Abstract
Localized sets of random point mutations generated by PCR amplification can be transferred efficiently to the chromosome of Acinetobacter ADP1 (also known as strain BD413) by natural transformation. The technique does not require cloning of PCR fragments in plasmids: PCR-amplified DNA fragments are internalized by cells and directly incorporated into their genomes by homologous recombination. Previously such procedures for random mutagenesis could be applied only to Acinetobacter genes affording the selection of mutant phenotypes. Here we describe the construction of a vector and recipient that allow for mutagenesis, recovery, and expression of heterologous genes that may lack a positive selection. The plasmid carries an Acinetobacter chromosomal segment interrupted by a multiple cloning site next to a kanamycin resistance marker. The insertion of heterologous DNA into the multiple cloning site prepares the insert as a target for PCR mutagenesis. PCR amplifies the kanamycin resistance marker and a flanking region of Acinetobacter DNA along with the insert of heterologous DNA. Nucleotide sequence identity between the flanking regions and corresponding chromosomal segments in an engineered Acinetobacter recipient allows homologous recombination of the PCR-amplified DNA fragments into a specific chromosomal docking site from which they can be expressed. The recipient strain contains only a portion of the kanamycin resistance gene, so donor DNA containing both this gene and the mutagenized insert can be selected by demanding growth of recombinants in the presence of kanamycin. The effectiveness of the technique was demonstrated with the relatively GC-rich Pseudomonas putida xylE gene. After only one round of PCR amplification (35 cycles), donor DNA produced transformants of which up to 30% carried a defective xylE gene after growth at 37°C. Of recombinant clones that failed to express xylE at 37°C, about 10% expressed the gene when grown at 22°C. The techniques described here could be adapted to prepare colonies with an altered function in any gene for which either a selection or a suitable phenotypic screen exists.
The analysis of how structure influences the function of a protein benefits from the availability of a spectrum of point mutations in the encoding gene. The randomness of nucleotide substitutions by thermostable polymerases in the PCR makes it a good candidate for the generation of such mutations (1, 15, 22, 23, 25). The investigation of how random mutations may change the properties of a protein benefits from a biological system in which their individual phenotypes may be either selected or screened in vivo, preferably expressed from a chromosomal background.
Recently it has been shown that PCR-generated mutations can be targeted to chromosomal Acinetobacter genes by the direct coupling of mutagenesis during PCR amplification to the uptake of the amplified DNA segments by natural transformation (10, 11). This procedure allows for the easy recovery of strains carrying nonpolar single nucleotide substitutions in chromosomal genes, provided there is a selection for the mutant phenotype (10, 11). Thus, a vast number of independently generated mutant alleles, many with conditional phenotypes, were recovered from the chromosomal pobR gene encoding the regulator of 4-hydroxybenzoate degradation in Acinetobacter (4, 5). Acinetobacter forms an ideal recipient for the chromosomal integration of PCR-generated alleles, as the natural transformation system of the organism (6–8, 18, 19) is highly efficient and accepts linear DNA fragments that have been amplified by PCR. Moreover, unlike many other transformable organisms, Acinetobacter displays a constitutive elevation of the level of recombination (RecA) activity that does not require specific induction upon induction of competence for natural transformation (21).
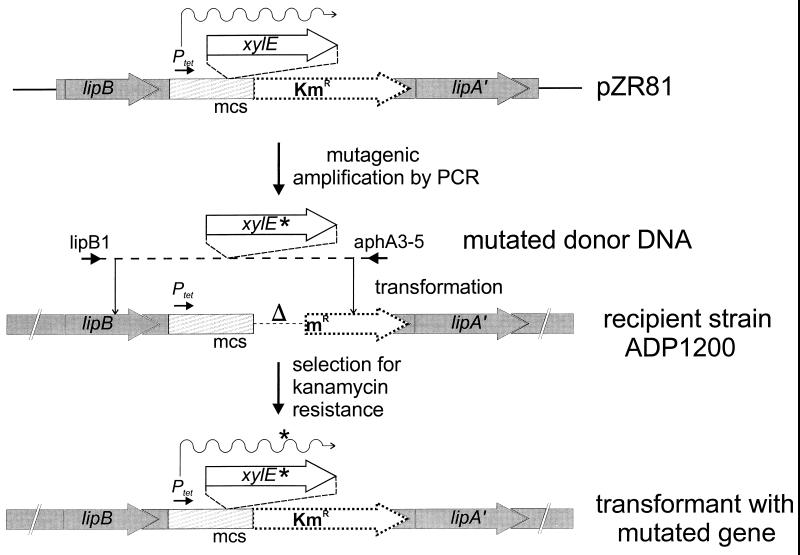
A limit to the mutagenesis system described above is that it requires a selection for strains containing mutant alleles. In addition, it can only be applied to genes from naturally transformable organisms. To overcome these limitations, we have constructed a specific Acinetobacter recipient (ADP1200) and a cloning vector (pZR80) that together allow for the easy chromosomal recovery of mutant alleles of virtually any gene of either homologous or heterologous origin (Fig. 1). The cloned gene is expressed from a constitutive promoter and is amplified together with a functional kanamycin marker by PCR. The PCR fragments are used directly as donor DNA in the transformation of the Acinetobacter recipient strain, leading to the integration of the PCR-amplified DNA into a chromosomal docking site formed by the Acinetobacter lipBA operon (13, 14). Selection for kanamycin resistance results in a population of Acinetobacter strains, each carrying a PCR-generated copy of the cloned gene. The procedure yields only cells that have incorporated the heterologous gene into their chromosomes. This allows for ready screening of colonies in which the gene product has an altered function. In the example given here, up to 30% of the strains retrieved after kanamycin selection expressed a defective mutant allele of the cloned heterologous xylE gene from Pseudomonas putida.
FIG. 1.
Random PCR mutagenesis of a heterologous gene (exemplified by P. putida xylE) and recovery of mutated genes for expression from a targeted site in the Acinetobacter chromosome. The plasmid pZR81 was prepared by the insertion of xylE into the multiple cloning site (mcs) of expression vector pZR80, downstream from the constitutive tet promoter (Ptet), and flanked by sequences homologous to the chromosome of recipient strain ADP1200. Strain ADP1200 lacks a functional aphA3 gene encoding kanamycin resistance. PCR was used to amplify xylE together with portions of lipB and aphA3, the latter encoding resistance to kanamycin. PCR DNA that may carry a PCR-generated mutation (∗) was used to transform ADP1200 to kanamycin resistance. A significant fraction of the kanamycin-resistant recombinants contained a mutant allele of the P. putida xylE docked into the chromosome of ADP1200. Thus, the procedure provides a way to produce and express single-copy mutant alleles of genes that lack a selectable mutant phenotype.
MATERIALS AND METHODS
Construction of pZR80 for PCR mutagenesis of DNA inserts.
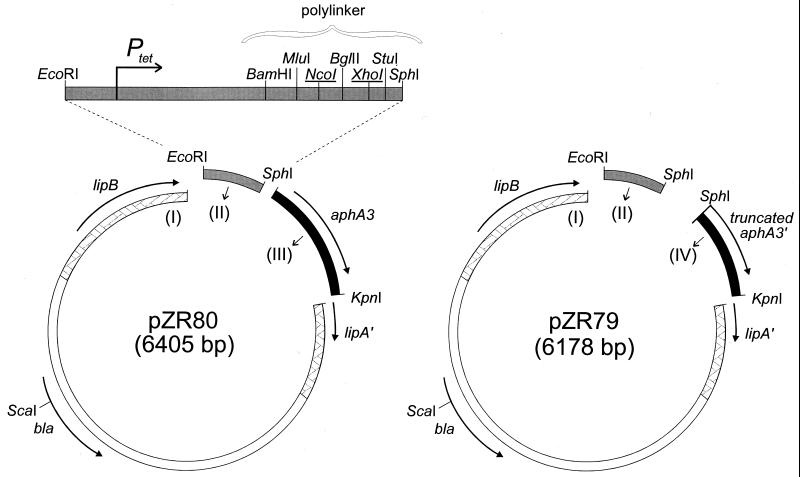
The basis of pZR80 is the ColE1 plasmid pALJA434 (12), which carries the lipBA operon of Acinetobacter ADP1 as a 3.0-kbp EcoRV-SalI insert. Plasmid pZR80 was constructed in a triple ligation step from three fragments (Fig. 2).
FIG. 2.
One-step construction of plasmids pZR80 and pZR79 from three independent restriction fragments, numbered I (white), II (grey), and III (black) for pZR80 and I, II, and IV (black) for pZR79. The individual fragments have been marked, as have the three relevant restriction sites used for their cloning. Acinetobacter DNA present on the ColE1 vector fragment I is crosshatched. Fragment II has been enlarged at the top of the figure to show the presence of the constitutive Ptet and the unique cloning sites present downstream of this promoter in the small polylinker. Plasmid pZR79 was used to generate transformation recipient ADP1200 and carries a truncated aphA3 gene (aphA3′; see the text).
(i) Fragment I.
Plasmid pALJA434 contains a unique EcoRI site engineered near the start of lipA, in addition to a unique KpnI site further downstream in lipA. pALJA434 was digested with EcoRI and KpnI, and a 5.2-kbp fragment was isolated.
(ii) Fragment II.
Vector pMTL21p (3) carries an asymmetric polylinker. The 185-bp EcoRI-EcoRV fragment of pBR322, carrying part of the tet gene with the constitutive tet promoter (Ptet), was cloned into pMTL21p, which had been digested with EcoRI and SmaI. The resulting plasmid was digested with EcoRI and SphI, and a 247-bp fragment that contained most of the polylinker of pMTL21p downstream of Ptet was isolated (Fig. 2).
(iii) Fragment III.
The kanamycin resistance gene (aphA3) of pZR80 was derived from pPJ1 (20). The aphA3 gene was amplified by PCR, with pPJ1 as the template DNA and with primer aphA3-1 (5′-GAATGCATGCAACAGTGAATTGGAGTTCG-3′), which anneals upstream of the aphA3 promoter and carries an SphI site (underlined) due to two base changes (double underlined), and aphA3-2 (5′-GCGGAGGTACCTCAGAAAAGATTAGATGTC-3′), which anneals downstream of aphA3 and contains a KpnI site due to two base changes. The resulting 963-bp PCR fragment was digested with SphI and KpnI to yield fragment III.
In a single forced-ligation step (Fig. 2), equimolar amounts of fragments I, II, and III were ligated to yield pZR80, with selection for resistance to ampicillin and kanamycin in transformed Escherichia coli DH5α. Vector pZR80 carries the constitutive promoter Ptet upstream of the polylinker of pMTL21p (with BamHI, MluI, NcoI, BglII, XhoI, StuI, and SphI sites left as unique cloning sites) and the functional aphA3 marker all cloned into the Acinetobacter lipBA operon (Fig. 2); the nucleotide sequence of part of pZR80 carrying Ptet and the polylinker (to the start of aphA3) is depicted in Fig. 3.
FIG. 3.
Nucleotide sequence of the region of pZR80 from EcoRI to the start of the functional kanamycin resistance gene aphA3. The fragment carries the promoter of the tet gene of pBR322 (Ptet) that is constitutive in Acinetobacter and part of the polylinker of pMTL21p (3) (doubly underlined). Unique restriction sites (in pZR80) that may be relevant for cloning have been marked above the polylinker. Relevant promoter elements have been marked and are shown in bold. The region that corresponds to the truncated tet′ gene of pBR322 has been underlined. The start of the aphA3 gene has been marked in italics, with the encoded initial methionine in AphA3 shown (Met).
Construction of the recipient, Acinetobacter ADP1200.
Acinetobacter ADP1200 was used as the recipient for transformation with PCR fragments amplified with pZR80-derived plasmids as template DNA (such as pZR81; Fig. 1). Strain ADP1200 carries an insert in the lipBA operon similar to the insert in pZR80, except that it lacks a 227-bp fragment carrying the aphA3 promoter and the first part of the aphA3 gene. A derivative of pZR80, designated pZR79, was used for the generation of ADP1200. Plasmid pZR79 was constructed essentially in the same way as that described for pZR80, except that an alternative to fragment III was created by using an internal aphA3 primer, aphA3 (5′-GACGGCATGCCGGTATAAAGGGACCAC-3′), which carries an engineered SphI site, in conjunction with primer aphA3-2. The resulting 736-bp PCR fragment was digested with SphI and KpnI to yield fragment IV, with a truncated aphA3 gene. The ligation of fragment IV with the above-described fragments I and II yielded plasmid pZR79 (Fig. 2). After linearization with ScaI (which cuts in the vector part), pZR79 was used for the transformation of Acinetobacter ADP1. Strains were plated onto a nonselective medium to yield a few hundred colonies per plate, and colonies were transferred to lipase indicator plates with egg yolk and NaCl to be screened for loss of lipase activity, as described previously (9). Among 554 strains, seven failed to produce a turbid zone around the colony, indicating a loss of lipase activity. These strains did not express the ampicillin resistance marker of pZR79. Through PCR, one strain, designated ADP1200, was verified to contain the proper insert of fragments II and IV in the lipBA operon on the chromosome.
Construction of pZR81 and mutagenesis of xylE.
Plasmid pZR81 was generated by cloning a 1.4-kbp NcoI-XhoI fragment from plasmid pUC18Sfi-HA, carrying the xylE gene from the no. 1 meta operon in the P. putida TOL plasmid pWW53 (17), between the unique NcoI and XhoI sites in the polylinker of pZR80 (Fig. 1 and 2), and selecting for resistance to ampicillin and kanamycin in transformed E. coli DH5α. The xylE gene does not carry its own promoter but is expressed from Ptet in pZR81 (Fig. 1). For the mutagenesis of the P. putida xylE gene, part of pZR81 was amplified with primers annealing in the Acinetobacter lipB gene, lipB1 (5′-TGCAGGGCTGTTCGGCTCAG-3′), and in aphA3, aphA3-5 (5′-GGCAATGTCATACCACTTGT-3′) (Fig. 1). The same primers were used to demonstrate that the inserts in recombinants failing to express xylE were the same size as the inserts in strains that expressed xylE.
Mutagenesis and screening differences in phenotypic expression of wild-type and mutant xylE genes after their introduction into Acinetobacter by transformation.
Taq polymerase was used, as indicated by the supplier (Boehringer Mannheim), for the amplification of xylE-aphA3 fragments for transformation. PCRs were carried out with 200 nM concentrations each of primers lipB1 and aphA3-5, 200 μM concentrations of each deoxynucleoside triphosphate, between 5 and 10 ng of template DNA (pZR81), and 0.5 U of polymerase in a final volume of 50 μl. The thermocycle protocol consisted of a total of 35 cycles, with a denaturation step at 94°C, primer annealing at 58°C, and elongation at 72°C. The DNA fragments produced by PCR were isolated from an agarose gel and directly used to transform ADP1200, according to the standard procedure for the natural transformation of ADP1-derived strains (10), followed by selection for recombinants on a mineral medium with 10 mM succinate supplemented with 30 μg of kanamycin per ml. Ten preparations of xylE-aph3A DNA were amplified separately with Taq polymerase and used to prepare kanamycin-resistant transformants from strain ADP1200. No more than 10 kanamycin-resistant recombinants were selected after each transformation to reduce the chance of picking identical mutants. Selected strains were subsequently screened for the expression of either functional or defective XylE by spraying colonies formed overnight with a 100 μM catechol solution.
Sequence analysis of mutations.
The primers lipB1 and aphA3-5 (Fig. 1) were used at concentrations of 40 nM to amplify mutant xylE DNA from the chromosome with Taq polymerase. Without further purification, 200 to 300 ng of the PCR DNA was directly used as template DNA in cycle sequence reactions, with the ABI PRISM dye terminator cycle sequencing kit with Amplitaq DNA polymerase (−FS) as recommended by the supplier (Perkin-Elmer). Further sequencing procedures were described previously (10).
Nucleotide sequence accession number.
The nucleotide sequence of the xylE insert in pZR81 has been deposited with GenBank under accession no. AF102891.
RESULTS
Integration of wild-type and mutated P. putida xylE genes into the Acinetobacter chromosomal docking site.
After the transformation of ADP1200 with pZR81 DNA that had replicated in vivo, all 270 tested kanamycin-resistant recombinants expressed the GC-rich P. putida xylE gene, as evidenced by α-hydroxymuconic semialdehyde formation. In contrast, only 70% (443 of 629 colonies tested) of recombinant colonies emerging after transformation with PCR-amplified DNA produced a functional enzyme, as judged by the formation of yellow color from catechol after growth of the cells at 37°C. Of the colonies that failed to express functional XylE at 37°C, 10% formed active XylE during growth at 22°C. It therefore is apparent that the xylE gene in these recombinant strains contained a conditional mutation allowing the formation of a functional gene product at the lower temperature.
The presence of a DNA insert with predicted size and location in the recombinant strains was demonstrated with primers lipB1 and aphA3-5 (Fig. 1). Ten strains that failed to express xylE produced a DNA fragment of 3.1 kb; strains lacking the xylE insert formed a DNA fragment of 0.6 kb (data not shown). Therefore, it can be concluded that the failure of these strains to express xylE is due to a defective xylE gene rather than the absence of the gene in strains that had acquired the kanamycin resistance marker.
Nucleotide sequences of mutant xylE genes.
In order to demonstrate the ease of phenotypic observation of PCR-generated mutations introduced into Acinetobacter by transformation, highly mutagenized xylE was used as a donor; about 30% of the resulting transformants exhibited defects in XylE (Fig. 1). As might be expected, a sample of xylE from strains with null phenotypes revealed a number of genes with multiple mutations (Table 1). Therefore, sequencing focused upon genes exhibiting either a heat-sensitive phenotype or a leaky phenotype, because it seemed likely that XylE from such organisms had undergone relatively subtle mutations impairing but not destroying enzyme activity. Most of these strains contained xylE with a single nucleotide substitution (Table 1).
TABLE 1.
Genotypes and phenotypes of Acinetobacter mutant strains
| Strain | Mutant gene(s) | xylE substi-tutionsa | XylE sub-stitutions | Phenotype |
|---|---|---|---|---|
| ADP1203 | None | None | None | Wild type |
| ADP1204 | xylE1204 | C559T | R187C | Heat-sensitive |
| ADP1205 | xylE1205 | A371G | E124G | Heat-sensitive |
| ADP1206 | xylE1206 | T208C | F70L | Heat-sensitive |
| xylE1306 | G853A | D285N | ||
| ADP1207 | xylE1207 | T893C | L298P | Heat-sensitive |
| ADP1208 | xylE1208 | A7G | K3E | Heat-sensitive |
| ADP1210 | xylE1210 | T502C | F168L | Heat-sensitive |
| ADP1212 | xylE1212 | T469A | Y157N | Null |
| ADP1213 | xylE1213 | C413T | A138V | Heat-sensitive |
| ADP1214 | xylE1214 | C12T (GCC → GCT) | Silent | Heat-sensitive |
| xylE1314 | A245G | E82G | ||
| ADP1215 | xylE1215 | A21G (CGA → CGG) | Silent | Leaky |
| ADP1216 | xylE1216 | G168A (GTG → GTA) | Silent | Heat-sensitive |
| xylE1316 | G782A | G261D | ||
| ADP1217 | xylE1217 | A703G | T244A | Heat-sensitive |
| ADP1219 | xylE1219 | C880T | H294Y | Heat-sensitive |
| ADP1220 | xylE1220 | A185G | E62P | Null |
| xylE1320 | T575C | L192P | ||
| ADP1221 | xylE1221 | T502C | F168L | Null |
| xylE1321 | G688A | D230N | ||
| ADP1222 | xylE1222 | G39C (CTG → CTC) | Silent | Null |
| xylE1322 | T542C | L181P | ||
| ADP1227 | xylE1227 | T14C | V5A | Null |
| xylE1327 | A730G | T244A | ||
| xylE1328 | A884G | D295G |
Silent codon changes are shown in parentheses.
DISCUSSION
Direct phenotypic identification of genetic defects generated by PCR.
A benefit offered by Acinetobacter natural transformation is that the phenotypic consequence of a mutation can be expressed in a recombinant colony shortly after the exposure of recipient cells to the modified DNA. In the present system, the mutated genes are expressed in a single copy from the recombinant chromosome, so that recessive alleles are unlikely to be masked by their dominant counterparts as might happen with genes expressed from multicopy plasmids. The tet promoter used here allows the constitutive expression of the cloned gene, so that defective genes can be identified in cells grown without an inducer. All that is required for the identification of phenotypic variants of the amplified gene is a simple screening procedure that monitors the activity of the gene product.
Comparison of amino acid substitutions causing XylE defects with amino acids that have been conserved during evolutionary divergence of XylE proteins.
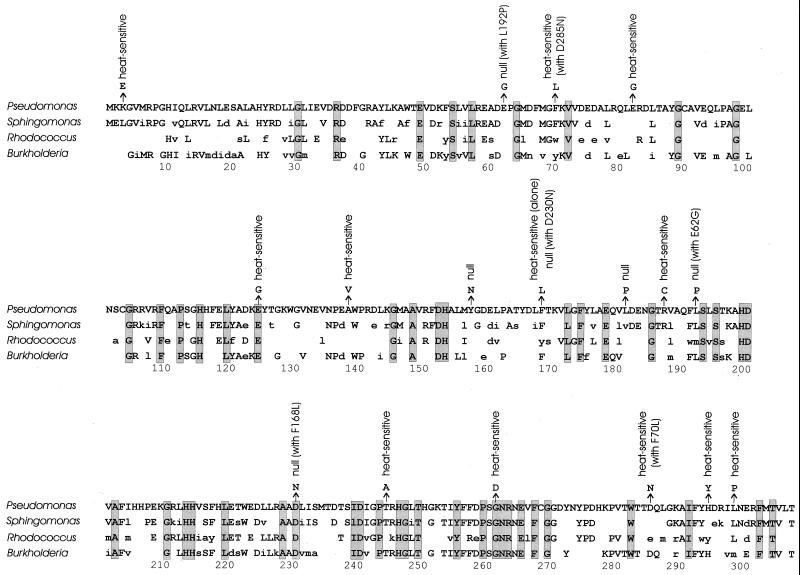
In order to assess the significance of PCR-generated amino acid substitutions in XylE, they were compared with residues that have been conserved in divergent proteins from three distant bacterial genera (Fig. 4). As a rough estimate, it might be assumed that highly conserved amino acid residues would be relatively sensitive to mutations causing defects that would be evident in the phenotypic screen. On the other hand, amino acid substitutions at loci where divergence had been accommodated during evolution might be expected to escape detection. To some extent these expectations were fulfilled. Thus, the substitutions E124G and G261D cause radical chemical changes in conserved residues and result in defective enzymes. Perhaps more noteworthy is the fact that the mutant enzymes are only partially defective, because they function at room temperature. Since the different cell lines are unlikely to have encountered elevated temperatures frequently during their evolution, the selective basis for the conservation of these residues becomes an open question.
FIG. 4.
Comparison of amino acid substitutions causing defects in Pseudomonas XylE with amino acids conserved during divergence of XylE in Sphingomonas (24), Rhodococcus (2), and Burkholderia (16). This presentation does not take into account gaps required for optimal alignment. Amino acid residues identical to those in Pseudomonas XylE are in uppercase, similar amino acids are in lowercase, and different amino acids are not shown. Shaded boxes mark amino acid residues conserved in all four proteins.
In some cases amino acid substitutions with seemingly minor chemical effects resulted in defective enzymes. Thus, A138V, a conservative substitution at a position where evolutionary divergence had been tolerated, rendered Pseudomonas XylE heat sensitive. In two cases, F70L and F168L, substitutions at positions where various aromatic acid substitutions had been tolerated by evolution resulted in heat-sensitive enzymes. The H294Y substitution renders Pseudomonas XylE defective by the introduction of an amino acid residue that is maintained in the Rhodococcus enzyme.
Limitation of expression of heterologous genes imposed by demands for coding in Acinetobacter.
A potential limitation of the procedure for analysis of heterologous genes was the possibility that Acinetobacter, generally possessing genes with G+C content in the range between 40 and 46%, might be unable to express genes with a relatively high G+C content. For this reason, the P. putida xylE gene with a G+C content of 59.6% was selected for investigation. At least in this particular instance, the high G+C content of the inserted wild-type gene presented no obvious barrier to its expression. A limit may have been pushed, however, by xylE1215, which causes the substitution of one arginine codon (CGA) by another (CGG) at the position encoding residue 7 within the protein. The usage of CGG is not unusual in Pseudomonas genes (wild-type xylE already contains three such codons) but is highly exceptional in Acinetobacter. A survey of 14 cat, qui, pob, and pca structural genes with typical Acinetobacter G+C contents revealed that of 191 arginine codons, only two are CGG (18 are CGA). It appears likely that the additional demand for CGG coding imposed by xylE1215 lowers the level of expression of this gene in Acinetobacter. Nevertheless, the expression of wild-type Pseudomonas xylE demonstrates that it should be possible to subject genes from a range of biological sources to structure-function analysis as assessed by a variation in activity as a consequence of mutations acquired by PCR mutagenesis. Such variations may include alterations in specificity (11) as well as a loss of function.
ACKNOWLEDGMENTS
This research was supported by the United States Army Research Office, the National Science Foundation, and the General Reinsurance Corporation. David Young was supported by a predoctoral fellowship from the Department of Education and the DuPont Corporation.
We thank Victor de Lorenzo for the plasmid pUC18Sfi-HA and Peter A. Williams for his stimulating suggestions during the course of the investigation.
Footnotes
This is publication 18 from the Biological Transformation Center in the Yale Institute for Biospheric Studies.
REFERENCES
- 1.Cadwell R C, Joyce G F. Randomization of genes by PCR mutagenesis. PCR Methods Appl. 1992;2:28–33. doi: 10.1101/gr.2.1.28. [DOI] [PubMed] [Google Scholar]
- 2.Candidus S, van Pee K H, Lingens F. The catechol 2,3-dioxygenase gene of Rhodococcus rhodochrous CTM: nucleotide sequence, comparison with isofunctional dioxygenases and evidence for an active-site histidine. Microbiology. 1994;140:321–330. doi: 10.1099/13500872-140-2-321. [DOI] [PubMed] [Google Scholar]
- 3.Chambers S P, Prior S, Barstow D B, Minton N P. The pMTL series of cloning vectors: I. Improved polylinker regions to facilitate the generation of sonicated DNA for nucleotide sequencing. Gene. 1988;68:139–149. doi: 10.1016/0378-1119(88)90606-3. [DOI] [PubMed] [Google Scholar]
- 4.DiMarco A A, Averhoff B, Ornston L N. Identification of the transcriptional activator pobR and characterization of its role in the expression of pobA, the structural gene for p-hydroxybenzoate hydroxylase in Acinetobacter calcoaceticus. J Bacteriol. 1993;175:4499–4506. doi: 10.1128/jb.175.14.4499-4506.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiMarco A A, Ornston L N. Regulation of p-hydroxybenzoate hydroxylase synthesis by PobR bound to an operator in Acinetobacter calcoaceticus. J Bacteriol. 1994;176:4277–4284. doi: 10.1128/jb.176.14.4277-4284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972;112:917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juni E. Simple genetic transformation assay for rapid diagnosis of Moraxella osloensis. Appl Microbiol. 1974;27:16–24. doi: 10.1128/am.27.1.16-24.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juni E, Janik A. Transformation of Acinetobacter calcoaceticus (Bacterium anitratum) J Bacteriol. 1969;98:281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kok R G, Christoffels V M, Vosman B, Hellingwerf K J. Growth-phase dependent expression of the lipolytic system of Acinetobacter calcoaceticus BD413: cloning of a gene encoding one of the esterases. J Gen Microbiol. 1993;139:2329–2342. doi: 10.1099/00221287-139-10-2329. [DOI] [PubMed] [Google Scholar]
- 10.Kok R G, D’Argenio D A, Ornston L N. Combining localized PCR mutagenesis and natural transformation in direct genetic analysis of a transcriptional regulator gene, pobR. J Bacteriol. 1997;179:4270–4276. doi: 10.1128/jb.179.13.4270-4276.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kok R G, D’Argenio D A, Ornston L N. Mutation analysis of PobR and PcaU, closely related transcriptional activators in Acinetobacter. J Bacteriol. 1998;180:5058–5069. doi: 10.1128/jb.180.19.5058-5069.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kok R G, Nudel C B, Gonzalez R H, Nugteren-Roodzant I M, Hellingwerf K J. Physiological factors affecting production of extracellular lipase (LipA) in Acinetobacter calcoaceticus BD413: fatty acid repression of lipA expression and degradation of LipA. J Bacteriol. 1996;178:6025–6035. doi: 10.1128/jb.178.20.6025-6035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kok R G, van Thor J J, Nugteren-Roodzant I M, Brouwer M B W, Egmond M R, Nudel C B, Vosman B, Hellingwerf K J. Characterization of the extracellular lipase, LipA, of Acinetobacter calcoaceticus BD413 and sequence analysis of the cloned structural gene. Mol Microbiol. 1995;15:803–818. doi: 10.1111/j.1365-2958.1995.tb02351.x. [DOI] [PubMed] [Google Scholar]
- 14.Kok R G, van Thor J J, Nugteren-Roodzant I M, Vosman B, Hellingwerf K J. Characterization of lipase-deficient mutants of Acinetobacter calcoaceticus BD413: identification of a periplasmic lipase chaperone essential for the production of extracellular lipase. J Bacteriol. 1995;177:3295–3307. doi: 10.1128/jb.177.11.3295-3307.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung D W, Chen E, Goeddel D V. A method for random mutagenesis of a defined DNA segment using a modified polymerase chain reaction. Technique. 1989;1:11–15. [Google Scholar]
- 16.Ma, Y., and D. S. Herson. Unpublished data.
- 17.Osborne D J, Pickup R W, Williams P A. The presence of two complete homologous meta pathway operons on TOL plasmid pWW53. J Gen Microbiol. 1988;134:2965–2967. doi: 10.1099/00221287-134-11-2965. [DOI] [PubMed] [Google Scholar]
- 18.Palmen R, Vosman B, Buijsman P, Breek C K D, Hellingwerf K J. Physiological characterization of natural transformation in Acinetobacter calcoaceticus. J Gen Microbiol. 1993;139:295–305. doi: 10.1099/00221287-139-2-295. [DOI] [PubMed] [Google Scholar]
- 19.Palmen R, Vosman B, Kok R, van der Zee J R, Hellingwerf K J. Characterization of transformation-deficient mutants of Acinetobacter calcoaceticus. Mol Microbiol. 1992;6:1747–1754. doi: 10.1111/j.1365-2958.1992.tb01347.x. [DOI] [PubMed] [Google Scholar]
- 20.Peeters B P H, de Boer J H, Bron S, Venema G. Structural plasmid instability in Bacillus subtilis: effect of direct and inverted repeats. Mol Gen Genet. 1988;212:450–458. doi: 10.1007/BF00330849. [DOI] [PubMed] [Google Scholar]
- 21.Rauch P J G, Palmen R, Burds A A, Gregg-Jolly L A, van der Zee J R, Hellingwerf K J. The expression of the Acinetobacter calcoaceticus recA gene increases in response to DNA damage independently of RecA and of development of competence for natural transformation. Microbiology. 1996;142:1025–1032. doi: 10.1099/00221287-142-4-1025. [DOI] [PubMed] [Google Scholar]
- 22.Spee J H, de Vos W M, Kuipers O P. Efficient random mutagenesis method with adjustable mutation frequency by use of PCR and dITP. Nucleic Acids Res. 1993;21:777–778. doi: 10.1093/nar/21.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tindall K R, Kunkel T A. Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry. 1988;27:6008–6013. doi: 10.1021/bi00416a027. [DOI] [PubMed] [Google Scholar]
- 24.Yrjala K, Paulin L, Kilpi S, Romantschuk M. Cloning of cmpE, a plasmid-borne catechol 2,3-dioxygenase-encoding gene from the aromatic- and chloroaromatic-degrading Pseudomonas sp. HV3. Gene. 1994;138:119–121. doi: 10.1016/0378-1119(94)90792-7. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Zhang X, Ebright R H. Random mutagenesis of gene-sized DNA molecules by use of PCR with Taq DNA polymerase. Nucleic Acids Res. 1991;19:6052. doi: 10.1093/nar/19.21.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]