Abstract
Background
Cinnamon (Cinnamomum zeylanicum) and Clove (Syzygium aromaticum) essential oils are two medicinally important plant-derived substances with a wide range of biological properties. Besides, nanoemulsion-based gels have been widely used to increase topical drug delivery and effectiveness.
Methods
This study aimed to explore the anti-inflammatory effect (paw edema test) and the anti-nociceptive effect (hot plate and formalin test) of nanoemulsion-based gels containing the essential oils in the animal model. Cinnamon and Clove essential oils nanoemulsions with droplet sizes of 28 ± 6 nm and 12 ± 3 nm were first prepared. By adding carboxymethylcellulose (3.5% w/v), the nanoemulsions were then gelified. Finally, the nanogels were characterized by ATR-FTIR analysis and were used as topical pre-treatment before induction of inflammation or pain in acute and chronic analgesic experimental studies.
Results
The paw edema and formalin findings showed that the nanogels formulations possess significant anti-nociceptive and anti-inflammatory effects.
Conclusion
The prepared nanogels could be considered as analgesic drugs for inhibiting the inflammation and pain of diseases.
Keywords: Analgesics, Nanomedicine, Painkiller, Paw edema test
Introduction
Inflammation, as a natural reaction at the site of external injurious (events) environments, plays a vital role in the pathogenesis of many chronic diseases such as rheumatoid arthritis, diabetes, and cancer [1, 2]. If the inflammation is severe at the tissue level, damage to nerves can cause pain signals that transmit through neurons to the brain [3, 4]. Nowadays, knowledge about pain and its mechanisms, especially neurophysiological and neuropathic pains, has increased significantly [5]. For example, emerging evidence suggests that inflammation and the release of inflammatory mediators from damaged tissues can cause pain [6]. Anti-inflammatory or painkiller nanodrugs drugs such as steroidal and non-steroidal are commonly used for inflammatory diseases and their related pains while having limited efficiency with side effects [7, 8]. Therefore, attempts to develop green nanodrugs as an important source of novel therapeutics have received more attention recently [9, 10].
Essential oils (EO)s as secondary metabolites of plants have recently been considered to treat inflammation and pain [11, 12]. For instance, Cinnamon EO (Cinnamomum zeylanicum), a spice derived from the inner bark of the genus Cinnamomum trees, has promising inflammatory effects [13, 14]. Some reports regarding Cinnamon’s anti-nociceptive and antipyretic effects in bronchitis, rheumatism, cold, fever, headache, and muscular pain [15, 16]. Also, the Cloves (Syzygium aromaticum) belongs to the Myrtaceae family, which is a nail-shaped dried flower bud [17, 18]; its EO has been traditionally applied in aromatherapy, relieving headaches, joint pain, toothaches, and oral antiseptic [19, 20]. Furthermore, Clove EO has also been used in dental emergencies as an asymptomatic reliever of toothache and anti-inflammatory in the mouth and throat [21, 22]. Also, other applications of Clove EO and Clove extracts have been reported, such as antimutagenic, antioxidant, antithrombotic, antiparasitic, antibacterial, antiviral, and antifungal activities [23, 24].
Formulating of EOs as nanoformulations is a promising approach for increasing their efficacy [25, 26]. Among the nanoformulations, nanoemulsions are more considered due to their relatively fewer side effects, bioavailability, and simpler preparation methods [27–29]. Nanoemulsions are biphasic transparent dispersions composed of oil and water phases stabilized by surfactants/co-surfactant. Such systems are stable droplets over aggregation or creaming processes because of their droplet size (less than 200 nm) [30, 31]. Nanoemulsions as effective topical delivery systems have demonstrated favorable characteristics, including enhanced permeability without skin irritations [32, 33]. However, transforming nanoemulsions to gel improves the topical administration and physical and thermal stability of EOs [34, 35].
To the best of our knowledge, the anti-nociceptive and anti-inflammatory effects of nanoemulsion-based gels of Cinnamon and Clove EOs have not been investigated. Therefore, their efficacy as a topical delivery system against inflammation and nociception was investigated in this study.
Materials and methods
Materials
λ-Carrageenan and carboxymethylcellulose (medium viscosity) were supplied from Sigma-Aldrich (Germany). Formalin (HCHO) and tween 20 were purchased from Merck Chemicals (Germany). Cinnamon and Clove EOs were bought from Green Plants of Life Co. and Zardband Pharmaceuticals Co. (Iran).
Twenty-four Wistar male rats weighing 180 ± 20 g were used. They were kept in the Standard Laboratory Animal Guidelines, and the experimental protocols were approved by the Tehran University of Medical Sciences Ethical Committee (code 91–01–87-17,072). Rats were randomly divided into 4 groups (n = 6). Each group was treated with 100 mg of the following samples, including distilled water as negative control (D.W.), blank gel, Cinnamon-nanogel (cinnamon- NG), and Clove-nanogel (clove-NG).
Preparation and characteristics of nanoemulsions-based gels of Cinnamon and Clove EOs
For the preparation of nanoemulsions, at first, Cinnamon and Clove EOs (2.5% v/v) were added to tubes containing tween-20 (7.5 and 10% v/v, respectively), which were on stirrer equipment at 2000 rpm at room temperature (MS-300HS, Protraction Intertrade Co., Korea). Then, distilled water was added at room temperature dropwise to the desired volume, stirring at 2000 rpm for 40 minutes. Next, nanogels were prepared by adding carboxymethylcellulose (CMC 3.5% w/v) to the as-prepared nanoemulsions while mixing in a mild condition (180 rpm) for 4 h. The prepared gels were abbreviated as cinnamon-NG and clove-NG. Similarly, a blank gel was also prepared with distilled water.
The mean droplets size of nanoemulsions was measured by dynamic light scattering (DLS) at a scattering angle of 90° using Scatteroscope (K-one Ltd. Korea). Droplet size distribution was calculated using the following equation, D90-D10/D50; D was the diameter of droplets, and D10, D50, and D90 were the percentile of droplets with a diameter lower than these values. The chemical spectra of nanoemulsions containing Cinnamon and Clove EOs, CMC powder, and nanogels were analyzed by Attenuated Total Reflection-Fourier-Transform InFrared (ATR-FTIR) using an infrared instrument in a wavenumber 400–4000 cm− 1 (Bruker, Tensor II, Germany) without any sample preparation process.
Acute anti-nociceptive studies
The hot plate test was carried out to assess the acute anti-nociceptive activity of cinnamon-NG and clove-NG [36]. For this purpose, topical pre-treatment was carried out with 100 mg of the nanogels for each rubbing on the left hind paw for an hour with intervals of 15 minutes. For topical adsorption of nanogels, the rats were allowed 1 h after pre-treatment. Then rats were then placed on a hot plate (53 ± 2 °C). The response latency to the thermal stimulus was considered as the sign of nociception. The response latency was measured as the time taken by the animal for nocifensive behaviors, including paw licking, jumping, or flinching. The first nocifensive behaviors were recorded, and rats were immediately removed from the hot plate. To prevent tissue damage, a cut-off period was taken as the 60 s.
Acute and chronic anti-nociceptive studies
Formalin test was used to assess gels’ acute and chronic anti-nociceptive effects [37]. After 2 weeks after the hot plate test, the rats were anesthetized, and topical pre-treatment was carried out with 100 mg of the nanogels (contains 2.5 mg of EOs) for each rubbing on the left hind paw for an hour with intervals of 15 minutes (totally 10 mg EO). For topical adsorption of nanogels, the rats were allowed 1 h after pre-treatment. Then acute pain was induced by subcutaneous injection of formalin 1% into the dorsal surface of the paw. Afterward, the rats were immediately placed into a flat plexiglass chamber (30 × 30 × 30 cm) with a mirror at 45° angles for observed the animals’ response to pain-related behaviors during 15 s periods for 1 h at 1 min intervals based on the numerical scale in Table 1 (Dubuisson, Dennis) [38]. Following formalin injection, anti-nociceptive effects were assessed in two phases: in phase-1 acute pain was recorded 5 min after the injection, and in phase-2 chronic pain was recorded 15–60 min after the injection were assessed. The weighted scores or rating scale method (Eq. 1) was used to quantify the pain [39].
| 1 |
Table 1.
Categories of formalin-induced nociception behaviors in rats
| Pain behavior scale | Pain score |
|---|---|
| Rat stands or walks firmly on injected paw | 0 |
| The injected paw was not fully lifted | 1 |
| The injected paw was wholly lifted off the floor | 2 |
| The rat licked or chewed the injected paw | 3 |
The second number of animals in each category is defined as T and multiplied by its given weight score for 5 min test. The total time was 300 s, and the pain scores were generated with a range of 0–3.
In addition to anti-nociceptive effects, the total licking times of the injected paw were recorded in two phases; the first phase 0–5 min (acute) and the second phase 15–60 min (chronic), which represent both neurogenic and inflammatory pain responses, respectively.
Anti-inflammatory studies
According to previous studies, the carrageenan-induced model performed the paw edema test to investigate the anti-inflammatory effects of the nanogels [9]. After deep anesthesia of rats, topical pre-treatment was carried out with 100 mg of the nanogels for each rubbing on the right hind paw for an hour with intervals of 15 minutes. For topical adsorption of nanogels, the rats were allowed 1 h after pre-treatment. Then inflammation was induced by injection of 0.1 mL freshly carrageenan solution (1% w/v in normal saline 0.9%) into the sub-plantar region. Then paw edema (average volume of paw swelling) was measured by using a digital caliper up to 5 h with 1 h intervals following carrageenan injection. Finally, the paw edema percentage was calculated by Eq. 2. Where Va indicates the paw diameters after injecting carrageenan and Vb denotes the paw diameters before injecting carrageenan
| 2 |
Statistical analysis
One-way analysis of variance and Tukey comparison were performed to assess the statistical significance of differences among groups. Results with a p value< 0.05 were considered statistically significant. Statistical analyses were carried out using the SPSS software, v. 21 (SPSS, Inc., USA).
Results
Determining the size of the nanoemulsions
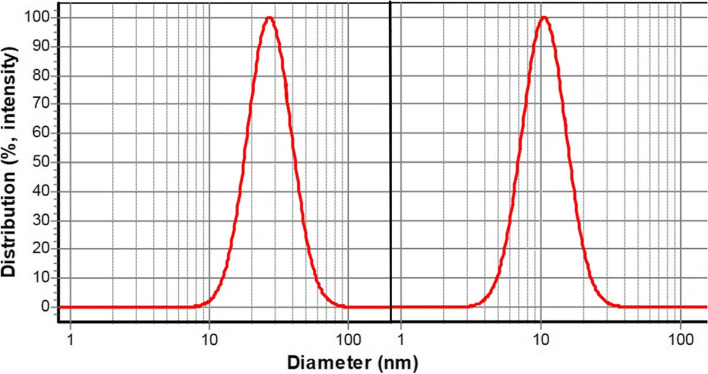
Figure 1 shows the mean droplets size and size distribution of nanoemulsions containing Cinnamon and Clove EOs, which had a size of less than 100 nm and droplet size distribution of less than 1.
Fig. 1.
Left curve: Cinnamon EO nanoemulsion with mean droplet size ± SD: 28 ± 6 nm; Right curve: Clove EO nanoemulsion with droplet size ± SD: 12 ± 3 nm
Chemical properties of the nanogels
Figure 2 shows the ATR-FTIR spectra of the samples in terms of transmittance rate (%). In the Cinnamon nanoemulsions spectrum, the peak at 2922 and 2854 cm− 1 are attributed to C-H stretching. The peak at 1732 cm− 1 exhibited C=O stretching, and the characteristic absorption at around 1450 cm− 1 shows CH2 bending. In the Clove nanoemulsions spectrum, the broadband at 3358 cm− 1 is attributed to O-H stretching vibration due to hydrogen bonding between Clove EO and tween 20 molecules. The peak at 2924 cm− 1 is attributed to C-H stretching, and the peak at 1731 and 1631 cm− 1 exhibited C=O stretching. The characteristic absorption at around 1463 cm− 1 showed CH2 bending, and the peak at 1003 cm− 1 is attributed to C-O stretching.
Fig. 2.
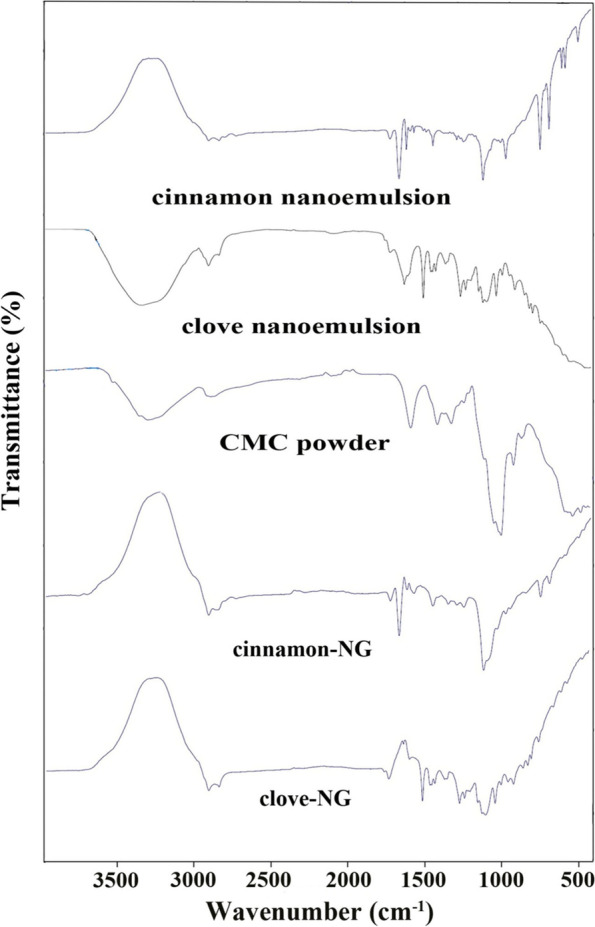
ATR-FTIR spectra of the samples (CMC: carboxymethylcellulose, NG: nanogel)
ATR-FTIR spectrum of CMC showed the broad bands at 3321 cm− 1 can be attributed to the stretching of the hydroxyl group O-H due to H-bonding, the strong band at 1589 cm− 1 related to COO- group (asymmetric stretching), and 1413 cm− 1 related to COO- (symmetric stretching). The characteristic absorption at around 993 cm− 1 is attributed to C-O stretching. Cinnamon-NG spectrum peak at about 2922 cm− 1 is attributed to C-H stretching due to EO, tween, and CMC. The peak at 1731 and 1674 cm− 1 exhibited C=O stretching, representing the carbonyl group in Cinnamon EO with tween molecules. The sharp and strong peak at 1120 cm− 1 is attributed to C-O stretching. Noticeably, the COO− band at 1589 cm− 1 in the presence of CMC was shifted toward the lower wavenumber at 1576 cm− 1, confirming the association of CMC with tween through intermolecular H-bonding.
Moreover, in the spectrum of clove-NG, the peak at 2922 and 2854 cm− 1 are attributed to C-H stretching due to Clove EO, tween, and CMC. The peak at 1735 cm− 1 exhibited C=O stretching, representing the overlap carbonyl group in Clove EO with tween molecules. The sharp and strong peak at 1097 cm− 1 is attributed to C-O stretching. Noticeably, the COO− band at 1589 cm− 1 in the presence of CMC was shifted toward a lower wavenumber at 1513 cm− 1, confirming the association of CMC with tween through intermolecular H-bonding.
Acute anti-nociceptive studies
Figure 3 shows the experimental results of the hot plate test, the thermal anti-nociceptive activity of formulations, and the central pain index. As the details direct, topical administration of cinnamon-NG and clove-NG failed to prolong latency time to the hot plate painful stimulus compared to the control group.
Fig. 3.
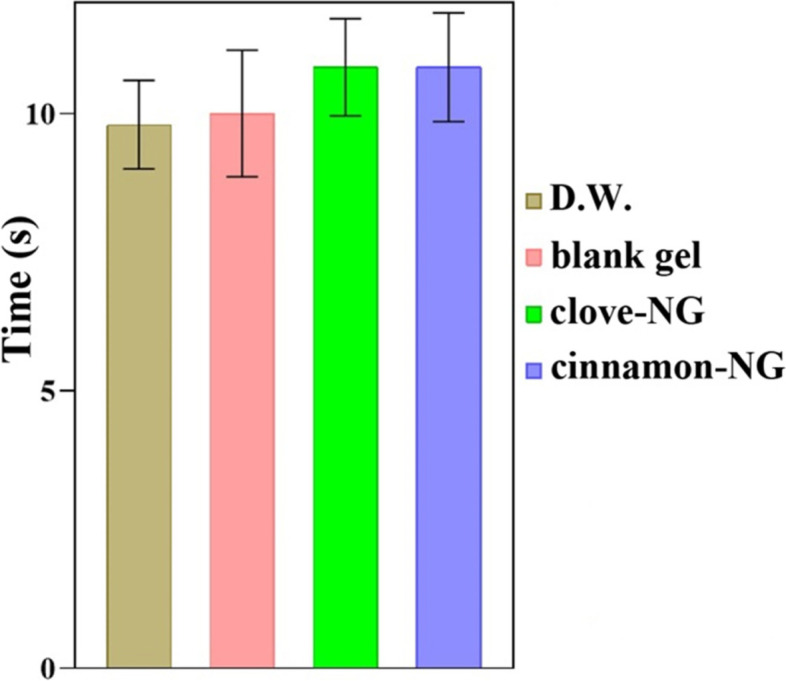
The acute anti-nociceptive activity of cinnamon-NG and clove-NG on reaction latency in hot plate test; no significant differences between samples. Investigated groups (n = 6) include distilled water (D.W.: control group), blank gel, cinnamon-NG, and clove-NG. The data were presented as mean ± SE
Acute and chronic anti-nociceptive studies
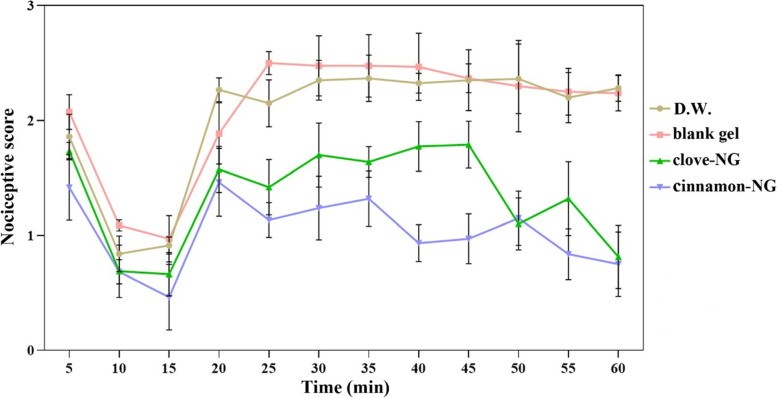
Formalin test was used to assess acute and chronic anti-nociceptive responses. The anti-nociceptive results of formulations through chemical stimuli of formalin were reported in Fig. 4 and Table 2. Noteworthy, no anesthesia, movement impairments, and respiratory insufficiency were seen in the animals during the test. In the second phase of the formalin test, pre-treatment rats with cinnamon-NG and clove-NG show significant anti-nociceptive activity and reduced nociceptive scores. However, this effect was more significant and noticeable in cinnamon-NG. In detail, as the graph shows, no significant difference was observed between groups up to 20 min following formalin injection. However, in the groups pre-treated with cinnamon-NG and clove-NG, an immediate reduction in nociceptive scores was observed at 25 min.
Fig. 4.
Comparison of acute and chronic phases results of formalin test after treatment with distilled water (D.W.: control group), cinnamon-NG, and clove-NG. Each value represents mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001
Table 2.
Statistical comparison between the groups on formalin test (chronic phase) including distilled water (D.W.: control group), cinnamon-NG, and clove-NG
| Time (min) | Groups | P-Value |
|---|---|---|
| 25 | D.W. vs. cinnamon-NG | 0.0194 |
| blank gel vs. clove-NG | 0.0072 | |
| blank gel vs. cinnamon-NG | 0.0017 | |
| 30 | D.W. vs. cinnamon-NG | 0.0019 |
| blank gel vs. cinnamon-NG | 0.0009 | |
| 35 | D.W. vs. cinnamon-NG | 0.0099 |
| blank gel vs. clove-NG | 0.0331 | |
| blank gel vs. cinnamon-NG | 0.0012 | |
| 40 | D.W. vs. cinnamon-NG | 0.0005 |
| blank gel vs. cinnamon-NG | 0.0003 | |
| 45 | D.W. vs. cinnamon-NG | 0.0003 |
| blank gel vs. cinnamon-NG | 0.0002 | |
| clove-NG vs. cinnamon-NG | 0.0241 | |
| 50 | D.W. vs. clove-NG | 0.0007 |
| D.W. vs. cinnamon-NG | 0.0006 | |
| blank gel vs. clove-NG | 0.0037 | |
| blank gel vs. cinnamon-NG | 0.0036 | |
| 55 | D.W. vs. clove-NG | 0.0418 |
| D.W. vs. cinnamon-NG | 0.0007 | |
| blank gel vs. clove-NG | 0.0135 | |
| blank gel vs. cinnamon-NG | 0.0001 | |
| 60 | D.W. vs. clove-NG | 0.0002 |
| D.W. vs. cinnamon-NG | 0.0001 | |
| blank gel vs. clove-NG | < 0.0001 | |
| blank gel vs. cinnamon-NG | < 0.0001 |
Moreover, these groups remained lower than the blank gel and control groups (D.W.) until the end of the test. Worth mentioning that the significant differences in nociceptive scores between clove-NG and control were seen from 50 min onwards, while the significant ones between clove-NG and gel were observed at 25, 35, 50, 55, and 60 min. Meanwhile, the significant anti-nociceptive effect in the cinnamon-NG group compared with the control and gel groups was noticed at all-time points after 20 min, indicating a strong anti-nociceptive effect.
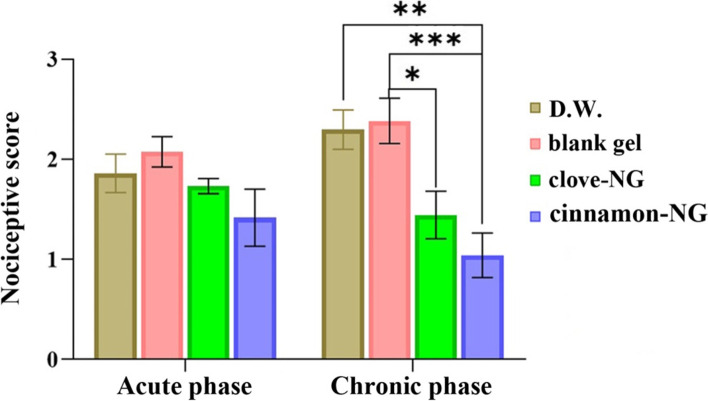
The pain response of formulations to both acute and chronic phases of the formalin test is illustrated in Fig. 5. Our results indicated no significant difference between the tested group in the acute phase, while chronic pain intensity was significantly decreased in the cinnamon-NG and clove-NG groups. These findings are consistent with the results in Fig. 4. Therefore, both cinnamon-NG and clove-NG significantly affect the second phase of the nociceptive response in the formalin test and not in the first phase.
Fig. 5.
The effect of cinnamon-NG and clove-NG on rat nociceptive behavior. Groups (n = 6) include distilled water (D.W.: control group), cinnamon-NG, and clove-NG. Each value represents mean ± SE
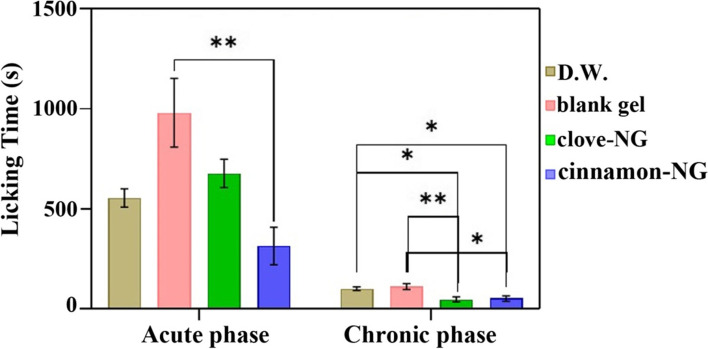
As shown in Table 3, the paw licking time revealed a significant (P < 0.05) reduction in nociceptive response between different treated groups compared to controls. The cinnamon-NG and clove-NG suppressed licking time more effectively in the late phase. However, cinnamon-NG also shortened licking time in the early phase (Fig. 6).
Table 3.
Statistical comparison of the groups on formalin-induced paw licking (in seconds) in acute and chronic phases, including distilled water (D.W.: control group), blank gel, cinnamon-NG, and clove-NG
| Phase | Groups | P-value |
|---|---|---|
| Acute | blank gel vs. cinnamon-NG | 0.0031 |
| Chronic | D.W. vs. clove-NG | 0.0205 |
| D.W. vs. cinnamon-NG | 0.0483 | |
| blank gel vs. clove-NG | 0.0091 | |
| blank gel vs. cinnamon-NG | 0.0213 |
Fig. 6.
Comparison of licking time in acute and chronic phases of the formalin test after treatment with distilled water (D.W.: control group), blank gel, clove-NG, and cinnamon-NG. Each value represents mean ± SEM, *P < 0.05, **P < 0.01
Anti-inflammatory studies
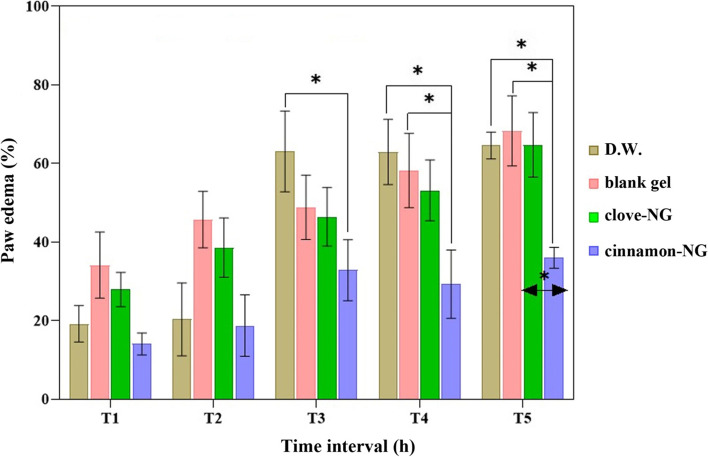
The inhibition of carrageenan-induced paw edema in rats is a model for establishing the efficacy of anti-inflammatory drugs [40]. As shown in Fig. 7, cinnamon-NG suppressed carrageenan-induced rat paw edema more than other groups, including clove-NG, control, and blank gel groups. A maximum inhibitory effect was seen in the cinnamon-NG group 4 h after treatment. The clove-NG inhibitory effect was more than D.W. and blank gel groups at the 1st and 2nd h of the experiment; however, the differences were insignificant. On the other hand, compared to D.W., the cinnamon-NG group was effective against carrageenan-induced inflammation, especially at the 4th and 5th h of the test, suggesting longer biological life cinnamon-NG compared to the carrageenan with the most activity at the 3rd h [41].
Fig. 7.
The effect of cinnamon-NG and clove-NG in a rat paw edema induced by carrageenan model. Rats (n = 6) were pre-treated with distilled water (D.W.: control group), blank gel, cinnamon-NG, and clove-NG. The measurements were obtained at 1, 2, 3, 4, and 5 hours after the carrageenan sub-plantar injection (1%, 100 μl). *P < 0.05
Discussions
In the current study, nanoemulsions containing cinnamon and clove EOs were prepared; as obtained droplet sizes were lower than 200 nm, their nanoscale sizes were confirmed. Moreover, a droplet size distribution of less than 1 confirms narrow size distribution and single sharp peaks referred to as monodisperse systems [26]. The low-Energy or spontaneous method is common for preparing EO-based nanoemulsions and preventing evaporating volatile compounds in EOs [42, 43]. This method involves mixing the components of nanoemulsions at room temperature without any external energies as a high-homogenizer or ultrasound [33].
Ingredients of the used EOs in the current study (i.e., Cinnamon and Clove) were identified using Gas Chromatography-Mass Spectrometry and reported in our previous reports. Cinnamaldehyde, with 62.04%, is the major component of Cinnamon EO, and linalool (6.96%), trans-caryophyllene (6.60%), trans-cinnamyl acetate (4.29%), and benzyl benzoate (3.32%) are other major constituents [44]. Besides, eugenol, with 65.41%, is the major component in Clove EO. trans-caryophyllene (12.06%), eugenol acetate (9.85%), caryophyllene oxide (3.00%), and α-humulene (1.73%) are other major constituents [45].
Pro-inflammatory agents, including carrageenan, can affect vascular permeability and blood cells migration through modulated nitric oxide (NO) secretion [46, 47]. On the other hand, the production of pro-inflammatory mediators is stimulated by inducing nitric synthase (iNOS) and cyclooxygenase-2 (COX-2) (50, 51). It has been reported that eugenol and cinnamaldehyde, the main component of Clove EO and Cinnamon EO, demonstrated anti-inflammatory effects similar to COX inhibitors, including indomethacin and celecoxib [17, 48]. In addition, cinnamaldehyde inhibits lipopolysaccharide-induced chondrocyte inflammation [49]. Besides, eugenol in LPS-stimulated mouse macrophages showed a COX-2 inhibitory effect [50, 51]. This result suggests that cinnamon-NG and clove-NG could be candidates for further developing anti-inflammatory drugs.
It has been proposed that the analgesic effect of EO of Clove and Cinnamon may be due to these main ingredients (eugenol and cinnamaldehyde) [52, 53]. Their administration as analgesic agents in experimental models of pain in mice was reported [54, 55]. Moreover, it has been reported that eugenol demonstrated a significant anti-nociceptive effect against chemical stimuli [56]. Therefore, it is proposed that eugenol predominantly prevents the peripheral pain mechanism. Two cinnamon-NG and clove-NG block the peripheral pain mechanism and cannot affect central pain through the hot plate test. The formalin (chemical stimuli) results are also inconsistent with these findings.
It is important to note that acute and chronic phases results of the formalin test showed that both cinnamon-NG and clove-NG significantly affect the second phase of the nociceptive response in the formalin test and not in the first phase. Similar observations have also been reported about other nanoemulsions [57]. In addition, it was found that topical application of Clove EO and intraperitoneal administration of Cinnamon extract significantly decreased acute and chronic pain in formalin tests [58, 59].
According to rat’s paw licking time results, cinnamon-NG inhibited the formalin-induced pain response in both phases, indicating the involvement of both peripheral and central mediated mechanisms. In one study, a significant reduction in pain response was found in Cinnamomum zeylanicum (200 and 400 mg/kg) treated groups during the first phase of the formalin test. However, during the second phase, a significant reduction in formalin-induced pain response was observed in 100, 200, and 400 mg/kg C. zeylanicum extract-treated groups compared to the control group [60]. Moreover, cinnamic alcohols (a phenylpropanoid of cinnamon)-treated animals (6.25, 12.5, 25 mg/kg) exhibited reduced paw licking behavior in the first and second phases of the formalin test. Cinnamic alcohol’s anxiolytic and antinociceptive-like effects were suggested to be due to GABAergic system modulation [61]. The clove-NG inhibited the rat’s paw licking time in the late phase. According to the literature, eugenol exhibited an antinociceptive effect more in the inflammatory phase than in the neurogenic phase in a formalin-induced licking pain model [56]. Clove oil also reduces pain response through a mainly peripheral action, as demonstrated by the formalin test and the tail-flick test, which indicated the participation of opioid receptors [62].
Conclusion
This study developed cinnamon-NG and clove-NG as a topical delivery system. Our work has led us to conclude that nanoemulsion-based gel formulations, especially cinnamon-NG, could apply as anti-nociceptive and anti-inflammatory agents or promising therapeutics in relieving diseases accompanied by inflammation and pain.
Acknowledgments
Not applicable.
Authors’ contributions
MO and FE conceived and designed the experiments. MO prepared and characterized nanogels. FE and MZ performed the experiments. MZ, YY, and HA rewrote the manuscript and analyzed the data, and performed the analysis with constructive discussions. All authors read and approved the final manuscript.
Funding
This project was supported by the Tehran University of Medical Sciences (grant No. 91–01–87-17072).
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Raw data are available from FE upon a reasonable request.
Declarations
Ethics approval and consent to participate
The Ethical Committee of Tehran University of Medical Sciences approved this study (91-01-87-17072), and all methods were carried out per relevant guidelines and regulations. Moreover, all methods are reported per ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
All authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhong J, Shi G. Regulation of inflammation in chronic disease. Front Immunol. 2019;10:737. doi: 10.3389/fimmu.2019.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jancalek R. Signaling mechanisms in mirror image pain pathogenesis. Ann Neurosci. 2011;18(3):123–127. doi: 10.5214/ans.0972.7531.11183010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee S-Y, Kim SH, Lee MG, Choi YH, Kim J. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2002;99(15):10150–10155. doi: 10.1073/pnas.152002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliveira CC. Understanding pain and human suffering. Rev Bioét. 2016;24:225–234. doi: 10.1590/1983-80422016242122. [DOI] [Google Scholar]
- 6.Ronchetti S, Migliorati G, Delfino DV. Association of inflammatory mediators with pain perception. Biomed Pharmacother. 2017;96:1445–1452. doi: 10.1016/j.biopha.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Lazzaroni M, Bianchi Porro G. Gastrointestinal side-effects of traditional non-steroidal anti-inflammatory drugs and new formulations. Aliment Pharmacol Ther. 2004;20:48–58. doi: 10.1111/j.1365-2036.2004.02037.x. [DOI] [PubMed] [Google Scholar]
- 8.Vongtau H, Abbah J, Ngazal I, Kunle O, Chindo B, Otsapa P, Gamaniel K. Anti-nociceptive and anti-inflammatory activities of the methanolic extract of Parinari polyandra stem bark in rats and mice. J Ethnopharmacol. 2004;90(1):115–121. doi: 10.1016/j.jep.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 9.Esmaeili F, Rajabnejhad S, Partoazar AR, Mehr SE, Faridi-Majidi R, Sahebgharani M, Syedmoradi L, Rajabnejhad MR, Amani A. Anti-inflammatory effects of eugenol nanoemulsion as a topical delivery system. Pharm Dev Technol. 2016;21(7):887–893. doi: 10.3109/10837450.2015.1078353. [DOI] [PubMed] [Google Scholar]
- 10.Subramanian B, Kuo F, Ada E, Kotyla T, Wilson T, Yoganathan S, Nicolosi R. Enhancement of anti-inflammatory property of aspirin in mice by a nano-emulsion preparation. Int Immunopharmacol. 2008;8(11):1533–1539. doi: 10.1016/j.intimp.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Aman RM, Hashim IIA, Meshali MM. Novel Clove essential oil nanoemulgel tailored by Taguchi’s model and scaffold-based nanofibers: Phytopharmaceuticals with promising potential as cyclooxygenase-2 inhibitors in external inflammation. Int J Nanomedicine. 2020;15:2171. doi: 10.2147/IJN.S246601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeengar MK, Rompicharla SVK, Shrivastava S, Chella N, Shastri NR, Naidu V, Sistla R. Emu oil based nano-emulgel for topical delivery of curcumin. Int J Pharm. 2016;506(1–2):222–236. doi: 10.1016/j.ijpharm.2016.04.052. [DOI] [PubMed] [Google Scholar]
- 13.Schink A, Naumoska K, Kitanovski Z, Kampf CJ, Fröhlich-Nowoisky J, Thines E, Pöschl U, Schuppan D, Lucas K. Anti-inflammatory effects of cinnamon extract and identification of active compounds influencing the TLR2 and TLR4 signaling pathways. Food Funct. 2018;9(11):5950–5964. doi: 10.1039/C8FO01286E. [DOI] [PubMed] [Google Scholar]
- 14.Hong J-W, Yang G-E, Kim YB, Eom SH, Lew J-H, Kang H. Anti-inflammatory activity of cinnamon water extract in vivo and in vitro LPS-induced models. BMC Complement Altern Med. 2012;12(1):1–8. doi: 10.1186/1472-6882-12-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vetal S, Bodhankar SL, Mohan V, Thakurdesai PA. Anti-inflammatory and anti-arthritic activity of type-A procyanidine polyphenols from bark of Cinnamomum zeylanicum in rats. Food Sci Hum Wellness. 2013;2(2):59–67. doi: 10.1016/j.fshw.2013.03.003. [DOI] [Google Scholar]
- 16.Ranasinghe P, Pigera S, Premakumara GAS, Galappaththy P, Constantine GR, Katulanda P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): a systematic review. BMC Complement Altern Med. 2013;13(1):275. doi: 10.1186/1472-6882-13-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniel AN, Sartoretto SM, Schmidt G, Caparroz-Assef SM, Bersani-Amado CA, Cuman RKN. Anti-inflammatory and antinociceptive activities A of eugenol essential oil in experimental animal models. Rev Bras Farmacogn. 2009;19(1B):212–217. doi: 10.1590/S0102-695X2009000200006. [DOI] [Google Scholar]
- 18.Agra MF, Silva KN, Basílio IJLD, Freitas PF, Barbosa-Filho JM. Survey of medicinal plants used in the region northeast of Brazil. Rev Bras Farmacogn. 2008;18(3):472–508. doi: 10.1590/S0102-695X2008000300023. [DOI] [Google Scholar]
- 19.Chaieb K, Hajlaoui H, Zmantar T, Kahla-Nakbi AB, Rouabhia M, Mahdouani K, Bakhrouf A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): a short review. Phytother Res. 2007;21(6):501–506. doi: 10.1002/ptr.2124. [DOI] [PubMed] [Google Scholar]
- 20.Cortés-Rojas DF, de Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014;4(2):90–96. doi: 10.1016/S2221-1691(14)60215-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milind P, Deepa K. Clove: a champion spice. Int J Res Ayurveda Pharm. 2011;2(1):47–54. [Google Scholar]
- 22.Prakash P, Gupta N. Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: a short review. Indian J Physiol Pharmacol. 2005;49(2):125. [PubMed] [Google Scholar]
- 23.Valizadeh A, Khaleghi AA, Alipanah H, Zarenezhad E, Osanloo M. Anticarcinogenic effect of chitosan nanoparticles containing syzygium aromaticum essential oil or eugenol toward breast and skin cancer cell lines. BioNanoScience. 2021;11(3):678–686. doi: 10.1007/s12668-021-00880-z. [DOI] [Google Scholar]
- 24.Zarenezhad E, Abdollahi A, Esmaeili F, Satvati S, Osanloo M. A fast-degradable nano-dressing with potent antibacterial effect. BioNanoScience. 2020;10(4):983–990. doi: 10.1007/s12668-020-00790-6. [DOI] [Google Scholar]
- 25.Osanloo M, Arish J, Sereshti H. Developed methods for the preparation of electrospun nanofibers containing plant-derived oil or essential oil: a systematic review. Polym Bull. 2020;77(11):6085–6104. doi: 10.1007/s00289-019-03042-0. [DOI] [Google Scholar]
- 26.Abedinpour N, Ghanbariasad A, Taghinezhad A, Osanloo M. Preparation of nanoemulsions of mentha piperita essential oil and investigation of their cytotoxic effect on human breast cancer lines. BioNanoScience. 2021;11(2):428–436. doi: 10.1007/s12668-021-00827-4. [DOI] [Google Scholar]
- 27.Maghbool M, Khosravi T, Vojdani S, Chaijan MR, Esmaeili F, Amani A, Rezayat F, Nasimi Doost Azgomi R, Mehraban SS, Hashempur MH. The effects of eugenol nanoemulsion on pain caused by arteriovenous fistula cannulation in hemodialysis patients: a randomized double-blinded controlled cross-over trial. Complement Ther Med. 2020;52:102440. doi: 10.1016/j.ctim.2020.102440. [DOI] [PubMed] [Google Scholar]
- 28.Ghiasi Z, Esmaeli F, Aghajani M, Ghazi-Khansari M, Faramarzi MA, Amani A. Enhancing analgesic and anti-inflammatory effects of capsaicin when loaded into olive oil nanoemulsion: an in vivo study. Int J Pharm. 2019;559:341–347. doi: 10.1016/j.ijpharm.2019.01.043. [DOI] [PubMed] [Google Scholar]
- 29.Sondari D, Tursiloadi S. The effect of surfactan on formulation and stability of nanoemulsion using extract of Centella Asiatica and Zingiber Officinale. In: AIP conference proceedings: 2018. AIP Publishing LLC; 2018. p. 030014. 10.1063/1.5082515.
- 30.Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ. Nano-emulsions. Curr Opin Colloid Interface Sci. 2005;10(3–4):102–110. doi: 10.1016/j.cocis.2005.06.004. [DOI] [Google Scholar]
- 31.Ee SL, Duan X, Liew J, Nguyen QD. Droplet size and stability of nano-emulsions produced by the temperature phase inversion method. Chem Eng J. 2008;140(1–3):626–631. doi: 10.1016/j.cej.2007.12.016. [DOI] [Google Scholar]
- 32.Tadros T, Izquierdo P, Esquena J, Solans C. Formation and stability of nano-emulsions. Adv Colloid Interf Sci. 2004;108:303–318. doi: 10.1016/j.cis.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 33.Abbasifard M, Yousefpoor Y, Amani A, Arababadi MK. Topical bee venom nano-emulsion ameliorates serum level of Endothelin-1 in collagen-induced rheumatoid arthritis model. BioNanoScience. 2021;11:810–815. doi: 10.1007/s12668-021-00871-0. [DOI] [Google Scholar]
- 34.Hussain A, Samad A, Singh S, Ahsan M, Haque M, Faruk A, Ahmed F. Nanoemulsion gel-based topical delivery of an antifungal drug: in vitro activity and in vivo evaluation. Drug Deliv. 2016;23(2):642–657. doi: 10.3109/10717544.2014.933284. [DOI] [PubMed] [Google Scholar]
- 35.Ghanbariasad A, Azadi S, Agholi M, Osanloo M. The nanoemulsion-based nanogel of Artemisia dracunculus essential oil with proper activity against Leishmania tropica and Leishmania major. J Nanomed Res. 2021;6(1):89–95. [Google Scholar]
- 36.Masocha W, Kombian SB, Edafiogho IO. Evaluation of the antinociceptive activities of enaminone compounds on the formalin and hot plate tests in mice. Sci Rep. 2016;6(1):1–9. doi: 10.1038/srep21582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tjølsen A, Berge O-G, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51(1):5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 38.Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4(2):161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 39.Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52(3):259–285. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- 40.Patil KR, Mahajan UB, Unger BS, Goyal SN, Belemkar S, Surana SJ, Ojha S, Patil CR. Animal models of inflammation for screening of anti-inflammatory drugs: implications for the discovery and development of phytopharmaceuticals. Int J Mol Sci. 2019;20(18):4367. doi: 10.3390/ijms20184367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silva F, Dore C, Marques C, Nascimento M, Benevides N, Rocha H, Chavante S, Leite E. Anticoagulant activity, paw edema and pleurisy induced carrageenan: action of major types of commercial carrageenans. Carbohydr Polym. 2010;79(1):26–33. doi: 10.1016/j.carbpol.2009.07.010. [DOI] [Google Scholar]
- 42.Ostertag F, Weiss J, McClements DJ. Low-energy formation of edible nanoemulsions: factors influencing droplet size produced by emulsion phase inversion. J Colloid Interface Sci. 2012;388(1):95–102. doi: 10.1016/j.jcis.2012.07.089. [DOI] [PubMed] [Google Scholar]
- 43.Bouchemal K, Briançon S, Perrier E, Fessi H. Nano-emulsion formulation using spontaneous emulsification: solvent, oil and surfactant optimisation. Int J Pharm. 2004;280(1–2):241–251. doi: 10.1016/j.ijpharm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 44.Ghanbariasad A, Valizadeh A, Ghadimi SN, Fereidouni Z, Osanloo M. Nanoformulating Cinnamomum zeylanicum essential oil with an extreme effect on Leishmania tropica and Leishmania major. J Drug Deliv Sci Technol. 2021;63:102436. doi: 10.1016/j.jddst.2021.102436. [DOI] [Google Scholar]
- 45.Moemenbellah-Fard MD, Abdollahi A, Ghanbariasad A, Osanloo M. Antibacterial and leishmanicidal activities of Syzygium aromaticum essential oil versus its major ingredient, eugenol. Flavour Fragr J. 2020;35(5):534–540. doi: 10.1002/ffj.3595. [DOI] [Google Scholar]
- 46.Costa JFO, David JP, David JM, Giulietti AM, Queiroz LP, Santos RR, Soares MBP. Immunomodulatory activity of extracts from Cordia superba Cham. and Cordia rufescens A. DC.(Boraginaceae), plant species native from Brazilian Semi-arid. Rev Bras Farmacogn. 2008;18:11–15. [Google Scholar]
- 47.Markov AV, Sen’kova AV, Babich VO, Odarenko KV, Talyshev VA, Salomatina OV, Salakhutdinov NF, Zenkova MA, Logashenko EB. Dual effect of soloxolone methyl on LPS-induced inflammation in vitro and in vivo. Int J Mol Sci. 2020;21(21):7876. doi: 10.3390/ijms21217876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao J-C, Deng J-S, Chiu C-S, Hou W-C, Huang S-S, Shie P-H, Huang G-J. Anti-inflammatory activities of Cinnamomum cassia constituents in vitro and in vivo. Evid Based Complement Alternat Med. 2012;2012:429320. doi: 10.1155/2012/429320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen P, Ruan A, Zhou J, Huang L, Zhang X, Ma Y, Wang Q. Cinnamic aldehyde inhibits lipopolysaccharide-induced chondrocyte inflammation and reduces cartilage degeneration by blocking the nuclear factor-kappa B signaling pathway. Front Pharmacol. 2020;11:949. doi: 10.3389/fphar.2020.00949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim SS, Oh O-J, Min H-Y, Park E-J, Kim Y, Park HJ, Han YN, Lee SK. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264. 7 cells. Life Sci. 2003;73(3):337–348. doi: 10.1016/S0024-3205(03)00288-1. [DOI] [PubMed] [Google Scholar]
- 51.Huss U, Ringbom T, Perera P, Bohlin L, Vasänge M. Screening of ubiquitous plant constituents for COX-2 inhibition with a scintillation proximity based assay. J Nat Prod. 2002;65(11):1517–1521. doi: 10.1021/np020023m. [DOI] [PubMed] [Google Scholar]
- 52.Hosseini M, Kamkar M, Rakhshandeh H. Analgesic effect of clove essential oil in mice. Avicenna J Phytomed. 2011;1(1):1–6. [Google Scholar]
- 53.Churihar R, Solanki P, Vyas S, Hemant Tanwani H, Shubham Atal S. Analgesic activity of cinnamaldehyde per se and it’s interaction with diclofenac sodium and pentazocine in swiss albino mice. Int J Phamacog. 2016;3:97–102. [Google Scholar]
- 54.Kamkar Asl M, Nazariborun A, Hosseini M. Analgesic effect of the aqueous and ethanolic extracts of clove. Avicenna J Phytomed. 2013;3(2):186–192. [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma M, Rauniar G, Das B. Experimental study of various central nervous system effects of eugenol in mice and rats. Health Renaissance. 2012;10(3):208–214. doi: 10.3126/hren.v10i3.7137. [DOI] [Google Scholar]
- 56.Kurian R, Arulmozhi D, Veeranjaneyulu A, Bodhankar S. Effect of eugenol on animal models of nociception. Indian J Pharmacol. 2006;38(5):341–345. doi: 10.4103/0253-7613.27702. [DOI] [Google Scholar]
- 57.Azizi M, Esmaeili F, Partoazar A, Ejtemaei Mehr S, Amani A. Efficacy of nano-and microemulsion-based topical gels in delivery of ibuprofen: an in vivo study. J Microencapsul. 2017;34(2):195–202. doi: 10.1080/02652048.2017.1316324. [DOI] [PubMed] [Google Scholar]
- 58.Dashti-Rahmatabadi M, Vahidi Merjardi A, Pilavaran A, Farzan F. Antinociceptive effect of cinnamon extract on formalin induced pain in rat. J Shahid Sadoughi Univ Med Sci. 2009;17(2):190–199. [Google Scholar]
- 59.Daroogari S, Parandin R, Yousofvand N, Shakibaie D. The analgesic effect of topical clove oil using formalin test in male mice. J Ardabil Univ Med Sci. 2017;17(2):252–260. [Google Scholar]
- 60.Jain S, Gupta S. Effects of Cinnamomum zeylanicum bark extract on nociception and anxiety like behavior in mice. Asian J Pharm Clin Res. 2019;12(9):236–241. doi: 10.22159/ajpcr.2019.v12i9.31287. [DOI] [Google Scholar]
- 61.de Andrade HHN, Monteiro ÁB, Braga RM, da Cruz RMD, Salvadori MGSS, Scotti MT, de Sousa DP, de Almeida RN. Anxiolytic and antinociceptive-like effects of cinnamic alcohol by possible GABAergic pathway modulation: in vivo and in silico studies. Braz J Dev. 2020;6(7):51372–51389. doi: 10.34117/bjdv6n7-690. [DOI] [Google Scholar]
- 62.Halder S, Mehta AK, Mediratta PK, Sharma KK. Acute effect of essential oil of Eugenia caryophyllata on cognition and pain in mice. Naunyn-Schmiedeb Arch Phar. 2012;385(6):587–593. doi: 10.1007/s00210-012-0742-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Raw data are available from FE upon a reasonable request.