Abstract
Background and objectives
B-cell-depleting therapies may affect the development of a protective immune response following vaccination against SARS-CoV-2. It is important to have a different strategy for creating immunity in this patient population. The objective of this study was to evaluate whether Evusheld (tixagevimab co-packaged with cilgavimab) affects the antibody response to SARS-CoV-2 following an attenuated response to the vaccines against SARS-CoV-2 in patients on b-cell depleters who have multiple sclerosis.
Methods
This was a single-center cohort study performed at Methodist Hospitals in Merrillville, IN, USA. It included patients with multiple sclerosis treated with ocrelizumab and ofatumumab. Patients had already received the mRNA vaccinations against SARS-CoV-2 and had demonstrated an attenuated response on baseline antibody testing. All participants received 150 mg of Evusheld. Follow-up antibody levels were measured at least two weeks following Evusheld injections.
Results
All patients (100%) developed the highest level of antibodies possible at least two weeks following Evusheld injections.
Discussion
In this study, patients with MS who had an attenuated antibody response to the COVID-19 vaccines due to exposure to b-cell depleters now had the highest antibody response possible after receiving Evusheld. This is important as it provides a different strategy for protection against COVID-19.
Keywords: SARS-CoV-2, COVID-19, Multiple sclerosis, Ocrelizumab, Ofatumumab, Evusheld
1. Introduction
Although vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been developed, immunocompromised patients or patients who are on medications that blunt the humoral response to vaccination remain a challenge. Multiple studies have shown that b-cell depleters, such as ocrelizumab (OCR) and ofatumumab (OFA) attenuate the humoral response to SARS-CoV-2 vaccines in patients with multiple sclerosis (MS) (Achiron et al., 2021; Apostolidis et al., 2021; Brill et al., 2021; Conte, 2022; Gallo et al., 2021; Georgieva et al., 2022; Jakubecz et al., 2022; Katz et al., 2022; Mariottini et al., 2022; Novak et al., 2021; Sormani et al., 2021; Tallantyre et al., 2022).
On December 8, 2021, the Food and Drug Administration (FDA) authorized Evusheld (tixagevimab co-packaged with cilgavimab) for pre-exposure prophylaxis in certain individuals. The indication includes patients who are immunocompromised, either due to a medical condition or due to taking medications that causes people to not mount an adequate immune response to vaccines. The authorization was based on the PROVENT study, which was a double-blind trial comparing Evusheld to placebo (Levin et al., 2022). The primary endpoint (symptomatic COVID-19) occurred in 8 patients who received antibodies and in 17 who received placebo which amounted to a statistically significant reduction in incidence of SARS-CoV-2 RT-PCR-positive symptomatic illness (relative risk reduction 76.7%, p<0.001) (FDA, 2021; Levin et al., 2022).
2. Methods
2.1. Study participants
Patients with MS were recruited at the Comprehensive MS Center at Methodist Hospitals, in Merrillville, IN, USA. Patients who were vaccinated against SARS-CoV-2 and were exposed to b-cell depleters (OCR and OFA) during their vaccination period were recruited. Patients were tested for antibodies against SARS-CoV-2, and if less than 150 U/mL (50% of the maximum assay value) were offered Evusheld. Post-Evusheld antibody testing was performed at least two weeks following injections of Evusheld.
2.2. Antibody testing
The immunoassay for the detection of IgG antibodies was performed using the Labcorp anti-SARS-CoV-2 semi-quantitative IgG ECLIA assay against the spike protein receptor binding domain. The assay ranged from <0.4 to >250 U/mL. Titers <0.8 U/mL were considered negative.
2.3. Evusheld injection
150 mg of tixagevimab and 150 mg of cilgavimab were given as an intramuscular injection (note that this study was completed prior to the FDA's update to 300 mg each of tixagevimab and cilgavimab).
2.4. Data collection
The study was approved by the institutional review board at Methodist Hospitals. Individual, deidentified participant data is available on request.
2.5. Statistical analysis
Descriptive statistics were calculated for demographic variables. To compare the difference on frequency pre and post Evusheld for antibodies higher or lower than 0.8 U/mL, a McNemar's test was performed. Additionally, Paired Wilcoxon test and permutation tests were performed to compare the level of antibodies pre and post Evusheld. All analyses for this project were run in R version 4.0.3 (R Core team, 2020) using RStudio (RStudio Team, 2020).
3. Results
3.1. Demographic characteristics
Baseline characteristics are reported in Table 1 . Eighteen patients were recruited, with 17 patients on OCR and 1 patient on OFA. The majority of patients were female (55.6%) and received the Pfizer vaccine (72.2%).
Table 1.
Baseline characteristics.
| Overall (N=18) |
|
|---|---|
| Age | |
| Mean (SD) | 49.9 (13.6) |
| Median [Min, Max] | 50.0 [27.0, 72.0] |
| Gender | |
| Female | 10 (55.6%) |
| Male | 8 (44.4%) |
| Race | |
| Black | 1 (5.6%) |
| White | 17 (94.4%) |
| Type of MS | |
| PPMS | 3 (16.7%) |
| RRMS | 15 (83.3%) |
| Med | |
| Ocrelizumab (OCR) | 17 (94.4%) |
| Ofatumumab (OFA) | 1 (5.6%) |
| Onset of Disease (years) | |
| Mean (SD) | 12.1 (9.02) |
| Median [Min, Max] | 10.0 [3.00, 40.0] |
| Lymph <1.2 | |
| Mean (SD) | 1.55 (0.620) |
| Median [Min, Max] | 1.45 [0.600, 2.70] |
| Time from infusion (m) | |
| Mean (SD) | 4.32 (2.49) |
| Median [Min, Max] | 4.00 [0.500, 11.0] |
| Missing | 1 (5.6%) |
| time from injection (d) | |
| Mean (SD) | 22.1 (7.78) |
| Median [Min, Max] | 18.0 [14.0, 38.0] |
| Vaccine | |
| Moderna | 5 (27.8%) |
| Pfizer | 13 (72.2%) |
| Boosted | |
| No | 1 (5.6%) |
| Yes | 17 (94.4%) |
3.2. Level of SARS-CoV-2 antibody response
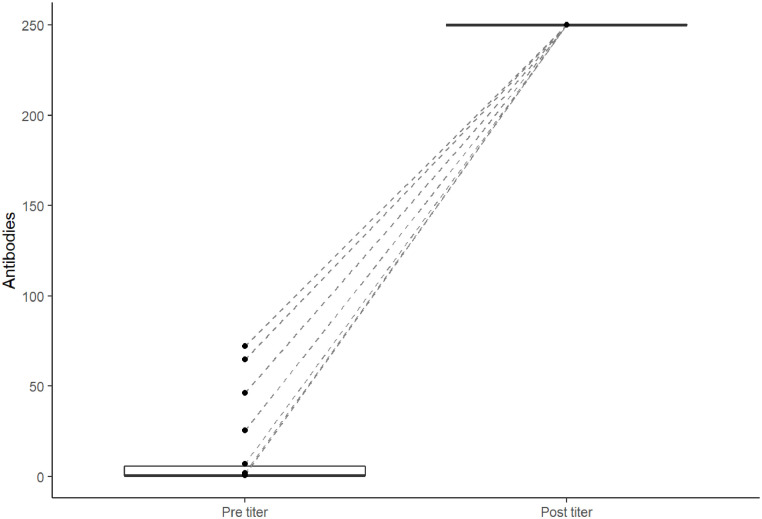
Prior to Evusheld, the mean antibody level was 12.38 U/mL and 12 patients had undetectable antibody levels (66%). At least two weeks following Evusheld injection, all patients in this cohort had an antibody response. Specifically, all patients had aa >250 U/mL level of antibody, which is the highest level the assay measures.
To compare the level of antibodies, it can be seen in Fig. 1 that all values increased to the highest level that can be recorded.
Fig. 1.
Paired plot showing pre- and post-titer.
At baseline there were 12 patients lower than 0.8 U/mL and 6 higher than the threshold. After Evusheld, all 18 subjects were above the threshold. When comparing the frequencies of samples higher or lower than 0.8 U/mL, there were significant differences between pre and post frequencies (p = 0.001) with McNemar's test.
When comparing the ranks of the level of antibodies between pre and post using a paired Wilcoxon test, there was a statistical difference (p<0.001). To look into the difference further and see how extreme the change was prior and after Evusheld injection, a Permutation Test was performed, where the mean observed difference in our sample was 237.62, which was statistically significant (p<0.001).
4. Discussion
It is concerning that certain medications, such as b-cell depleters, attenuate the antibody response to SARS-CoV-2. Therefore, it is important to have other options that do not depend on the humoral immune system. As demonstrated in the data, we have shown that Evusheld creates a 100% antibody response in this patient population.
This study is limited by a relatively small sample size. It is also worth mentioning that it is still unknown what antibody level denotes immunity to SARS-CoV-2. Further, due to time constraints, we did not measure clinical outcomes after Evusheld. A future study should look at clinical outcomes following Evusheld in patients with MS on b-cell depleters. In addition, we will continue to monitor patients’ antibody levels in order to assess the longitudinal effects of Evusheld on antibody levels in this patient population.
CRediT authorship contribution statement
William L. Conte: Conceptualization, Methodology, Data curation, Writing – original draft, Writing – review & editing. Lilian Golzarri-Arroyo: Formal analysis.
Declaration of Competing Interest
WLC: Research funding from Novartis and Sanofi Genzyme, unrelated to the present study. Consultant fees from Bayer, Biogen, Bristol Meyers Squib, Genentech, Novartis, and Sanofi Genzyme. Speaker fees from AbbVie, Alexion, Biogen, Bristol Meyers Squib, EMD Serono, Genentech, Horizon, Janssen, and Sanofi Genzyme
LGA: None pertinent.
References
- Achiron A., Mandel M., Dreyer-Alster S., Harari G., Dolev M., Menascu S., Magalashvili D., Flechter S., Givon U., Guber D., Sonis P., Zilkha-Falb R., Gurevich M. Humoral immune response in multiple sclerosis patients following PfizerBNT162b2 COVID19 vaccination: Up to 6 months cross-sectional study. J. Neuroimmunol. 2021;361 doi: 10.1016/j.jneuroim.2021.577746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C., Markowitz D.M., C E.M., Mexhitaj I., Jacobs D., Babb A., Betts M.R., Prak E.T.L., Weiskopf D., Grifoni A., Lundgreen K.A., Gouma S., Sette A., Bates P., Hensley S.E., Greenplate A.R., Wherry E.J., Li R., Bar-Or A. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27(11):1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill L., Rechtman A., Zveik O., Haham N., Oiknine-Djian E., Wolf D.G., Levin N., Raposo C., Vaknin-Dembinsky A. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol. 2021;78(12):1510–1514. doi: 10.1001/jamaneurol.2021.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte W.L. B-cell depleters attenuate the humoral response to SARS-CoV-2 vaccines in multiple sclerosis patients: A case-control study. Mult. Scler. Relat. Disord. 2022;57 doi: 10.1016/j.msard.2021.103413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA, 2021. Fact sheet for health care providers–Emergency use authorization for Evusheld (tixagevimab co-packaged with cilgavimab). (Accessed 4/5/2022).
- Gallo A., Capuano R., Donnarumma G., Bisecco A., Grimaldi E., Conte M., d'Ambrosio A., Coppola N., Galdiero M., Tedeschi G. Preliminary evidence of blunted humoral response to SARS-CoV-2 mRNA vaccine in multiple sclerosis patients treated with ocrelizumab. Neurol. Sci. 2021;42(9):3523–3526. doi: 10.1007/s10072-021-05397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieva Z.G., Dӧffinger R., Kumararatne D., Coles A.J., McCarthy C. Diminished seroconversion following a single SARS-COV-2 vaccine in ocrelizumab-treated relapsing-remitting multiple sclerosis patients. Mult. Scler. 2022:1126–1130. doi: 10.1177/13524585211046786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubecz C., Zhang X.S., Woodson S., Serra A., Abboud H. The humoral response to SARS-COV-2 vaccines in MS patients: A case series exploring the impact of DMT, lymphocyte count, immunoglobulins, and vaccine type. Mult. Scler. Relat. Disord. 2022;61 doi: 10.1016/j.msard.2022.103785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J.D., Bouley A.J., Jungquist R.M., Douglas E.A., O'Shea I.L., Lathi E.S. Humoral and T-cell responses to SARS-CoV-2 vaccination in multiple sclerosis patients treated with ocrelizumab. Mult. Scler. Relat. Disord. 2022;57 doi: 10.1016/j.msard.2021.103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M.J., Ustianowski A., De Wit S., Launay O., Avila M., Templeton A., Yuan Y., Seegobin S., Ellery A., Levinson D.J., Ambery P., Arends R.H., Beavon R., Dey K., Garbes P., Kelly E.J., Koh G.C.K.W., Near K.A., Padilla K.W., Psachoulia K., Sharbaugh A., Streicher K., Pangalos M.N., Esser M.T. Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for prevention of COVID-19. N. Engl. J. Med. 2022 doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariottini A., Bertozzi A., Marchi L., Di Cristinzi M., Mechi C., Barilaro A., Massacesi L., Repice A.M. Effect of disease-modifying treatments on antibody-mediated response to anti-COVID19 vaccination in people with multiple sclerosis. J. Neurol. 2022;269:2840–2847. doi: 10.1007/s00415-022-11003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak F., Nilsson A.C., Nielsen C., Holm D.K., Østergaard K., Bystrup A., Byg K.E., Johansen I.S., Mittl K., Rowles W., McPolin K., Spencer C., Sagan S., Gerungan C., Wilson M.R., Zamvil S.S., Bove R., Sabatino J.J., Sejbaek T. Humoral immune response following SARS-CoV-2 mRNA vaccination concomitant to anti-CD20 therapy in multiple sclerosis. Mult. Scler. Relat. Disord. 2021;56 doi: 10.1016/j.msard.2021.103251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Inglese M., Schiavetti I., Carmisciano L., Laroni A., Lapucci C., Da Rin G., Serrati C., Gandoglia I., Tassinari T., Perego G., Brichetto G., Gazzola P., Mannironi A., Stromillo M.L., Cordioli C., Landi D., Clerico M., Signoriello E., Frau J., Ferrò M.T., Di Sapio A., Pasquali L., Ulivelli M., Marinelli F., Callari G., Iodice R., Liberatore G., Caleri F., Repice A.M., Cordera S., Battaglia M.A., Salvetti M., Franciotta D., Uccelli A. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72 doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallantyre E.C., Vickaryous N., Anderson V., Asardag A.N., Baker D., Bestwick J., Bramhall K., Chance R., Evangelou N., George K., Giovannoni G., Godkin A., Grant L., Harding K.E., Hibbert A., Ingram G., Jones M., Kang A.S., Loveless S., Moat S.J., Robertson N.P., Schmierer K., Scurr M.J., Shah S.N., Simmons J., Upcott M., Willis M., Jolles S., Dobson R. COVID-19 vaccine response in people with multiple sclerosis. Ann. Neurol. 2022;91(1):89–100. doi: 10.1002/ana.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]