https://www.fda.gov/drugs/news-events-human-drugs/fdas-decision-approve-new-treatment-alzheimers-disease (accessed 2021-08-16). [Google Scholar]; (c) Mullard A Landmark Alzheimer’s drug approval confounds research community. Nature 2021, 594, 309–310. [DOI] [PubMed] [Google Scholar]
- (3).Sofroniew MV; Howe CL; Mobley WC Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci 2001, 24, 1217–1281. [DOI] [PubMed] [Google Scholar]
- (4).(a) Wilson RW; Danishefsky SJ Applications of Total Synthesis to Problems in Neurodegeneration: Fascinating Chemistry along the Way. Acc. Chem. Res 2006, 39, 539–549. [DOI] [PubMed] [Google Scholar]; (b) Xu J; Lacoske MH; Theodorakis EA Neurotrophic Natural Products: Chemistry and Biology. Angew. Chem. Int. Ed 2014, 53, 956–987. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Shenvi RA Neurite Outgrowth Enhancement by Jiadifenolide: Possible Targets. Nat. Prod. Rep 2016, 33, 535–539. [DOI] [PubMed] [Google Scholar]
- (5).Ma X; Gang DR The Lycopodium alkaloids. Nat. Prod. Rep 2004, 21, 752–772. [DOI] [PubMed] [Google Scholar]
- (6).(a) Yang G; Wang Y; Tian J; Liu J-P Huperzine A for Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. PLoS One 2013, 8, e74916. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Herzon SB; Tun MKM The pharmacology and therapeutic potential of (−)-huperzine A. J. Exp. Pharmacol 2012, 4, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Kobayashi J; Hirasawa Y; Yoshida N; Morita H Lyconadin A, a Novel Alkaloid from Lycopodium complanatum. J. Org. Chem 2001, 66, 5901–5904. [DOI] [PubMed] [Google Scholar]
- (8).(a) Kobayashi J; Hirasawa Y; Yoshida N; Morita H Complanadine A, a new dimeric alkaloid from Lycopodium complanatum. Tetrahedron Lett. 2000, 41, 9069–9073. [Google Scholar]; (b) Morita H; Ishiuchi K; Haganuma A; Hoshino T; Obara Y; Nakahata N; Kobayashi J Complanadine B, obscurumines A and B, new alkaloids from two species of lycopodium. Tetrahedron 2005, 61, 1955–1960. [Google Scholar]
- (9).Yang Y; Haskins CW; Zhang W; Low PL; Dai M Divergent total syntheses of lyconadins A and C. Angew. Chem. Int. Ed 2014, 53, 3922–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) Ishiuchi K; Kubota T; Ishiyama H; Hayashi S; Shibata T; Mori K; Obara Y; Nakahata N; Kobayashi J Lyconadins D and E, and complanadine E, new Lycopodium alkaloids from Lycopodium complanatum. Bioorg. Med. Chem 2011, 19, 749–753. [DOI] [PubMed] [Google Scholar]; (b) Ishiuchi K; Kubota T; Mikami Y; Obara Y; Nakahata N; Kobayashi J Complanadines C and D, new dimeric alkaloids from Lycopodium complanatum. Bioorg. Med. Chem 2007, 15, 413–417. [DOI] [PubMed] [Google Scholar]
- (11).(a) Ishiuchi K; Kubota T; Hayashi S; Shibata T; Kobayashi J Lycopladines F and G, new C16N2-type alkaloids with an additional C4N unit from Lycopodium complanatum. Tetrahedron Lett. 2009, 50, 4221–4224. [Google Scholar]; (b) Yeap JS-Y; Lim K-H; Yong K-T; Lim S-H; Kam T-S; Low Y-Y Lycopodium Alkaloids: Lycoplatyrine A, an Unusual Lycodine-Piperidine Adduct from Lycopodium platyrhizoma and the Absolute Configurations of Lycoplanine D and Lycogladine H. J. Nat. Prod 2019, 82, 324–329. [DOI] [PubMed] [Google Scholar]; (c) Haley HMS; Payer SE; Papidocha SM; Clemens S; Nyenhuis J; Sarpong R Bioinspired Diversification Approach Toward the Total Synthesis of Lycodine-Type Alkaloids. J. Am. Chem. Soc 2021, 143, 4732–4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).For a recent biosynthesis study and references therein: Wang J; Zhang Z-K; Jiang F-F; Qi B-W; Ding N; Hnin SYY; Liu X; Li J; Wang X-H, Tu P-F; Abe I; Morita H; Shi S-P Deciphering the Biosynthetic Mechanism of Pelletierine in Lycopodium Alkaloid Biosynthesis. Org. Lett, 2020, 22, 8725–8729. [DOI] [PubMed] [Google Scholar]
- (13).Heathcock CH; Kleinman EF; Binkley ES Total Synthesis of Lycopodium Alkaloids: (±)-lycopodine, (±)-lycodine, and (±)-lycodoline. J. Am. Chem. Soc 1982, 104, 1054–1068. [Google Scholar]
- (14).Zhao L; Tsukano C; Kown E; Shirakawa H; Kaneko S; Takemoto Y; Hiram M Competent Route to Unsymmetric Dimer Architectures: Total Syntheses of (−)-lycodine and (−)-Complanadines A and B, and Evaluation of Their Neurite Outgrowth Activities. Chem. Eur. J 2017, 23, 802–812. [DOI] [PubMed] [Google Scholar]
- (15).Azuma M; Yoshikawa T; Kogure N; Kitajima M; Takayama H Biogenetically Inspired Total Syntheses of Lycopodium Alkaloids, (+)-Flabellidine and (−)-Lycodine. J. Am. Chem. Soc 2014, 136, 11618–11621. [DOI] [PubMed] [Google Scholar]
- (16).(a) Yuan CX; Chang CT; Axelrod A; Siegel D Synthesis of (+)-Complanadine A, an Inducer of Neurotrophic Factor Excretion. J. Am. Chem. Soc 2010, 132, 5924–5925. [DOI] [PubMed] [Google Scholar]; (b) Yuan CX; Chang CT; Siegel D Syntheses of (+)-Complanadine A and Lycodine Derivatives by Regioselective [2+2+2] Cycloadditions. J. Org. Chem 2013, 78, 5647–5668. [DOI] [PubMed] [Google Scholar]
- (17).(a) Fischer DF; Sarpong R Total Synthesis of (+)-Complanadine A Using an Iridium-Catalyzed Pyridine C-H Functionalization. J. Am. Chem. Soc 2010, 132, 5926–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Newton JN; Fischer DF; Sarpong R Synthetic Studies on Pseudo-Dimeric Lycopodium Alkaloids: Total Synthesis of Complanadine B. Angew. Chem. Int. Ed 2013, 52, 1726–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Zhao L; Tsukano C; Kwon E; Takemoto Y; Hirama M Total Syntheses of Complanadines A and B. Angew. Chem. Int. Ed 2013, 52, 1722–1725. [DOI] [PubMed] [Google Scholar]
- (19).Johnson T; Siegel D Complanadine A, a selective agonist for the Mas-related G protein-coupled receptor X2. Bioorg. Med. Chem. Lett 2014, 24, 3512–3515. [DOI] [PubMed] [Google Scholar]
- (20).Ciamician GL; Dennstedt M Ueber Die Einwirkung Des Chloroforms Auf Die Kaliumverbindung Pyrrols. Ber. Dtsch. Chem. Ges 1881, 14, 1153–1163. [Google Scholar]
- (21).(a) Dhanak D; Reese C Synthesis of [6](2,4)pyridinophanes. J. Chem. Soc., Perkin Trans 1 1987, 2829–2832. [Google Scholar]; (b) Raheem IT; Thiara PS Jacobsen, E. N. Regio- and Enantioselective Catalytic Cyclization of Pyrroles onto N-Acyliminium Ions. Org. Lett 2008, 10, 1577–1580. [DOI] [PubMed] [Google Scholar]; (c) Dherange BD; Kelly PQ; Liles JP; Sigman MS; Levin MD Carbon Atom Insertion into Pyrroles and Indoles Promoted by Chlorodiazirines. J. Am. Chem. Soc 2021, 143, 11337–11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Wynberg H The Reimer-Tiemann Reaction. Chem. Rev 1960, 60, 169–184. [Google Scholar]
- (23).(a) Yang Y; Bai Y; Sun S; Dai M Biosynthetically Inspired Divergent Approach to Monoterpene Indole Alkaloids: Total Synthesis of Mersicarpine, Leuconodines B and D, Leuconoxine, Melodinine E, Leuconolam, and Rhazinilam. Org. Lett 2014, 16, 6216–6219. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Nakahara K; Hirano K; Maehata R; Kita Y; Fujioka H Asymmetric Total Synthesis of Clavolonine. Org. Lett 2011, 13, 2015–2017. [DOI] [PubMed] [Google Scholar]; (c) Chen J; Forsyth CJ Synthesis of the Apratoxin 2,4-Disubstituted Thiazoline via an Intramolecular Aza-Wittig Reaction. Org. Lett 2003, 5, 1281–1283. [DOI] [PubMed] [Google Scholar]
- (24).Meng L Total Synthesis of (−)-Carinatine A and (+)-Lycopladine A. J. Org. Chem 2016, 81, 7784–7789. [DOI] [PubMed] [Google Scholar]
- (25).Linghu X; Kennedy-Smith JJ; Toste FD Total Synthesis of (+)-Fawcettimine. Angew. Chem. Int. Ed 2007, 46, 7671–7673. [DOI] [PubMed] [Google Scholar]
- (26).Li H; Shen S-J; Zhu C-L; Xu H Direct Intermolecular Anti-Markovnikov Hydroazidation of Unactivated Olefins. J. Am. Chem. Soc 2019, 141, 9415–9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Li X; Chen P; Liu G Iodine(III) reagent (ABN-N3)-induced intermolecular anti-Markovnikov hydroazidation of unactivated alkenes. Sci. China Chem 2019, 62, 1537–1541. [Google Scholar]
- (28).Aube J; Milligan GL Intramolecular Schmidt reaction of alkyl azides. J. Am. Chem. Soc 1991, 113, 8965–8966. [Google Scholar]
- (29).CCDC 2101755 contains the supplementary crystallographic data for compound 36.
- (30).Gros P; Choppin S; Mathieu J; Fort Y Lithiation of 2-Heterosubstituted Pyridines with BuLi-LiDMAE: Evidence of Regiospecificity at C-6. J. Org. Chem 2002, 67, 234–237. [DOI] [PubMed] [Google Scholar]
- (31).Campeau L-C; Schipper DJ; Fagnou K Site-Selective sp2 and Benzylic sp3 Palladium-Catalyzed Direct Arylation. J. Am. Chem. Soc 2008, 130, 3266–3267. [DOI] [PubMed] [Google Scholar]
- (32).Welin ER; Ngamnithiporn A; Klatte M; Lapointe G; Pototschnig GM; McDermott MSJ; Conklin D; Gilmore CD; Tadross PM; Haley CK; Negoro K; Glibstrup E; Grünanger CU; Allan KM; Virgil SC; Slamon DK; Stoltz BM Concise total syntheses of (−)-jorunnamycin A and (−)-jorumycin enabled by asymmetric catalysis. Science 2019, 363, 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.