Abstract
Purpose
Anlotinib, a novel multi-target tyrosine kinase inhibitor, has shown encouraging antitumor effects in advanced hepatocellular carcinoma (HCC). This study evaluated the effectiveness and safety of anlotinib with or without programmed death-1 (PD-1) blockades for patients with advanced primary HCC in a real-world setting in China.
Patients and Methods
Between July 2019 and May 2021, 27 patients with advanced primary HCC who received at least 2 cycles of anlotinib were included in this retrospective study. Primary endpoint was objective response rate (ORR). Secondary endpoints were disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and safety.
Results
Of the 27 patients, ORR and DCR were 25.93% and 74.07%, respectively. The median follow-up time was 6.27 months (range: 1.30–17.40) with a median PFS and OS of 3.29 months (95% CI: 1.31–15.47) and 6.21 months (95% CI: 2.23–15.87), respectively. A total of 14 patients received anlotinib and PD-1 blockade combination therapy, and 13 received anlotinib monotherapy. No significant differences were observed in ORR (28.57 vs 23.08%), DCR (71.43 vs 76.92%), PFS (3.38 [95% CI: 2.66–13.14] vs 11.86 months [95% CI: 4.27–15.93]) and OS (4.90 [95% CI: 2.56–13.60] vs 11.04 months [95% CI: 1.31–17.18]) between the two groups (all p>0.05). Treatment-related AEs were reported in 88.89% of patients. Grade 3 AE was bleeding, which occurred in 3 patients (11.11%).
Conclusion
Anlotinib yielded a promising efficacy and manageable safety in patients with advanced primary HCC irrespective of whether patients received PD-1 blockades, indicating that anlotinib might be a promising treatment option for this patient population.
Keywords: anlotinib, objective response rate, PD-1 blockades, primary hepatocellular carcinoma
Introduction
Primary liver cancer is the second leading cause of tumor-related death and the fourth most common malignancy in China, and around 90% of cases are hepatocellular carcinoma (HCC).1 There are approximately 466,000 new cases and 422,000 death cases of HCC in China annually, accounting for over half of the total incidence and mortality worldwide.2 Since it is difficult to detect this malignant tumor at an early stage, the prognosis of HCC is poor.3 In advanced HCC, radical surgery becomes infeasible as the tumor has spread beyond the liver.4 For more than one decade, sorafenib is the only systemic therapy to improve the survival of advanced HCC.5 However, the median survival of advanced HCC was prolonged by fewer than 3 months from sorafenib, and less than 5% of patients achieved an objective response rate (ORR).6,7 Although several new drugs (lenvatinib, regorafenib, and cabozantinib) have been approved for the treatment of advanced HCC since 2017, the clinical efficacy of these drugs is not superior to that of sorafenib.8–10 Therefore, it is extremely crucial to develop a novel and effective therapy for advanced HCC.
In recent years, anti-programmed cell death-1 (PD-1)/anti-programmed death ligand-1 (PD-L1)-based immune checkpoint inhibitor (ICI) therapy has represented a breakthrough in tumor drug development.11,12 Anti-PD-1/anti-PD-L1 monotherapy has been approved for the treatment of more than ten cancers, with an ORR of 15–20% and acceptable safety profiles.13,14 Despite the initial enthusiasm for ICI therapy in the treatment of advanced HCC, anti-PD-1 monotherapy did not demonstrate significant improvement in overall survival (OS).15,16 A Phase III randomized trial investigated first-line single-agent anti–PD-1/PD-L1 therapy (nivolumab monotherapy) compared with sorafenib for patients with advanced HCC and found that the OS was identical between two groups (16.4 months vs 14.7 months, p=0.075).15 Similarly, patients receiving pembrolizumab (anti-PD-1 monotherapy) plus best supportive care as second-line treatment did not report significant improvement in OS (13.9 months vs 10.6 months, p=0.0238) compared to placebo plus best supportive care.16 Therefore, it is necessary to explore combinational strategies with other treatments to enhance the efficacy of ICIs in the treatment of advanced HCC.
Angiogenesis is one of the crucial events during cancer progression as it is required for sustained tumor growth and metastasis.17 It has been proved that anti-angiogenic drugs can regulate immune-related cells in the tumor microenvironment, thus affecting the clinical efficacy of ICIs.18 Anlotinib is a newly developed oral angiogenesis inhibitor that selectively targets the vascular endothelial growth factor 2 (VEGF2).19 A preclinical study demonstrated that anlotinib significantly prompted the apoptosis, inhibited the proliferation of HCC cells, and alleviated HCC progression possibly by inhibiting B cell CLL/lymphoma-2 (BCL-2) and Survivin expression and promoting Bax expression via inactivating extracellular signal-regulated kinase (ERK) and protein kinase B (Akt) pathways.20 Based on the above results, a Phase II study was performed to assess the efficacy and safety of anlotinib as a first- or second-line treatment for patients with advanced HCC.21 The results showed that anlotinib showed promising efficacy and safety in advanced HCC, with a 12-week progression-free survival (PFS) rate of 80.8% and 72.5%, respectively, in patients who received anlotinib with or without prior tyrosine kinase inhibitors (TKIs).21 As the sample size of the phase II study was limited, the efficacy of anlotinib in advanced HCC still needed to be further verified by expanded clinical research. Moreover, a preclinical study has shown that anlotinib can ameliorate the tumor immune microenvironment by downregulating PD-L1 expression to inhibit tumor growth.22 In a mouse model of lung cancer, anlotinib combined with PD-1 inhibitor can promote the infiltration of the innate immune cells, and confer potentially synergistic antitumor activity.23 Although several phase II studies have confirmed the clinical benefit of anti-PD-1 monotherapy (sintilimab or penpulimab) plus anlotinib for advanced or unresectable HCC, both studies were performed in the first-line setting.24,25 Evidence of anlotinib combined with anti-PD-1 monotherapy as second-line or further therapy in advanced HCC is still scanty currently, especially in the real-world setting. Thus, a retrospective, real-world study was conducted to evaluate the effectiveness and safety of anlotinib with or without PD-1 blockades as first-line or further therapy for Chinese patients with advanced primary HCC.
Materials and Methods
Study Design and Patients
This was a retrospective, single-center, real-world study (http://www.chictr.org.cn; registration number: ChiCTR2100054806) to evaluate the effectiveness and safety of anlotinib for patients with advanced primary HCC. A total of 27 patients with advanced primary HCC were included between July 2019 and May 2021. The study was conducted following the Declaration of Helsinki and approved by the ethic committee institutional review board of The First Affiliated Hospital of Henan University of Traditional Chinese Medicine (number: 2021HL-266). Informed consent from patients was exempted by the ethical review.
Patients who were aged 18–80 years, had histopathologically or cytologically confirmed advanced primary HCC according to the Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2019 edition)26 in China, and received at least 2 cycles of anlotinib were eligible for the study. Patients were also required to have survival data, adverse events (AEs), and at least one follow-up radiological information (computed tomography). Patients who underwent radical surgery, as well as pregnant or lactating patients, were excluded from this study.
Therapy
Anlotinib was given orally once daily with an initial dose of 8–12 mg (day 1–14, every 3 weeks per cycle; Chia-tai Tianqing Pharmaceutical Co., Ltd., Nanjing, China). Discontinuation, suspension, and dose modification of anlotinib were allowed according to the disease progression or AEs.
Whether to receive PD-1 blockades was the choice of physicians according to the comprehensive evaluation of the patient’s condition, concomitant diseases (such as hepatitis), and economic situation. The PD-1 blockades including camrelizumab (200 mg every 3 weeks; Jiangsu Hengrui Pharmaceutical Co., Ltd., Lianyungang, China), tislelizumab (200 mg every 3 weeks; BeiGene Co. Ltd, Shanghai, China), nivolumab (200 mg every 3 weeks; Bristol-Myers Squibb), and pembrolizumab (100 mg every 3 weeks; MSD R&D [China] Co., Ltd, Shanghai, China) were administrated via an intravenous drip.
Treatment Evaluation
Information on the patients’ demographic characteristics, laboratory test results, radiological information, survival data, and AEs were collected retrospectively. The tumor response was assessed by the investigator according to the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 using computed tomography scans.27
The primary endpoint was the ORR, which was defined as the proportion of patients who achieved complete response (CR) or partial response (PR). Secondary endpoints included disease control rate (DCR), PFS, OS, and safety. The ORR, DCR, PFS, and OS were also analyzed in subgroups of patients who had received different therapeutic regimens (anlotinib monotherapy vs anlotinib and PD-1 blockade combination therapy). The DCR was defined as the proportion of confirmed CR, PR, or stable disease (SD) at the best response. The PFS was defined as the time from the start of therapy to radiographic disease progression or death from any cause. The OS was defined as the time from the start of therapy until the date of death from any cause. Safety was assessed by AEs according to the Common Terminology Criteria Adverse Events version 5.0 (CTCAE 5.0).
Statistical Analysis
The SPSS software (version 21.0, SPSS Institute. IL., USA) was used for statistical analysis. The Shapiro–Wilk test was used to confirm the normality of data distribution. The continuous variables were present by mean with standard deviation (normally distributed data) or median and interquartile range (skewed distributed data), while categorical variables were expressed as numbers and percentages. Between-group comparisons of normally distributed continuous variables were analyzed using Student’s t-test, and skewed distributed continuous variables were analyzed using Mann–Whitney U-test. Between-group comparisons of categorical variables were analyzed using χ2 of Fisher’s exact tests. PFS and OS were calculated by the Kaplan-Meier method and compared using a stratified Log rank test. The analysis for ORR and DCR was based on the best overall response. P<0.05 was considered significant.
Results
Patient Characteristics
Between July 2019 and May 2021, 27 patients (mean age, 57.89±12.03 years; 21 male, 6 female; mean body weight, 64.31±11.00 kg) with advanced primary HCC were included in this retrospective study. The baseline characteristics of the 27 patients are shown in Table 1. The majority of patients were older than 60 years of age (15/27, 53.6%), had extrahepatic metastasis (17/27, 62.96%), hepatic cirrhosis (23/27, 85.19%), and hepatitis B (22/27, 81.48%). Five patients (18.52%) had Barcelona Clinic Liver Cancer (BCLC) B stage disease, 15 patients (55.56%) had BCLC C stage, and 7 patients (25.93%) had BCLC D stage. The mean tumor size at baseline was 8.21±5.85 cm.
Table 1.
Baseline Characteristics of All Patients
| Total (n=27) | Combination Group (n=14) | Monotherapy Group (n=13) | P value | |
|---|---|---|---|---|
| Age (years; n, %), ≤60/>60 | 13 (48.15)/14 (51.85) | 6 (42.86)/8 (57.14) | 8 (61.54)/5 (38.46) | 0.280 |
| Gender (n, %), male/female | 21 (77.78)/6 (21.4) | 10 (71.43)/4 (28.57) | 11 (84.62)/2 (15.38) | 0.362 |
| Height (cm), Mean±SD | 168.15±6.78 | 167.35±2.09 | 169.00±1.56 | 0.540 |
| Body weight (kg), Mean±SD | 64.31±11.00 | 65.54±3.52 | 63.00±2.32 | 0.560 |
| Tumor size (cm), Mean±SD | 8.21±5.85 | 8.97±1.03 | 7.41±2.27 | 0.517 |
| Drinking history (n, %) | 3 (11.11) | 1 (7.14) | 2 (15.38) | 0.471 |
| AFP (n, %), ≤400/>400 | 14 (51.85)/13 (48.15) | 6 (42.86)/8 (57.14) | 8 (61.54)/5 (38.46) | 0.280 |
| Extrahepatic metastasis (n, %) | 17 (62.96) | 8 (57.14) | 9 (69.23) | 0.402 |
| Hepatic cirrhosis (n, %) | 23 (85.19) | 13 (92.86) | 10 (76.92) | 0.269 |
| Hepatitis (n, %) | ||||
| None | 2 (7.41) | 1 (7.14) | 1 (7.69) | 0.741 |
| B | 22 (81.48) | 11 (78.57) | 11 (84.62) | 0.538 |
| C | 3 (11.11) | 2 (14.29) | 1 (7.69) | 0.529 |
| Child-Pugh class (n, %) | 0.188 | |||
| A | 16 (59.26) | 9 (64.29) | 7 (53.85) | |
| B | 8 (29.63) | 5 (35.71) | 3 (23.08) | |
| C | 3 (11.11) | 0 | 3 (23.08) | |
| Previous therapy (n, %) | ||||
| Surgery | 5 (18.52) | 2 (14.29) | 3 (23.08) | 0.455 |
| Radiofrequency ablation | 8 (29.63) | 5 (35.71) | 3 (23.08) | 0.385 |
| TACE | 18 (66.67) | 8 (57.14) | 10 (76.92) | 0.279 |
| Chemotherapy | 3 (11.11) | 1 (7.14) | 2 (15.38) | 0.730 |
| Radiotherapy | 6 (22.22) | 4 (28.57) | 2 (15.38) | 0.048 |
| Immunotherapy | 12 (44.44) | 11 (78.57) | 1 (7.69) | 0.000 |
| Targeted therapy | 18 (66.67) | 10 (71.43) | 8 (61.54) | 0.445 |
| Antiviral therapy | 22 (81.48) | 11 (78.57) | 11 (84.62) | 0.538 |
| Traditional Chinese medicine | 24 (88.89) | 12 (85.71) | 12 (92.31) | 0.529 |
| BCLC stage (n, %) | 0.452 | |||
| B | 5 (18.52) | 3 (21.43) | 2 (15.38) | |
| C | 15 (55.56) | 9 (64.29) | 6 (46.15) | |
| D | 7 (25.93) | 2 (14.29) | 5 (38.46) | |
| CNLC stage (n, %) | 0.193 | |||
| II | 5 (18.52) | 2 (14.29) | 3 (23.08) | |
| III | 15 (55.56) | 10 (71.43) | 5 (38.46) | |
| IV | 7 (25.93) | 2 (14.29) | 5 (38.46) |
Abbreviations: AFP, alpha-fetoprotein; TACE, transhepatic arterial chemotherapy and embolization; BCLC, Barcelona Clinic Liver Cancer; CNLC, China Liver Cancer Staging.
Treatment
All patients received anlotinib as first-line (11/27, 40.74%), second-line (9/27, 33.33%), third-line (5/27, 18.52%), or fourth-line treatment (2/27, 7.41%). The initial doses of anlotinib were 8 mg (12/27, 44.44%), 10 mg (6/27, 22.22%), and 12 mg (9/27, 33.33%), respectively. At the time of data cutoff (September 15, 2021), the median duration of anlotinib was 4.20 months (range, 0.77–17.40). No patient experienced the dose modification of anlotinib. Discontinuations of anlotinib were observed in 18 patients (disease progression, n=6; AEs, n=6; unknown, n=6).
Among the 27 patients, 14 (51.85%) received anlotinib and PD-1 blockade combination therapy, and 13 (48.15%) received anlotinib monotherapy (Table 1). Combination therapy with PD-1 blockades in 14 patients included camrelizumab, tislelizumab, nivolumab, and pembrolizumab in 11 (78.57%), 1 (7.14%), 1 (7.14%), and 1 (7.14%) patients, respectively.
Clinical Outcomes
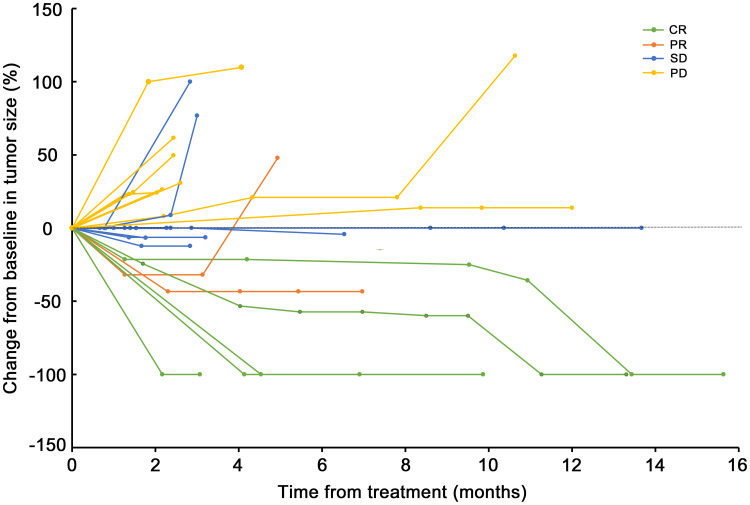
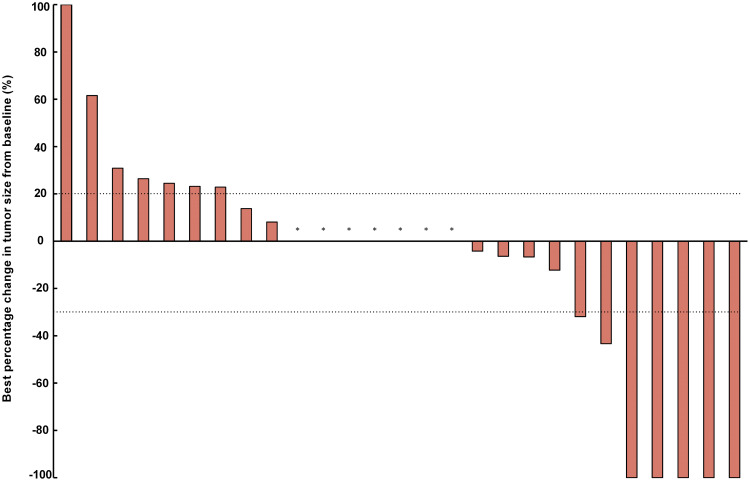
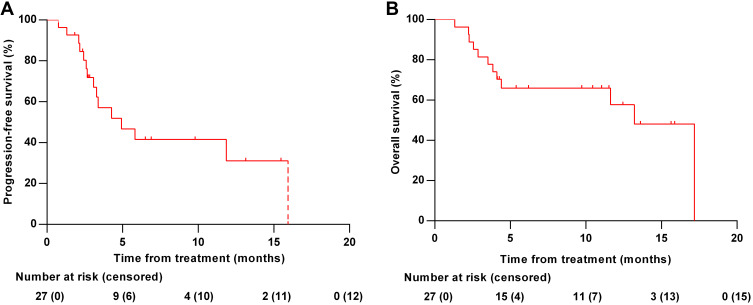
At the time of data cutoff, the median follow-up time was 6.27 months (range: 1.30–17.40). Of all 27 patients, 5 (18.52%) met the RECIST criteria for attaining a CR as their best response (Figure 1). Two patients (7.41%) had PR, 13 (48.15%) had SD, and 7 (25.93%) had progression disease (PD). The ORR and DCR were 25.93% and 74.07%, respectively. The median best percentage change in tumor size was −0.0% (IQR, −31.91 to +22.86%; Figure 2). At data cut-off, OS data were still evolving, with deaths occurring in a total of 12 patients (44.44%). The median PFS was 3.29 months (95% CI: 1.31–15.47), and the median OS was 6.21 months (95% CI: 2.23–15.87) (Figure 3).
Figure 1.
Percentage change in tumor size from baseline.
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progression disease.
Figure 2.
Best percentage change in tumor size from baseline. *The best percentage change in tumor size from baseline was 0.0%.
Figure 3.
Kaplan–Meier curves. (A) Progression-free survival of all patients. (B) Overall survival of all patients.
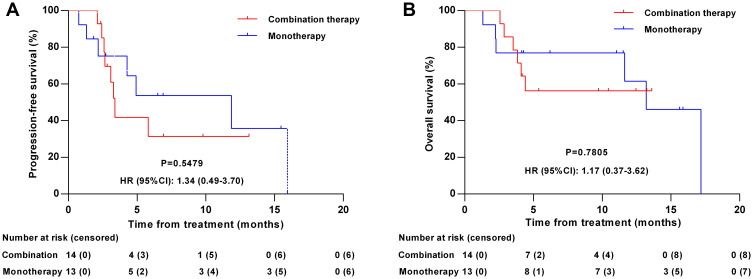
Of the 14 patients who received anlotinib and PD-1 blockade combination therapy, 3 (21.43%) achieved a CR, 1 (7.14%) achieved a PR, 6 (42.86%) had SD, and 4 (28.57%) had PD. The ORR and DCR were 28.57% and 71.43%, respectively. The median PFS and OS were 3.38 months (95% CI: 2.66–13.14) and 4.90 months (95% CI: 2.56–13.60), respectively (Figure 4). For patients who received anlotinib monotherapy, the ORR, DCR, median PFS, and median OS were 23.08% (2 CR, 1 PR), 76.92% (2 CR, 1 PR, 7 SD), 11.86 months (95% CI: 4.27–15.93) and 11.04 months (95% CI: 1.31–17.18), respectively. No statistical difference existed in the ORR, DCR, PFS, and OS between the two groups (all p>0.05).
Figure 4.
Kaplan–Meier curves according to therapeutic regimens. (A) Progression-free survival according to therapeutic regimens (anlotinib monotherapy vs anlotinib and PD-1 blockade combination therapy). (B) Overall survival according to therapeutic regimens (anlotinib monotherapy vs anlotinib and PD-1 blockade combination therapy).
Safety
As shown in Table 2, treatment-related AEs were reported in 24 patients (88.89%). The most frequently observed AEs of any grade were anorexia (16/27, 59.26%), thrombocytopenia (10/27, 37.04%), hypertension (9/27, 33.33%), diarrhea (8/27, 29.63%), fatigue (6/27, 22.22%), and anaemia (5/27, 18.52%). Most treatment-related AEs were regarded as grade 1–2 (21/27, 77.78%). Treatment-related grade 3 AE was bleeding, which occurred in 3 patients (11.11%). No treatment-related grade 4 AEs or serious AEs were observed. Safety profiles (Table 2) of the anlotinib and PD-1 blockade combination group and anlotinib monotherapy group were comparable (p>0.05), except for anorexia of any grade (85.71% vs 30.77%, p=0.005).
Table 2.
Summary of Treatment-Related Adverse Events in All Patients
| Adverse Events | Total (n=27) | Combination Group (n=14) | Monotherapy Group (n=13) | P value* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any Grade | Grade 1–2 | Grade 3 | Any Grade | Grade 1–2 | Grade 3 | Any Grade | Grade 1–2 | Grade 3 | ||
| Anorexia | 16 (59.26) | 16 (59.26) | 0 | 12 (85.71) | 12 (85.71) | 0 | 4 (30.77) | 4 (30.77) | 0 | 0.005 |
| Thrombocytopenia | 10 (37.04) | 10 (37.04) | 0 | 7 (50.00) | 7 (50.00) | 0 | 3 (23.08) | 3 (23.08) | 0 | 0.147 |
| Hypertension | 9 (33.33) | 9 (33.33) | 0 | 6 (42.86) | 6 (42.86) | 0 | 3 (23.08) | 3 (23.08) | 0 | 0.249 |
| Diarrhoea | 8 (29.63) | 8 (29.63) | 0 | 4 (28.57) | 4 (28.57) | 0 | 4 (30.77) | 4 (30.77) | 0 | 0.615 |
| Fatigue | 6 (22.22) | 6 (22.22) | 0 | 4 (28.57) | 4 (28.57) | 0 | 2 (15.38) | 2 (15.38) | 0 | 0.362 |
| Anaemia | 5 (18.52) | 5 (18.52) | 0 | 2 (14.29) | 2 (14.29) | 0 | 3 (23.08) | 3 (23.08) | 0 | 0.462 |
| Proteinuria | 3 (11.11) | 3 (11.11) | 0 | 2 (14.29) | 2 (14.29) | 0 | 1 (7.69) | 1 (7.69) | 0 | 0.529 |
| Hand-foot syndrome | 3 (11.11) | 3 (11.11) | 0 | 3 (21.43) | 3 (21.43) | 0 | 0 | 0 | 0 | 0.124 |
| Bleeding | 4 (14.71) | 1 (3.70) | 3 (11.11) | 3 (21.43) | 1 (7.14) | 2 (14.29) | 1 (7.69) | 0 | 1 (7.69) | 0.327 |
Notes: Data were expressed as n (%); *p value of any grade between combination and monotherapy group.
Subgroup Analyses According to the Line of Therapy
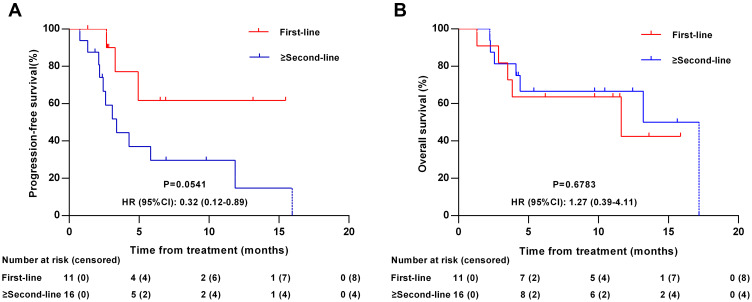
Subgroup analyse was performed according to the line of therapy (first-line vs ≥second-line treatment). Among the 27 patients, 11 (40.74%) received anlotinib as first-line treatment, and 16 (59.26%) received anlotinib as ≥second-line treatment. For patients who received anlotinib as first-line treatment, the ORR, DCR, median PFS and median OS was 36.36% (3 CR, 1 PR), 90.91% (3 CR, 1 PR, 6 SD), 3.32 months (95% CI: 1.31–15.47) and 11.63 months (95% CI: 3.52–15.87), respectively. Of the 16 patients who received anlotinib as ≥second-line treatment, 2 (12.50%) achieved a CR, 1 (6.25%) achieved a PR, 7 (43.75%) had SD, and 6 (37.50%) had PD, with an ORR and DCR of 18.75% and 62.50%, respectively. The median PFS and OS was 3.38 months (95% CI: 2.17–11.86) and 13.21 months (95% CI: 4.11–17.18), respectively. No significant difference was found in the ORR, DCR, PFS, and OS between the two groups (all p>0.05, Figure 5).
Figure 5.
Kaplan–Meier curves according to the line of therapy. (A) Progression-free survival according to the line of therapy (first-line vs ≥second-line treatment). (B) Overall survival according to the line of therapy (first-line vs ≥second-line treatment).
Discussion
The retrospective, real-world study demonstrated that patients with advanced primary HCC who received at least 2 cycles of anlotinib as first-line or further therapy had a promising efficacy and acceptable safety irrespective of whether received PD-1 blockades. The high ORR (25.93%) and DCR (74.07%), as well as long median PFS (3.29 months [95% CI: 1.31–15.47]) and OS (6.21 months [95% CI: 2.23–15.87]), observed in the present study, indicated that anlotinib might be a promising treatment option for advanced primary HCC.
Anlotinib is a novel multi-target TKI that inhibits both tumor cell proliferation and tumor angiogenesis.28 Compared with other TKIs (sorafenib, pazopanib, and sunitinib), anlotinib can inhibit more targets including VEGFR1, VEGFR2, VEGFR3, fibroblast growth factor receptors (FGFR1, FGFR2, and FGFR3), platelet-derived growth factor receptor (PDGFR-α and PDGFR-β), and c-Kit.29 A preclinical study has shown that the anti-angiogenic activity of anlotinib is better than that of sorafenib, sunitinib, and nintedanib, which are the three main anti-angiogenesis agents in the clinic.30 In the present study, we evaluated the effectiveness of anlotinib monotherapy for patients with advanced primary HCC. As expected, our study revealed encouraging results with an ORR of 23.08% and an OS of 11.04 months. Previous studies have reported that the ORR and OS achieved in sorafenib, sunitinib, and lenvatinib were 2.0–6.9% and 6.3–10.9 months,6,7,31 2.7–2.9% and 8.0–9.8 months,32,33 18.8% and 13.6 months,8 respectively. Besides, our result was also higher than that reported by Sun et al, which indicated an ORR of 8.3% and 3.9%, respectively, in patients who received anlotinib with or without prior TKIs.21 The reason for the dramatic improvement in ORR with anlotinib monotherapy over previous reports might result from a bias introduced by the small sample size in these studies. Moreover, the baseline characteristics of included patients (Child-Pugh class, BCLC stage, and CNLC stage), the initial dose of anlotinib, and previous treatment history may also contribute to the discrepancy in ORR. Anyway, based on the above results, anlotinib was comparable to other TKIs in patients with advanced HCC, suggesting anlotinib is a promising treatment option for advanced HCC. Certainly, further studies with a larger sample size are necessary to make a definitive conclusion regarding the efficacy of anlotinib in advanced HCC.
Increasing evidence has confirmed that anti-angiogenic therapy plays an immunomodulatory role in the tumor microenvironment, which seems to enhance the efficacy of PD-1 blockades by increasing the infiltration of immune effector cells into tumors and normalizing abnormal tumor vessels.34–36 Anlotinib conferred significantly synergistic therapeutic benefits when combined with ICIs in a preclinical study.23 A meta-analysis demonstrated that PD-1/PD-L1 monoclonal antibodies resulted in an ORR of 20%, a DCR of 60%, a PFS of 3.58 months, and an OS of 12.24 months in patients with advanced HCC.37 Phase II study of sintilimab (a novel selective anti-PD-1 monoclonal antibody) plus anlotinib yielded an improvement in ORR (42.9%) and DCR (92.9%) in patients with advanced HCC.24 Besides, penpulimab (a humanized anti-PD-1 antibody) plus anlotinib demonstrated an ORR of 31.0%, a DCR of 82.8%, and a median PFS of 8.8 months (95% CI, 4.0–12.3) after the first-line treatment in Chinese patients with unresectable HCC.25 In the present study, anlotinib and PD-1 blockades combination therapy resulted in an encouraging ORR of 28.57% and a DCR of 71.43%, which was comparable to previously combination therapy or PD-1/PD-L1 monotherapy. However, anlotinib and PD-1 blockade combination therapy in our study not tended to yield a favorable efficacy than anlotinib monotherapy in terms of ORR, DCR, PFS, and OS (all p>0.05). Overall, these results suggested that anlotinib yielded a promising efficacy in patients with advanced primary HCC irrespective of whether patients received PD-1 blockades. However, large sample size clinical trials should be conducted to further evaluate the efficacy difference of anlotinib with or without PD-1 blockades for patients with advanced primary HCC.
Anlotinib was generally well tolerated for our patients with advanced primary HCC. Moreover, anlotinib tended to yield a slightly higher safety profile (88.89%) compared with sorafenib (98.5%) and lenvatinib (100.0%).31,38 Similar to other TKIs (sorafenib, sunitinib, and lenvatinib),31,32,38 the most frequently observed AEs were anorexia, thrombocytopenia, hypertension, diarrhea, fatigue, and anemia. These AEs were also similar to those described in other studies for anlotinib,39–41 and no unexpected AEs were observed in our study. Of special concern was that majority of AEs were regarded as grade 1–2 (77.78%). As bleeding was the main concern of any antiangiogenic treatment, the only grade 3 AE reported in our study was bleeding, but the occurrence was low (11.11%). Besides, no treatment-related grade 4 AEs or serious AEs were observed in the present study. Overall, our study demonstrated the acceptable safety and tolerability for anlotinib.
In our study, the following limitations should be taken into account. Firstly, the retrospective study design might cause selection bias. Secondly, this study was conducted at a single institute in China, which might affect the generalizability of the results to a broader population. Thirdly, the subgroup analysis with sufficient power was limited by the small sample size. Finally, patients’ characteristics were not analyzed to determine which patients benefited more from anlotinib. Therefore, a double-blind randomized controlled trial with larger sample size is planned to be carried out to further evaluate the efficacy and safety of anlotinib with or without PD-1 blockades for patients with advanced primary HCC.
Conclusion
The retrospective, real-world study in China demonstrated that anlotinib yielded a promising efficacy and manageable safety in patients with advanced primary HCC irrespective of whether patients received PD-1 blockades, indicating that anlotinib might be a promising treatment option for this patient population. Clinical trials with a large sample size should be carried out to further evaluate the efficacy of anlotinib with or without PD-1 blockades for advanced primary HCC.
Acknowledgments
The abstract of this paper was presented at the American Association for Cancer Research (AACR) as an abstract presentation with interim findings. The poster’s abstract will be published in “Poster Abstracts” in Cancer Research. Xiao-Qi Chen and Yun-Xia Zhao are co-first authors for this study.
Funding Statement
This work was supported by the National Science and Technology Major Project [number 2018ZX10303502], Henan Provincial Key project of Traditional Chinese Medicine scientific research [number 2018JDZX110], and Construction project of Traditional Chinese Medicine with Characteristic backbone discipline in Henan Province [number STG-ZYXKY-2020019].
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethical Statement
The study was conducted following the Declaration of Helsinki and approved by the ethic committee institutional review board of The First Affiliated Hospital of Henan University of Traditional Chinese Medicine (number: 2021HL-266). Written informed consent was not sought from the legally authorized representatives of the patients directly since we used a medical record. Therefore, informed consent from patients was exempted by the ethical review. When the data was recorded from their medical chart’s identification numbers were used for each individual patient so as to make it easy to identify the individuals’ profile while keeping patient’s medical secret.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9(6):682–720. doi: 10.1159/000509424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Imai K, Takai K, Miwa T, et al. Rapid depletion of subcutaneous adipose tissue during sorafenib treatment predicts poor survival in patients with hepatocellular carcinoma. Cancers. 2020;12(7):1795. doi: 10.3390/cancers12071795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Li J, Tang G, et al. Clinical outcomes and influencing factors of PD-1/PD-L1 in hepatocellular carcinoma. Oncol Lett. 2021;21(4):279. doi: 10.3892/ol.2021.12540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huppert LA, Gordan JD, Kelley RK. Checkpoint inhibitors for the treatment of advanced hepatocellular carcinoma. Clin Liver Dis. 2020;15(2):53–58. doi: 10.1002/cld.879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 7.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 8.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 9.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9 [DOI] [PubMed] [Google Scholar]
- 10.Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi: 10.1056/NEJMoa1717002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee PC, Chao Y, Chen MH, et al. Risk of HBV reactivation in patients with immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J Immunother Cancer. 2020;8(2):e001072. doi: 10.1136/jitc-2020-001072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng AL, Hsu C, Chan SL, et al. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 2020;72(2):307–319. doi: 10.1016/j.jhep.2019.09.025 [DOI] [PubMed] [Google Scholar]
- 13.Callahan MK, Postow MA, Wolchok JD. Targeting T cell co-receptors for cancer therapy. Immunity. 2016;44(5):1069–1078. doi: 10.1016/j.immuni.2016.04.023 [DOI] [PubMed] [Google Scholar]
- 14.Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov. 2016;15(4):235–247. doi: 10.1038/nrd.2015.35 [DOI] [PubMed] [Google Scholar]
- 15.Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2021;23(1):77–90. doi: 10.1016/S1470-2045(21)00604-5 [DOI] [PubMed] [Google Scholar]
- 16.Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, Phase III trial. J Clin Oncol. 2020;38(3):193–202. doi: 10.1200/JCO.19.01307 [DOI] [PubMed] [Google Scholar]
- 17.Marmé D. Tumor angiogenesis: a key target for cancer therapy. Oncol Res Treat. 2018;41(4):164. doi: 10.1159/000488340 [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto K, Shiroyama T, Kuge T, et al. Impact of treatment timing and sequence of immune checkpoint inhibitors and anti-angiogenic agents for advanced non-small cell lung cancer: a systematic review and meta-analysis. Lung Cancer. 2021;162:175–184. doi: 10.1016/j.lungcan.2021.11.008 [DOI] [PubMed] [Google Scholar]
- 19.Jin Z, Lu Y, Wu X, et al. The cross-talk between tumor cells and activated fibroblasts mediated by lactate/BDNF/TrkB signaling promotes acquired resistance to anlotinib in human gastric cancer. Redox Biol. 2021;46:102076. doi: 10.1016/j.redox.2021.102076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He C, Wu T, Hao Y. Anlotinib induces hepatocellular carcinoma apoptosis and inhibits proliferation via Erk and Akt pathway. Biochem Biophys Res Commun. 2018;503(4):3093–3099. doi: 10.1016/j.bbrc.2018.08.098 [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Zhou A, Zhang W, et al. Anlotinib in the treatment of advanced hepatocellular carcinoma: an open-label phase II study (ALTER-0802 study). Hepatol Int. 2021;15(3):621–629. doi: 10.1007/s12072-021-10171-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S, Qin T, Liu Z, et al. Anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis. 2020;11(5):309. doi: 10.1038/s41419-020-2511-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y, Li L, Jiang Z, et al. Anlotinib optimizes anti-tumor innate immunity to potentiate the therapeutic effect of PD-1 blockade in lung cancer. Cancer Immunol Immunother. 2020;69(12):2523–2532. doi: 10.1007/s00262-020-02641-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Li W, Wu X, et al. Sintilimab plus anlotinib as first-line therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC). J Clin Oncol. 2021;39(15_suppl):e16146–e16146. doi: 10.1200/JCO.2021.39.15_suppl.e16146 [DOI] [Google Scholar]
- 25.Han C, Ye S, Hu C, et al. Clinical activity and safety of penpulimab (Anti-PD-1) with anlotinib as first-line therapy for unresectable hepatocellular carcinoma: an open-label, multicenter, Phase Ib/II trial (AK105-203). Front Oncol. 2021;11:684867. doi: 10.3389/fonc.2021.684867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu G, Jin Z, Chen X, et al. Interpretation of guidelines for the diagnosis and treatment of primary liver cancer (2019 edition) in China. Global Health Med. 2020;2(5):306–311. doi: 10.35772/ghm.2020.01051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 28.Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer. 2018;118(5):654–661. doi: 10.1038/bjc.2017.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11(1):120. doi: 10.1186/s13045-018-0664-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin B, Song X, Yang D, et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene. 2018;654:77–86. doi: 10.1016/j.gene.2018.02.026 [DOI] [PubMed] [Google Scholar]
- 31.Cainap C, Qin S, Huang WT, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33(2):172–179. doi: 10.1200/JCO.2013.54.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faivre S, Raymond E, Boucher E, et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol. 2009;10(8):794–800. doi: 10.1016/S1470-2045(09)70171-8 [DOI] [PubMed] [Google Scholar]
- 33.Zhu AX, Sahani DV, Duda DG, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27(18):3027–3035. doi: 10.1200/JCO.2008.20.9908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52(Pt 2):117–124. doi: 10.1016/j.semcancer.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 35.Fukumura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340. doi: 10.1038/nrclinonc.2018.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Datta M, Coussens LM, Nishikawa H, et al. Reprogramming the tumor microenvironment to improve immunotherapy: emerging strategies and combination therapies. Am Soc Clin Oncol Educ Book. 2019;39(39):165–174. doi: 10.1200/EDBK_237987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao Q, Li M, Xu W, et al. Clinical benefits of PD-1/PD-L1 inhibitors in advanced hepatocellular carcinoma: a systematic review and meta-analysis. Hepatol Int. 2020;14(5):765–775. doi: 10.1007/s12072-020-10064-8 [DOI] [PubMed] [Google Scholar]
- 38.Ikeda K, Kudo M, Kawazoe S, et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52(4):512–519. doi: 10.1007/s00535-016-1263-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Y, Du F, Gao M, et al. Anlotinib for the treatment of patients with locally advanced or metastatic medullary thyroid cancer. Thyroid. 2018;28(11):1455–1461. doi: 10.1089/thy.2018.0022 [DOI] [PubMed] [Google Scholar]
- 40.Wang HY, Chu JF, Zhang P, et al. Safety and efficacy of chemotherapy combined with anlotinib plus anlotinib maintenance in Chinese patients with advanced/metastatic soft tissue sarcoma. Onco Targets Ther. 2020;13:1561–1568. doi: 10.2147/OTT.S235349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han B, Li K, Wang Q, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 Phase 3 randomized clinical trial. JAMA Oncol. 2018;4(11):1569–1575. doi: 10.1001/jamaoncol.2018.3039 [DOI] [PMC free article] [PubMed] [Google Scholar]