Abstract
Purpose of Review
Involvement of the central nervous system (CNS) in HIV-1 infection is commonly associated with neurological disorders and cognitive impairment, commonly referred to as HIV-associated neurocognitive disorders (HAND). Severe and progressive neurocognitive impairment is rarely observed in the post-cART era; however, asymptomatic and mild neurocognitive disorders still exist, despite viral suppression. Additionally, comorbid conditions can also contribute to the pathogenesis of HAND.
Recent Findings
In this review, we summarize the characterization of HAND, factors contributing, and the functional impairments in both preclinical and clinical models. Specifically, we also discuss recent advances in the animal models of HAND and in in vitro cultures and the potential role of drugs of abuse in this model system of HAND. Potential peripheral biomarkers associated with HAND are also discussed.
Summary
Overall, this review identifies some of the recent advances in the field of HAND in cell culture studies, animal models, clinical findings, and the limitations of each model system, which can play a key role in developing novel therapeutics in the field.
Keywords: HIV, HAND, Synaptodendritic injury, Neurodegeneration, Drug abuse
Introduction
Infection of the central nervous system (CNS) is an early event observed in the course of HIV infection/AIDS [1]. HIV-associated neurocognitive disorders (HAND) are an outcome of the HIV-1 infection and persist in the era of combined antiretroviral therapy (cART). It is estimated that approximately 30–50% of the HIV-1 infected individuals on cART therapy develop a range of HAND symptomatology [2]. HAND comprises a spectrum of neurological disorders ranging from asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), to its severe, although rare form, HIV-associated dementia (HAD) [3]. In the era of antiretroviral therapy (cART), while there are very low incidences of HAD observed in HIV-1 infected individuals, the incidence of milder forms of HAND is on the rise. While neuronal cell death has been observed in the postmortem brain of HAND patients, the evidence suggests that neuronal cell death does not correspond to the severity of cognitive impairment [4]. Although the introduction of cART has transformed HIV-1 into a chronic but manageable disease, the prevalence and development of HAND remain comorbidity of HIV-1 infection. Interestingly, HAND patients undergoing cART treatment exhibit negligible neuronal loss but show evidence of neuronal injury, suggesting the possibility of more subtle neuropathological changes underpinning the disease pathogenesis and progression [5]. The current thinking underlying the persistence of HAND is thought to involve multiple factors including but not limited to aging, poor penetration of the antiretroviral drugs across the blood-brain barrier, and residual chronic inflammation, all of which can result in persistent low-level viral replication in the CNS with the accumulation of cytotoxic viral proteins such as HIV-1 transactivator of transcription (Tat) and gp120 and potential viral escape and emergence of resistant viral species [6]. It has also been shown that long-term cART exposure itself can contribute to the process of aging and neurodegeneration [7]. Recent clinical and preclinical findings have also demonstrated various novel mechanisms of neuronal damage underlying the neuropathogenesis of HAND.
Overall, a deeper understanding of the mechanism(s) involved in neurocognitive impairment in patients living with HIV-1 could generate credible norms to evaluate the neurocognitive outcomes. To date, there is paucity in our understanding on the mechanisms underlying HIV-induced neuropathogenesis and cognitive decline. This review is focused on the role of HIV-1 and HIV-1 viral proteins in the onset and progression of HAND neuropathogenesis and its functional impairments, involving both the preclinical and clinical models.
Clinical Study
Cognitive Impairment in HAND
Many cross-sectional studies have demonstrated that almost 50% of treated HIV patients are afflicted with varying levels of cognitive impairment [8, 9]. Although the introduction of cART has successfully eliminated the prevalence of HIV-associated dementia from about 20 % [10] to less than 5% [11], the milder forms of neurocognitive impairment continue to persist. Sorting out the etiology, prognosis, and optimal cART regimen for treated HAND patients remains a major incomplete task. The most well-accepted HAND subclassification was articulated in 2006 at Frascati, Italy [12]. Based on this articulate, HAND was subclassified to HIV-associated dementia (HAD), mild neurocognitive disorder (MND), and asymptomatic neurocognitive impairment (ANI).
HAD is the serious consequence of HIV infection with the involvement of neurological and psychiatric impairments. It was shown that the more spreading of HIV in the brain, the worse development of dementia symptoms [13]. HAD manifestations include loss of memory, reduced ability to think and speak clearly or accurately, difficulty in concentration or staying focused, apathy, gradual loss of motor skills, and many variable behavioral components, which, ultimately, leads to death [14].
Mild neurocognitive disease (MND) and asymptomatic neurocognitive impairment (ANI)—the most prevalent form of HAND—have been reported in 40–56% of HIV+ individuals [15, 16]. MND and ANI are now characterized by impairment based on neurocognitive testing with or without obviously associated interference in daily functioning. ANI is the most common form of HAND [16] and has been reported in approximately 70% of HAND cases [12]. However, the current definitions for distinguishing ANI and MND based on neuropsychometric performance are found to be challenging and likely imprecise.
For example, the longitudinal CHARTER (CNS HIV Antiretroviral Therapy Effects Research) study recruited 436 HIV-infected participants in the CHARTER cohort, followed for 16–72 months, and measured the comprehensive laboratory neuromedical and neurocognitive parameters every 6 months [17]. This study found that almost 61% of HIV patients with cART remained stable, 16.5% improved neurocognitive status, and 22.7% declined, which indicated that neurocognitive change is pervasive in HIV infection and driven risk factors are very complex. Another comparative study also reported classifying neurocognitive impairments in large groups of HIV-infected and HIV-negative individuals from the pre-cART era (1988–1995; N = 857) and cART era (2000–2007; N = 937). This study reported more significant impairments in motor skills, cognitive speed, and verbal fluency in HIV-infected individuals from the pre-cART era, whereas more impairments in learning memory and executive function in HIV-infected individuals in the cART era [18]. This study also reported the high prevalence of mild NCI that persists in all stages of HIV infection, despite improved viral suppression and immune reconstitution with cART [18]. Emerging studies have also correlated the prospective memory deficits in conferring an increased risk of unemployment in HIV-infected individuals and ultimately increased morbidity in relevance to systemic diseases [19, 20].
Neuropathology of HAND
There is scant information available on the association of specific viral determinants to the development of milder forms of HAND. HIV RNA and DNA found in the postmortem brains have primarily been associated with the severe form of multinucleated cell encephalitis linked with HIV-associated dementia (HAD). On the other hand, the milder forms of HAND are not associated with pathological findings and viral recovery. Notably, there are no neuropathological correlates of either ANI or MND [21, 22]. Several preclinical and clinical studies have demonstrated a strong correlation of synaptic alterations and synaptodendritic abnormalities with the severity of cognitive impairment in HAND patients [23]. In fact, before the antiretroviral era also, there were reports of correlations of the brain viral load with the degree of synaptodendritic damage in HIV-1 patients [24]. Earlier reports have also shown a strong association of milder neurocognitive impairment with microinjury to the synaptic structures [25]. Several other studies have also reported region-specific presynaptic and synaptic damages as the major pathobiological mechanisms underlying the development of HAND [23, 26]. Several reports indicate neuronal damage or injury as a key phenomenon in patients with HAND, despite the fact that neurons are not infectable by HIV-1 [27]. Along these lines, several studies have implicated viral proteins such as transactivator of transcription (Tat) and gp120, to play a key role in neuronal damage in HAND, likely via direct interactions with the neuronal N-methyl-D-aspartate receptors (NMDAR), the low-density lipoprotein receptor-related protein (LRP), chemokine receptors CCR5 and CXCR4, and dopamine transporter and indirectly via activation of the glial cells [28]. Although the clinical utility of inflammatory markers in the CSF has been limited, it is suggested that these markers could serve as potential biomarkers for patients that are either at risk for developing or have ongoing HAND.
Recent studies have also shown that patients on long-term suppressive cART have been reported to exhibit mildly elevated CSF neopterin and IgG index [29]. Interestingly, persistent immune activation markers including IL-6, IL-8, and CCL2 (MCP-1) have been found to be constantly elevated in cART patients [30]. Apart from inflammation, specific histopathological features including the presence of microglial nodules (MGN) comprising of activated microglial cells arranged in clusters of variable sizes [22], multinucleated giant cells (MNGC) around the blood vessels [31], astrocyte hypertrophy, and glial scarring in the brain white matter [32] and myelin loss in the perivascular areas with focal white matter anomalies are relatively common in HIV-infected patients despite successful viral suppression following cART therapy [33, 34]. Blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging (fMRI) studies have revealed perturbations in blood flow in specific brain regions of cART-treated patients [35]. Additionally, it has also been reported that GABAergic transmission is abnormally low in the HIV-infected brain tissue [36], contributing to changes in microvascular blood flow in virally suppressed patients.
Intriguingly, recent studies have shown activation of stress pathways and accumulation of amyloid-beta (Aβ) fibrils in specific brain regions of HAND patients on cART therapy, which, in turn, could lead to neuronal dysfunction [37•]. It has been reported that individuals with HIV encephalitis (HIVE) display higher levels of intraneuronal Aβ accumulation, thus suggesting that HIV-1 infection impacts the clearance of Aβ in the brain [38]. It has also been shown that the APOE ε4 allele is associated with a genetic predisposition for Alzheimer’s disease (AD) [39]. Interestingly, HIV+ individuals carrying this etiologic risk factor (APOE4) for AD exhibit increased dementia compared to patients negative for the APOE4 allele [40]. In the same study, it was also shown that in the presence of APOE4, there was enhanced entry/fusion of HIV (R5 and X4) in T cells [40]. Increased neurofilament light protein (NF-L) has been reported in untreated HAND patients and is shown to decline following successful cART treatment. One of the hallmark features of AD-like pathology is the presence of phosphorylated Tau as deposition of tangles. Interestingly, expression levels of phosphorylated Tau were observed to be elevated in HAD but not in ANI or MND patients [41, 42].
Recent studies have shown that phosphorylation of Tau proteins was significantly increased in various brain regions of treated HAND patients exhibiting either ANI or MND [37•]. There are also reports of perturbed neurotransmitter systems in HAND patients. For example, a study conducted on a cohort of 449 HIV patient samples with or without HAND showed significantly decreased GABAergic markers with a concomitant increase in the expression of endothelial cell markers, thereby suggesting the role of dysfunctional inhibitory neurotransmission and impaired synaptic plasticity in HAND [43]. Targeting the above-discussed components of neuronal toxicity could thus be considered potential therapeutic strategies for alleviating the symptomatology of HAND and for improving the quality of life of those suffering from HAND (Table 1). Recent reports from Pulliam group have demonstrated that plasma neuronal-derived EVs (NDE) found enrichment of neuronal markers—neurofilament light (NF-L) and synaptophysin (SYP)—in a cohort of HIV-seropositive and HIV-seronegative controls that had mild cognitive impairment. Interestingly, neuropsychologically impaired (NPI) individuals had fewer NDEs than neuropsychological normal subjects (NPN), suggesting thereby that there were increased numbers of damaged neurons than the healthy ones, and likely that, in these patients, EV biogenesis is strongly associated with neuronal dysfunction. NDEs isolated from NPI subjects exhibited significantly higher levels of high mobility group box 1 (HMGB1), NF-L, and Aβ proteins compared with NDEs from NPN individuals. It can thus be concluded that cargoes (HMGB1, NF-L, and Aβ) in plasma NDE can serve as a biomarker for ongoing cognitive impairments [44]. Another study by the same group has shown that NDE from HIV+ men and women had divergent results, i.e., HMGB1 and NF-L were significantly increased in NDE from cognitively impaired men, while no difference was observed in women. Cargoes for p-Tau181 were found to be decreased in NDE from men but did not change in NDEs isolated from women. NDEs from HIV-infected women with mild impairment exhibited significantly decreased levels of cathepsin S, total tau, neuronal cell adhesion molecule, and contactin 5. On the other hand, NDEs from HIV+ men with cognitive impairment exhibited increased expression of carboxypeptidase M, cadherin 3, colony-stimulating factor 2 receptor alpha subunit, and mesencephalic astrocyte-derived neurotropic factor. It could thus be concluded that NDE cargoes from HIV-infected individuals differ in men and women, thereby underscoring mechanistic sex differences associated with HAND. It must be noted that several NDE targets during HIV infection differ from those reported for AD [45]. Overall studies from the Pulliam group have shown that although lower numbers of NDEs were present in the NPI group compared to NPN, higher expression of protein cargoes, e.g., neurofilament light chain, Aβ, etc., was evident, indicating thereby that neurons in the NPI group were likely synthesizing toxic proteins that could likely contribute to the neuronal injury in HAND. Furthermore, this study also indicated that the assessment of CNS-derived EVs in the periphery could serve as biomarkers for ongoing neurological disorders in the brain.
Table 1.
Neuropathogenesis and potential biomarkers of HAND: the potential biomarkers or neuropathologic changes of HAND are listed
| Potential biomarkers or neuropathology | Region | Cognitive status | ART | Bibliography |
|---|---|---|---|---|
| Elevated neopterin and IgG index | CSF | N/A | Long-term suppressive cART | [29] |
| Elevated IL-6, IL-8, CCL2, sCD14 | CSF | Impaired neurocognitive | cART | [30] |
| MGN (microglial nodules) | Brain | HAND | cART | [22, 31] |
| MNGC (multinucleated giant cell) | ||||
| Astrocyte hypertrophy | Brain white matter | HIV-D | HAART | [32] |
| Glial scarring | MCMD | |||
| Myelin loss | Brain perivascular sectors | N/A | HAART | [33, 34] |
| Blood flow perturbed | Brain | N/A | cART | [35] |
| Low GABAergic transmission | Brain | NCI (neurocognitive impairment) | cART | [36] |
| Amyloid-beta (Aβ) fibril accumulation; | Brain | HAND | cART | [37•, 38] |
| Intraneuronal Aβ accumulation | ||||
| Phosphorylation of Tau | Brain | ANI or MND | cART | [37] |
| HMGB1 | Brain | NPI (neuropsychologically impaired) | cART | [44, 45] |
NCI neurocognitive impairment; ANI asymptomatic neurocognitive impairment; MND mild neurocognitive disorder; HIV-D HIV-associated dementia; NPI neuropsychologically impaired; HMGB1 high mobility group box 1; HAART highly active antiretroviral therapy
Neuropathological and Behavioral Studies in Animal Models of HAND
Neuropathological studies have provided insights into some salient features of HAND, underpinning the essential role of synaptodendritic injuries and altered neurotransmission. Studies on the postmortem brain tissues from HIV-infected individuals provide a snapshot view of several interconnected systems and implicate the role of key neuronal and synaptic genes responsible for synaptodendritic injury as well as other genes responsible for neuroinflammation and bioenergetics in the pathology of HAND [46]. Several clinical studies of HAND suggest that well-controlled HIV infection can still dysregulate neuronal functions in specific brain regions, thus providing a basis for the underlying cognitive impairment observed in cART-treated patients. However, it is still unclear how controlled HIV infection promotes more subtle and region-specific neuronal dysfunction. Studies in HIV-transgenic rats that have examined synaptodendritic injury have reported deficits of the thin spine and the presence of increased stubby spine on proximal dendrites [47]. In another study, synaptodendritic injury was also evident in the Tat-transgenic mice where dopamine subtype 2 (D2) receptor-expressing medium spiny neurons (MSNs) were found to be selectively vulnerable to Tat exposure [48]. Furthermore, another study in the inducible Tat-transgenic mice demonstrated that dendritic spine deficits were sex-dependent, with more pronounced cellular spine deficit and cognitive impairment observed in the male versus the female mice [49]. While the utility of human clinical samples cannot be overemphasized for the study of HAND, there has been immense advancement in our understanding of the neurological complications and behavioral deficits of HAND using the existing preclinical animal models.
Several reports are available on dendritic spine alterations in HAND in the HIV-transgenic rat model [50–56]. This animal model is ideally suited to understand the role of specific viral proteins in mediating CNS neuropathology and, to some extent, mimics the human scenarios analogous to HIV-infected individuals on cART, wherein in the face of virus suppression, viral protein expression continues unabated [57–59]. Like humans with HAND, HIV-transgenic rats exhibit cognitive impairment, including defective executive function, temporal processing, long-term episodic memory, and spatial learning [50–56, 60]. Furthermore, the HIV-transgenic rats also exhibit deficits in dendritic spines and branches in both the layer of II and III neurons in the motor cortical region, thereby suggesting a correlation of neuronal defects with cognitive impairment and motor dysfunction [50]. In addition to deficits in the dendritic spine in the HIV-transgenic rats, there is also chronic immune activation in these animals, which could be implicated as one of the driving factors for initiation of synaptodendritic injury [61–64]. Similar to the HIV-transgenic rats, HIV Tat-transgenic male mice expressing Tat 1-86 also exhibit reduced dendritic spine density in the layer II and III neuronal branches [48]. Similar changes have also been reported in the doxycycline-inducible HIV Tat-transgenic male and female mice in which both mice exhibit dendritic spine deficits in the striatal medium spiny neurons as well as impairment in motor learning [49]. There are limited studies on the HIV gp120 animal models. A single intracerebroventricular injection of gp120 to adult male rats resulted in decreased dendritic spine density in various cortical regions and was correlated with decreased cognitive flexibility, thereby contributing to motor deficits observed in these rats [50, 65]. Other reports have also investigated dendritic spine deficits in the hippocampus and the dorsal striatum of both sexes of HIV gp120 transgenic mice and found that the activation of the p75 neurotrophin receptor is critical for the dendritic spine loss in this mouse model [66, 67] (Fig. 1).
Fig. 1.
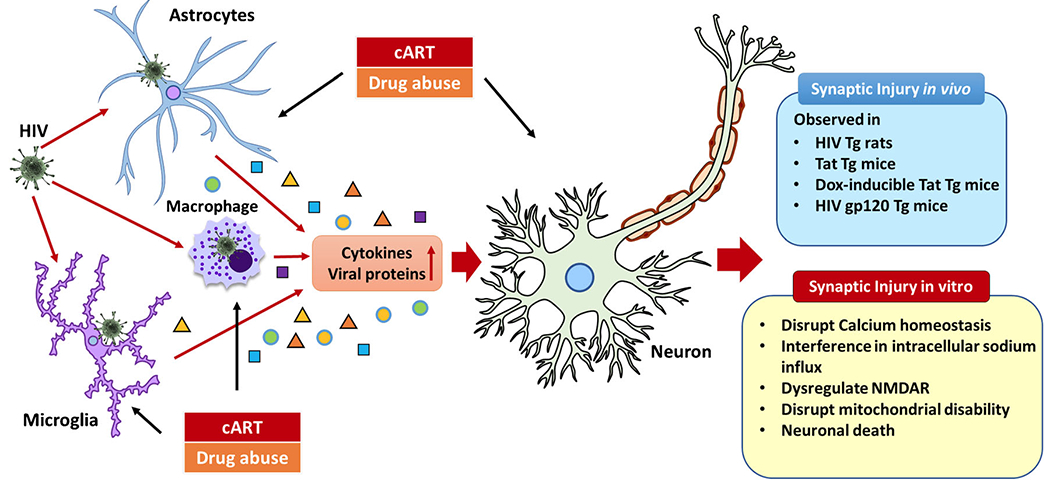
The mechanisms of neuronal dysfunction caused by HIV-1, cART, and drug abuse. Although HIV-1 cannot infect neuron itself, specific HIV viral proteins including envelope glycoprotein (gp)120, gp41, gp 160, Nef, Rev, TAT, and Vpr and inflammatory factors secreted from HIV-1-infected CNS macrophages, microglia, and astrocytes which can cause synaptodendritic damage in neurons leading to HIV-1-associated neuropathogenesis; drug abuse and cART could directly induce synaptodendritic injury and indirectly alter neuronal morphology and functions by modulating the glial cells in the CNS; the neurocognitive impairment or synaptic injury was found in HIV-transgenic rats, Tat-transgenic mice, Dox-inducible Tat-transgenic mice, and HIV gp120-transgenic mice
The observed neuronal alterations observed in various preclinical HAND models have been suggested to be exacerbated in the presence of comorbid conditions such as drug abuse. For example, it has been reported that psychostimulant cocaine significantly elevated the excitability of layer V and VI pyramidal neurons in the prefrontal cortices of male HIV-transgenic rats [68]. Opioids exposure also notably resulted in decline of the GABAergic neurotransmission in the striatal regions of both male and female mice injected with HIV Tat protein [69•, 70]. Studies have also reported the effects of methamphetamine on the expression of synapse and neuronal plasticity markers in both the sexes in HIV-1-transgenic rats and found a region-specific activation of ERK with increased expression of ΔFosB and D1R [71] and decreased expression in synapsin 1, synaptophysin, Arg3.1, PSD-95, and BDNF by HIV-1 Tat protein and methamphetamine [72]. These preclinical findings align with the complex and multifactorial nature of HAND that is observed in the clinical setting and highlight the need to better understand the etiology and pathogenesis of HAND. It has also been shown that drug abuse can indirectly alter neuronal morphology and functioning by impacting BBB integrity and transmigration of HIV-infected and HIV-uninfected monocytes across the BBB, as well as their effects on monocytes/macrophages, microglia, astrocytes, endothelial cells, and pericytes within the CNS [73–76]. In addition to drug abuse, ART has also been shown to contribute to synaptodendritic injury, increased glial activation, as well as neuroinflammation [77–79]. For example, it has been reported that SIV-infected macaques on cART therapy elicited reduced synaptophysin levels compared with the SIV-infected macaques without therapy [80]. Increased glial activation and neuroinflammation were also observed in 15-month-old HIV-transgenic rats treated with ART compared with those without cART [78, 79].
Since HIV-1 does not infect rodents, recapitulating the human disease in a small animal model has been a major limitation in the field [81, 82]. Over the last three decades, various approaches have been employed to develop novel rodent models of HAND. While none of these truly represent HAND, they mimic “some aspects” of HAND. These models include severe combined immunodeficient model involving direct stereotactic injection of HIV+ human monocytes into the CNS [83–85]; direct injection of HIV proteins either into the CNS or via IV route [86–90]; EcoHIV mouse comprising of inoculation of mice with a chimeric HIV-1 that uses species-specific cellular receptors to enter mouse cells [91]; HIV Tg rat model expressing all HIV proteins except gag and pol [54, 57]; transgenic (Tg) mice with constitutive/inducible expression of HIV proteins [54]; and Dox-inducible HIV Tat Tg mouse model, developed by Drs. A. Nath and K. Hauser, and in this model, Tat is expressed following induction with Dox in the feed. These mice express HIV-1 Tat1-86 in astrocytes throughout the brain when induced with dox. Additionally, this model is characterized to exhibit neurologic abnormalities that mimic the human condition of chronic HIV-1 exposure with cART, including glial activation [92], dendritic damage and reduced spine density in striatal neurons [93], abnormal oligodendrocytes [94], and importantly, behavioral abnormalities [95]. Importantly, it is now well recognized that Tat persists in various tissues, including the brain, despite virological control by cART and humanized mice generated by transplanting hu cells/tissues into genetically modified immunodeficient mice [96]. Although none of the rodent models truly represent HAND, each of these models has proved to be a valuable tool for exploring “specific aspects” of HAND. As a result, most of these models have cumulatively helped galvanize the field by exhibiting relevance for studies of human disease diagnosis and therapies.
Mechanisms of Neuronal Damage In Vitro HIV and Viral Proteins
Despite the absence of productive infection in neurons, neuropathological abnormalities, including synaptodendritic damage, neuronal dysfunction, and neurodegeneration in the brains of HIV-infected patients, seem to be the underlying pathology for HAND. In the post-cART era, it has been shown that there is low-level viral replication as well as infiltration of infected monocytes/macrophages in the CNS, likely due to a leaky blood-brain barrier. Other factors, such as viral proteins including HIV Tat, gp120, inflammatory cytokines, and small metabolites [such as reactive oxygen species (ROS) and nitric oxide (NO)] that are secreted by the infected cells in the CNS, can also contribute to neurotoxicity in the brain. Consorted effects of all these factors play a key role in neurodegeneration, leading to cognitive decline associated with HAND [27, 97–100]. The interactions between viral proteins and neurons describe the direct effect of HIV-mediated neurotoxicity that leads to neuronal damage. Of these, the viral protein Tat was found to be taken up by the neurons and mediate injury to the neurons not only in close vicinity to the protein but also at distant sites after its release into the extracellular space from HIV-infected cells [101]. The deleterious effects of Tat on neurons include disruption of calcium homeostasis [102], interference in intracellular sodium influx [103], dysregulation of NMDA receptor [104], disruption of microRNAs [105], and mitochondrial disability [106]. In the face of low-level viremia observed in the presence of cART, presence of specific HIV viral proteins, including envelope glycoprotein (gp)120, gp41, gp 160, Nef, Rev, TAT, and Vpr, as well as inflammatory factors secreted from HIV-1-infected CNS macrophages and microglia, has been implicated in mediating the synaptodendritic damage in neurons and thereby contributing to HIV-1-associated neuropathogenesis [98, 100, 107, 108]. Furthermore, the neuronal loss observed in the HIV-1-infected patients is likely possible due to HIV-1-mediated dysregulation of several neuronal cell surface receptors, including N-methyl-D-aspartate receptors (NMDAR), the low-density lipoprotein receptor-related protein (LRP), chemokine receptors CCR5 and CXCR4, and the dopamine transporter, leading to induction of necrosis and apoptosis [109, 110]. Several reports have demonstrated that HIV-1-associated neurologic and cognitive dysfunction is primarily due to injury to dendrites and synapses that often occur much before neuronal cell death [111].
Although HIV-1 cannot infect neurons, HIV-1-infected macrophages and microglia that are target cells of the virus can release a plethora of proinflammatory and neurotoxic mediators, resulting in neuronal injury [27, 99]. A recent study by Dos Reis et al. [112•] using a three-dimensional human brain organoids (hBORG) model comprising of microglia, astrocytes, and neurons infected with HIV-1 to mimic the human brain reported identical changes, including synaptic damage and loss of neurons, microgliosis, and astrocytosis, as observed in the postmortem brain tissues of HIV-infected individuals. Furthermore, this study also reported loss of synaptic integrity in HIV-infected hBORGs with microglia (Mg) as evidenced by a significant 10.6-fold decrease in the mean co-localization intensity of PSD95/SYN stained puncta compared mock hBORGs. Another study by Guha et al. [113] demonstrated that the culture supernatants obtained from HIV-infected monocytederived macrophages (MDM) showed a significant increase in proinflammatory and neurotoxic cytokines, including IL-1β and IL-8. Exposure of neural progenitor cells to the supernatants obtained from HIV-infected MDMs resulted in neuronal apoptosis [113]. Intriguingly, deletion of Vpr using HIV-1ΔVpr virus restricted neuronal apoptosis by decreasing the release of proinflammatory cytokines from the infected target cells either directly or indirectly by suppressing viral replication, thereby suggested that targeting HIV-1 Vpr could be one possible approach to dampen the risk of developing HAND. Not only inflammatory cytokines but several small molecules, including platelet-activating factor (PAF) and quinolinic acid, have also been reported to be released by HIV-1-infected macrophages and have been implicated as key mediators of neurotoxicity via NMDAR dysregulation [114].
The mechanisms underlying HIV-associated synaptodendritic abnormalities are likely due to the bystander effect impacted by the neighboring infected cells since HIV-1 does not directly infect neurons. It is believed that HIV-infected blood monocytes and macrophages cross the blood-brain barrier and subsequently infect glial cells in the brain. Infected microglia or astrocytes, in turn, release viral proteins, cytokines, chemokines, and other neurotoxic substances that likely mediate alterations in synaptic activity with enhanced calcium influx, disturbed energy metabolism, and increased production of reactive oxygen species that eventually disturb normal neuronal homeostasis and functioning [115]. The normal synaptodendritic network is highly complex with several branches, which in the injured network appears trimmed with retracted dendritic spines with aberrant sprouting, in turn disturbing the normal synaptodendritic communication [23]. HIV-1 proteins including the envelope glycoproteins gp120, gp41, and gp 160; the non-structural protein Nef; the trans-activating gene regulatory protein Tat; viral replication regulatory protein Rev; and HIV-1 accessory protein vpr are reported to be neurotoxic and have been documented to induce neurodegeneration [116]. Studies on human autopsy brains of HIV-1 infected individuals have documented the existence of mRNA for these proteins in the brains of infected individuals [117]. Notably, a transmembrane protein gp41 has been shown to be expressed on the cell surface of infected cells, and similarly, Nef was also reported to be present intracellularly in infected brain cells [118]. A study by Lannuzel et al. [119] using the human embryonic neurons has reported that HIV-1 gp120 can directly bind with NMDAR resulting in a lethal influx of calcium ions. In another study, it has been reported that HIV-1 gp120 can directly bind with both CCR5 and CXCR4, thereby resulting in the induction of cell death in neuroblastoma cells [120].
Additionally, it has been shown that in hippocampal neurons, HIV gp120 elicits neuroinflammation resulting in synaptic alterations, including increased numbers of inhibitory synapses with a concomitant loss of excitatory synapses, coordinately involving both p38 MAPK and Src-NMDAR pathways and the Src-NMDAR pathway [121]. Several findings report that gp-120 exposure also induces dendritic and synaptic loss in primary neurons [107]. It has also been shown that gp-120 induced synaptic loss by altering endocannabinoid signaling and by decreased prostaglandin (PG) [122]. Furthermore, it was reported that inhibition of monoacylglycerol lipase using the selective inhibitor JZL184 prevented gp-120-mediated synapse loss via activating cannabinoid type 2 receptor and by decreasing PG signaling, ultimately resulting in decreased production of the inflammatory cytokine IL-1β [122] (Fig. 1).
Similarly, other viral protein HIV Tat has also been reported to bind with several surface receptors, including NMDA receptors and phospholipase-C, resulting in toxic influx of Ca2+ ions, dysregulation of calcium homeostasis, and ultimately neuronal cell death [123]. Tat has been shown to bind LRP followed by internalization of Tat into the cells, which, in turn, prevents the uptake of natural LRP ligands such as apolipoprotein E in neurons [124]. Additionally, the binding of Tat to LRP has also been reported to induce apoptosis-promoting complex formation and has been shown to involve neuronal death [125]. Another study showed HIV Tat-mediated neurotoxicity via apoptosis and neurite and MAP-2 loss in neuroblastoma SH-SY5Y cells. Furthermore, pretreatment with platelet-derived growth factor-CC (PDGF-CC) abrogated these effects of Tat in SH-SY5Y cells [126]. It has also been reported that Tat induces synaptodendritic injury and swelling along dendrites in mouse primary striatal neurons involving focal disruptions in Na+ influx, mitochondrial instability, and excessive Ca2+ influx [103]. Additionally, this study also reported that morphine co-exposure aggravated these effects of Tat in primary neurons [103]. Tat has also been shown to induce neurite shortening and changes in synapse formation involving the miR-132/CREB signaling. Furthermore, this study also reported that Tat-induced miR-132 led to the repression of MecP2 and BDNF, which, in turn, contributed to changes in neurite outgrowth [127]. Another study reported that in mouse primary neurons isolated from the prefrontal cortex, Tat exposure resulted in dendritic spine deficits while promoting excitotoxicity. Additionally, it has been documented that these effects were abrogated by cannabinoid receptor 1 (CB1) agonists, thereby suggested that CB1 signaling could function as a feedback mechanism against Tat-induced excitotoxicity [128].
Ample research evidence has shown that HIV viral protein Nef could also lead to neuronal dysfunction, synaptodendritic injury, and neuronal loss [129]. A multielectrode array study in rat hippocampal neurons by Saribas et al. [130] has reported that Nef viral protein from astrocyte-derived extracellular vesicles resulted in progressive reduction of neuronal activity with almost no network activity post 48–96 h Nef exposure [130]. In another study, it was shown that exposure of differentiated SH-SY5Y human neuroblastoma cells to Nef-transfected HEK293 EVs resulted in reduced cellular ABC transporter A1 (ABCA1), which, in turn, leads to increased abundance and modification of lipid rafts. Furthermore, it has been shown that modification of lipid drafts potentiates accumulation as well as misfolding of amyloidogenic proteins, APP, and Tau, thereby aggravating the inflammatory signaling and thereby contributing to apoptosis and neurodegeneration [131]. Furthermore, several in vitro studies have also reported that HIV-1 accessory protein viral protein R (Vpr) induces neuronal apoptosis in cultured rat hippocampal neurons [132], rat cortical and striatal neurons [133], as well as human neuronal cell lines [134], rat fetal neurons, and human cholinergic neuroblastoma cells [135]. Taken together, these studies showed that development of therapeutics targeting low-level HIV-1 replication, HIV viral proteins, and/or neuroinflammation could restore neuronal functioning including reversing synaptodendritic damage in HAND.
Role of Glial Cells in HAND
Glial cells play a major role in neuronal synaptodendritic injury leading to HAND. Microglia and macrophages in the CNS are productively infected by HIV-1. Following virus infection, proinflammatory cytokines are released from the infected/reactivated microglial cells/macrophages [130, 136], which, in turn, have been known to contribute to synaptic degeneration via multiple pathways, including NMDAR hyperactivation, in the neurons. Additionally, the proinflammatory cytokines have also been shown to induce synaptic abnormalities by aberrantly activating cytokine receptors in the neurons [137, 138], leading to activation of several cytotoxic signaling pathways, including but not limited to the nuclear factor-kappa B (NF-κB), ERK, p38 MAPK, the c-Jun N-terminal kinase, and caspase pathways [139, 140]. In addition to cytokines, chemokines also play a key role in neuronal damage. For example, CXCL12/SDF-1α is elevated in the brain and CSF of HIV patients with HAD [141, 142]. Cleavage of CXCL12, which results in switching its preferred receptor from CXCR4 to CXCR3, leads to enhanced neurotoxic effects [143]. Interestingly, the chemokine CXCL10, which is upregulated in both human and macaque brains following HIV/SIV infection, has also been shown to promote neuronal damage by stimulating Ca2+ flux [144, 145]. Interestingly, chemokines also cause neuronal damage by modulating the infiltration of peripheral monocytes. For example, monocyte chemoattractant protein-1 (MCP1/ CCL2), which is increased in the CSF of HIV-1 patients with cognitive impairment [146], mediates neuronal injury by promoting migration and infiltration of monocytes/macrophages [147, 148]. The neuronal released chemokine fractalkine (FKN; a.k.a. CX3CL1), which is also observed to be upregulated in HIV patients [149, 150], has been implicated in HIV-associated dementia [151, 152], and may also modulate monocyte migration and neuron damage [150, 153]. Recent studies have shown that HIV viral proteins can also cause microglial activation and neuroinflammation via activation of the NLRP3 inflammasome pathway [63, 154•], defective mitophagy [155], senescence [156•], overexpression of CX3CR1 [157], activation of the p38 MAPK pathway [158], reduced levels of dopamine and transporters [159], and nitrosative stress [160]. Studies have shown that HIV-1 Nef-containing EVs can be taken up by neurons, resulting in oxidative stress, degeneration of axons, and impairment of functional neuronal action potential [130]. In addition to viral proteins, cART exposure has also been implicated in microglial activation via the dysregulated autophagy and lysosomal dysfunction [79]. Since astrocytes lack CD4 receptor [161], they do not support the classical entry of HIV via CD4/gp120 fusion; however, several other reports have shown that the HIV entry such as cell-to-cell contact with infected CD4+ T cells [162, 163] and through receptor-mediated endocytosis could lead to astrocytic infection [164, 165]. Reports are suggesting that a small population of astrocytes can be infected by HIV-1 [166, 167], leading to the premise that these infected, activated astrocytes can, in turn, indirectly mediate synaptic injury [32, 168]. It has also been found that integrated HIV-1 was present in nearly 25% of astrocytes isolated directly from the autopsied brain tissues of HIV-1-infected subjects [167]. HIV-infected astrocytes produce and secrete viral proteins such as gp120, Tat, Vpr, Rev, and Nef, even though they support limited viral replication [169, 170]. It is well known that both HIV Tat and gp120 activate astrocytes, thereby producing a plethora of proinflammatory cytokines such as TNFα, IL6, and ILβ [171–173] and neurotoxic nitric oxide [174], which can lead to neuronal injury. HIV Tat-transfected human astrocytic cell line was also shown to induce neurotoxicity via the microRNA (miR)-132 [127]. HIV-infected human astrocytes can also secrete increased levels of glutamate and CCL2, eventually contributing to the dysregulation of the BBB integrity, increased monocyte influx, and impaired CNS immune responses [175]. Accumulating studies also suggest that exosomes released from astrocytes stimulated with both HIV Tat and morphine can shuttle miR-29b, which, in turn, can be taken up by neurons, resulting in neuronal death [176]. Another study has shown that we show that EVs isolated from the brains of SIV-infected monkeys activate TLR7 and were neurotoxic compared to EVs from control animals. Interestingly, these EVs derived from in vitro culture of macrophages contain miR-21 and are neurotoxic compared to EVs derived from miR-21−/− knockout animals [177]. Taken together, HIV/HIV protein/cART/drugs of abuse-mediated activation of the glial cells can induce neuronal dysfunction via both the release of inflammatory factors and via EVs carrying the toxic cargoes.
Drugs of Abuse and HIV in Neuronal Damage
Substantial clinical and preclinical reports have been instrumental in defining the factors that contribute to neurocognitive impairment in HIV-1-infected patients, and drug abuse is well-identified as key comorbidity that adversely influences the severity of HAND [178•]. Reports have shown that the extent of synaptodendritic injury could be heightened by comorbid abuse of opioid drugs. Since opioid receptors are present in various brain regions, opioids could potentiate region-specific synaptodendritic damage associated with HAND [179]. A preclinical study has demonstrated that morphine exacerbates the excitotoxic effects of Tat, including disrupted calcium and iron homeostasis and mitochondrial instability, thereby promoting synaptodendritic injury in primary neuronal cultures or mu-opioid-receptor (MOR) knockout mouse [103]. Another group found that chronic administration of morphine significantly reduced dendritic spine density in the somatosensory cortex, hippocampal CA1, and dentate gyrus of adult rats [180]. Morphine administration for over 3 to 6 days resulted in collapse of preexisting dendritic spines, whereas treatment with naloxone, an opioid antagonist, was found to increase the density of spines.
Interestingly, in transgenic mice lacking the μ-opioid receptor, morphine failed to exert any effect on dendritic spines, thereby implicating that abnormal alterations of the dendritic spine could manifest as a sequela of drug addiction [181]. Follow-up studies reported that morphine administration caused significant reductions in dendritic spine density [182]. A recent review has discussed this in greater detail regarding the region-specific dendritic spine effects and synaptodendritic damage in animal models and the cerebral cortex of HAND patients. The mechanisms by which μ-opioid agonists mediate region-specific synaptodendritic damage involving the opioid signaling in specific neuronal populations with particular emphasis on the CXCR4 signaling cascade are intriguing. Apart from CXCR4 signaling, the crucial involvement of calcium signaling and ERK and Akt signaling has also been targeted for reversing dendritic spine deficits. It was shown that the binding of opioids to opioid receptors in the neurons increased intracellular iron levels that subsequently increased the production of iron storage proteins—ferritin heavy chain (FHC) and ferritin light chain (FLC). FHC functions in association with CXCR4 and blocks its downstream signaling. Activation of CXCR4, in turn, increases dendritic spine density in the cortical neurons. Novel therapeutics aimed at restoring dendritic spine morphology could thus help in aiding functional recovery in patients with HAND [50]. Comorbid use of stimulant drugs such as cocaine or methamphetamine could also contribute to synaptodendritic abnormalities and altered neuronal excitability. Repeated exposure with amphetamine or cocaine was shown to result in increased dendritic branching with altered synaptic connectivity in the prefrontal cortex of rats [183]. A recent study showed that methamphetamine potentiated HIV-1 Tat-induced memory deficits by reducing the expression of neuroplasticity markers and pre- and postsynaptic proteins, thereby promoting neurocognitive impairments [72].
Conclusion and Future Perspective
Neuropathogenesis associated with HIV infection remains an essential topic in the field, requiring continued research to understand the key determinants and mediators. In the post-cART era, brain changes are slow and subtle; thus, HAND remains challenging to study. However, considering the cognitive health of HIV patients, which is intricately related to the quality of life, there is an ongoing need to halt the further cognitive decline and improve these patients’ functioning. Studies confirm that although cART has decreased the prevalence of HAD, there is increased prevalence and persistence of HAND, which could likely be due to combined multifactorial components including but not limited to aging, poor penetration of the antiretroviral drugs across the blood-brain barrier, and residual chronic inflammation, all of which can result in persistent CNS low-level viral replication with accumulation of cytotoxic viral proteins. Thus, research in the area of anticipated changes in aging, neurotoxic cART, the continued accumulation of viral proteins and chronic residual inflammation, and the interactions among all these factors needs further attention. Additionally, the association of Alzheimer’s-like pathology has been an area of upcoming interest in the field of HAND. Interestingly, it has been shown that there is an increased genetic predisposition for Alzheimer’s-like pathology in HIV patients with APOE ε4 allele [39]. Studies have shown that the presence of this etiologic risk factor (APOE4) for AD in HIV-1 patients resulted in a more exacerbated dementia phenotype compared to that seen in patients negative for APOE4 [40]. Interestingly, the same study showed that in the presence of APOE4, there was enhanced entry/fusion of HIV (R5 and X4) in T cells [40]. While these studies depict the interconnection of AD pathology with HAND, the cause-effect relationship still needs to be determined. Additionally, the complexity of drug abuse comorbidity in HIV-1 infection has also been shown to converge to induce synaptodendritic abnormalities and cognitive impairment in HAND patients. Further comprehensive studies are warranted to identify specific neuron and glial cell-derived biomarkers in the periphery, along with MRI studies to track HIV-induced neuropathogenesis. Taken together, advanced research attempts are needed to identify novel multifaceted therapeutics that offer protection against different aspects of HIV-induced neuropathogenesis for functional recovery in HAND patients.
Funding
This research was funded by NIH NIDA, grant numbers MH112848, DA050545, DA044586, DA040397, DA047156, DA043138, DA052266, and AG069541. The support by CHAIN (Chronic HIV infection and Aging in NeuroAIDS) Center grant (MH062261) and NCSAR (Nebraska Center for Substance Abuse Research) is also highly acknowledged.
Footnotes
Conflict of Interest The authors declare no competing interests.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Spudich S, Gonzalez-Scarano F. HIV-1-related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harbor perspectives in medicine. 2012;2(6):a007120. 10.1101/cshperspect.a007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh AK, Sarkar A, Mitsuya H. HIV-associated neurocognitive disorder (HAND) and the prospect of brain-penetrating protease inhibitors for antiretroviral treatment. Medical research archives. 2017;5(4). [PMC free article] [PubMed] [Google Scholar]
- 3.Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13(11):976–86. 10.1016/S1473-3099(13)70269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherner M, Masliah E, Ellis RJ, Marcotte TD, Moore DJ, Grant I, et al. Neurocognitive dysfunction predicts postmortem findings of HIV encephalitis. Neurology. 2002;59(10):1563–7. 10.1212/01.wnl.0000034175.11956.79. [DOI] [PubMed] [Google Scholar]
- 5.Gelman BB. Neuropathology of HAND with suppressive antiretroviral therapy: encephalitis and neurodegeneration reconsidered. Current HIV/AIDS reports. 2015;12(2):272–9. 10.1007/s11904-015-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langford D, Grigorian A, Hurford R, Adame A, Ellis RJ, Hansen L, et al. Altered P-glycoprotein expression in AIDS patients with HIV encephalitis. J Neuropathol Exp Neurol. 2004;63(10):1038–47. 10.1093/jnen/63.10.1038. [DOI] [PubMed] [Google Scholar]
- 7.Smith RL, de Boer R, Brul S, Budovskaya Y, van Spek H. Premature and accelerated aging: HIV or HAART? Front Genet. 2012;3:328. 10.3389/fgene.2012.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haddow LJ, Laverick R, Daskalopoulou M, McDonnell J, Lampe FC, Gilson R, et al. Cognitive impairment in people with HIVitERSG. Multicenter European Prevalence Study of Neurocognitive Impairment and Associated Factors in HIV Positive Patients. AIDS Behav. 2018;22(5):1573–83. 10.1007/s10461-017-1683-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nightingale S, Winston A, Letendre S, Michael BD, McArthur JC, Khoo S, et al. Controversies in HIV-associated neurocognitive disorders. The Lancet Neurology. 2014;13(11)):1139–51. 10.1016/S1474-4422(14)70137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brew BJ, Chan P. Update on HIV dementia and HIV-associated neurocognitive disorders. Current neurology and neuroscience reports. 2014;14(8):468. 10.1007/s11910-014-0468-2. [DOI] [PubMed] [Google Scholar]
- 11.McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. The Lancet Neurology. 2005;4(9):543–55. 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- 12.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snider WD, Simpson DM, Nielsen S, Gold JW, Metroka CE, Posner JB. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol. 1983;14(4):403–18. 10.1002/ana.410140404. [DOI] [PubMed] [Google Scholar]
- 14.Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features Annals of neurology. 1986;19(6):517–24. 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 15.Grant I, Franklin DR Jr, Deutsch R, Woods SP, Vaida F, Ellis RJ, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;82(23):2055–62. 10.1212/WNL.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–96. 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heaton RK, Franklin DR Jr, Deutsch R, Letendre S, Ellis RJ, Casaletto K, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60(3):473–80. 10.1093/cid/ciu862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heaton RK, Franklin DR, Ellis RJ, JA MC, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods SP, Weber E, Weisz BM, Twamley EW, Grant I, Group HIVNRP. Prospective memory deficits are associated with unemployment in persons living with HIV infection. Rehabilitation psychology. 2011;56(1):77–84. 10.1037/a0022753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoare J, Westgarth-Taylor J, Fouche JP, Spottiswoode B, Paul R, Thomas K, et al. A diffusion tensor imaging and neuropsychological study of prospective memory impairment in South African HIV positive individuals. Metab Brain Dis. 2012;27(3):289–97. 10.1007/s11011-012-93110. [DOI] [PubMed] [Google Scholar]
- 21.Everall I, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, et al. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol. 2009;15(5-6):360–70. 10.3109/13550280903131915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelman BB, Lisinicchia JG, Morgello S, Masliah E, Commins D, Achim CL, et al. Neurovirological correlation with HIV-associated neurocognitive disorders and encephalitis in a HAART-era cohort. J Acquir Immune Defic Syndr. 2013;62(5):487–95. 10.1097/QAI.0b013e31827f1bdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8(1):33–44. 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- 24.Masliah E, Achim CL, Ge N, DeTeresa R, Terry RD, Wiley CA. Spectrum of human immunodeficiency virus-associated neocortical damage. Ann Neurol. 1992;32(3):321–9. 10.1002/ana.410320304. [DOI] [PubMed] [Google Scholar]
- 25.Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42(6):963–72. 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- 26.Sa MJ, Madeira MD, Ruela C, Volk B, Mota-Miranda A, Paula-Barbosa MM. Dendritic changes in the hippocampal formation of AIDS patients: a quantitative Golgi study. Acta Neuropathol. 2004;107(2):97–110. 10.1007/s00401-003-0781-3. [DOI] [PubMed] [Google Scholar]
- 27.Everall IP, Luthert PJ, Lantos PL. Neuronal number and volume alterations in the neocortex of HIV infected individuals. J Neurol Neurosurg Psychiatry. 1993;56(5):481–6. 10.1136/jnnp.56.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367(6459):188–93. 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- 29.Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis. 2007;196(12):1779–83. 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- 30.Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012;60(3):234–s. 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Budka H, Wiley CA, Kleihues P, Artigas J, Asbury AK, Cho ES, et al. HIV-associated disease of the nervous system: review of nomenclature and proposal for neuropathology-based terminology. Brain Pathol. 1991;1(3):143–52. 10.1111/j.1750-3639.1991.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 32.Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, et al. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res. 2003;1(4):463–73. 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- 33.Power C, Kong PA, Crawford TO, Wesselingh S, Glass JD, McArthur JC, et al. Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood-brain barrier. Ann Neurol. 1993;34(3):339–50. 10.1002/ana.410340307. [DOI] [PubMed] [Google Scholar]
- 34.Langford TD, Letendre SL, Marcotte TD, Ellis RJ, McCutchan JA, Grant I, et al. Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. Aids. 2002;16(7):1019–29. 10.1097/00002030-200205030-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ances BM, Sisti D, Vaida F, Liang CL, Leontiev O, Perthen JE, et al. Resting cerebral blood flow: a potential biomarker of the effects of HIV in the brain. Neurology. 2009;73(9):702–8. 10.1212/WNL.0b013e3181b59a97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelman BB, Chen T, Lisinicchia JG, Soukup VM, Carmical JR, Starkey JM, et al. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS One. 2012;7(9):e46178. 10.1371/journal.pone.0046178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.•.Sil S, Hu G, Liao K, Niu F, Callen S, Periyasamy P, et al. HIV-1 Tat-mediated astrocytic amyloidosis involves the HIF-1alpha/lncRNA BACE1-AS axis. PLoS Biol. 2020;18(5):e3000660. 10.1371/journal.pbio.3000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Achim CL, Adame A, Dumaop W, Everall IP, Masliah E, Neurobehavioral RC. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2009;4(2):190–9. 10.1007/s114810099152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–18. 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burt TD, Agan BK, Marconi VC, He W, Kulkarni H, Mold JE, et al. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proc Natl Acad Sci USA. 2008;105(25):8718–23. 10.1073/pnas.0803526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gisslen M, Krut J, Andreasson U, Blennow K, Cinque P, Brew BJ, et al. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol. 2009;9:63. 10.1186/1471-2377-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellis RJ, Seubert P, Motter R, Galasko D, Deutsch R, Heaton RK, et al. Cerebrospinal fluid tau protein is not elevated in HIV-associated neurologic disease in humans. HIV Neurobehavioral Research Center Group (HNRC). Neurosci Lett. 1998;254(1):1– 10.1016/s0304-3940(98)00549-7. [DOI] [PubMed] [Google Scholar]
- 43.Buzhdygan T, Lisinicchia J, Patel V, Johnson K, Neugebauer V, Paessler S, et al. Neuropsychological, Neurovirological and Neuroimmune Aspects of Abnormal GABAergic Transmission in HIV Infection. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2016;11(2):279–93. 10.1007/s11481-016-9652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J Neurovirol. 2019;25(5):702–9. 10.1007/s13365-018-0695-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun B, Fernandes N, Pulliam L. Profile of neuronal exosomes in HIV cognitive impairment exposes sex differences. Aids. 2019;33(11):1683–92. 10.1097/QAD.0000000000002272. [DOI] [PubMed] [Google Scholar]
- 46.Borjabad A, Volsky DJ. Common transcriptional signatures in brain tissue from patients with HIV-associated neurocognitive disorders, Alzheimer’s disease, and Multiple Sclerosis. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2012;7(4):914–26. 10.1007/s11481-012-9409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLaurin KA, Li H, Booze RM, Mactutus CF. Disruption of Timing: NeuroHIV Progression in the Post-cART Era. Sci Rep. 2019;9(1):827. 10.1038/s41598-018-36822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schier CJ, Marks WD, Paris JJ, Barbour AJ, McLane VD, Maragos WF, et al. Selective Vulnerability of Striatal D2 versus D1 Dopamine Receptor-Expressing Medium Spiny Neurons in HIV-1 Tat Transgenic Male Mice. J Neurosci. 2017;37(23):5758–69. 10.1523/JNEUROSCI.0622-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hahn YK, Podhaizer EM, Farris SP, Miles MF, Hauser KF, Knapp PE. Effects of chronic HIV-1 Tat exposure in the CNS: heightened vulnerability of males versus females to changes in cell numbers, synaptic integrity, and behavior. Brain Struct Funct. 2015;220(2):605–23. 10.1007/s00429-013-0676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nash B, Festa L, Lin C, Meucci O. Opioid and chemokine regulation of cortical synaptodendritic damage in HIV-associated neurocognitive disorders. Brain Res. 2019;1723:146409. 10.1016/j.brainres.2019.146409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.•.Festa LK, Irollo E, Platt BJ, Tian Y, Floresco S, Meucci O. CXCL12-induced rescue of cortical dendritic spines and cognitive flexibility. eLife. 2020;9. 10.7554/eLife.49717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McLaurin KA, Booze RM, Mactutus CF. Evolution of the HIV-1 transgenic rat: utility in assessing the progression of HIV-1-associated neurocognitive disorders. J Neurovirol. 2018;24(2):229–45. 10.1007/s13365-017-0544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moran LM, Booze RM, Webb KM, Mactutus CF. Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp Neurol. 2013;239:139–47. 10.1016/j.expneurol.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vigorito M, LaShomb AL, Chang SL. Spatial learning and memory in HIV-1 transgenic rats. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2007;2(4):319–28. 10.1007/s114810079078-y. [DOI] [PubMed] [Google Scholar]
- 55.Lashomb AL, Vigorito M, Chang SL. Further characterization of the spatial learning deficit in the human immunodeficiency virus-1 transgenic rat. J Neurovirol. 2009;15(1):14–24. 10.1080/13550280802232996. [DOI] [PubMed] [Google Scholar]
- 56.Huynh YW, Thompson BM, Larsen CE, Buch S, Guo ML, Bevins RA, et al. Male HIV-1 transgenic rats show reduced cocaine-maintained lever-pressing compared to F344 wildtype rats despite similar baseline locomotion. J Exp Anal Behav. 2020;113(2):468–84. 10.1002/jeab.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reid W, Sadowska M, Denaro F, Rao S, Foulke J Jr, Hayes N, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci USA. 2001;98(16):9271–6. 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.•.Denaro F, Benedetti F, Worthington MD, Scapagnini G, Krauss CC, Williams S, et al. The HIV-1 Transgenic Rat: Relevance for HIV Noninfectious Comorbidity Research. Microorganisms. 2020;8(11). 10.3390/microorganisms8111643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng J, Vigorito M, Liu X, Zhou D, Wu X, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010;218(1-2):94–101. 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 60.Moran LM, Booze RM, Mactutus CF. Modeling deficits in attention, inhibition, and flexibility in HAND. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2014;9(4):508–21. 10.1007/s11481-014-9539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Royal W 3rd, Zhang L, Guo M, Jones O, Davis H, Bryant JL. Immune activation, viral gene product expression and neurotoxicity in the HIV-1 transgenic rat. J Neuroimmunol. 2012;247(1-2):16–24. 10.1016/j.jneuroim.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rowson SA, Harrell CS, Bekhbat M, Gangavelli A, Wu MJ, Kelly SD, et al. Neuroinflammation and Behavior in HIV-1 Transgenic Rats Exposed to Chronic Adolescent Stress. Frontiers in psychiatry. 2016;7:102. 10.3389/fpsyt.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chivero ET, Guo ML, Periyasamy P, Liao K, Callen SE, Buch S. HIV-1 Tat Primes and Activates Microglial NLRP3 Inflammasome-Mediated Neuroinflammation. J Neurosci. 2017;37(13):3599–609. 10.1523/JNEUROSCI.3045-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Periyasamy P, Thangaraj A, Guo ML, Hu G, Callen S, Buch S. Epigenetic Promoter DNA Methylation of miR-124 Promotes HIV-1 Tat-Mediated Microglial Activation via MECP2-STAT3 Axis. J Neurosci. 2018;38(23):5367–83. 10.1523/JNEUROSCI.3474-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Festa L, Gutoskey CJ, Graziano A, Waterhouse BD, Meucci O. Induction of Interleukin-1beta by Human Immunodeficiency Virus-1 Viral Proteins Leads to Increased Levels of Neuronal Ferritin Heavy Chain, Synaptic Injury, and Deficits in Flexible Attention. J Neurosci. 2015;35(29):10550–61. 10.1523/JNEUROSCI.4403-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Speidell A, Asuni GP, Wakulski R, Mocchetti I. Up-regulation of the p75 neurotrophin receptor is an essential mechanism for HIV-gp120 mediated synaptic loss in the striatum. Brain Behav Immun. 2020;89:371–9. 10.1016/j.bbi.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bachis A, Wenzel E, Boelk A, Becker J, Mocchetti I. The neurotrophin receptor p75 mediates gp120-induced loss of synaptic spines in aging mice. Neurobiol Aging. 2016;46:160–8. 10.1016/j.neurobiolaging.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wayman WN, Chen L, Hu XT, Napier TC. HIV-1 Transgenic Rat Prefrontal Cortex Hyper-Excitability is Enhanced by Cocaine Self-Administration. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41(8):1965–73. 10.1038/npp.2015.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.•.Barbour AJ, Hauser KF, McQuiston AR, Knapp PE. HIV and opiates dysregulate K(+)- Cl(−) cotransporter 2 (KCC2) to cause GABAergic dysfunction in primary human neurons and Tat-transgenic mice. Neurobiol Dis. 2020;141:104878. 10.1016/j.nbd.2020.104878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu C, Fitting S. Inhibition of GABAergic Neurotransmission by HIV-1 Tat and Opioid Treatment in the Striatum Involves mu-Opioid Receptors. Front Neurosci. 2016;10:497. 10.3389/fnins.2016.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohene-Nyako M, Persons AL, Napier TC. Region-specific changes in markers of neuroplasticity revealed in HIV-1 transgenic rats by low-dose methamphetamine. Brain Struct Funct. 2018;223(7):3503–13. 10.1007/s0042901817016. [DOI] [PubMed] [Google Scholar]
- 72.Nookala AR, Schwartz DC, Chaudhari NS, Glazyrin A, Stephens EB, Berman NEJ, et al. Methamphetamine augment HIV-1 Tat mediated memory deficits by altering the expression of synaptic proteins and neurotrophic factors. Brain Behav Immun. 2018;71: 37–51. 10.1016/j.bbi.2018.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chilunda V, Calderon TM, Martinez-Aguado P, Berman JW. The impact of substance abuse on HIV-mediated neuropathogenesis in the current ART era. Brain Res. 2019;1724:146426. 10.1016/j.brainres.2019.146426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cai Y, Yang L, Callen S, Buch S. Multiple Faceted Roles of Cocaine in Potentiation of HAND. Curr HIV Res. 2016;14(5):412–6. 10.2174/1570162x14666160324125158. [DOI] [PubMed] [Google Scholar]
- 75.Sil S, Periyasamy P, Guo ML, Callen S, Buch S. Morphine-Mediated Brain Region-Specific Astrocytosis Involves the ER Stress-Autophagy Axis. Mol Neurobiol. 2018;55(8):6713–33. 10.1007/s12035018-878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sil S, Niu F, Tom E, Liao K, Periyasamy P, Buch S. Cocaine Mediated Neuroinflammation: Role of Dysregulated Autophagy in Pericytes. Mol Neurobiol. 2019;56(5):3576–90. 10.1007/s12035-018-1325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanchez AB, Varano GP, de Rozieres CM, Maung R, Catalan IC, Dowling CC, et al. Antiretrovirals, Methamphetamine, and HIV-1 Envelope Protein gp120 Compromise Neuronal Energy Homeostasis in Association with Various Degrees of Synaptic and Neuritic Damage. Antimicrob Agents Chemother. 2016;60(1):168–79. 10.1128/AAC.01632-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tripathi A, Thangaraj A, Chivero ET, Periyasamy P, Burkovetskaya ME, Niu F, et al. N-Acetylcysteine Reverses Antiretroviral-Mediated Microglial Activation by Attenuating Autophagy-Lysosomal Dysfunction. Front Neurol. 2020;11:840. 10.3389/fheur.2020.00840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tripathi A, Thangaraj A, Chivero ET, Periyasamy P, Callen S, Burkovetskaya ME, et al. Antiretroviral-Mediated Microglial Activation Involves Dysregulated Autophagy and Lysosomal Dysfunction. Cells. 2019;8(10). 10.3390/cells8101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Akay C, Cooper M, Odeleye A, Jensen BK, White MG, Vassoler F, et al. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neurovirol. 2014;20(1):39–53. 10.1007/s13365-013-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jaeger LB, Nath A. Modeling HIV-associated neurocognitive disorders in mice: new approaches in the changing face of HIV neuropathogenesis. Dis Model Mech. 2012;5(3):313–22. 10.1242/dmm.008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gorantla S, Poluektova L, Gendelman HE. Rodent models for HIV-associated neurocognitive disorders. Trends Neurosci. 2012;35(3):197–208. 10.1016/j.tins.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Persidsky Y, Gendelman HE. Murine models for human immunodeficiency virus type 1-associated dementia: the development of new treatment testing paradigms. J Neurovirol. 2002;8(Suppl 2):49–52. 10.1080/13550280290167993. [DOI] [PubMed] [Google Scholar]
- 84.Persidsky Y, Limoges J, McComb R, Bock P, Baldwin T, Tyor W, et al. Human immunodeficiency virus encephalitis in SCID mice. Am J Pathol. 1996;149(3):1027–53. [PMC free article] [PubMed] [Google Scholar]
- 85.Tyor WR, Power C, Gendelman HE, Markham RB. A model of human immunodeficiency virus encephalitis in scid mice. Proc Natl Acad Sci USA. 1993;90(18):8658–62. 10.1073/pnas.90.18.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aksenov MY, Hasselrot U, Bansal AK, Wu G, Nath A, Anderson C, et al. Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci Lett. 2001;305(1):5–8. 10.1016/s03043940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- 87.Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879(1-2):42–9. 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- 88.Hayman M, Arbuthnott G, Harkiss G, Brace H, Filippi P, Philippon V, et al. Neurotoxicity of peptide analogues of the transactivating protein tat from Maedi-Visna virus and human immunodeficiency virus. Neuroscience. 1993;53(1):1–6. 10.1016/0306-4522(93)90278-n. [DOI] [PubMed] [Google Scholar]
- 89.Jones M, Olafson K, Del Bigio MR, Peeling J, Nath A. Intraventricular injection of human immunodeficiency virus type 1 (HIV-1) tat protein causes inflammation, gliosis, apoptosis, and ventricular enlargement. J Neuropathol Exp Neurol. 1998;57(6):563–70. 10.1097/00005072-199806000-00004. [DOI] [PubMed] [Google Scholar]
- 90.Philippon V, Vellutini C, Gambarelli D, Harkiss G, Arbuthnott G, Metzger D, et al. The basic domain of the lentiviral Tat protein is responsible for damages in mouse brain: involvement of cytokines. Virology. 1994;205(2):519–29. 10.1006/viro.1994.1673. [DOI] [PubMed] [Google Scholar]
- 91.Lee PH, Ohtake T, Zaiou M, Murakami M, Rudisill JA, Lin KH, et al. Expression of an additional cathelicidin antimicrobial peptide protects against bacterial skin infection. Proc Natl Acad Sci U S A. 2005;102(10):3750–5. 10.1073/pnas.0500268102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin T, Chauhan A, Reid R, et al. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia. 2008;56(13):1414–27. 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fitting S, Zou S, Chen W, Vo P, Hauser KF, Knapp PE. Regional heterogeneity and diversity in cytokine and chemokine production by astroglia: differential responses to HIV-1 Tat, gp120, and morphine revealed by multiplex analysis. J Proteome Res. 2010;9(4): 1795–804. 10.1021/pr900926n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hauser KF, Hahn YK, Adjan VV, Zou S, Buch SK, Nath A, et al. HIV-1 Tat and morphine have interactive effects on oligodendrocyte survival and morphology. Glia. 2009;57(2):194–206. 10.1002/glia.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, et al. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry. 2013;73(5):443–53. 10.1016/j.biopsych.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gorantla S, Makarov E, Finke-Dwyer J, Castanedo A, Holguin A, Gebhart CL, et al. Links between progressive HIV-1 infection of humanized mice and viral neuropathogenesis. Am J Pathol. 2010;177(6):2938–49. 10.2353/ajpath.2010.100536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaul M, Lipton SA. Mechanisms of neuroimmunity and neurodegeneration associated with HIV-1 infection and AIDS. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2006;1(2):138–51. 10.1007/s11481-006-9011-9. [DOI] [PubMed] [Google Scholar]
- 98.Ketzler S, Weis S, Haug H, Budka H. Loss of neurons in the frontal cortex in AIDS brains. Acta Neuropathol. 1990;80(1):92–4. 10.1007/BF00294228. [DOI] [PubMed] [Google Scholar]
- 99.Weis S, Haug H, Budka H. Neuronal damage in the cerebral cortex of AIDS brains: a morphometric study. Acta Neuropathol. 1993;85(2):185–9. 10.1007/BF00227766. [DOI] [PubMed] [Google Scholar]
- 100.Rao VR, Ruiz AP, Prasad VR. Viral and cellular factors underlying neuropathogenesis in HIV associated neurocognitive disorders (HAND). AIDS Res Ther. 2014;11:13. 10.1186/1742-6405-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A. Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J Neurosci. 2003;23(23):8417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li W, Li G, Steiner J, Nath A. Role of Tat protein in HIV neuropathogenesis. Neurotox Res. 2009;16(3):205–20. 10.1007/s126400099047-8. [DOI] [PubMed] [Google Scholar]
- 103.Fitting S, Knapp PE, Zou S, Marks WD, Bowers MS, Akbarali HI, et al. Interactive HIV-1 Tat and morphine-induced synaptodendritic injury is triggered through focal disruptions in Na(+) influx, mitochondrial instability, and Ca(2)(+) overload. J Neurosci. 2014;34(38):12850–64. 10.1523/JNEUROSCI.5351-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Magnuson DS, Knudsen BE, Geiger JD, Brownstone RM, Nath A. Human immunodeficiency virus type 1 tat activates non-N-methyl-D-aspartate excitatory amino acid receptors and causes neurotoxicity. Ann Neurol. 1995;37(3):373–80. 10.1002/ana410370314. [DOI] [PubMed] [Google Scholar]
- 105.Chang JR, Mukerjee R, Bagashev A, Del Valle L, Chabrashvili T, Hawkins BJ, et al. HIV-1 Tat protein promotes neuronal dysfunction through disruption of microRNAs. J Biol Chem. 2011;286(47):41125–34. 10.1074/jbc.M111.268466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Norman JP, Perry SW, Kasischke KA, Volsky DJ, Gelbard HA. HIV-1 trans activator of transcription protein elicits mitochondrial hyperpolarization and respiratory deficit, with dysregulation of complex IV and nicotinamide adenine dinucleotide homeostasis in cortical neurons. J Immunol. 2007;178(2):869–76. 10.4049/jimmunol.178.2.869. [DOI] [PubMed] [Google Scholar]
- 107.Iskander S, Walsh KA, Hammond RR. Human CNS cultures exposed to HIV-1 gp120 reproduce dendritic injuries of HIV-1-associated dementia. J Neuroinflammation. 2004;1(1):7. 10.1186/17422094-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, et al. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 1999;9(2):209–17. 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11(3):327–35. 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 110.Hayashi H, Campenot RB, Vance DE, Vance JE. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J Neurosci. 2007;27(8):1933–41. 10.1523/JNEUROSCI.5471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alam MZ, Alam Q, Kamal MA, Jiman-Fatani AA, Azhar EI, Khan MA, et al. Infectious Agents and Neurodegenerative Diseases: Exploring the Links. Curr Top Med Chem. 2017;17(12):1390–9. 10.2174/1568026617666170103164040. [DOI] [PubMed] [Google Scholar]
- 112.•.Dos Reis RS, Sant S, Keeney H, Wagner MCE, Ayyavoo V. Modeling HIV-1 neuropathogenesis using three-dimensional human brain organoids (hBORGs) with HIV-1 infected microglia. Sci Rep. 2020;10(1):15209. 10.1038/s41598-020-72214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guha D, Nagilla P, Redinger C, Srinivasan A, Schatten GP, Ayyavoo V. Neuronal apoptosis by HIV-1 Vpr: contribution of proinflammatory molecular networks from infected target cells. J Neuroinflammation. 2012;9:138. 10.1186/1742-2094-9-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kerr SJ, Armati PJ, Guillemin GJ, Brew BJ. Chronic exposure of human neurons to quinolinic acid results in neuronal changes consistent with AIDS dementia complex. Aids. 1998;12(4):355–63. 10.1097/00002030-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 115.Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4(3):307–18. 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- 116.Nath A, Geiger J. Neurobiological aspects of human immunodeficiency virus infection: neurotoxic mechanisms. Prog Neurobiol. 1998;54(1):19–33. 10.1016/s0301-0082(97)00053-1. [DOI] [PubMed] [Google Scholar]
- 117.Wesselingh SL, Power C, Glass JD, Tyor WR, McArthur JC, Farber JM, et al. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol. 1993;33(6):576–82. 10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- 118.Adamson DC, Wildemann B, Sasaki M, Glass JD, McArthur JC, Christov VI, et al. Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science. 1996;274(5294): 1917–21. 10.1126/science.274.5294.1917. [DOI] [PubMed] [Google Scholar]
- 119.Lannuzel A, Lledo PM, Lamghitnia HO, Vincent JD, Tardieu M. HIV-1 envelope proteins gp120 and gp160 potentiate NMDA-induced [Ca2+]i increase, alter [Ca2+]i homeostasis and induce neurotoxicity in human embryonic neurons. Eur J Neurosci. 1995;7(11):2285–93. 10.1111/j.1460-9568.1995.tb00649.x. [DOI] [PubMed] [Google Scholar]
- 120.Catani MV, Corasaniti MT, Navarra M, Nistico G, Finazzi-Agro A, Melino G. gp120 induces cell death in human neuroblastoma cells through the CXCR4 and CCR5 chemokine receptors. J Neurochem. 2000;74(6):2373–9. 10.1046/j.1471-4159.2000.0742373.x. [DOI] [PubMed] [Google Scholar]
- 121.Zhang X, Green MV, Thayer SA. HIV gp120-induced neuroinflammation potentiates NMDA receptors to overcome basal suppression of inhibitory synapses by p38 MAPK. J Neurochem. 2019;148(4):499–515. 10.1111/jnc.14640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang X, Thayer SA. Monoacylglycerol lipase inhibitor JZL184 prevents HIV-1 gp120-induced synapse loss by altering endocannabinoid signaling. Neuropharmacology. 2018;128:269–81. 10.1016/j.neuropharm.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, et al. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol. 1996;70(3):1475–80. 10.1128/JVI.70.3.1475-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, et al. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6(12):1380–7. 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- 125.Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MV, et al. HIV-tat induces formation of an LRP-PSD-95-NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci USA. 2007;104(9):3438–43. 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peng F, Yao H, Akturk HK, Buch S. Platelet-derived growth factor CC-mediated neuroprotection against HIV Tat involves TRPC-mediated inactivation of GSK 3beta. PLoS One. 2012;7(10):e47572. 10.1371/journal.pone.0047572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rahimian P, He JJ. HIV-1 Tat-shortened neurite outgrowth through regulation of microRNA-132 and its target gene expression. J Neuroinflammation. 2016;13(1):247. 10.1186/s12974-01607162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu C, Hermes DJ, Nwanguma B, Jacobs IR, Mackie K, Mukhopadhyay S, et al. Endocannabinoids exert CB1 receptor-mediated neuroprotective effects in models of neuronal damage induced by HIV-1 Tat protein. Mol Cell Neurosci. 2017;83:92–102. 10.1016/j.mcn.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van Marle G, Henry S, Todoruk T, Sullivan A, Silva C, Rourke SB, et al. Human immunodeficiency virus type 1 Nef protein mediates neural cell death: a neurotoxic role for IP-10. Virology. 2004;329(2):302–18. 10.1016/j.virol.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 130.Sami Saribas A, Cicalese S, Ahooyi TM, Khalili K, Amini S, Sariyer IK. HIV-1 Nef is released in extracellular vesicles derived from astrocytes: evidence for Nef-mediated neurotoxicity. Cell Death Dis. 2017;8(1):e2542. 10.1038/cddis.2016.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ditiatkovski M, Mukhamedova N, Dragoljevic D, Hoang A, Low H, Pushkarsky T, et al. Modification of lipid rafts by extracellular vesicles carrying HIV-1 protein Nef induces redistribution of amyloid precursor protein and Tau, causing neuronal dysfunction. J Biol Chem. 2020;295(38):13377–92. 10.1074/jbc.RA120.014642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Piller SC, Jans P, Gage PW, Jans DA. Extracellular HIV-1 virus protein R causes a large inward current and cell death in cultured hippocampal neurons: implications for AIDS pathology. Proc Natl Acad Sci U S A. 1998;95(8):4595–600. 10.1073/pnas.95.8.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sabbah EN, Roques BP. Critical implication of the (70-96) domain of human immunodeficiency virus type 1 Vpr protein in apoptosis of primary rat cortical and striatal neurons. J Neurovirol. 2005;11(6):489–502. 10.1080/13550280500384941. [DOI] [PubMed] [Google Scholar]
- 134.Patel CA, Mukhtar M, Pomerantz RJ. Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells. J Virol. 2000;74(20):9717–26. 10.1128/jvi.74.20.9717-9726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jones GJ, Barsby NL, Cohen EA, Holden J, Harris K, Dickie P, et al. HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J Neurosci. 2007;27(14):3703–11. 10.1523/JNEUROSCI.5522-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brabers NA, Nottet HS. Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. Eur J Clin Investig. 2006;36(7):447–58. 10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 137.Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–92. 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 138.Kovalevich J, Langford D. Neuronal toxicity in HIV CNS disease. Futur Virol. 2012;7(7):687–98. 10.2217/fvl.12.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–94. 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 140.Olmos G, Llado J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediat Inflamm. 2014;2014:861231. 10.1155/2014/861231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Peng H, Erdmann N, Whitney N, Dou H, Gorantla S, Gendelman HE, et al. HIV-1-infected and/or immune activated macrophages regulate astrocyte SDF-1 production through IL-1beta. Glia. 2006;54(6):619–29. 10.1002/glia.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rostasy K, Egles C, Chauhan A, Kneissl M, Bahrani P, Yiannoutsos C, et al. SDF-1alpha is expressed in astrocytes and neurons in the AIDS dementia complex: an in vivo and in vitro study. J Neuropathol Exp Neurol. 2003;62(6):617–26. 10.1093/jnen/62.6.617. [DOI] [PubMed] [Google Scholar]
- 143.Vergote D, Butler GS, Ooms M, Cox JH, Silva C, Hollenberg MD, et al. Proteolytic processing of SDF-1alpha reveals a change in receptor specificity mediating HIV-associated neurodegeneration. Proc Natl Acad Sci USA. 2006;103(50):19182–7. 10.1073/pnas.0604678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sui Y, Stehno-Bittel L, Li S, Loganathan R, Dhillon NK, Pinson D, et al. CXCL10-induced cell death in neurons: role of calcium dysregulation. Eur J Neurosci. 2006;23(4):957–64. 10.1111/j.14609568.2006.04631.x. [DOI] [PubMed] [Google Scholar]
- 145.Sui Y, Potula R, Dhillon N, Pinson D, Li S, Nath A, et al. Neuronal apoptosis is mediated by CXCL10 overexpression in simian human immunodeficiency virus encephalitis. Am J Pathol. 2004;164(5):1557–66. 10.1016/S0002-9440(10)63714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol. 1998;44(5):831–5. 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- 147.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2009;29(6):313–26. 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG. Monocyte chemoattractant protein-1 correlates with subcortical brain injury in HIV infection. Neurology. 2006;66(8):1255–7. 10.1212/01.wnl.0000208433.34723.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Letendre SL, Zheng JC, Kaul M, Yiannoutsos CT, Ellis RJ, Taylor MJ, et al. Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. J Neurovirol. 2011;17(1):63–9. 10.1007/s13365-010-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tong N, Perry SW, Zhang Q, James HJ, Guo H, Brooks A, et al. Neuronal fractalkine expression in HIV-1 encephalitis: roles for macrophage recruitment and neuroprotection in the central nervous system. J Immunol. 2000;164(3):1333–9. 10.4049/jimmunol.164.3.1333. [DOI] [PubMed] [Google Scholar]
- 151.Pereira CF, Middel J, Jansen G, Verhoef J, Nottet HS. Enhanced expression of fractalkine in HIV-1 associated dementia. J Neuroimmunol. 2001;115(1-2):168–75. 10.1016/s0165-5728(01)00262-4. [DOI] [PubMed] [Google Scholar]
- 152.Cotter R, Williams C, Ryan L, Erichsen D, Lopez A, Peng H, et al. Fractalkine (CX3CL1) and brain inflammation: Implications for HIV-1-associated dementia. J Neurovirol. 2002;8(6):585–98. 10.1080/13550280290100950. [DOI] [PubMed] [Google Scholar]
- 153.Limatola C, Lauro C, Catalano M, Ciotti MT, Bertollini C, Di Angelantonio S, et al. Chemokine CX3CL1 protects rat hippocampal neurons against glutamate-mediated excitotoxicity. J Neuroimmunol. 2005;166(1-2):19–28. 10.1016/j.jneuroim.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 154.•.He X, Yang W, Zeng Z, Wei Y, Gao J, Zhang B, et al. NLRP3-dependent pyroptosis is required for HIV-1 gp120-induced neuropathology. Cell Mol Immunol. 2020;17(3):283–99. 10.1038/s41423-019-0260-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Thangaraj A, Periyasamy P, Liao K, Bendi VS, Callen S, Pendyala G, et al. HIV-1 TAT-mediated microglial activation: role of mitochondrial dysfunction and defective mitophagy. Autophagy. 2018;14(9):1596–619. 10.1080/15548627.2018.1476810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.•.Thangaraj A, Chivero ET, Tripathi A, Singh S, Niu F, Guo ML, et al. HIV TAT-mediated microglial senescence: Role of SIRT3-dependent mitochondrial oxidative stress. Redox Biol. 2021;40:101843. 10.1016/j.redox.2020.101843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ru W, Liu X, Bae C, Shi Y, Walikonis R, Mo Chung J, et al. Microglia Mediate HIV-1 gp120-Induced Synaptic Degeneration in Spinal Pain Neural Circuits. J Neurosci. 2019;39(42):8408–21. 10.1523/JNEUROSCI.2851-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Medders KE, Sejbuk NE, Maung R, Desai MK, Kaul M. Activation of p38 MAPK is required in monocytic and neuronal cells for HIV glycoprotein 120-induced neurotoxicity. J Immunol. 2010;185(8):4883–95. 10.4049/jimmunol.0902535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Acharjee S, Branton WG, Vivithanaporn P, Maingat F, Paul AM, Dickie P, et al. HIV-1 Nef expression in microglia disrupts dopaminergic and immune functions with associated mania-like behaviors. Brain Behav Immun. 2014;40:74–84. 10.1016/j.bbi.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 160.Mangino G, Famiglietti M, Capone C, Veroni C, Percario ZA, Leone S, et al. HIV-1 Myristoylated Nef Treatment of Murine Microglial Cells Activates Inducible Nitric Oxide Synthase, NO2 Production and Neurotoxic Activity. PLoS One. 2015;10(6):e0130189. 10.1371/journal.pone.0130189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Russell RA, Chojnacki J, Jones DM, Johnson E, Do T, Eggeling C, et al. Astrocytes Resist HIV-1 Fusion but Engulf Infected Macrophage Material. Cell Rep. 2017;18(6):1473–83. 10.1016/j.celrep.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Do T, Murphy G, Earl LA, Del Prete GQ, Grandinetti G, Li GH, et al. Three-dimensional imaging of HIV-1 virological synapses reveals membrane architectures involved in virus transmission. J Virol. 2014;88(18):10327–39. 10.1128/JVI.00788-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Luo X, He JJ. Cell-cell contact viral transfer contributes to HIV infection and persistence in astrocytes. J Neurovirol. 2015;21(1):66–80. 10.1007/s13365-014-0304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chauhan A, Mehla R, Vijayakumar TS, Handy I. Endocytosis-mediated HIV-1 entry and its significance in the elusive behavior of the virus in astrocytes. Virology. 2014;456-457:1–19. 10.1016/j.virol.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hao HN, Chiu FC, Losev L, Weidenheim KM, Rashbaum WK, Lyman WD. HIV infection of human fetal neural cells is mediated by gp120 binding to a cell membrane-associated molecule that is not CD4 nor galactocerebroside. Brain Res. 1997;764(1-2):149–57. 10.1016/s0006-8993(97)00441-1. [DOI] [PubMed] [Google Scholar]
- 166.Conant K, Tornatore C, Atwood W, Meyers K, Traub R, Major EO. In vivo and in vitro infection of the astrocyte by HIV-1. Adv Neuroimmunol. 1994;4(3):287–9. 10.1016/s0960-5428(06)80269-x. [DOI] [PubMed] [Google Scholar]
- 167.Churchill MJ, Gorry PR, Cowley D, Lal L, Sonza S, Purcell DF, et al. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol. 2006;12(2):146–52. 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- 168.Ton H, Xiong H. Astrocyte Dysfunctions and HIV-1 Neurotoxicity. Journal of AIDS & clinical research. 2013;4(11):255. 10.4172/2155-6113.1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ranki A, Nyberg M, Ovod V, Haltia M, Elovaara I, Raininko R, et al. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. Aids. 1995;9(9):1001–8. 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 170.Gorry P, Purcell D, Howard J, McPhee D. Restricted HIV-1 infection of human astrocytes: potential role of nef in the regulation of virus replication. J Neurovirol. 1998;4(4):377–86. 10.3109/13550289809114536. [DOI] [PubMed] [Google Scholar]
- 171.Shah A, Verma AS, Patel KH, Noel R, Rivera-Amill V, Silverstein PS, et al. HIV-1 gp120 induces expression of IL-6 through a nuclear factor-kappa B-dependent mechanism: suppression by gp120 specific small interfering RNA. PLoS One. 2011;6(6):e21261. 10.1371/journal.pone.0021261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Williams R, Yao H, Dhillon NK, Buch SJ. HIV-1 Tat co-operates with TFN-gamma and TNF-alpha to increase CXCL10 in human astrocytes. PLoS One. 2009;4(5):e5709. 10.1371/journal.pone.0005709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Williams R, Yao H, Peng F, Yang Y, Bethel-Brown C, Buch S. Cooperative induction of CXCL10 involves NADPH oxidase: Implications for HIV dementia. Glia. 2010;58(5):611–21. 10.1002/glia.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Reddy PV, Gandhi N, Samikkannu T, Saiyed Z, Agudelo M, Yndart A, et al. HIV-1 gp120 induces antioxidant response element-mediated expression in primary astrocytes: role in HIV associated neurocognitive disorder. Neurochem Int. 2012;61(5):807–14. 10.1016/j.neuint.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Eugenin EA, Clements JE, Zink MC, Berman JW. Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. J Neurosci. 2011;31(26):9456–65. 10.1523/JNEUROSCI.1460-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hu G, Yao H, Chaudhuri AD, Duan M, Yelamanchili SV, Wen H, et al. Exosome-mediated shuttling of microRNA-29 regulates HIV Tat and morphine-mediated neuronal dysfunction. Cell Death Dis. 2012;3:e381. 10.1038/cddis.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yelamanchili SV, Lamberty BG, Rennard DA, Morsey BM, Hochfelder CG, Meays BM, et al. MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease. PLoS Pathog. 2015;11(7):e1005032. Epub 2015/07/15. 10.1371/journal.ppat.1005032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.•.Sil S, Niu F, Chivero ET, Singh S, Periyasamy P, Buch S. Role of Inflammasomes in HIV-1 and Drug Abuse Mediated Neuroinflammaging. Cells. 2020;9(8). 10.3390/cells9081857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fitting S, Xu R, Bull C, Buch SK, El-Hage N, Nath A, et al. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol. 2010;177(3):1397–410. 10.2353/ajpath.2010.090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002;46(4):271–9. 10.1002/syn.10146. [DOI] [PubMed] [Google Scholar]
- 181.Liao D, Lin H, Law PY, Loh HH. Mu-opioid receptors modulate the stability of dendritic spines. Proc Natl Acad Sci U S A. 2005;102(5):1725–30. 10.1073/pnas.0406797102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pitcher J, Abt A, Myers J, Han R, Snyder M, Graziano A, et al. Neuronal ferritin heavy chain and drug abuse affect HIV-associated cognitive dysfunction. J Clin Invest. 2014;124(2):656–69. 10.1172/JCI70090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11(5):1598–604. 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]


