Abstract
Background
Antimicrobial resistance of H. pylori can lead to treatment failure. Importantly, several studies have reported on heteroresistance, i.e. the presence of resistant and susceptible H. pylori populations in the same sample and/or a difference in the susceptibility patterns between biopsy samples. This meta-analysis aims to provide comprehensive data on the prevalence of metronidazole and clarithromycin heteroresistance and the approaches to their detection.
Material and methods
A systematic review was performed after the search of MEDLINE, Scopus and Web of Science. The study outcomes were the weighted pooled prevalence of heteroresistance to clarithromycin and metronidazole in H. pylori positive samples and/or isolates with a subanalysis by continent.
Results
A total of 22 studies that had investigated 3852 H. pylori positive patients were included in the meta-analysis. Heteroresistance to clarithromycin was reported in 20 studies, with a weighted pooled prevalence of 6.8% (95% CI 5.1–8.6; 3654 H. pylori positive patients; the substantial heterogeneity I2 = 55.6%). Heteroresistance to metronidazole was reported in 12 studies, with a weighted pooled prevalence of 13.8% (95% CI 8.9–18.6; 1670 H. pylori positive patients; the substantial heterogeneity I2 = 60.9%). The weighted pooled prevalence of clarithromycin heteroresistance was similar in Asia and Europe (p = 0.174584), however, metronidazole heteroresistance was detected more often in Europe (p < 0.00001). Clarithromycin heteroresistance was detected more often by phenotype rather than by using genotyping methods (12 vs 8 studies), whereas heteroresistance to metronidazole was detected only by phenotype.
Conclusion
The prevalence of heteroresistance to clarithromycin and/or metronidazole is not negligible and can be detected in approximately 7 and 14% of H. pylori positive samples, respectively. These findings highlight the need to raise the awareness of gastroenterologists and microbiologists to the heteroresistance to clarithromycin and metronidazole in patients with a H. pylori infection.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12941-022-00509-3.
Introduction
Helicobacter pylori, a gram-negative spiral-shaped bacterium, is one of the most prevalent pathogens worldwide [1]. Peptic ulcer disease, or non-ulcer dyspepsia, are the most common clinical conditions of H. pylori infection [2]. H. pylori is classified as a group 1 carcinogen that causes gastric adenocarcinoma [3].
Helicobacter pylori eradication treatment decreases the incidence of duodenal or gastric ulceration and gastric cancer [2]. A combination of proton pump inhibitors, different antimicrobials and/or bismuth are used for H. pylori eradication, however, the increasing antimicrobial resistance can lead to treatment failure [4].
Antimicrobial susceptibility testing for H. pylori should be performed using the minimal inhibitory concentration method as recommended by the European Committee on Antimicrobial Susceptibility Testing (EUCAST). However, H. pylori is a fastidious organism that requires specific culture conditions [5]. Moreover, a delay in the transport of biopsy samples to a laboratory, or the use of proton pump inhibitors before biopsy, can result in a failure to culture H. pylori [6]. To overcome difficulties with H. pylori cultures, molecular assays for the detection of H. pylori have been developed. In addition to pathogen detection, several assays were designed for the identification of mutations associated with clarithromycin and/or levofloxacin [7].
Metronidazole and clarithromycin are included in non-bismuth quadruple H. pylori eradication regimens (concomitant, sequential and hybrid) and in triple therapy when metronidazole can be replaced by amoxicillin. In addition, metronidazole is a part of the bismuth quadruple H. pylori eradication regimen; [4] resistance to any of these drugs can lead to treatment failure. The wide spectrum of mechanisms of metronidazole resistance in H. pylori have been described, e.g. genetic rearrangements in the rdxA gene (insertions and deletions of transposons, and missense and frameshift mutations) and point mutations in the frxA and frxB genes that can further increase the level of metronidazole resistance in the presence of mutations in the rdxA gene [8, 9]. The resistance to clarithromycin is caused by point mutations in the 23S ribosomal subunit (23S rRNA). Four conserved efflux systems families have been also described in H. pylori strains [10].
In addition to resistance, the occurrence of heteroresistance in H. pylori isolates or samples has been reported [11]. Heteroresistance, a mixture of susceptible and resistant patterns, was found in H. pylori isolates and/or samples from the same site of biopsy (intraniche) or from H. pylori isolates and/or samples from different biopsy sites (interniche) [12]. Interestingly, heteroresistant H. pylori causative strains demonstrate similar fingerprinting patterns [13–17] suggesting the presence of the same strain with and without resistance mechanisms (monoclonal heteroresistance) rather than a co-infection with different strains (polyclonal heteroresistance) [11].
This study aims to summarize data on the prevalence of metronidazole and clarithromycin heteroresistance and the approaches to their detection.
Methods
Search strategy and study selection
Three databases including MEDLINE [PubMed], Scopus, and Web of Science were searched for relevant articles (Up to February 3rd, 2020) by using the following keywords: “Helicobacter pylori” OR “H. pylori” AND “heterogeneous resistance” OR “resistance heterogeneity” OR “heteroresistance” OR “antimicrobial heteroresistance” OR “metronidazole heteroresistance” OR “clarithromycin heteroresistance” in the Title/Abstract/Keywords fields. Only studies written in English were included. Reference lists of all related studies were also reviewed for any other related publications. The records found by searching the database were merged and the duplicates were removed using EndNote X7 (Thomson Reuters, New York, NY, USA).
Selection criteria and data extraction
The information extracted from each study included: (1) first author; (2) publication year; (3), patient gender and age (mean, range, paediatrics vs. adults); (4) biopsy site; (5) number of samples; (6) the method of heteroresistance detection; (7) heteroresistance rates; and (8) a definition of heteroresistance. A summary of the extracted data is shown in the Additional file 1: Table S1.
Exclusion criteria were: (1) heteroresistance was not detected; (2) the results of heteroresistance were not clearly reported; and (3) data on heteroresistance were from a meta-analysis and/or systematic review, non-original research or conference abstract.
Statistical analysis
Studies presenting data on metronidazole and/or clarithromycin heteroresistance were included in the meta-analysis which was performed by computing the pooled prevalence of heteroresistance for each antimicrobial agent using a random-effects model [18]. Inconsistencies across studies were examined by the forest plot as well as the I2 statistic. Values of I2 (25%, 50% and 75%) were interpreted as the presence of low, medium, or high heterogeneity, respectively.
Study outcomes
The main outcome of the study was the weighted pooled prevalence of heteroresistance to clarithromycin and metronidazole with subgroup analysis for the continent (Asia and Europe).
Results
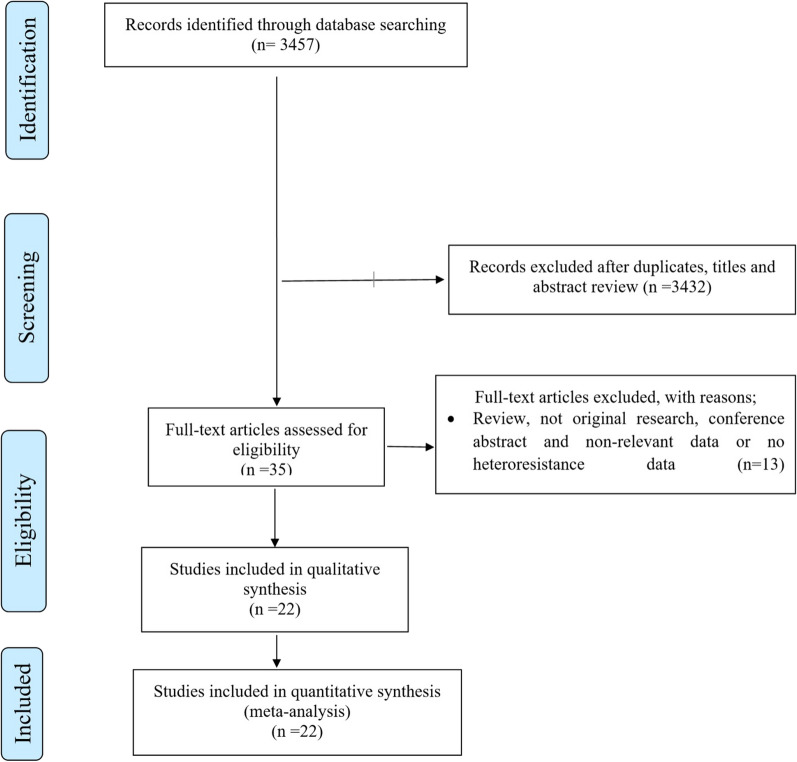
A total of 3457 records were identified in the initial search. From these, 3432 articles were excluded after an initial screening of the title and abstract due to their irrelevance or duplication. The full texts of the remaining 35 articles were reviewed. From the 35 articles, 13 were excluded for the following reasons: reviews; non-original researches; conference abstract; and non-relevant data or that no heteroresistance data were reported. Finally, 22 studies were included in this systematic review and meta-analysis (Fig. 1, Additional file 1) [12–17, 19–34].
Fig. 1.
Flow chart of study selection
In 22 studies, 3852 H. pylori positive patients were investigated. According to the continent, the majority of studies were from Europe (n = 10, 2172 patients), followed by Asia (n = 7, 1331 patients), America (Argentina, Mexico and Colombia 195 patients, Africa (Tunisia, 21 patients) and the Middle East (Turkey and Iran, 133 patients), Table 1, Aditional File 1.
Table 1.
Studies detecting heteroresistance to metronidazole and/or clarithromycin in Helicobacter pylori isolates/positive samples
| Study | Country | H. pylori-positive patients | Testing method | MTZ_R | MTZ_HR | CLR_R | CLR_HR |
|---|---|---|---|---|---|---|---|
| Kocsmár et al., 2020 [12] | Hungary | 305 | rRNA‐targeted FISH | NA | NA | 35 | 38 |
| Güven et al., 2019 [19] | Turkey | 93 | HelicoDR test | NA | NA | 22 | 12 |
| Farzi et al., 2019 [16] | Iran | 40 | Sequencing of 23S rDNA | NA | NA | 7 | 7 |
| Arévalo-Jaimes et al., 2019 [17] | Colombia | 63 | Sequencing 23S rDNA | NA | NA | 19 | 5 |
| Sun et al., 2018 [20] | China | 49 | Droplet digital PCR | NA | NA | 11 | 13 |
| Mascellino et al., 2018 [21] | Italy | 40 (30) | E-test, Real-Time PCR | 15 | 1 | 15 | 4 |
| Aguilera-Correa et al., 2017 [22] | Spain | 111 | HelicoDR test | NA | NA | 53 | 11 |
| Mansour et al., 2016 [23] | Tunisia, France | 42 | E-test | 21 | 7 | 12 | 6 |
| Navarro-Jarabo et al., 2015 [24] | Spain | 401 | HelicoDR test | NA | NA | 35 | 37 |
| Kao et al., 2014 [14] | Taiwan | 412 | E-test | NA | 16 | NA | 1 |
| Selgrad et al., 2014 [25] | Germany | 66 | E-test | 30 | 4 | 27 | 2 |
| Ayala et al., 2011 [26] | Mexico | 90 | E-test | 47 | 17 | 4 | 5 |
| Marzio et al., 2011 [27] | Italy | 68 | Agar dilution | NA | NA | 12 | 15 |
| Norazah et al., 2009 [28] | Malaysia | 22 | E-test | 6 | 6 | 0 | 1 |
| Matteo et al., 2006 [15] | Argentina | 42 | Agar dilution | 6 | 8 | NA | NA |
| Raymond et al., 2005 [29] | France | 28 | E-test | 22 | 14 | 13 | 3 |
| Lee et al., 2005 [30] | South Korea | 21 | Agar dilution | 4 | 12 | 2 | 5 |
| Rimbara et al., 2005 [31] | Japan | 542 (541) | Agar dilution | 4 | 4 | 39 | 25 |
| Kim et al., 2003 [13] | South Korea | 220 | Agar dilution | 72 | 28 | 7 | 6 |
| Masuda et al., 2003 [32] | Japan | 65 | PCR-SSCP, sequencing | NA | NA | 5 | 11 |
| van der Ende et al., 2001 [33] | Netherlands | 976 | E-test | NA | NA | 45 | 6 |
| Weel et al., 1996 [34] | Netherlands | 156 | Disk diffusion test, E-test | 37 | 52 | NA | NA |
The number in brackets indicates the number of samples investigated for metronidazole resistance when it differed from the number of samples investigated for clarithromycin resistance
R resistance (samples/isolates with heteroresistance are not included), HR heteroresistance
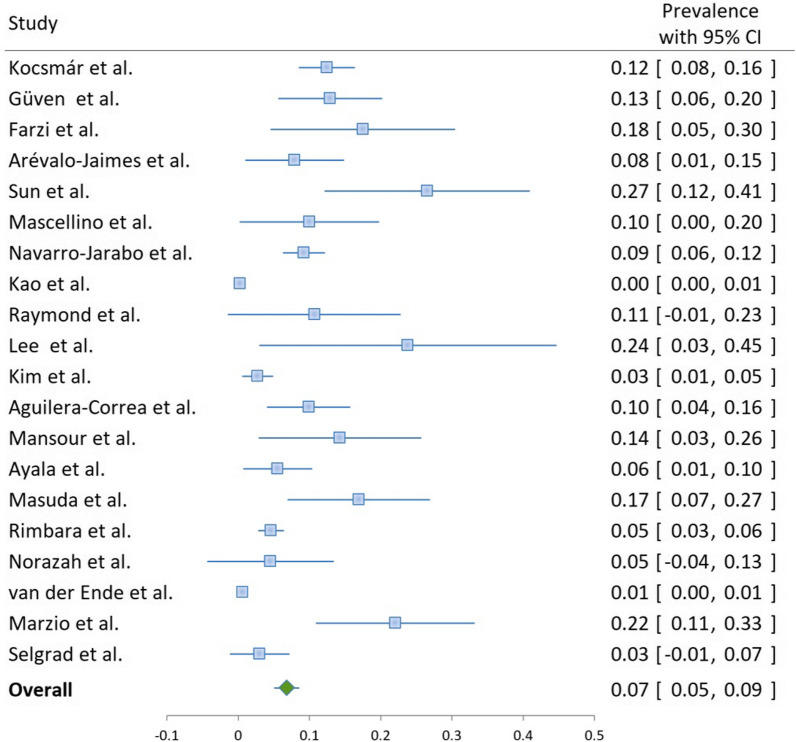
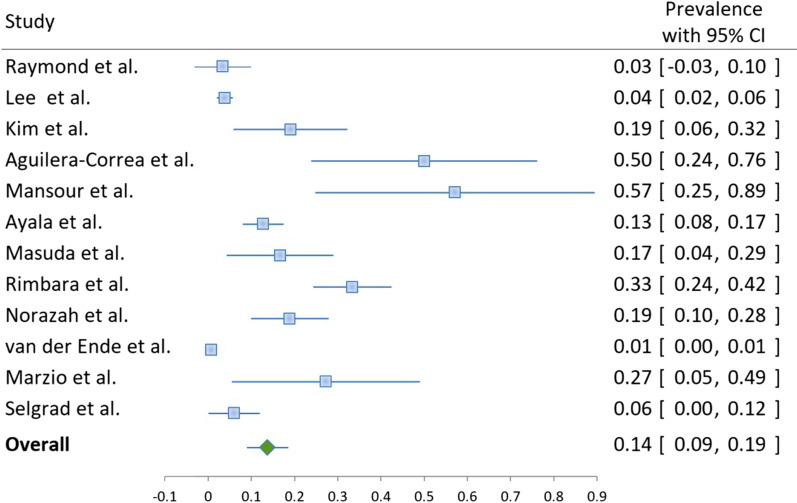
Heteroresistance to clarithromycin was reported in 20 studies, with a weighted pooled prevalence of 6.8% (95% CI 5.1–8.6) among 3654 H. pylori positive patients; the substantial heterogeneity was I2 = 55.6%. (Table 1, Fig. 2). Heteroresistance to metronidazole was reported in 12 studies, with a weighted pooled prevalence of 13.8% (95% CI 8.9–18.6) among 1670 H. pylori positive patients and substantial heterogeneity (I2 = 60.9%) (Table 1, Fig. 3).
Fig. 2.
Clarithromycin resistance in Helicobacter pylori-positive samples or isolates
Fig. 3.
Metronidazole resistance in Helicobacter pylori-positive samples or isolates
The weighted pooled prevalence of clarithromycin heteroresistance was similar in Europe (8.4%; 95% CI 3.8–12.9%; I2 = 0), and Asia (5.6%; 2.1–9.1%; I2 = 61.8%, p-value 0.174584); however, when compared to Asia, the metronidazole heteroresistance was detected more often in European H. pylori positive patients (19.6%; 95% CI 5.6–33.6%; I2 = 24.7% vs. (7.6%; 95% CI 2.4–12.8%; I2 = 73.3%), Additional files 2, 3, 4, 5: Fig. S1-S4. Data for the Middle East, Africa and America were not calculated due to the small number of studies.
Clarithromycin heteroresistance was detected by phenotype in 12 studies (agar dilution n = 5, E-test n = 7) and by genotype in eight studies (Table 1). Three studies used the same commercial molecular assay HelicoDR test (Hain Lifescience, Germany). In the study of Navarro-Jarabo et al., this assay was applied to H. pylori isolates and in the studies of Aguillera-Correa et al. and Güven et al., DNA extracted from biopsy samples was tested [19, 22, 24]. Another commercial assay, BACTfish H. pylori Combi kit (Izinta Kft., Hungary) was used to analyse the biopsy specimens [12]. Two other studies designed their molecular assay: one was based on Real-Time PCR followed by a melting curve analysis using fluorochrome-labelled probes in DNA from H. pylori isolates; the second analysed DNA from gastric brushes samples using droplet digital PCR [20, 21]. In contrast to the heteroresistance to clarithromycin, the heteroresistance to metronidazole was detected only by phenotype (agar dilution n = 4, E-test n = 7, disk diffusion followed by E-test n = 1), (Table 1).
Discussion
Antimicrobial susceptibility testing is essential for the administration of effective antibiotic treatment and the control of antimicrobial resistance, however, antimicrobial susceptibility testing in causative H. pylori strains is recommended after second-line treatment failure. Given that a combination of antimicrobials is used for the treatment of H. pylori infections, antimicrobial susceptibility testing should be performed to reduce the burden on the patient and decrease the risk of eradication treatment failure through the administration of ineffective antimicrobial drugs [35].
Global data gathered by the World Health Organization (WHO) on the resistance of antimicrobials used for the eradication of H. pylori show an alarming upward trend in all WHO regions [36]. In addition to resistance, the occurrence of heteroresistance in H. pylori isolates or samples has been described [11]. The heteroresistance can be detected intraniche by the presence of susceptible and resistant patterns in one strain and/or sample and interniche when differences in susceptibility patterns are observed between strains and/or samples from different biopsy sites [12]. As was shown, the interniche heteroresistance can be undetected in one-fifth of cases when only one antrum biopsy site approach is used [12]. Two biopsy sites, where preferably multiple biopsies are taken, can increase the probability of differences in antimicrobial susceptibility patterns [12].
In our meta-analysis that included 22 studies, a weighted pooled prevalence of heteroresistance to clarithromycin was 6.8% (95% CI 5.1–8.6) and heteroresistance to metronidazole was shown to be greater than two times higher at 13.8% (95% CI 8.9–18.6). These data are consistent with the latest WHO data on resistance of H. pylori where resistance to metronidazole was found to occur approximately twice as often as resistance to clarithromycin in all WHO regions, except for the Americas [36].
Interestingly, in several studies, the heteroresistant phenotype was detected rarely in several isolates [25], however, other studies have shown an equal or even higher number of heteroresistant samples compared to resistant phenotype [12, 24, 27].
The subgroups analysis of the methods for heteroresistance showed that a majority of studies detected heteroresistance by phenotype; E-test was the most common. Recently, the E-test performance was compared to agar dilution on 72 clinical H. pylori isolates and a high essential agreement (> 90.0%) was found for amoxicillin, erythromycin, tetracycline and levofloxacin, but it was only 84.7% for metronidazole. However, higher detected rates of resistance by the E-test were not statistically significant [37].
Genotyping methods were used for the detection of mutations in the 23S rRNA gene associated with resistance to clarithromycin [9]. In our meta-analysis, four studies used a commercial molecular assay [12, 19, 22, 24]. Two other studies used lab-developed molecular assays [20, 21]. None of the studies used a genotyping method for the detection of heteroresistance to metronidazole probably due to the nature of the molecular mechanisms. The wide spectrum of genetic changes in the rdxA, the main mechanisms of resistance to metronidazole, complicates the design of a molecular assay and, for now, the detection of resistance to metronidazole relies on phenotype-based susceptibility testing [9].
Conclusion
The prevalence of heteroresistance to clarithromycin and/or metronidazole is not negligible and can be detected approximately in 7 and 14% of H. pylori positive samples, respectively. These findings highlight the need to raise the awareness of gastroenterologists and microbiologists to the heteroresistance to clarithromycin and metronidazole in patients with a H. pylori infection. Therefore, data on heteroresistance should be included in a new guidance document for the diagnosis and treatment of H. pylori infections [38]. This meta-analysis can serve as solid evidence for this purpose.
Supplementary Information
Additional file 1. Summary of included studies.
Additional file 2: Figure S1. Clarithromycin resistance in Helicobacter pylori-positive samples/isolates in Europe.
Additional file 3: Figure S2. Clarithromycin resistance in Helicobacter pylori-positive samples or isolates in Asia.
Additional file 4: Figure S3. Metronidazole resistance in Helicobacter pylori-positive samples or isolates in Europe.
Additional file 5: Figure S4. Metronidazole resistance in Helicobacter pylori-positive samples or isolates in Asia.
Acknowledgements
Not applicable.
Abbreviations
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- 23S rRNA
23S ribosomal subunit
- WHO
World Health Organization
Author contributions
HV, SG, EK, AK, TA, NS: conception and design of the study; the acquisition of data. HS: analysis and interpretation of data. MK: drafting the first version of the manuscript and editing subsequent versions according to the comments of the other authors. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Consent for publication
Informed consent was obtained from all individual participants included in the review.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Calvet X, Ramírez Lázaro MJ, Lehours P, Mégraud F. Diagnosis and epidemiology of Helicobacter pylori infection. Helicobacter. 2013;18:5–11. doi: 10.1111/hel.12071. [DOI] [PubMed] [Google Scholar]
- 2.Crowe SE. Helicobacter pylori infection. N Engl J Med. 2019;380(12):1158–1165. doi: 10.1056/NEJMcp1710945. [DOI] [PubMed] [Google Scholar]
- 3.IARC Working Group: Schistosomes liver flukes and Helicobacter pylori IARC working group on the evaluation of carcinogenic risks to humans Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 4.Fallone CA, Moss SF, Malfertheiner P. Reconciliation of recent Helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology. 2019;157(1):44–53. doi: 10.1053/j.gastro.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard TG, Nedrud JG. Laboratory maintenance of Helicobacter species. Curr Protoc Microbiol. 2006 doi: 10.1002/9780471729259.mc08b01s00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saniee P, Shahreza S, Siavoshi F. Negative effect of proton-pump inhibitors (PPIs) on Helicobacter pylori growth, morphology, and urease test and recovery after ppi removal–an in vitro study. Helicobacter. 2016;21(2):143–152. doi: 10.1111/hel.12246. [DOI] [PubMed] [Google Scholar]
- 7.Smith SM, O'Morain C, McNamara D. Antimicrobial susceptibility testing for Helicobacter pylori in times of increasing antibiotic resistance. World J Gastroenterol. 2014;20(29):9912–9921. doi: 10.3748/wjg.v20.i29.9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matteo MJ, Pérez CV, Domingo MR, Olmos M, Sanchez C, Catalano M. DNA sequence analysis of rdxA and frxA from paired metronidazole-sensitive and-resistant Helicobacter pylori isolates obtained from patients with heteroresistance. Int J Antimicrob Agents. 2006;27(2):152–158. doi: 10.1016/j.ijantimicag.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Nishizawa T, Suzuki H. Mechanisms of Helicobacter pylori antibiotic resistance and molecular testing. Front Mol Biosci. 2014;1:19. doi: 10.3389/fmolb.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bina JE, Alm RA, Uria-Nickelsen M, Thomas SR, Trust TJ, Hancock RE. Helicobacter pylori uptake and efflux: basis for intrinsic susceptibility to antibiotics in vitro. Antimicrob Agents Chemother. 2000;44(2):248–254. doi: 10.1128/AAC.44.2.248-254.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tshibangu-Kabamba E, Yamaoka Y. Helicobacter pylori infection and antibiotic resistance - from biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2021;18(9):613–629. doi: 10.1038/s41575-021-00449-x. [DOI] [PubMed] [Google Scholar]
- 12.Kocsmár É, Kocsmár I, Buzás GM, Szirtes I, Wacha J, Takáts A, Hritz I, Schaff Z, Rugge M, Fassan M, Kiss A, Lotz G. Helicobacter pylori heteroresistance to clarithromycin in adults-new data by in situ detection and improved concept. Helicobacter. 2020;25(1):e12670. doi: 10.1111/hel.12670. [DOI] [PubMed] [Google Scholar]
- 13.Kim JJ, Kim JG, Kwon DH. Mixed-infection of antibiotic susceptible and resistant Helicobacter pylori isolates in a single patient and underestimation of antimicrobial susceptibility testing. Helicobacter. 2003;8(3):202–206. doi: 10.1046/j.1523-5378.2003.00145.x. [DOI] [PubMed] [Google Scholar]
- 14.Kao CY, Lee AY, Huang AH, Song PY, Yang YJ, Sheu SM, Chang WL, Sheu BS, Wu JJ. Heteroresistance of Helicobacter pylori from the same patient prior to antibiotic treatment. Infect Genet Evol. 2014;23:196–202. doi: 10.1016/j.meegid.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Matteo MJ, Pérez CV, Domingo MR, Olmos M, Sanchez C, Catalano M. DNA sequence analysis of rdxA and frxA from paired metronidazole-sensitive and -resistant Helicobacter pylori isolates obtained from patients with heteroresistance. Int J Antimicrob Agents. 2006;27(2):152–158. doi: 10.1016/j.ijantimicag.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Farzi N, Behzad C, Hasani Z, Alebouyeh M, Zojaji H, Zali MR. Characterization of clarithromycin heteroresistance among Helicobacter pylori strains isolated from the antrum and corpus of the stomach. Folia Microbiol. 2019;64(2):143–151. doi: 10.1007/s12223-018-0637-9. [DOI] [PubMed] [Google Scholar]
- 17.Arévalo-Jaimes BV, Rojas-Rengifo DF, Jaramillo CA, de Molano BM, Vera-Chamorro JF, Del Pilar DM. Genotypic determination of resistance and heteroresistance to clarithromycin in Helicobacter pylori isolates from antrum and corpus of colombian symptomatic patients. BMC Infect Dis. 2019;19(1):546. doi: 10.1186/s12879-019-4178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and forest plots using a microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5:52. doi: 10.1186/1756-0500-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Güven B, Gülerman F, Kaçmaz B. Helicobacter pylori resistance to clarithromycin and fluoroquinolones in a pediatric population in Turkey: a cross-sectional study. Helicobacter. 2019;24(3):e12581. doi: 10.1111/hel.12581. [DOI] [PubMed] [Google Scholar]
- 20.Sun L, Talarico S, Yao L, He L, Self S, You Y, Zhang H, Zhang Y, Guo Y, Liu G, Salama NR, Zhang J. Droplet digital pcr-based detection of clarithromycin resistance in Helicobacter pylori isolates reveals frequent heteroresistance. J Clin Microbiol. 2018;56(9):e00019–e118. doi: 10.1128/JCM.00019-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mascellino MT, Oliva A, De Angelis M, Pontone S, Porowska B. Helicobacter pylori infection: antibiotic resistance and eradication rate in patients with gastritis showing previous treatment failures. New Microbiol. 2018;41(4):306–309. [PubMed] [Google Scholar]
- 22.Aguilera-Correa JJ, Urruzuno P, Barrio J, Martinez MJ, Agudo S, Somodevilla A, Llorca L, Alarcón T. Detection of Helicobacter pylori and the genotypes of resistance to clarithromycin and the heterogeneous genotype to this antibiotic in biopsies obtained from symptomatic children. Diagn Microbiol Infect Dis. 2017;87(2):150–153. doi: 10.1016/j.diagmicrobio.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Ben Mansour K, Fendri C, Battikh H, Garnier M, Zribi M, Jlizi A, Burucoa C. Multiple and mixed Helicobacter pylori infections: comparison of two epidemiological situations in Tunisia and France. Infect Genet Evol. 2016;37:43–48. doi: 10.1016/j.meegid.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 24.Navarro-Jarabo JM, Fernández-Sánchez F, Fernández-Moreno N, Hervas-Molina AJ, Casado-Caballero F, Puente-Gutierrez JJ, Pallares-Manrique H, Rodríguez-Ramos C, Fernández-Gutierrez C, Pérez-Aisa A, Rivas-Ruiz F, Montiel Q-G. Prevalence of primary resistance of Helicobacter pylori to clarithromycin and levofloxacin in Southern Spain. Digestion. 2015;92(2):78–82. doi: 10.1159/000435949. [DOI] [PubMed] [Google Scholar]
- 25.Selgrad M, Tammer I, Langner C, Bornschein J, Meißle J, Kandulski A, Varbanova M, Wex T, Schlüter D, Malfertheiner P. Different antibiotic susceptibility between antrum and corpus of the stomach, a possible reason for treatment failure of Helicobacter pylori infection. World J Gastroenterol. 2014;20(43):16245–16251. doi: 10.3748/wjg.v20.i43.16245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayala G, Galván-Portillo M, Chihu L, Fierros G, Sánchez A, Carrillo B, Román A, López-Carrillo L, Silva-Sánchez J, Study Group Resistance to antibiotics and characterization of Helicobacter pylori strains isolated from antrum and body from adults in Mexico. Microb Drug Resist. 2011;17(2):149–155. doi: 10.1089/mdr.2010.0154. [DOI] [PubMed] [Google Scholar]
- 27.Marzio L, Cellini L, Amitrano M, Grande R, Serio M, Cappello G, Grossi L. Helicobacter pylori isolates from the proximal and distal stomach of patients never treated and already treated show genetic variability and discordant antibiotic resistance. Eur J Gastroenterol Hepatol. 2011;23(6):467–472. doi: 10.1097/MEG.0b013e328345d40f. [DOI] [PubMed] [Google Scholar]
- 28.Norazah A, Rasinah WZ, Zaili Z, Aminuddin A, Ramelah M. Analysis of PCR-RAPD DNA and antibiotic susceptibility profiles of the antrum and corpus isolates of Helicobacter pylori from Malaysian patients. Malays J Pathol. 2009;31(1):29–34. [PubMed] [Google Scholar]
- 29.Raymond J, Nguyen B, Bergeret M, Dupont C, Kalach N. Heterogeneous susceptibility to metronidazole and clarithromycin of Helicobacter pylori isolates from a single biopsy in adults is confirmed in children. Int J Antimicrob Agents. 2005;26(4):272–278. doi: 10.1016/j.ijantimicag.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Lee YC, Lee SY, Pyo JH, Kwon DH, Rhee JC, Kim JJ. Isogenic variation of Helicobacter pylori strain resulting in heteroresistant antibacterial phenotypes in a single host in vivo. Helicobacter. 2005;10(3):240–248. doi: 10.1111/j.1523-5378.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 31.Rimbara E, Noguchi N, Tanabe M, Kawai T, Matsumoto Y, Sasatsu M. Susceptibilities to clarithromycin, amoxycillin and metronidazole of Helicobacter pylori isolates from the antrum and corpus in Tokyo, Japan, 1995–2001. Clin Microbiol Infect. 2005;11(4):307–311. doi: 10.1111/j.1469-0691.2005.01099.x. [DOI] [PubMed] [Google Scholar]
- 32.Masuda H, Hiyama T, Yoshihara M, Tanaka S, Shimamoto F, Haruma K, Chayama K. Necessity of multiple gastric biopsies from different sites for detection of clarithromycin-resistant Helicobacter pylori strains. Scand J Gastroenterol. 2003;38(9):942–946. doi: 10.1080/00365520310004731. [DOI] [PubMed] [Google Scholar]
- 33.van der Ende A, van Doorn LJ, Rooijakkers S, Feller M, Tytgat GN, Dankert J. Clarithromycin-susceptible and -resistant Helicobacter pylori isolates with identical randomly amplified polymorphic DNA-PCR genotypes cultured from single gastric biopsy specimens prior to antibiotic therapy. J Clin Microbiol. 2001;39(7):2648–2651. doi: 10.1128/JCM.39.7.2648-2651.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weel JF, van der Hulst RW, Gerrits Y, Tytgat GN, van der Ende A, Dankert J. Heterogeneity in susceptibility to metronidazole among Helicobacter pylori isolates from patients with gastritis or peptic ulcer disease. J Clin Microbiol. 1996;34(9):2158–2162. doi: 10.1128/JCM.34.9.2158-2162.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM, European Helicobacter and Microbiota Study Group and consensus panel Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017;66(1):6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 36.Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in world health organization regions. Gastroenterology. 2018;155(5):1372–1382.e17. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miftahussurur M, Fauzia KA, Nusi IA, Setiawan PB, Syam AF, Waskito LA, Doohan D, Ratnasari N, Khomsan A, Adnyana IK, Akada J, Yamaoka Y. E-test versus agar dilution for antibiotic susceptibility testing of Helicobacter pylori: a comparison study. BMC Res Notes. 2020;13(1):22. doi: 10.1186/s13104-019-4877-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizvanov AA, Haertlé T, Bogomolnaya L, Talebi Bezmin Abadi A. Helicobacter pylori and its antibiotic heteroresistance: a neglected issue in published guidelines. Front Microbiol. 2019;10:1796. doi: 10.3389/fmicb.2019.01796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Summary of included studies.
Additional file 2: Figure S1. Clarithromycin resistance in Helicobacter pylori-positive samples/isolates in Europe.
Additional file 3: Figure S2. Clarithromycin resistance in Helicobacter pylori-positive samples or isolates in Asia.
Additional file 4: Figure S3. Metronidazole resistance in Helicobacter pylori-positive samples or isolates in Europe.
Additional file 5: Figure S4. Metronidazole resistance in Helicobacter pylori-positive samples or isolates in Asia.
Data Availability Statement
Not applicable.