Abstract
Background
Fluorescence optical imaging (FOI) enables visualisation of inflammation in both hands in rheumatoid arthritis (RA).
Objective
To investigate the usefulness of FOI in treatment monitoring under anti-TNFα therapy with certolizumab pegol (CZP) in patients with RA in comparison to clinical and laboratory outcome parameters.
Methods
CZP-naïve patients with RA were eligible for this open-label study with an observational period of 52 weeks. Disease activity was monitored by the clinical score DAS28, tender/swollen joint count (TJC-28/SJC-28) and laboratory outcomes for systemic inflammation (CRP and ESR). FOI results were analysed in three different phases (P1-3) and PrimaVistaMode (PVM) by the FOI activity score (FOIAS).
Results
Twenty-eight RA patients (median age 52.5 years, 26 females, thirteen with a history of other biologic therapy) were included. DAS28 (CRP) decreased from moderate disease activity at baseline (median 4.6, IQR 1.8) to low disease activity at week (w)52 (median 2.7, IQR 2.1; p < 0.001). Statistically significant decreases could also be demonstrated for SJC-28 and TJC-28. CRP/ESR were reduced numerically from baseline to w52. FOIAS in P1 (early phase) showed a continuous decrease of enhancement during the course of treatment period: from baseline (median 1.5, IQR 9.3) over w6 (median 1.0, IQR 3.0; p = 0.069), w12 (median 0.5, IQR 3.0; p = 0.171), w24 (n = 27, median 0.0, IQR 3.0; p = 0.004), until w52 (n = 18, median 0.0, IQR 2.8; p = 0.091), which could not be presented for FOIAS in P2, P3 and PVM.
Conclusion
FOI in P1 appears to be a valuable tool for fast and easy monitoring of treatment response to certolizumab in a clinical setting.
Keywords: Fluorescence optical imaging, Anti-TNFα therapy, Certolizumab, Rheumatoid arthritis, Inflammation
Introduction
Rheumatic and musculoskeletal diseases (RMDs) represent a heterogeneous group with a high prevalence of around 22% in adults in the United States (US) [1]. Even though there is a wide range of novel therapeutics, inflammatory arthritis still causes reduced mobility, limited quality of life and high health care costs [1, 2]. Among RMDs, rheumatoid arthritis (RA) is the most common, with a prevalence of 0.5–1.0% [3, 4]. To reduce the effect of chronic joint inflammation, present therapeutic strategies focus on early recognition during the “window of opportunity“ for effective therapy to improve short and long term outcomes significantly [5, 6].
Inhibitors of tumour necrosis factor alpha (TNFα) were the first developed among biological medications and they dramatically changed the therapeutic perspectives of RA patients [7]. Follow-up studies investigating their clinical and radiographic effects have shown that TNFα inhibitors, especially in combination with conventional synthetical disease-modifying antirheumatic drugs (csDMARD), lead to significant improvements in clinical status and significant inhibition of radiographic progression.
In order to monitor the therapeutic response in RA, several options are applied, such as different clinical disease activity scores, e.g. DAS28 [8]. For early and more objective monitoring of RA disease activity, musculoskeletal ultrasound (MSUS) and magnetic resonance imaging (MRI) are used in clinical practice [9–12]. In several clinical studies, MSUS presented sensitivity in disease changes under therapy, especially in power Doppler mode (PD)-detected synovitis and tenosynovitis [13, 14]. Different joint combinations have been used for MSUS scoring. The US7-joint score first proposed by Backhaus et al. has been the most utilised score in the literature and includes five hand and two foot joints of the clinically dominant side [15].
More recently, after its first report in 2006 [13] and the launch of a commercial device in 2009 (Xiralite GmbH, Berlin; Germany), fluorescence optical imaging (FOI) has been shown to detect inflammation in preclinical studies as well as in humans in the joint regions of both hands [16–21]. The basis of the Xiralite method is the illustration of impaired microcirculation caused by the inflammatory process of arthritis. For that, the enhancement of the intravenously applied dye indocyanine green (ICG) is evaluated. Accordingly, FOI is a non-ionising technique that can examine both hands in a single session of 6 min.
The aim of this study was to investigate FOI’s ability to monitor the treatment response to TNFα inhibitor therapy with certolizumab pegol (CZP) in RA patients and to compare FOI findings to clinical outcome parameters such as DAS28 (CRP), tender joint count (TJC-28), swollen joint count (SJC-28) and laboratory parameters. The US7 score was set as a further imaging method with high sensitivity to inflammation.
Patients and methods
Patients with RA according to 2010 ACR/EULAR classification criteria [22] have been enrolled after written informed consent to participate in the study. They were examined at baseline (before CZP) and after 6, 12, 24 and 52 weeks. Eligible for participation in the study were adults (age of ≥ 18 years), CZP-naïve (not naïve for other biologic therapies) patients with active RA (DAS28 (CRP)) with tolerability of the drug’s ingredients and no known heart failure (NYHA III/IV).
Clinical and laboratory examination
During all visits, the disease activity score 28 (DAS28) [23, 24] including clinical assessments of tender (TJC-28), swollen joints (SJC-28) and patient’s self-reported global disease activity on a visual analogue scale (VAS) from 0 to 10 cm was assessed. Furthermore, physician’s global assessment (PGA) and morning stiffness (in minutes) were evaluated. The laboratory investigation included the assessment of erythrocyte sedimentation rate (ESR; normal value < 20 mm/h) and C-reactive protein (CRP; normal value < 5.0 mg/l). Clinical and laboratory examination was accomplished on the same day as the imaging examinations (FOI; US7 score).
Fluorescence optical imaging (FOI)
The FOI system (Xiralite) was used according to a standardised procedure with an examination term of 6 min, recording one image per second, and resulting in a cluster of 360 images. One single bolus of indocyanine green (ICG) as fluorescence optical dye with a dose of 0.1 mg/kg body weight was injected intravenously ten seconds after beginning of the examination [20, 21]. To evaluate the distribution of ICG, the image sequence in the film modus was evaluated on one hand. For this purpose, three phases in position to the fingertips were defined regarding development of signal intensities that depend on the dye flooding of the individual patient [21]. Phase 1 (P1) includes the period between starting the examination, application of the dye and increased signal intensities in the fingertips [21]. When the dye leaves the fingertips from distal to proximal, phase 2 (P2) begins as the period of persisting high signal intensities in the fingertips [21]. It can be identified by the red colour in the fingertips. Phase 3 (P3) starts when no signal intensity can be determined in the fingertips, i.e. the time point without signal intensity (only yellow sparkles) in the fingertips as signal for clearance [21]. In addition, an electronically generated composite image (Prima Vista Mode, PVM) automatically obtained by means of 240 images with the integrated software XiraView was analysed.
For analysing the joint activity shown by FOI, ‘FOIAS’ (fluorescence optical imaging activity score) as a semiquantitative grading system was applied, based on individual joint scores from 0 to 3. The scores in P1-3 and in PrimaVistaMode (PVM) were assessed for 30 joints per patient, including the bilateral wrist, metacarpophalangeal joints (MCP) I-V, proximal interphalangeal joints (PIP) II-V, distal interphalangeal joints (DIP) II-V and interphalangeal joint of the thumb (IP). In addition, the sum scores of all affected joints of the left hand and the right hand were individually calculated and represented as one sum score (sum score both hands) [25]. FOIAS was performed by an agreement-based consensus of two investigators (SO, SH).
Musculoskeletal ultrasound (MSUS)
Ultrasonographic examination was performed in greyscale (GS) and power Doppler (PD) mode with a linear 10-18 MHz transducer (Esaote Mylab Twice,Genova; Italy). The examined level of the wrist included the dorsomedian, palmomedian and ulnar plane. The metacarpophalangeal (MCP) 2,3, the proximal interphalangeal (PIP) 2,3 and the metatarsophalangeal (MTP) 2,5 joints were examined from the palmar/plantar and dorsal view in the longitudinal plane. Tenosynovitis was measured on longitudinal and transverse scans only at the wrist and MCP 2,3 joint level. The wrist, MCP/PIP2,3 and MTP 2,5 joints were examined for synovial effusion and/or proliferation (indicating synovitis) according to Scheel et al. [26] in GSUS and according to Szkudlarek et al. [27] in PDUS (each 0–3) and for signs of tenosynovitis in GS (0–1)/PDUS (0–3) [15]. The US7 score was used in a modified way also including MCP2,3 and PIP2,3 from the dorsal view in GS [28]. The scoring range was 0–39 for GS and PD synovitis, 0–5 for GS tenosynovitis (0-5) and 0–15 for PD tenosynovitis
Statistical analyses
A two-sided Wilcoxon signed rank test was used to explore the difference in central tendency of the clinical data, FOI and MSUS scores (each 0–3, except tenosynovitis in GS US7 score) between baseline and follow-up visits. In addition, we examined whether the FOI was correlated with the clinical outcome using Spearman correlation, since a considerable number of tied values were to be expected. Furthermore, the resulting correlation coefficients were tested for their difference from zero, i.e. no correlation. Statistical analyses were performed with the statistical program SPSS. If not specified otherwise, the descriptive statistics provided median values (min–max; interquartile range [IQR]). For FOI scores, the sum of all joints and both hands were evaluated (sumscore both hands). For US7 scores, the sum of all subscores were evaluated. Whenever meaningful, p-values were provided based on the underlying methods. These explorative p-values were used to highlight statistical noticeable differences.
Results
In this study, 28 patients with RA were included and treated with 400 mg CZP s.c. at weeks 0, 2 and 4 and every 2 weeks thereafter with 200 mg s.c.. Of these patients, all received CZP for at least 12 weeks, n = 27 (96.4%) until w24, and n = 18 (64.3 %) received CZP throughout the entire 52 weeks. The reasons for drop-out were withdrawal of CZP due to lack of efficacy (n = 9) or worsening of symptoms (n = 1). Fifteen patients were biologic naïve at inclusion. Thirteen patients had a history of biologic treatment. Sixteen patients were on background therapy with methotrexate, who received a mean dosage of 16 ± 4.28 mg per week and 23 patients with prednisolone. During the course of the study, the mean dosage of prednisolone decreased from 6.0 ± 4.75 mg per day to 3.9 ± 2.60 mg/d at w24 and 2.3 ± 2.26 mg/d at w52.
Patients’ characteristics at baseline are presented in Table 1.
Table 1.
Baseline characteristics
| N | ||
|---|---|---|
| Age (years) | 28 | 52.5 (22–75; 20.0) |
| Sex (female), n (%) | 28 | 26 (92.9) |
| Weight (kg) | 28 | 75.5 (50–105;24.3) |
| Disease duration (months) | 28 | 31.5 (4–180) |
| Medication | ||
| Methotrexate, n (%) | 28 | 16 (57.1) |
| Prednisolone, n (%) | 28 | 23 (82.1) |
| Biologics naïve, n (%) | 25a | 15 (60.0) |
| Clinical parameters | ||
| DAS28 (CRP) | 28 | 4.61 (2.65–6.49; 1.75) |
| Tender joint count (28-TJC) | 28 | 7.0 (0–22;8.75) |
| Swollen joint count (28-SJC) | 28 | 2.5 (0–9;4.0) |
| VAS patient (0–10 cm) | 28 | 6.0 (2.4–10;3.0) |
| VAS physician (0–10 cm) | 28 | 4.0 (1.9–8.5;1.7) |
| Morning stiffness (min.) | 28 | 30 (0–240;63.75) |
| Laboratory parameters | ||
| ESR (mm/h) | 28 | 27 (8–66;21.0) |
| CRP (mg/l) | 27b | 5.4 (0.3–52;17.4) |
| Fluorescence optical imagingc | ||
| P1 | 28 | 1.5 (0–16;9.25) |
| P2 | 28 | 19.5 (0–36;14.5) |
| P3 | 28 | 1.0 (0–20;3.75) |
| PVM | 28 | 5 (0–27;6.25) |
| Musculoskeletal ultrasound (US7 Score) | ||
| GS US7 synovitis (range 0–39) | 28 | 11 (4–22;8.25) |
| PD US7 synovitis (range 0–39) | 28 | 2.0 (0–17;3.0) |
| GS US7 tenosynovitis (range 0–5) | 28 | 0.5 (0–3;1.0) |
| PD US7 tenosynovitis (range 0–15) | 28 | 0 (0–2;1.0) |
Data represent median (min–max; interquartile range) unless otherwise stated. aStatus for 3 patients unknown; bone sample not analysed; csum scores of both hands. DAS28 disease activity score of 28 joints, CRP C-reactive protein, VAS visual analogue scale, ESR erythrocyte sedimentation rate, GS grey scale, PD power Doppler, P phase, PVM prima vista mode, US7 ultrasound score of 7 selected joints
Clinical and laboratory outcome parameters
Disease activity measured by DAS28 (CRP) continuously decreased from baseline to w52. At baseline, patients had a moderate to high disease activity with a median (min-max; IQR) DAS28 (CRP) of 4.6 (2.7–6.5; 1.8), which decreased under treatment to 3.2 (1.3–6.4;1.8; p = 0.001) at w24 and to 2.7 (1.2–4.9; 2.1; p < 0.001) at w52.
Similar results were observed for SJC-28 and TJC-28, which are also part of the DAS28 (CRP). Both outcome parameters continuously decreased from baseline to w52. For SJC-28, a decrease from median of 2.5 (0–9, 4.0) at baseline to 0.0 (0–6, 2.0; p = 0.005) at w24 and to 0.0 (0-6–1.8; p = 0.012) at w52 was observed. For TJC-28, a decrease from median of 7.0 (0–22, 8.8) at baseline to 3.0 (0–17, 4.0; p = 0.001) at w24 and to 2.0 (0–15, 3.5; p = 0.004) at w52 was observed.
Patient global assessment (VAS) decreased during the course of the study. Significant differences were observed at w6, w12 and w52. Similar results were observed for the global assessment performed by the physician.
Morning stiffness decreases continuously from baseline to w52 with significant findings at w6, w12 and w24.
CRP and ESR values continuously decreased from baseline to w52. The median CRP decreased from 5.4 mg/l (0.3–52; 17.4) to 2.3 mg/l (0.3–41; 5.5; p = 0.028) at w24 and to 2.0 mg/l (0.2–99; 5.0; p = 0.156) at w52. The median ESR decreased from 27 mm/h (8–66; 21) to 21 mm/h (2–70; 25.5; p = 0.203) at w24 and to 18 mm/h (2–54; 20; p = 0.177) at w52.
A summary of all measured clinical and laboratory outcome parameters is listed in Table 2.
Table 2.
Summary statistics of clinical and laboratory outcome parameters at baseline and after w6, w12, w24 and w52
| Clinical outcome parameters | N | Median (min–max, IQR) | p-value |
|---|---|---|---|
| DAS28 (CRP) | |||
| Baseline | 28 | 4.61 (2.65–6.49, 1.76) | |
| w6 | 28 | 3.80 (1.49–6.60, 1.88) | 0.002 |
| w12 | 27 | 3.26 (1.39–6.65, 2.04) | < 0.001 |
| w24 | 27 | 3.20 (1.27–6.37, 1.81) | 0.001 |
| w52 | 18 | 2.73 (1.22–4.85, 2.06) | < 0.001 |
| Tender Joint Count-28 | |||
| Baseline | 28 | 7.00 (0–22, 8.75) | |
| w6 | 28 | 3.00 (0–22, 7.00) | 0.009 |
| w12 | 28 | 4.00 (0–21, 5.00) | < 0.001 |
| w24 | 27 | 3.00 (0–17, 4.00) | 0.001 |
| w52 | 18 | 2.00 (0–15, 3.50) | 0.004 |
| Swollen Joint Count-28 | |||
| Baseline | 28 | 2.50 (0–9, 4.00) | |
| w6 | 28 | 2.00 (0–6, 2.00) | 0.052 |
| w12 | 28 | 1.00 ((0–7, 3.00) | 0.009 |
| w24 | 27 | 0.00 (0–6, 2.00) | 0.005 |
| w52 | 18 | 0.00 (0–6, 1.75) | 0.012 |
| CRP (mg/l) | |||
| Baseline | 28 | 5.40 (0.30–52.00, 17.36) | |
| w6 | 27 | 4.00 (0.30–61.87, 11.85) | 0.687 |
| w12 | 26 | 3.25 (0.30–28.90, 6.95) | 0.091 |
| w24 | 25 | 2.30 (0.30–41.40, 5.50) | 0.028 |
| w52 | 18 | 2.00 (0.20–98.70, 5.02) | 0.156 |
| ESR (mm/h) | |||
| Baseline | 28 | 27 (8–66, 21,00) | |
| w6 | 28 | 20 (4–52, 24,50 | 0.027 |
| w12 | 28 | 18 (6–56, 23.25) | 0.059 |
| w24 | 27 | 21 (2–70, 25.50) | 0.203 |
| w52 | 18 | 18 (2–54, 20.00) | 0.177 |
| VAS (0–10 cm) patient | |||
| Baseline | 28 | 6.00 (2.40–10.00, 3.00) | |
| w6 | 28 | 2.90 (0.00–8.50, 3.95) | 0.003 |
| w12 | 28 | 4.20 (0.10–8.90, 3.00) | 0.003 |
| w24 | 27 | 5.00 (0.30–9.00, 3.70) | 0.112 |
| w52 | 18 | 2.95 (0–8.00, 3.63) | 0.002 |
| VAS (0–10 cm) physician | |||
| Baseline | 28 | 4.00 (1.9–8.50, 1.70) | |
| w6 | 28 | 3.00 (1.0–7.50, 2.00) | 0.002 |
| w12 | 28 | 2.25 (1.0–8.00, 1.63) | 0.001 |
| w24 | 27 | 2.00 (0.5–10.00, 2.25) | 0.066 |
| w52 | 18 | 1.50 (0.0–6.00, 2.00) | 0.014 |
| Morning stiffness (min.) | |||
| Baseline | 28 | 30.00 (0–240, 63,75) | |
| w6 | 28 | 2.50 (0–120, 30.00)) | 0.003 |
| w12 | 28 | 7.50 (0–90, 33.75) | 0.025 |
| w24 | 27 | 0.00 (0–120, 45.00) | 0.042 |
| w52 | 18 | 0.00 (0–150, 12.50) | 0.091 |
IQR interquartile range, DAS28 disease activity score of 28 joints, ESR erythrocyte sedimentation rate, CRP C-reactive protein, VAS visual analogue scale; significance level of p < 0.05
Fluorescence optical imaging (FOI)
The sum score of both hands in P1 of FOI continuously decreased from baseline to w52. A decrease in P1 of FOI sum score was already observed at w6, with a reduction from a median of 1.5 (0–16, 9.3) at baseline to 1.0 (0–12, 3; p = 0.069) at w6. The FOI P1 1 sum score further decreased to 0.5 (0–46, 3; p = 0.171), 0.0 (0-–0, 3; p = 0.004) and 0.0 (0–13, 2.8; p = 0.091) at w12, w24 and w52, respectively.
A statistically significant reduction was detected at w24. Although the sumscore from baseline to w52 decreased, the applied test did not yield a significant result. This likely occurred due to the reduced number of patients at that time point.
All other sum scores of both hands (PVM, P2 and P3) did not show a median reduction during the treatment.
The results of the FOI are presented in Table 3.
Table 3.
Summary statistics of FOI phases 1-3 and PMV at baseline, w6, w12, w24 and w52
| N | Median (min-max, IQR) | p-value | |
|---|---|---|---|
| Phase 1 | |||
| Baseline | 28 | 1.5 (0–16, 9.25) | |
| w6 | 27 | 1.0 (0–12, 3.00) | 0.069 |
| w12 | 28 | 0.5 (0–46, 3.00) | 0.171 |
| w24 | 27 | 0.0 (0–10, 3.00) | 0.004 |
| w52 | 18 | 0.0 (0–13, 1.75) | 0.091 |
| Phase 2 | |||
| Baseline | 28 | 19.5 (0–36, 14.50) | |
| w6 | 27 | 19.0 (0–43, 13.50) | 0.451 |
| w12 | 28 | 19.0 (0–50, 13.25) | 0.071 |
| w24 | 27 | 15.0 (1–48, 15.50) | 0.310 |
| w52 | 18 | 19.0 (0–35, 12–75) | 0.256 |
| Phase 3 | |||
| Baseline | 28 | 1.0 (0–20, 3.75) | |
| w6 | 27 | 2.0 (0.22, 3.50) | 0.501 |
| w12 | 28 | 2.5 (0–32, 6.25) | 0.089 |
| w24 | 27 | 1.0 (0–17, 3.00) | 0.191 |
| w52 | 18 | 0.5 (0–12, 2.00) | 0.959 |
| PMV | |||
| Baseline | 28 | 5.0 (0–27, 7.00) | |
| w6 | 27 | 8.0 (0–25, 7.00) | 0.018 |
| w12 | 28 | 5.5 (0–36, 9.25) | 0.472 |
| w24 | 27 | 7.0 (0–23, 8.50) | 0.204 |
| w52 | 18 | 5.5 (0–23, 5.75) | 0.717 |
FOI fluorescence optical imaging, PMV PrimaVistaMode, IQR interquartile range; significance level of p < 0.05
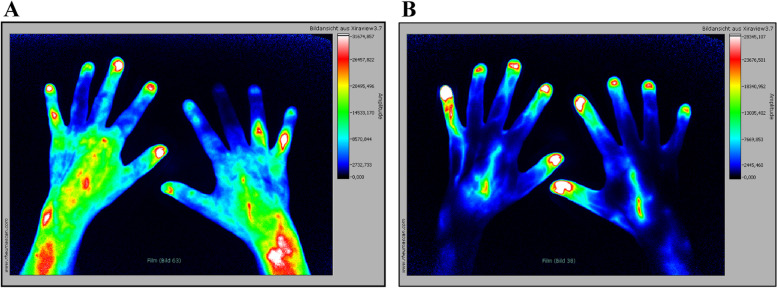
Figure 1 shows an example of a patient with good response to the CZP therapy which is displayed as a signal attenuation in comparison from baseline, visit 1 to week 52 in P1. The improvement of the inflammatory process can be seen in the significant decrease of early enhancement in both hands during the treatment period.
Fig. 1.
Fluorescence optical imaging illustrations in phase 1. A Baseline visit. FOI findings with clinical active RA. Increased signal intensities as a sign of active inflammation in P1. High signal intensities (FOIAS grade 3) in PIP5 of the right hand, moderate signal intensities (FOIAS grade 2) in both wrists and PIP4 of the right and PIP5 of the left hand. B After 52 weeks of CZP treatment; no increased signal intensities are detectable
Musculoskeletal ultrasound
For GS, a numerical decrease of the US7 synovitis sum score was only observed at w52 (from 11.0 at baseline to 8.5 at w52).
For PD, a numerical decrease of the US7 synovitis score was detected at w12 (from 2.0 to 1.5) with a further decrease at w52 (to 0.5).
For GS tenosynovitis und PD tenosynovitis US7 scores, no relevant changes could be detected throughout the study.
The outcome parameters of the GS and PD US7 synovitis/tenosynovitis scores are presented in the Supplement Table S1 at baseline and after 6, 12, 24 and 52 weeks of therapy with CZP.
A visual summary of the results is provided in Supplement Figure S2 which presents box plots of DAS28, FOIAS P1, GS, PD, SJC-28 and TJC-28 at baseline, w6, w12, w24 and w52.
Correlation of FOIAS with selected clinical and US7 score outcome parameters
Regarding baseline data, FOIAS in PMV and P1, P2 P3 showed no significant positive correlations with any clinical outcome parameter. There was a moderate correlation between FOIAS P3 with PD US7 synovitis score. All other US7 Score parameters showed a weak or no positive correlation with FOIAS in PMV and P1, P2, P3.
At week 24, there was a moderate correlation between FOIAS in P2 with DAS28 and with TJC-28. All other clinical parameters showed weak or no positive correlation with FOIAS in PMV and P1, P2, P3. No significant correlation could be presented between FOIAS in PMV and P1, P2, P3 with all US7 Score parameters.
At week 52, FOIAS P2 showed a moderate correlation with TJC-28. There was a moderate correlation between FOIAS P2, P3, PVM and PD synovitis score and a moderate correlation between FOIAS P3, PVM and PD tenosynovitis score.
Pairwise correlations between clinical and laboratory outcomes with FOIAS P1-P3 and PMV as well as with US7 Score parameters are presented in Figure S1 (supplement).
Discussion
In this open-label study, we are presenting for the first time that FOI assessments by means of short-term study visits allow early monitoring of treatment response after initiation of anti-TNFα therapy. Already after a 6-week treatment period, FOI in P1 showed a reduction of ICG enhancement based on the FOIAS and at w24; the reduction of the FOIAS compared to baseline was statistically significant (p = 0.004). However, at w52, no significant reduction of the FOIAS compared to baseline has been observed—most probably due to the low number of patients (n = 18) at w52. Furthermore, comparing the results between w24 and w52, no additional effect in the FOIAS could be observed. The reason is unclear but could indicate a total remission of inflammation already seen after w24. However, the sample size is small and further studies are necessary to draw a final conclusion.
According to the results of this analysis, FOI in P1 seems to be the most useful FOI phase for monitoring treatment response.
These results are in line with previous results by Meier et al. [29] and Glimm et al. [25] presenting significant reductions in early signal intensities after 24 weeks and after 1 year of treatment, respectively. In addition, in the study of Glimm et al., FOI P1 was the only FOI phase with significant changes over the treatment period of 1 year. In another study on osteoarthritis [17] as the primary non-inflammatory joint disease, OA patients exhibited less activity in FOI P 1 compared to joint inflammation in RA (though, high signal accumulation in P2). These results support the hypothesis that FOI P1 detects an acute inflammation.
Regarding clinical disease activity, DAS28 (CRP) decreased significantly from a median of 4.6 to a median of 3.2 at w24. After 52 weeks of treatment, a low disease activity (median of 2.7) was observed. The good response of CZP treatment in RA patients could also be shown by a reduction of the prednisolone dosage, which decreased from 6.0 ± 4.75 mg per day to 2.3 ± 2.26 mg/day at w52.
A correlation between DAS28 (CRP) and P1 could not be observed, which again is in line with the findings by Glimm et al. [25]. The lack of correlation may be caused by measuring different outcome parameters assessed through the clinical examination by DAS28 compared to the FOI method. Clinical disease activity is mainly assessed by swollen and tender joints and by patients’ self-reported VAS. In contrast, FOI via the Xiralite method detects disturbed microcirculation in the joints of both hands. Hypervascularisation and neoangiogenesis of the synovial membrane are considered to be primary pathogenic mechanisms for the development of rheumatoid pannus on the joint leading to bone destruction [30].
Because of the subjective component of DAS28 (CRP) in decision making during RA treatment, which leads to a high variance of the DAS28 (CRP) score, FOI represents a more objective method for therapy monitoring.
Another explanation for the lack of correlation between FOI P1 and clinical outcome parameters could be the positive findings in FOI in asymptomatic joints, indicating subclinical inflammation. Werner et al. [20] found that FOI showed positive findings in 45% of clinically asymptomatic joints.
In our study, we also included the US7 score for detection of synovitis and tenosynovitis. The US7 synovitis sum score decreased, but not initially after start of CZP therapy. For GS, a numerical decrease of the synovitis sum score was observed only at w52 and for PD, a decrease of the synovitis score was detected at w12 with further decrease at w52. Sarzi-Puttini et al. [31] already found an improvement in PD ultrasound after w8 of CZP therapy, which continued to w52. Overall, the MSUS results are in line with other studies. In contrast to the fast response in PD scoring, which identifies active inflammation, the GS usually identifies chronic or later changes such as synovial proliferation, which explains the late response in our study [15, 32–34]. In spite of the positive results in clinical studies, the operator-dependency and time-consumption are potential limitations of MSUS [33].
An advantage of FOI is the ability to do fast imaging in a comfortable sitting position by using a machine which can be installed in every clinical setting. It is also a delegable tool that can performed by a medical assistance with a physician in the background. FOI can be used in both diagnostic and therapy monitoring setting. Therapy monitoring by means of short-term FOI study visits is likely the more important role because biological therapies such as anti-TNFα therapies are highly effective but expensive. Thus, to keep costs as low as possible and to provide the patient with the optimal treatment, it is important to obtain early objective evidence of a response or non-response.
The use of the chosen imaging method depends on the treating physician’s preference and the indication for imaging. Information on bone erosions will likely remain the domain of MRI, MSUS and X-ray. However, determination of the degree and extent of joint inflammation as well as for the diagnostic of subclinical inflammation are indication for the use of FOI method. Because of the detection of early synovitis in symptomatic and asymptomatic patients, FOI might also be a tool in early clinical studies to detect treatment response.
A limitation of the current study is the open design and the small sample size. Treatment responders could also be influenced by other factors that were not considered into the analysis, e.g. wounds located over the joint. Another limitation of the FOI device is that the system can only image the hands, so far. Furthermore, the intravenous ICG administration before initiating the FOI procedure might limit the routine use in clinical practice.
Conclusion
To our knowledge, this is the first study showing that FOI can be used to monitor early treatment response after initiating anti-TNFα therapy. Significant reductions in FOIAS were found in P1 which appears to be the most relevant phase for monitoring treatment response.
In contrast to clinical outcome parameters, FOI represents—in our opinion—a more objective method for therapy-monitoring and may provide an additional tool to assess early therapy response after initiating therapy with biologics. It may also provide the opportunity for an additional evaluation of inflammation of wrist and finger joints of RA patients in clinical studies. Consequently, decrease in disease activity can easily be monitored with the Xiralite system by quantifying the fluorescence signals in the early phase (= phase 1) of signal acquisition.
Fluorescence optical imaging with the Xiralite system is an interesting alternative to other imaging methods, not only in terms of providing a fast and easy assessment but also its pictorial output, making it easier for patients to follow and understand, enabling higher acceptance of treatment measures.
Acknowledgements
We would like to acknowledge all participating patients.
Abbreviations
- ACR
American College of Rheumatology
- ACPA
Anti-cyclic citrullinated peptides
- bDMARD
Biological disease-modifying anti-rheumatic drug
- CRP
C-reactive protein
- csDMARD
Conventional synthetic disease-modifying anti-rheumatic drug
- CZP
Certolizumab pegol
- DAS28
Disease activity score of 28 joints
- ESR
Erythrocyte sedimentation rate
- EULAR
European alliance of associations for rheumatology
- FOI
Fluorescence optical imaging
- FOIAS
Fluorescence optical imaging activity score
- GS
Greyscale (ultrasound)
- ICG
Indocyanine green
- IgM-RF
Immunoglobulin M-rheumatoid factor
- IP
Interphalangeal joint of the thumb
- IQR
Interquartile range
- MCP
Metacarpophalangeal (joint)
- MRI
Magnetic resonance imaging
- MSUS
Musculoskeletal ultrasound
- MTP
Metatarsophalangeal (joint)
- NSAIDs
Non-steroidal anti-inflammatory drugs
- NYHA
New York Heart Association
- OMERACT
Outcome measures in rheumatology clinical trial
- P
Phase
- PGA
Physician’s Global Assessment
- PIP
Proximal interphalangeal (joint)
- PD
Power Doppler (ultrasound)
- PVM
PrimaVistaMode
- RA
Rheumatoid arthritis
- RMD
Rheumatic and musculoskeletal diseases
- SJC-28
Swollen joint count-28
- TNFα
Tumour necrosis factor α
- TJC-28
Tender joint count-28
- US7 Score
Ultrasound Seven Score
- VAS
Visual analogue scale
Authors’ contributions
SH, JK, QS, MB and SO made substantial contributions to the conception or design of the work or the acquisition, analysis or interpretation of data for the work; SH, JK, QS, AMG, GRB, PH, GS, TH, SH, MB and SO were drafting the work or revising it critically for important intellectual content; all authors provide approval for publication of the content and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was financially supported by UCB Pharma GmbH. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the local Medical Ethical Committee of the Charité – Universitätsmedizin, Berlin, Germany. All patients provided informed consent to participate in the study.
Consent for publication
All patients provided informed consent to publish the data.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cheng Y, Hootman J, Murphy L. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation: United States, 2007-2009. MMWR Morb Mortal Wkly Rep. 2010;62(44):869. [PMC free article] [PubMed]
- 2.Fazal SA, Khan M, Nishi SE, Alam F, Zarin N, Bari MT, et al. A clinical update and global economic burden of rheumatoid arthritis. Endocr Metab Immune Disord Drug Targets. 2018. 10.2174/1871530317666171114122417. [DOI] [PubMed]
- 3.Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin N Am. 2001. 10.1016/S0889-857X(05)70201-5. [DOI] [PubMed]
- 4.Silman AJ, Pearson JE. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002. 10.1186/ar578. [DOI] [PMC free article] [PubMed]
- 5.Wilke WS, Sweeney TJ, Calabrese LH. Early, aggressive therapy for rheumatoid arthritis: concerns, descriptions, and estimate of outcome. Semin Arthritis Rheum. 1993;23:26–41. doi: 10.1016/S0049-0172(10)80005-8. [DOI] [PubMed] [Google Scholar]
- 6.Burgers LE, Raza K, A. H. Van Der helm-Van mil, window of opportunity in rheumatoid arthritis-definitions and supporting evidence: from old to new perspectives. RMD Open. 2019. 10.1136/rmdopen-2018-000870. [DOI] [PMC free article] [PubMed]
- 7.Radner H, Aletaha D. Anti-TNF in rheumatoid arthritis: an overview. Wien Med Wochenschr. 2015. 10.1007/s10354-015-0344-y. [DOI] [PubMed]
- 8.Prevoo MLL, Van’T Hof MA, Kuper HH, Van Leeuwen MA, Van De Putte LBA, Van Riel PLCM. Modified disease activity scores that include twenty-eight-joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995. 10.1002/art.1780380107. [DOI] [PubMed]
- 9.Backhaus M, Kamradt T, Sandrock D, Loreck D, Fritz J, Wolf KJ, et al. Arthritis of the finger joints: a comprehensive approach comparing conventional radiography, scintigraphy, ultrasound, and contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 1999. https://doi.org/10.1002/1529-0131(199906)42:6<1232::AID-ANR21>3.0.CO;2-3. [DOI] [PubMed]
- 10.Wakefield RJ, Gibbon WW, Conaghan PG, O’Connor P, McGonagle D, Pease C, et al. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthritis Rheum. 2000. https://doi.org/10.1002/1529-0131(200012)43:12<2762::aid-anr16>3.0.co;2-%23. [DOI] [PubMed]
- 11.Colebatch AN, Edwards CJ, Østergaard M, Van Der Heijde D, Balint PV, D’Agostino MA, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013. 10.1136/annrheumdis-2012-203158. [DOI] [PubMed]
- 12.Maier A, Ratanski M, Renelt M, Hammer M, Gehlen M. Sonography of the foot in rheumatology: ultrasound diagnostics of the ankle joint and foot in the rheumatological routine. Z Rheumatol. 2020. 10.1007/s00393-020-00823-1. [DOI] [PubMed]
- 13.Fischer T, Gemeinhardt I, Wagner S, Stieglitz DV, Schnorr J, Hermann KGA, et al. Assessment of unspecific near-infrared dyes in laser-induced fluorescence imaging of experimental arthritis. Acad Radiol. 2006. 10.1016/j.acra.2005.07.010. [DOI] [PubMed]
- 14.Bhasin S, Cheung PP. The role of power Doppler ultrasonography as disease activity marker in rheumatoid arthritis. Dis Markers. 2015. 10.1155/2015/325909. [DOI] [PMC free article] [PubMed]
- 15.Backhaus M, Ohrndorf S, Kellner H, Strunk J, Backhaus TM, Hartung W, et al. Evaluation of a novel 7-joint ultrasound score in daily rheumatologic practice: a pilot project. Arthritis Care Res. 2009. 10.1002/art.24646. [DOI] [PubMed]
- 16.Fischer T, Ebert B, Voigt J, Macdonald R, Schneider U, Thomas A, et al. Detection of rheumatoid arthritis using non-specific contrast enhanced fluorescence imaging. Acad Radiol. 2010. 10.1016/j.acra.2009.09.016. [DOI] [PubMed]
- 17.Glimm AM, Werner SG, Burmester GR, Backhaus M, Ohrndorf S. Analysis of distribution and severity of inflammation in patients with osteoarthitis compared to rheumatoid arthritis by ICG-enhanced fluorescence optical imaging and musculoskeletal ultrasound: a pilot study. Ann Rheum Dis. 2016;75:566–570. doi: 10.1136/annrheumdis-2015-207345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krohn M, Ohrndorf S, Werner SG, Schicke B, Burmester GR, Hamm B, et al. Near-infrared fluorescence optical imaging in early rheumatoid arthritis: a comparison to magnetic resonance imaging and ultrasonography. J Rheumatol. 2015. 10.3899/jrheum.141244. [DOI] [PubMed]
- 19.Meier R, Krug C, Golovko D, Boddington S, Piontek G, Rudelius M, et al. Indocyanine green - enhanced imaging of antigen-induced arthritis with an integrated optical imaging/radiography system. Arthritis Rheum. 2010. 10.1002/art.27542. [DOI] [PMC free article] [PubMed]
- 20.Werner SG, Langer HE, Ohrndorf S, Bahner M, Schott P, Schwenke C, et al. Inflammation assessment in patients with arthritis using a novel in vivo fluorescence optical imaging technology. Ann Rheum Dis. 2012. 10.1136/annrheumdis-2010-148288. [DOI] [PMC free article] [PubMed]
- 21.Werner SG, Langer HE, Schott P, Bahner M, Schwenke C, Lind-Albrecht G, et al. Indocyanine green-enhanced fluorescence optical imaging in patients with early and very early arthritis: a comparative study with magnetic resonance imaging. Arthritis Rheum. 2013. 10.1002/art.38175. [DOI] [PubMed]
- 22.Kay J, Upchurch KS. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology. 2012;51:vi5–vi9. doi: 10.1093/rheumatology/kes279. [DOI] [PubMed] [Google Scholar]
- 23.Prevoo ML, Van't Hof M, Kuper HH, Van Leeuwen MA, Van De Putte LB, Van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 24.Fransen J, Stucki G, van Riel PL. Rheumatoid arthritis measures: disease activity score (DAS), disease activity Score-28 (DAS28), rapid assessment of disease activity in rheumatology (RADAR), and rheumatoid arthritis disease activity index (RADAI) Arthritis Care Res. 2003;49:S214–S224. doi: 10.1002/art.11407. [DOI] [Google Scholar]
- 25.Glimm AM, Sprenger LI, Haugen IK, Mansmann U, Hermann S, Häupl T, et al. Fluorescence optical imaging for treatment monitoring in patients with early and active rheumatoid arthritis in a 1-year follow-up period. Arthritis Res Ther. 2019. 10.1186/s13075-019-1989-5. [DOI] [PMC free article] [PubMed]
- 26.Scheel AK, Hermann KGA, Ohrndorf S, Werner C, Schirmer C, Detert J, et al. Prospective 7 year follow up imaging study comparing radiography, ultrasonography, and magnetic resonance imaging in rheumatoid arthritis finger joints. Ann Rheum Dis. 2006. 10.1136/ard.2005.041814. [DOI] [PMC free article] [PubMed]
- 27.Szkudlarek M, Court-Payen M, Jacobsen S, Klarlund M, Thomsen HS, Østergaard M. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 2003. 10.1002/art.10877. [DOI] [PubMed]
- 28.Mendonça JA, Yazbek MA, Costallat BL, Gutiérrez M, Bértolo MB. The modified US7 score in the assessment of synovitis in early rheumatoid arthritis. Rev Bras Reumatol. 2014. 10.1016/j.rbr.2014.03.026. [DOI] [PubMed]
- 29.Meier R, Thuermel K, Noël PB, Moog P, Sievert M, Ahari C, et al. Synovitis in patients with early inflammatory arthritis monitored with quantitative analysis of dynamic contrast-enhanced optical imaging and MR imaging. Radiology. 2014;270:176–185. doi: 10.1148/radiol.13130039. [DOI] [PubMed] [Google Scholar]
- 30.Pap T, Distler O. Linking angiogenesis to bone destruction in arthritis. Arthritis Rheum. 2005. 10.1002/art.21015. [DOI] [PubMed]
- 31.Sarzi-Puttini P, Filippucci E, Adami S, Meroni PL, Batticciotto A, Idolazzi L, et al. Clinical, ultrasound, and predictability outcomes following certolizumab pegol treatment (with methotrexate) in patients with moderate-to-severe rheumatoid arthritis: 52-week results from the CZP-SPEED study. Adv Ther. 2018;35:1153–1168. doi: 10.1007/s12325-018-0751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali SLR, Glimm A-M, Burmester GR, Hoff P, Schmittat G, Hermann S, et al. Fluorescence optical imaging is helpful in the decision for rituximab (RTX) re-therapy in patients with rheumatoid arthritis. 2020. [Google Scholar]
- 33.do Prado AD, Staub HL, Bisi MC, da Silveira IG, Mendonça JA, Polido-Pereira J, et al. Ultrasound and its clinical use in rheumatoid arthritis: where do we stand? Adv Rheumatol (London, England). 2018. 10.1186/s42358-018-0023-y. [DOI] [PubMed]
- 34.Razmjou AA, Brook J, Elashoff D, Kaeley G, Choi S, Kermani T, et al. Ultrasound and multi-biomarker disease activity score for assessing and predicting clinical response to tofacitinib treatment in patients with rheumatoid arthritis. BMC Rheumatol. 2020. 10.1186/s41927-020-00153-4. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.