Abstract
Background:
Given the psychological stress associated with managing type 2 diabetes (T2D), resilience-promoting interventions may particularly benefit populations experiencing high levels of stress (e.g., racial/ethnic minority and lower-income individuals). Federally qualified Community Health Centers (CHCs) primarily serve these patients and are therefore ideal settings for resilience-promoting T2D programs. This proof-of-concept study tested the Resilience-Based Diabetes Self-Management Education (RB-DSME) intervention within a CHC.
Methods:
Thirty-five patients with T2D (M age = 51 years, 71% female, 60% Hispanic, 69% annual household income <$20,000) at two clinics within the CHC completed the RB-DSME, consisting of eight bi-weekly classes and two monthly support groups. In this treatment-only design, resilience resources, self-management behaviors, and physical and mental health outcomes were measured at baseline and 6-months.
Results:
Attendance (M = 7.66/10) and program satisfaction (M = 6.79/7) were high. Participants improved adaption to stress (d = .67), adaptive coping (d = .60), diabetes empowerment (d = .57) and finding positive meaning (d = .85). Large increases in self-management behaviors (d = 1.38) and number of steps (d = 1.11) were also observed. Participants lowered A1C from baseline (M = 8.79%) to 6 months (M = 8.11%; d = .50), along with diabetes distress (d = 1.31), depressive symptoms (d = .80), and general perceived stress (d = .55).
Conclusions:
This study demonstrated the ability of the RB-DSME to improve resilience resources, self-management behaviors, and health outcomes among racial/ethnic minority and lower-income patients with T2D at clinics within a CHC. A larger, randomized trial should more rigorously test the RB-DSME in this clinical setting.
Keywords: Resilience, diabetes, stress, health disparities, self-management
Introduction
Type 2 diabetes (T2D) is the 6th leading cause of disability [1] and 7th leading cause of death in the United States (U.S.) [2], accounting for the highest healthcare spending among all disease categories for adults [3]. Racial/ethnic minority and lower socioeconomic status (SES) individuals have a higher prevalence of T2D and morbidity and mortality associated with the disease [4,5]. Diabetes-related complications are relatively common among these groups, often with dire consequences (e.g., high prevalence of end-stage renal disease, retinopathy, and depression) [6]. Yet, this unremitting accumulation of damage is largely preventable. Impactful interventions addressing T2D disparities are urgently needed and align with the National Institutes of Health, World Health Organization, and United Nations’ goals to develop effective, tailored behavioral interventions to promote health in populations that experience health disparities [7,8].
The Obesity-Related Behavioral Intervention Trials (ORBIT) model for behavioral treatment development is a systematic framework for designing, refining, and testing such interventions [9]. The transdisciplinary ORBIT model facilitates the translation of basic behavioral science results to clinical application through a series of phases including defining, refining, proof-of-concept, pilot testing, efficacy testing, and effectiveness research [9]. The remainder of this introduction discusses phase Ia (define) and phase Ib (refine) before introducing the current study (phase IIa: proof-of-concept).
Literature Review: ORBIT Phase Ia (Define)
Health disparities in T2D may be partially explained by stress-related difficulties in practicing self-management behaviors. Acute and chronic stressors associated with T2D are often compounded by general life stressors (e.g., financial challenges, difficulty securing employment, discrimination), which worsen T2D by reducing adherence to diabetes self-management behaviors [10,11], causing insulin resistance, T2D-related complications, and subsequently diminishing physical and mental health [12,13]. In addition to these life stressors, perceived stress is also a risk factor for disparities in T2D [12,14]. Low-income individuals with T2D report perceived stress as a primary obstacle to healthy eating [15], and African American women with T2D serving multiple caregiver roles report high levels of perceived stress (e.g., difficulty saying “no”, putting family needs ahead of self) that inhibit self-management behaviors [16]. Along with perceived stress, depression is also associated with poorer diet, less physical activity, and lower adherence to diabetes medication [17].
In addition to inhibiting self-management behaviors, perceived stress can also impair T2D control via biological pathways, which in turn negatively impact individuals’ physical and mental health. According to the allostatic load model, the hypothalamic pituitary adrenal (HPA) axis is the primary biological pathway by which perceived stress influences disease [18]. The stress response, although initially adaptive, can become problematic if engaged excessively over the long-term [19]. For example, elevated levels of cortisol in scalp hair, a biomarker of chronic stress exposure, are associated with higher A1C among African American adults with T2D [20]. If a perceived threat persists long-term, the stress can contribute to the onset of depression via endocrine and immune system pathways [21,22], and impair health [23].
Although perceived stress can contribute to elevated diabetes risk, it also represents a modifiable T2D determinant [12]. The impact of perceived stress on T2D self-management can be attenuated by resilience resources, offering an innovative approach to self-management aligned with global strategic plans for more research examining the role of resilience in health [7,8]. Resilience is the ability to adapt in the face of adversity to promote positive outcomes [24,25], and describes a constellation of attributes [26–30] that reflect one’s resolve to succeed despite adversity (e.g., determination, confidence, personal strength [27], positive adaptation to stress [28,29], emotional regulation [31,32], and supportive relationships [24,33]). Higher levels of resilience resources are associated with enhanced diabetes self-management behaviors and improved A1C in the face of diabetes distress [34–36].
Intervention Development and Target Population: ORBIT Phase Ib (Refine)
Resilience can be developed to improve adaptation to stress and utilized in interventions to improve diabetes-related health outcomes. Our Resilience-Based Diabetes Self-Management Education (RB-DSME) intervention helps participants transfer learned resilience skills that they employ in other facets of their lives to their daily diabetes self-management. Racial/ethnic minority and lower-SES individuals may particularly benefit from resilience-infused diabetes programming because they experience high levels of life stressors and perceived stress [37] which can compound T2D-associated stressors and compromise T2D outcomes [38–40]. The RB-DSME has previously demonstrated outstanding recruitment and retention in predominantly African-American church settings and has shown clinically meaningful improvements in T2D health indicators [41,42].
In the U.S., federally qualified Community Health Centers (CHCs) serve a high proportion of racial/ethnic minority individuals and patients with lower SES and are therefore ideal locations to deliver resilience-based diabetes programming. Furthermore, diabetes programs offered by CHCs can improve glucose control and decrease medical expenditures by reducing emergency department visits and lowering inpatient costs due to diabetic complications. Thus, conducting the RB-DSME intervention in CHCs is a promising opportunity to integrate resilience programming in a high-risk clinical setting to address disparities in T2D.
In order to tailor the existing RB-DSME curriculum to the CHC setting and patient population, we conducted informal RB-DSME sessions in the context of group medical visits with a small group of patients (N=9). Classes were led by a board-certified endocrinologist, with assistance from the clinical extended health team (i.e., nurse, clinical pharmacist, behavioral health counselor). The RB-DSME was well-received, and clinically significant improvements were found over a six-month period (i.e., average change in A1C from 9.7% to 8.0%; unpublished data). Patients valued the instruction from a physician, the assistance from the extended health team, and the partnership with CHC staff, and suggested that bi-weekly classes were an optimal meeting frequency for the program.
The Current Study: ORBIT Phase IIa (Proof-of-Concept)
The findings above suggested that the RB-DSME may be feasible in CHCs. According to the ORBIT model, the next appropriate step is a proof-of-concept using a treatment-only design to determine if the treatment merits more rigorous and costly testing. This type of study can determine if clinically significant improvements under ideal conditions can be produced that warrant using a randomized design. Thus, our next step was to conduct the current proof-of-concept study, which tested the RB-DSME intervention’s ability to improve clinically relevant outcomes for patients with T2D at two clinics within a large federally qualified CHC in central Texas. We hypothesized that participants receiving the RB-DSME would have enhanced resilience resources, T2D self-management behaviors, and T2D physical and mental health outcomes at 6 months post-study entry compared to baseline.
Methods
Study Design
The proof-of-concept of the RB-DSME intervention at two clinics within a federally qualified CHC was examined using a treatment-only pretest-posttest design. Primary health outcomes were measured at baseline prior to the start of the intervention, and again at six months post-study entry. Two clinics participated, each hosting two small groups, for a total of four groups. The intervention was designed to help participants use resilience resources to effectively manage their T2D. All classes were taught by a board-certified endocrinologist with assistance from the extended health team (i.e., nurse, clinical pharmacist, behavioral health counselor). The program included eight bi-weekly class sessions followed by two monthly support group sessions.
Participants and Procedures
Participants were a convenience sample (N=42) of patients diagnosed with T2D who received care at two clinics within a CHC in Central Texas. Recruitment flyers were posted onsite and given to health care providers to share with their patients. Participants either contacted study staff directly or were referred by their physician. Participants were required to meet the following inclusion criteria: 1) diagnosed with T2D; 2) between 18 and 75 years old; and 3) not currently participating in another diabetes self-management program. Individuals were excluded if they were pregnant/lactating or had a medical condition for which changes in diet and activity would be contraindicated (e.g., kidney failure requiring dialysis and special dietary requirements, peripheral vascular disease severe enough to preclude walking three times/week). We did not experience difficulty in enrolling participants; over 80% of individuals who expressed a desire to participate in the program attended baseline data collection and participated.
In total, 42 participants completed the baseline assessment, comprising the four small groups of approximately 10 people per group. Seven participants discontinued the intervention and did not complete the 6-months data collection, resulting in a final analytic sample of N=35 (Figure 1). Eighty-six percent of completers had a high school degree/GED or higher educational attainment, while 43% of non-completers had a high school degree/GED or higher. The analytic sample was 71% female (25 women, 10 men), diagnosed with T2D an average of 13 years (range: 1 month-39 years), and ranged in age from 26 to 72 years with a mean age of 51 years. The most frequently reported demographic characteristics of participants were: Hispanic (60%), separated/divorced (40%), completed high school (46%), household income $19,999 or less (69%), and unemployed and not looking for work (37%). Descriptive information of the study sample is presented in Table 1.
Figure 1.
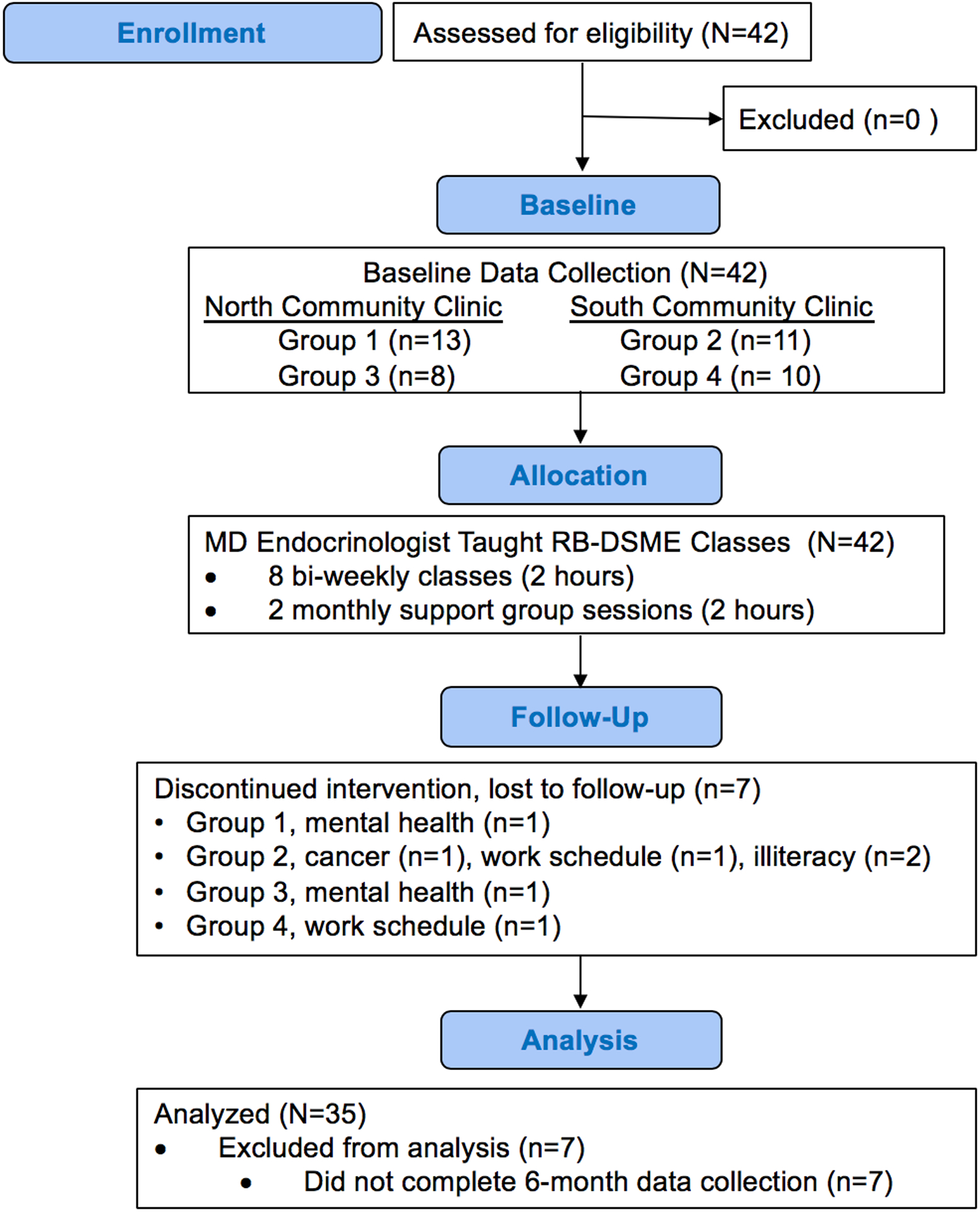
Participant flowchart
Table 1.
Descriptive statistics of the study sample
| Participant Characteristics | N | M (SD) or % |
|---|---|---|
| Age | 35 | 51.03 (11.09) |
| Sex (females) | 25 | 71.4% |
| Race/Ethnicity | ||
| Non-Hispanic White | 6 | 17.1% |
| Hispanic or Latino | 21 | 60.0% |
| African American | 5 | 14.3% |
| Other | 3 | 8.6% |
| Marital Status | ||
| Never Married | 5 | 14.3% |
| Married | 13 | 37.1% |
| Separated/ Divorced | 14 | 40.0% |
| Widowed | 3 | 8.6% |
| Education | ||
| Less than High School | 5 | 14.3% |
| High School Diploma | 16 | 45.7% |
| Some College/Technical School | 9 | 25.7% |
| College Graduate (Degree) | 2 | 5.7% |
| Graduate Degree | 3 | 8.6% |
| Employment | ||
| Employed Full or Part Time | 7 | 20.0% |
| Unemployed/Laid Off & Looking for Work | 7 | 20.0% |
| Unemployed & Not Looking for Work | 13 | 37.1% |
| Homemaker | 2 | 5.7% |
| Retired | 6 | 17.1% |
| Household Income | ||
| $19,999 or less | 24 | 68.6% |
| $20,000 to $39,000 | 9 | 25.7% |
| $40,000 to $59,000 | 1 | 2.9% |
| $60,000 to $79,000 | 1 | 2.9% |
| Diabetes Diagnosis Length (years) | 35 | 12.58 (8.49) |
| Diabetes-Related Medication Use | ||
| Oral Meds and/or Non-insulin Injectable | 8 | 23% |
| Insulin | 10 | 28% |
| Both | 14 | 40% |
| None | 3 | 9% |
Collection of survey and physiological data at baseline and at 6-months took approximately one hour to complete. All study variables for each participant were assessed during a single study visit. Questionnaires were self-administered, with assistance provided to those with literacy limitations. Physiological data were collected by a trained technician. Participants received $20 cash for completing baseline testing and an additional $20 for completing the 6-month follow-up testing, in compensation for their time. If needed, a one-day bus pass valued at $2.50 was also provided for each trip to the clinic to participate in the study. Written informed consent was obtained from all participants, and the study protocol was approved by the Institutional Review Board of the sponsoring university.
Measures
Participant characteristics included age, sex, race/ethnicity, marital status, education, employment, household income, length of T2D diagnosis, and diabetes-related medication use. A measure of participants’ satisfaction with the RB-DSME intervention was assessed with the following item answered on a 7-point Likert scale ranging from 1 (extremely dissatisfied) to 7 (extremely satisfied): “How satisfied were you with the diabetes program?” All survey measures (resilience resources, T2D self-management behaviors, and T2D physical and mental health outcomes) are listed below. Unless stated otherwise, these measures were calculated as the mean of all items and asked participants to reflect over the past three months, corresponding with the three months indexed by retrospective A1C assessment.
Resilience Resources
Resilience resources were assessed using four indicators: 1) adaptation to stress; 2) adaptive and maladaptive coping strategies; 3) finding positive meaning; and 4) diabetes empowerment. Adaptation to stress was measured using the 6-item Brief Resilience Scale (BRS) [43], which assesses the capacity of an individual to bounce back or recover from stress. Respondents reported the degree to which they felt statements such as, “I tend to bounce back quickly after hard times,” and “It does not take me long to recover from a stressful event,” described themselves. Response options ranged from 1 (strongly disagree) to 5 (strongly agree). Internal consistency for the BRS was high at baseline (α = .88) and follow-up (α = .85).
Coping strategies were measured using a shortened version of the Brief Coping Orientations to Problems Experienced Scale (Brief COPE), including one item from each of the fourteen subscales included in the Brief COPE [44]. Participants were asked to what extent they utilized specific cognitive and behavioral coping strategies on a scale from 1 (not at all) to 4 (a lot). Items were grouped into two commonly used subsets: adaptive and maladaptive coping strategies [45]. Adaptive coping strategies included planning, positive reframing, active coping, religion, acceptance, instrumental support, and emotional support, and maladaptive coping strategies included venting, behavioral disengagement, self-distraction, humor, substance use, denial, and self-blame. Partitioning the Brief COPE into these two broader coping strategies is consistent with prior research [45]. In patients with T2D, adaptive coping strategies have been associated with greater resilience and maladaptive coping strategies with lower resilience [46]. Adaptive coping strategies have also been associated with enhanced T2D self-management behaviors, whereas maladaptive coping strategies have been associated with greater depressive symptoms [47]. Internal consistency alpha coefficients for the adaptive and maladaptive coping subscales were .80 and .63, respectively, at baseline and .82 and .71, respectively, at follow-up.
Finding positive meaning was measured using a shortened version of the Positive Meaning Scale (PMS) [48]. Two items measured the extent to which participants found positive meaning in the context of their disease: “Did anything good come out of dealing with your diabetes?” and “Do you think it is likely that there is something to learn from your experience with diabetes?”. Response options ranged from 0 (definitely no) to 3 (definitely yes), and internal consistency was marginal at baseline (α = .59) and high at follow-up (α = .81).
Diabetes empowerment was measured using the 8-item Diabetes Empowerment Scale – Short Form (DES-SF) [49], operationalized as participants’ self-efficacy in managing their diabetes. Sample items included, “I can try out different ways of overcoming barriers to my diabetes goals,” and “I know the positive ways I cope with diabetes related stress.” Participants responded on a 5-point Likert scale from 1 (strongly disagree) to 5 (strongly agree). Reliability was high at baseline (α = .82) and follow-up (α = .87).
T2D Self-Management Behaviors
Self-management behaviors were assessed using two indicators: a self-report survey of self-management, and a pedometer to measure physical activity. Diabetes self-management was assessed using the 13-item Self-Care Inventory-Revised (SCI-R) [50], which measured perceived adherence during the past two months to self-management behaviors such as checking blood glucose levels, eating correct food portions, reading food labels, and keeping clinic appointments. Responses were assessed on a 5-point Likert scale ranging from 1 (never) to 5 (always). The SCI-R’s internal consistency was marginal at baseline (α = .69) and acceptable at follow-up (α = .71).
Physical activity was measured with wrist-worn MOVABLE pedometers (Brecksville, OH), computer-synced at the beginning of each T2D class. Average steps per day were assessed over a 2-week period at baseline and at 6 months follow-up.
T2D Health Outcomes
Health outcomes were assessed using six indicators. A1C, blood pressure, and body mass index (BMI) served as indicators of physical health. Diabetes distress, general perceived stress, and depressive symptoms served as indicators of mental health.
A1C was determined through finger prick capillary blood collection measured on a DCA Vantage Analyzer (Siemens, Tarrytown, NY). This method meets the National Glycohemoglobin Standardization Program certification criteria for a total coefficient of variation <3% in the clinically relevant range [51]. Blood pressure was measured using an Omron model HEM-712C Automatic Inflation Blood Pressure Monitor (Omincron, Philadelphia, PA). Following a 5-minute rest period, three measurements were taken using the subject’s right arm and averaged. BMI was calculated using the formula: BMI = (weight in kg)/(height in m)2. Body weight was measured using a Tanita Professional Digital Scale (Model BF350). Participants wore street clothes and no shoes; coats and belts were removed. Height was determined using a portable stadiometer (Seca 214).
Indicators of mental health included diabetes distress, general perceived stress, and depressive symptoms. The brief Diabetes Distress Scale (DDS2) [52] was used to measure patients’ level of distress related to their diabetes care. Participants responded to two items on a Likert scale from 1 (not a problem) to 6 (serious problem): “Feeling overwhelmed by the demands of living with diabetes” and “Feeling that I am often failing with my diabetes regimen.” Internal consistency was high at baseline (α = .90) and follow-up (α = .84).
General perceived stress was measured using the 4-item Perceived Stress Scale (PSS-4) [53], which measured the appraised stressfulness of the respondent’s life situations on a Likert scale from 0 (never) to 4 (very often). Sample items included, “How often have you felt that you were unable to control the important things in your life?” and “How often have you felt difficulties were piling up so high that you could not overcome them?” The internal consistency of the PSS-4 was high (α = .81) at baseline and adequate at follow-up (α = .68).
Depressive symptoms were measured using the 9-item Patient Health Questionnaire (PHQ9) [54], which assessed depressive symptoms experienced (e.g., depressed mood, feelings of guilt, worthlessness, restless sleep). Sample items included, “feeling tired or having little energy,” “little interest or pleasure in doing things,” and “trouble concentrating on things, such as reading the newspaper or watching television,” with responses ranging from 0 (not at all) to 3 (nearly every day). The PHQ9 measure had strong internal consistency at baseline (α = .89) and follow-up (α = .90).
Resilience-Based Diabetes Self-Management Education (RB-DSME) Intervention
The RB-DSME contains all core aspects of DSME [55], including signs and symptoms of T2D, long-term diabetes complications, how to use a glucometer, monitoring and interpreting blood glucose values, carbohydrate counting, healthy eating in social situations, benefits of physical activity, understanding medications, and daily T2D self-management (Table 2, left column). The RB-DSME builds on foundational resilience resources (e.g., self-efficacy, social support) in standard DSME, infusing novel resilience resources (adaptation to stress, finding positive meaning, adaptive coping, coping with discrimination, emotional regulation) into the RB-DSME curriculum (see Table 2, right column). Throughout the classes, resilience resources are integrated into each DSME component to overcome barriers to implementation and adherence.
Table 2.
Overview of the resilience-based diabetes self-management education intervention
| The RB-DSME Includes All Aspects of Traditional DSME (left column) Infused with Resilience Resources (right column) | |
|---|---|
| Week 1. What is Diabetes? | |
|
|
| Week 2. Getting Your Glucometer to Work for You | |
|
|
| Week 3. Carbs Count! | |
|
|
| Week 4. Eating Made Easy! The Healthy Plate | |
|
|
| Week 5. A Step Towards Success | |
|
|
| Week 6. Understanding Medications | |
|
|
| Week 7. Grocery Store Shopping and Dining Out | |
|
|
| Week 8. Putting it All Together | |
|
|
| Support Groups | |
|
|
Active learning strategies demonstrate how stressful situations can be used as opportunities to grow and improve control over T2D self-management. The RB-DSME uses the phrase Please Take Care of Self to describe core strategies individuals use to enhance their resilience resources. These strategies include having a Positive mindset [56], focusing on Tiny behavior changes [57], creating Community support via cheerleaders (e.g., family/ friends) and coaches (e.g., healthcare providers) [24,58], and fostering a Supportive internal (e.g., emotional regulation) and external (e.g., home, work, community) environment [59,60]. In the support group sessions, participants discuss personal challenges and solutions to long-term control of T2D using a more informal approach.
Statistical Analysis
Paired-samples t-tests were conducted to assess changes in all study variables from baseline to 6 months post-study entry, with p-values adjusted using the Benjamini-Yekutieli procedure [61]. All analyses were performed using SPSS Statistics version 25 (IBM Corporation, Armonk, NY) and were conducted using the 35 participants who completed both data collections. Demographic characteristics were compared between those who dropped out and those who completed the study. Participants completing the study had higher education attainment than those who dropped out (t[40] = 2.25, p < .05). No other demographic differences were found between completers and non-completers.
Treatment effect sizes (Cohen’s d) were calculated by dividing the mean paired difference by the standard deviation paired difference. Bootstrap resampling (5000 iterations) was used [62]. In an exploratory test of dose-response to the intervention, multiple linear regression was performed with number of classes attended as the predictor and A1C at 6-months as the outcome, controlling for baseline A1C.
Results
The average program attendance across all 4 RB-DSME intervention groups was high (M = 7.66 sessions out of 10, SD = 1.49). Participants’ rating of overall class satisfaction was highly positive (M = 6.79 on a 7-point scale, SD = .48), with 94.3% of participants indicating a continued interest in attending monthly support group sessions. Approximately 25% percent (n = 9) of participants reported a decrease in their prescription diabetes medication (e.g., lower dosage, fewer medications) after completing the program.
As shown in Table 3, significant increases in most resilience resources (adaption to stress, adaptive coping, finding positive meaning, and diabetes empowerment) were reported; however, maladaptive coping did not improve significantly. Treatment effect sizes for adaption to stress (d = .67), adaptive coping (d = .60), and diabetes empowerment (d = .57) exceeded Cohen’s convention of .50 for a medium effect. The effect size of d =.85 for finding positive meaning exceeded Cohen’s .80 cutoff for a large effect [63]. Participants also reported large significant increases in self-reported diabetes management behaviors (d = 1.38) and average steps per day (d = 1.11).
Table 3.
Baseline and six months means, standard deviations, and effect sizes for all study variables
| Baseline | Six Months | |||||
|---|---|---|---|---|---|---|
| Study Variables | M | SD | M | SD | t | Cohen’s d |
| Resilience Resources | ||||||
| Adaption to Stress | 3.06 | .91 | 3.67 | .66 | 3.92*** | .67 |
| Adaptive Coping | 2.79 | .73 | 3.20 | .59 | 3.47*** | .60 |
| Maladaptive Coping | 1.85 | .57 | 1.79 | .56 | −.78 | .13 |
| Finding Positive Meaning | 1.87 | .67 | 2.44 | .56 | 5.03*** | .85 |
| Diabetes Empowerment | 3.57 | .83 | 4.08 | .70 | 3.32** | .57 |
| T2D Self-Management Behaviors | ||||||
| Self-Report Behaviors | 3.15 | .59 | 3.87 | .55 | 8.02*** | 1.38 |
| Physical Activity - Steps | 3950 | 2181 | 5625 | 2419 | 6.59*** | 1.11 |
| T2D Health Outcomes | ||||||
| Physical | ||||||
| A1C | 8.79 | 1.97 | 8.11 | 1.64 | −2.92** | .50 |
| Systolic Blood Pressure | 131.74 | 15.26 | 129.50 | 17.64 | −.62 | .11 |
| Diastolic Blood Pressure | 76.38 | 11.47 | 77.35 | 10.74 | .40 | .07 |
| Body Mass Index | 68.24 | 19.71 | 68.25 | 19.78 | .00 | .00 |
| Mental | ||||||
| Diabetes Distress | 3.90 | 1.34 | 2.57 | 1.16 | −7.61*** | 1.31 |
| Perceived Stress | 1.83 | .83 | 1.33 | .70 | −3.23** | .55 |
| Depressive Symptoms | 12.41 | 7.30 | 6.59 | 5.64 | −4.68*** | .80 |
Note.
p < .05,
p <.01,
p <.001
Of the T2D physical health outcomes assessed, only A1C improved significantly, decreasing from baseline (M = 8.79%, SD = 1.97, range: 5.2–14.2%) to six months (M = 8.11%, SD = 1.64, range: 5.7–11.4%), indicating a medium-sized treatment effect (d = .50). Participants reported a significant decrease in all three mental health measures from baseline to six months, including large effects for diabetes distress (d = 1.31) and depressive symptoms (d = .80) and a medium effect for general perceived stress (d =.55). In the assessment of a dose-response relationship between intervention participation and A1C, RB-DSME attendance was not associated with A1C, although the standardized coefficient was of the expected direction and magnitude (β = −0.38, p = 0.13).
Discussion
This study tested the proof-of-concept of the RB-DSME intervention delivered to lower income and racial/ethnically diverse minority adults attending two clinics within a large federally qualified CHC. Attendance, retention, and program satisfaction were high for all groups. Participants noted that having the classes and support groups taught by the CHC endocrinologist with assistance from the extended health team contributed to their high attendance and satisfaction with the program. The convenient location of classes in CHC clinics, where participants regularly visited for their medical appointments, enhanced comfort and ease of attendance. Significant improvements were observed for resilience resources (adaptation to stress, finding positive meaning, adaptive coping, diabetes empowerment), self-management behaviors (self-management behaviors, physical activity), and T2D physical (A1C) and mental (diabetes distress, perceived stress, depressive symptoms) health outcomes. Collectively, these results provide support for the ability of the RB-DSME to improve T2D-related psychosocial, behavioral, and clinical outcomes when delivered in a CHC setting.
Individuals with T2D engage in chronic disease management in the face of a plethora of daily challenges; thus, the use of resilience resources are vital to effectively control the disease. In the present study, RB-DSME participants increased adaptation to stress, a resilience resource and dynamic process in which individuals exhibit positive adaptation despite stressful experiences [43,64], which may reduce adverse effects and complications from the disease. An advantage of the RB-DSME curriculum, in addition to addressing T2D risks and vulnerabilities, is the focal emphasis on identifying and fostering strengths that support healthy behaviors. This benefit was exemplified in the present study by participants significantly enhancing their ability to find positive meaning within the context of T2D. Consistent with our previous work [41, 65], and that of adult survivors of various types of cancer [66], finding positive meaning may help sustain coping efforts and facilitate long-term adjustment. Indeed, positive emotional health is consistently associated with T2D self-management behaviors and improved health outcomes [67].
In addition to improvements in adaptation to stress and finding positive meaning, RB-DSME participants also reported engaging in more adaptive coping strategies (e.g., taking action, reframing, planning, seeking social support) at the conclusion of the intervention. Despite differences in age, gender, ethnicity, cultural background, education, and SES, participants shared their struggles openly with others in the program and worked collaboratively to reframe their thinking and recommit to their T2D self-management goals. Adaptive coping strategies can improve a patient’s psychological adjustment to having T2D [68]. We hypothesize that reframing difficult situations into opportunities for personal growth fostered a belief in participants’ ability to positively impact their life circumstances. This speculation is aligned with the significant increase in participants’ diabetes empowerment, and is consistent with literature noting the importance of empowering patients to enhance their diabetes self-care and advocate for themselves when interacting with medical providers [69,70].
Individuals possessing high levels of resilience resources have the capacity to maintain diabetes self-management behaviors in the face of mounting diabetes distress [71]. Supporting this assertion, RB-DSME participants in the current study increased self-reported T2D self-management behaviors and physical activity, both with large effect sizes. This finding is consistent with our previous work in African-American church settings, in which RB-DSME participants enhanced their physical activity and diabetes self-care [41, 72]. In those studies, we posited that participants’ high resilience resource scores combined with a resilience resource-promoting environment (e.g., focus on finding positive meaning from setbacks through storytelling) accounted for behavioral improvements. In addition to the resilience-promoting environment of the RB-DSME, the CHC setting in the present study enabled participants access to members of the expanded health team (i.e., clinical pharmacist, nurse, behavior health counselor) who provided valuable educational information and social support. This enhanced network of support staff may have further contributed to behavioral improvements of RB-DSME participants.
Although the only significant improvement in physical health outcomes in the present study was for A1C, the magnitude of the reduction (from 8.8% to 8.1%) is promising. This improvement is superior to the mean A1C reduction of −0.31% found in a meta-analysis of 20 randomized controlled trials of DSME delivered among racial/ethnic minority groups [73]. In considering results of resilience interventions, we are aware of only one other study testing a resilience intervention to enhance T2D health outcomes, which also lowered A1C among participants. However, baseline A1C was already at recommended levels (6.7%) and the sample consisted overwhelmingly of non-Hispanic Whites [74]. Importantly, participants in the current study who also decreased medication use (n=9) lowered their A1C from 8.7% to 7.2%, suggesting that behavior modification, rather than intensification of medications by the physician or clinical pharmacist was the primary driver of improvement in A1C. In sum, preliminary clinical benefits of the RB-DSME were supported.
Complementing the impact of the RB-DSME intervention on A1C are the benefits on mental health outcomes. Participation in the RB-DSME intervention resulted in significant decreases in diabetes distress, general perceived stress, and depressive symptoms from baseline to 6-months. This is noteworthy, as individuals with T2D experience high levels of stress and depressive symptoms [13,23]. These adverse mental health consequences are associated with impaired T2D self-management behaviors, higher risk for cardiovascular disease, and mortality [75]. Reducing stress-related outcomes may thus allow individuals with T2D to adopt and adhere to self-management behaviors more easily, ultimately improving T2D health outcomes and associated complications.
Given the clinically significant A1C reduction achieved in the current proof-of-concept study, the next phase of research according to the ORBIT model is a larger, randomized controlled pilot trial (Phase IIb) [9]. In this future study, the RB-DSME would be tested against a control group receiving usual diabetes care in an attempt to replicate the clinically significant A1C reduction over and above important confounding factors (e.g., passage of time, nonspecific attention). The randomized Phase IIb pilot study will also test whether the usual diabetes care control group is appropriate for a larger efficacy trial (e.g., minimal dropouts, credible to participants). The positive findings of the present study will allow us to proceed confidently and systematically to a more rigorous and resource-intensive test of the RB-DSME’s impact on A1C among individuals with T2D in CHCs.
The study findings may have broader implications for T2D treatment in underserved communities and group medical visits in the U.S. and abroad. Our RB-DSME intervention was delivered in the format of group medical visits, which shift the power relationship between patients and health-care providers, creating a patient-centered, interprofessional environment that promotes patient confidence in disease management [76]. Group medical visits often lower A1C [77] and have been successfully used for diabetes treatment in Canada, Europe, and China [78], so the RB-DSME could be integrated into existing group medical visit infrastructure in those countries. Interventions adopting the group medical visit format, such as the RB-DSME, may be especially useful for CHCs, which often lack the resources of traditional clinical settings. The results of our study support a need for expanded and sustainable T2D programming in CHCs, the adoption of which has been hampered by funding constraints in the U.S. Although CHC-equivalent agencies are less common in countries with free and/or universal healthcare, increasing healthcare reach to marginalized communities is a growing global priority. For instance, the European Union (EU) is currently undertaking substantial efforts toward greater health equity for its citizens [79], so healthcare access for underserved populations in the EU will likely increase in the future. This initiative may result in more opportunities to treat underserved patients with T2D in a similar manner to CHCs. Policy changes supporting viable funding and reimbursement models for resilience-promoting diabetes programs could lead to a greater reach in underserved communities to achieve health equity [80].
The findings and implications of this proof-of-concept study should be considered in light of several study limitations. First, the lack of a control group does not provide strong causal evidence for observed treatment effects, given that participants could have improved on variables of interest due to factors outside of the program. Second, the use of self-report survey data has inherent limitations, such as the potential for untruthful or inaccurate responses due to the lack of self-awareness or fatigue. Third, response rate for participation in the study was not assessed, which could have informed future recruitment efforts for similar programs. Finally, the study may have been underpowered to test the large number of variables in the relatively small sample size. Despite these limitations, this study documented the ability of the RB-DSME intervention to enhance resilience resources, T2D self-management behaviors, and T2D health outcomes among lower SES and racial/ethnic minority patients at two clinics within a federally qualified CHC. A larger, randomized study is necessary to more rigorously test the impact of the RB-DSME in this setting.
Funding:
This study was funded by the Seton Healthcare Family Center for Health and Social Policy, Lyndon B. Johnson School of Public Affairs, The University of Texas at Austin. H. Matthew Lehrer was partially supported by NIH grant T32HL082610.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Courtney-Long EA, Carroll DD, Zhang QC, et al. Prevalence of disability and disability type among adults—United States, 2013. MMWR. Morbid Mortal Wkly Rep 2015;64:777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu J, Murphy SL, Kochanek KD, Bastian B, Arias E. Deaths: Final data for 2016. Natl Center Health Stat, Division of Vital Stat. 2018;67(5):1–176. [PubMed] [Google Scholar]
- 3.American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021–1029. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. National diabetes statistics report, 2017: Estimates of diabetes and its burden in the United States. Atlanta, GA: National Center for Chronic Disease Prevention and Health Promotion, Division of Diabetes Translation; 2017. [Google Scholar]
- 6.Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13:814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institutes of Health. NIH-wide strategic plan: Fiscal years 2016–2020. Turning discovery into health Washington, DC: US Government Printing Office; 2016. [Google Scholar]
- 8.World Health Organization. Strengthening Resilience: a Priority Shared by Health 2020 and the Sustainable Development Goals. World Health Organization Regional Office for Europe; 2017. [Google Scholar]
- 9.Czajkowski SM, Powell LH, Adler N, et al. From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol. 2015;34:971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayberry LS, Bergner EM, Chakkalakal RJ, Elasy TA, Osborn CY. Self-care disparities among adults with type 2 diabetes in the USA. Curr Diab Rep. 2016;16:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Storey M, Anderson P. Income and race/ethnicity influence dietary fiber intake and vegetable consumption. Nutr Res 2014;34:844–850. [DOI] [PubMed] [Google Scholar]
- 12.Hackett RA, Steptoe A. Type 2 diabetes mellitus and psychological stress—a modifiable risk factor. Nat Rev Endocrinol. 2017;13:547–560. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd C, Smith J, Weinger K. Stress and diabetes: a review of the links. Diabetes Spectr. 2005;18:121–127. [Google Scholar]
- 14.Wiernik E, Nabi H, Thomas F, Pannier B, Hanon O, Simon T, … & Lemogne C. (2016). Association between current perceived stress and incident diabetes is dependent on occupational status: Evidence from the IPC cohort study. Diabetes & Metabolism, 42(5), 328–335. [DOI] [PubMed] [Google Scholar]
- 15.Marcy TR, Britton ML, Harrison D. Identification of barriers to appropriate dietary behavior in low-income patients with type 2 diabetes mellitus. Diabetes Therapy. 2011;2:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black AR, Peacock N. Pleasing the masses: Messages for daily life management in African American women’s popular media sources. Am J Public Health. 2011;101:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin EH, Katon W, Von Korff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27:2154–2160. [DOI] [PubMed] [Google Scholar]
- 18.Juster RP, McEwen BS, Lupien SJ. (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav R 2010;35:2–16. [DOI] [PubMed] [Google Scholar]
- 19.Adler NE. Health disparities through a psychological lens. American Psychologist. 2009;64,663–673. [DOI] [PubMed] [Google Scholar]
- 20.Lehrer HM, Dubois SK, Maslowsky J, Laudenslager ML, Steinhardt MA. Hair cortisol concentration and glycated hemoglobin in African American adults. PNE. 2016;72:212–218. [DOI] [PubMed] [Google Scholar]
- 21.Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1:607–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali S, Stone MA, Peters JL, Davies MJ, Khunti, K. The prevalence of co-morbid depression in adults with type 2 diabetes: A systematic review and meta-analysis. Diabet Med. 2006;23:1165–1173. [DOI] [PubMed] [Google Scholar]
- 24.Schetter CD, Dolbier C. Resilience in the context of chronic stress and health in adults. Soc Personal Psychol Compass. 2011;5:634–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Southwick SM, Charney DS. The science of resilience: Implications for the prevention and treatment of depression. Science. 2012;338:79–82. [DOI] [PubMed] [Google Scholar]
- 26.Connor KM, Davidson JRT. Development of a new resilience scale: The Connor-Davidson resilience scale (CD-RISC). Depress Anxiety. 2003;18:76–82. [DOI] [PubMed] [Google Scholar]
- 27.Masten AS. Ordinary magic: Resilience processes in development. Am Psychol. 2001;56:227–238. [DOI] [PubMed] [Google Scholar]
- 28.Brooks R, Goldstein S. The power of resilience. New York: McGraw-Hill; 2003. [Google Scholar]
- 29.O’Leary VE, Ickovics JR. Resilience and thriving in response to challenge: An opportunity for a paradigm shift in women’s health. Women’s Health. 1995;1:121–142. [PubMed] [Google Scholar]
- 30.Carver CS. Resilience and thriving: Issues, models, and linkages. J Soc Issues. 1998;54:245–266. [Google Scholar]
- 31.Fredrickson BL, Branigan C. Positive emotions broaden the scope of attention and thought‐action repertoires. Cogn Emot. 2005;19:313–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Troy AS, Mauss IB. Resilience in the face of stress: Emotion regulation as a protective factor. In: Southwick SM, Litz BT, Charney D, Friedman MJ, editors. Resilience and mental health: Challenges across the lifespan. Cambridge, UK: Cambridge University Press; 2011. pp. 30–44. [Google Scholar]
- 33.Kok BE, Coffey KA, Cohn MA, et al. How positive emotions build physical health: Perceived positive social connections account for the upward spiral between positive emotions and vagal tone. Psychol Sci. 2014;24:1123–1132. [DOI] [PubMed] [Google Scholar]
- 34.King DK, Glasgow RE, Toobert DJ, et al. Self-efficacy, problem solving, and social-environmental support are associated with diabetes self-management behaviors. Diabetes Care. 2010;33:751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moskowitz JT, Epel ES, Acree M. Positive affect uniquely predicts lower risk of mortality in people with diabetes. Health Psychol. 2008;27(1):S73–S82. [DOI] [PubMed] [Google Scholar]
- 36.DeNisco S Exploring the relationship between resilience and diabetes outcomes in African Americans. J Am Assoc Nurse Pract. 2011;23:602–610. [DOI] [PubMed] [Google Scholar]
- 37.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Newbury Park, CA: Sage; 1988. [Google Scholar]
- 38.Hajat A, Diez-Roux A, Franklin TG, et al. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology. 2010;35:932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayberry LS, Bergner EM, Chakkalakal RJ, Elasy TA, Osborn CY. Self-care disparities among adults with type 2 diabetes in the USA. Curr Diab Rep. 2010;16:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Storey M, Anderson P. Income and race/ethnicity influence dietary fiber intake and vegetable consumption. Nutr Res. 2014;34:844–850. [DOI] [PubMed] [Google Scholar]
- 41.Steinhardt MA, Brown SA, Dubois SK, Harrison L Jr, Lehrer HM, Jaggars SS. A resilience intervention in African-American adults with type 2 diabetes. Am J Health Behav. 2015;39:507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehrer HM, Dubois SK, Brown SA, Steinhardt MA. Resilience-based diabetes self-management education: Perspectives from African American participants, community leaders, and healthcare providers. Diabetes Educ. 2017;43:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, Bernard, J. The brief resilience scale: Assessing the ability to bounce back. International Journal of Behavioral Medicine. 2008;15:194–200. [DOI] [PubMed] [Google Scholar]
- 44.Carver CS. You want to measure coping but your protocol’s too long: Consider the Brief COPE. International Journal of Behavioral Medicine. 1997;4:92–100. [DOI] [PubMed] [Google Scholar]
- 45.Zeidner M, Saklofske D. Adaptive and maladaptive coping. In: Zeidner M, Endler NS, editors. Handbook of coping: Theory, research, applications Oxford, England: John Wiley & Sons, 1996. pp. 505–531. [Google Scholar]
- 46.Yi‐Frazier JP, Smith RE, Vitaliano PP, Yi JC, Mai S, Hillman M, & Weinger K (2010). A person‐focused analysis of resilience resources and coping in patients with diabetes. Stress and health: Journal of the International Society for the Investigation of Stress, 26(1), 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miles SR, Khambaty T, Petersen NJ, Naik AD, & Cully JA (2018). The role of affect and coping in diabetes self-management in rural adults with uncontrolled diabetes and depressive symptoms. Journal of Clinical Psychology in Medical Settings, 25(1), 55–65. [DOI] [PubMed] [Google Scholar]
- 48.Frederickson BL, Tugade MM, Waugh CE, Larkin GR. What good are positive emotions in crises? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. J Pers Soc Psychol. 2003;84:365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson RM, Fitzgerald JT, Gruppen LD, Funnell MM, Oh MS. The diabetes empowerment scale-short form (DES-SF). Diabetes Care. 2003;26:1641–1642. [DOI] [PubMed] [Google Scholar]
- 50.Weinger K, Butler HA, Welch GW, La Greca AM. Measuring diabetes self-care: A psychometric analysis of the self-care inventory-revised with adults. Diabetes Care. 2005;28:1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lenters-Westra E, Slingerland RJ. Six of eight hemoglobin A1C point-of-care instruments do not meet the general accepted analytical performance criteria. Clinical Chemistry. 2010;56:44–52. [DOI] [PubMed] [Google Scholar]
- 52.Fisher L, Glasgow RE, Mullan JT, Skaff MM, Polonsky WH. Development of a brief diabetes distress screening instrument. The Annals of Family Medicine. 2008;6:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen S, Kamarck T, & Mermelstein R (1994). Perceived stress scale. Measuring stress: A guide for health and social scientists, 10. [Google Scholar]
- 54.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. Journal of General Internal Medicine. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beck J, Greenwood DA, Blanton L, et al. 2017 National standards for diabetes self-management education and support. Diabetes Educ. 2018;44:35–50. [DOI] [PubMed] [Google Scholar]
- 56.Dweck CS. Mindset: The new psychology of success. New York: Random House; 2006. [Google Scholar]
- 57.Oinas-Kukkonen H A foundation for the study of behavior change support systems. Pers Ubiquitous Comput. 2013;17:1223–1235. [Google Scholar]
- 58.Kok BE, Coffey KA, Cohn MA, et al. How positive emotions build physical health: Perceived positive social connections account for the upward spiral between positive emotions and vagal tone. Psychol Sci. 2013;24:1123–1132. [DOI] [PubMed] [Google Scholar]
- 59.Story M, Kaphingst KM, Robinson-O’Brien R, Glanz K. Creating healthy food and eating environments: Policy and environmental approaches. Annu Rev Public Health. 2008;29:253–272. [DOI] [PubMed] [Google Scholar]
- 60.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits: A meta-analysis. J Psychosom Res. 2004;57:35–43. [DOI] [PubMed] [Google Scholar]
- 61.Benjamini Y, & Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–1188. [Google Scholar]
- 62.Efron B, & Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;Feb:54–75. [Google Scholar]
- 63.Cohen J Statistical power analysis for the behavioral sciences. 2nd. New York, NY: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 64.Luthar SS, & Cicchetti D. The construct of resilience: Implications for interventions and social policies. Dev Psychopathol. 2000;12:857–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steinhardt MA, Dubois SK, Brown SA, Harrison L Jr., Dolphin KE, Park W, Lehrer HM. Positivity and indicators of health among African Americans with diabetes. Am J Health Behav. 2015;39:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park CL, Chmielewski J, Blank TO. Post‐traumatic growth: finding positive meaning in cancer survivorship moderates the impact of intrusive thoughts on adjustment in younger adults. Psycho oncology. 2010;19:1139–1147. [DOI] [PubMed] [Google Scholar]
- 67.Robertson SM, Stanley MA, Cully JA, Naik AD. Positive emotional health and diabetes care: concepts, measurement, and clinical implications. Psychosomatics. 2012;53(1),1–12. [DOI] [PubMed] [Google Scholar]
- 68.Macrodimitris SD, Endler NS. Coping, control, and adjustment in type 2 diabetes. Health Psychol. 2001;20:208–216. [PubMed] [Google Scholar]
- 69.Betancourt JR, Duong JV, Bondaryk MR. Strategies to reduce diabetes disparities: an update. Curr Diab Rep. 2012;12:762–768. [DOI] [PubMed] [Google Scholar]
- 70.Peek ME, Wilkes AE, Roberson TS, et al. Early lessons from an initiative on Chicago’s South Side to reduce disparities in diabetes care and outcomes. Health Affairs. 2012;31:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chew BH, Shariff-Ghazali S, Fernandez A. Psychological aspects of diabetes care: Effecting behavioral change in patients. World J Diabetes. 2014;5:796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steinhardt MA, Mamerow MM, Brown SA, Jolly CA. A resilience intervention in African American adults with type 2 diabetes: A pilot study of efficacy. Diabetes Educ. 2009;35:274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ricci-Cabello I, Ruiz-Pérez I, Rojas-García A, Pastor G, Rodríguez M, Gonçalves DC. Characteristics and effectiveness of diabetes self-management educational programs targeted to racial/ethnic minority groups: A systematic review, meta-analysis and meta-regression. BMC Endocr Disord. 2014;14(1), 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yi JP, Vitaliano PP, Smith RE, Yi JC, & Weinger K. The role of resilience on psychological adjustment and physical health in patients with diabetes. Br J Health Psychol. 2008;13:311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katon WJ, Rutter C, Simon G, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care. 2005;28:2668–2672. [DOI] [PubMed] [Google Scholar]
- 76.Housden L, Browne AJ, Wong ST, Dawes M. Attending to power differentials: How NP‐led group medical visits can influence the management of chronic conditions. Health Expectations. 2017;20:862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ. 2013;185: E635–E644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Housden L, Wong ST. Using group medical visits with those who have type 2 diabetes: examining the evidence. Curr Diabetes Rep. 2016;16:134. [DOI] [PubMed] [Google Scholar]
- 79.Scholz N Addressing health inequalities in the European Union: Concepts, action, state of play. Brussels: European Union. 2020;1–37. [Google Scholar]
- 80.Spencer MS, Kieffer EC, Sinco B, et al. Outcomes at 18 months from a community health worker and peer leader diabetes self-management program for Latino adults. Diabetes Care. 2018;41:1414–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]


