Abstract
Anaerobic treatment of a volatile fatty acid (VFA) mixture was investigated under psychrophilic (3 to 8°C) conditions in two laboratory-scale expanded granular sludge bed reactor stages in series. The reactor system was seeded with mesophilic methanogenic granular sludge and fed with a mixture of VFAs. Good removal of fatty acids was achieved in the two-stage system. Relative high levels of propionate were present in the effluent of the first stage, but propionate was efficiently removed in the second stage, where a low hydrogen partial pressure and a low acetate concentration were advantageous for propionate oxidation. The specific VFA-degrading activities of the sludge in each of the modules doubled during system operation for 150 days, indicating a good enrichment of methanogens and proton-reducing acetogenic bacteria at such low temperatures. The specific degradation rates of butyrate, propionate, and the VFA mixture amounted to 0.139, 0.110, and 0.214 g of chemical oxygen demand g of volatile suspended solids−1 day−1, respectively. The biomass which was obtained after 1.5 years still had a temperature optimum of between 30 and 40°C.
Anaerobic treatment of industrial wastewaters can be considered a well-established technology with a wide range of applications (26). So far, practically all full-scale applications of anaerobic treatment are restricted to concentrated wastewaters with a temperature exceeding 18°C. However, under moderate climate conditions, many dilute wastewaters, including domestic and industrial wastewaters, are discharged at low ambient temperatures. Besides low concentrations of organic matter, typically 0.3 to 1.0 g of chemical oxygen demand (COD) liter−1, these wastewaters usually contain a high dissolved oxygen concentration, sometimes even up to 10 mg of O2 liter−1. Thus far, attempts to treat such dilute wastewaters under psychrophilic conditions have not been very successful (7, 13, 21, 22). Because temperature strongly affects the rates of the anaerobic conversion processes, some essential improvements must be made to the design of the conventional high-rate reactors to enrich for microbial methanogenic consortia able to efficiently degrade dilute wastewaters at a low temperature. Because of the numerous advantages of anaerobic treatment systems in comparison with the conventional aerobic treatment systems (12), the development of psychrophilic high-rate anaerobic treatment systems undoubtedly will have a great economic and ecological impact. The feasibility of high-rate anaerobic reactor systems for cold wastewaters depends primarily on (i) the quality of the seed sludge in the reactors used and its development under psychrophilic conditions, (ii) the nature of the organic pollutants in the wastewater, and (iii) the reactor configuration, especially its capacity to retain viable sludge. Single- or multicompartment (staged) granular sludge reactors can be applied for psychrophilic anaerobic wastewater treatment. In many cases, multicompartment reactors offer better prospects than single-compartment reactors (23, 27).
Methanogenesis at low temperature has been studied mainly in natural environments such as tundra soil, pond sediments (10, 15), and sediments of deep lakes (14). From these environments, psychrotrophic hydrogen-consuming methanogens and psychrotrophic homoacetogenic bacteria have been isolated (5, 11, 18). There are indications that, in natural habitats with a low temperature, homoacetogens but not methanogens consume hydrogen (4, 10). This would mean that acetate is the main precursor for methanogenesis. However, this may hamper the degradation of higher fatty acids. In methanogenic environments, syntrophic consortia of proton-reducing acetogenic bacteria and methanogens degrade fatty acids like propionate and butyrate. For mesophilic methanogenic processes, it is generally accepted that the affinity of homoacetogens for hydrogen is too low to allow propionate and butyrate oxidizers to grow (19, 20). However, autotrophic hydrogen-consuming methanogens can be enriched at low temperature (15).
It is not clear if a stable psychrophilic fatty acid-degrading microbial consortium can be obtained and maintained in anaerobic wastewater treatment systems. The present article describes a novel approach for the start-up and the operation of an anaerobic high-rate staged expanded granular sludge bed (EGSB) system for the treatment of cold (3 to 8°C), dilute wastewater (0.5 to 0.9 g of COD liter−1), containing 12 mg of O2 liter−1. The operational performance and the temperature characteristics of the biomass are described.
MATERIALS AND METHODS
Experimental conditions.
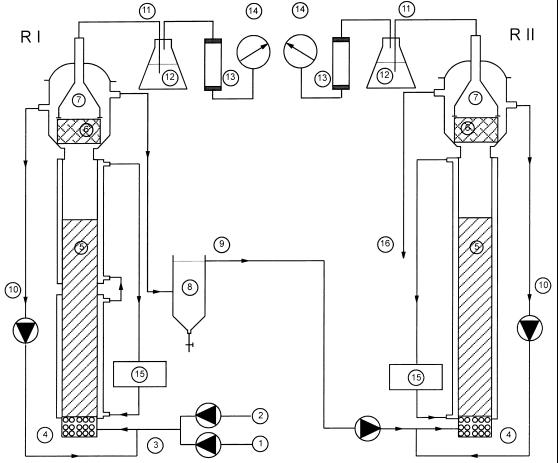
Experiments were performed with a two-stage EGSB system, consisting of two 0.05-m-diameter glass EGSB reactors operated in series (Fig. 1) with a total volume of 8.6 liters (internal settlers included). The same reactor system as that described by Rebac et al. (17) was used. In the experiments, the temperature in the sludge bed was measured with thermocouples (type SD 10; Shimaden, Tokyo, Japan) and controlled by thermostat cooling devices connected to the house cooling system.
FIG. 1.
Schematic diagram of the 8.6-liter two-stage EGSB reactor system (reactors I [RI] and II [RII]) used in this study. 1, feed; 2, tap water; 3, influent; 4, stones; 5, expanded sludge bed; 6, screen; 7, gas-liquid-solid separator; 8, external settler; 9, effluent from first module = influent for second module; 10, effluent recirculation; 11, biogas; 12, sodium hydroxide (10%); 13, soda lime pellets; 14, wet test gas meter; 15, cooling bath circulator; 16, effluent from system.
Biomass.
The reactor system was inoculated with 260 g of volatile suspended solids (VSS) methanogenic granular sludge, cultivated in a 225.5-liter pilot-scale EGSB reactor treating malting wastewater (16) at temperatures of between 12 and 20°C.
Medium.
The reactor was fed with a concentrated stock solution of 33.36 g of COD liter−1, consisting of a volatile fatty acid (VFA) mixture with a pH of 6.5 composed of acetate, propionate, and butyrate in the ratio 1:1.5:1.8, based on the COD. The concentrations of basal nutrients in the concentrated stock solution were as follows (in grams per liter): NH4Cl, 7.5; MgSO4 · 7H2O, 1.5; NaH2PO4 · 2H2O, 27.6; K2HPO4, 21.2; CaCl2 · 2H2O, 0.3; yeast extract, 0.5. To each liter of stock solution, 4.5 ml of a trace element solution was added containing the following (in grams per liter [unless otherwise noted]): FeCl2 · 4H2O, 2,000; H3BO3, 50; ZnCl2, 50; CuCl2 · 2H2O, 30; MnCl2 · 4H2O, 500; (NH4)6Mo7O24 · 4H2O, 50; AlCl3 · 6H2O, 90; CoCl2 · 6H2O, 2,000; NiCl2 · 6H2O, 92; Na2SeO3 · 5H2O, 164; EDTA, 1,000; resazurin, 200; and 36% HCl (1 ml liter−1). All chemicals were of analytical grade and were purchased from Merck (Darmstadt, Germany).
Start-up of the system.
The operation of reactor system was started immediately after inoculation with granular sludge, by feeding the synthetic wastewater at an organic loading rate (OLR) of 3 g of COD liter−1 day−1 and a hydraulic retention time (HRT) of 5.3 h. Each reactor was equipped with an external water circuit in which water of the desired temperature was pumped through the jacket of the reactor. From the start of the experiment, the temperature of the system was set at 9°C.
Batch experiments.
Specific substrate-degrading activities were examined as described previously (17). In order to determine the apparent Km (substrate half-saturation constant) value of the sludge from the second module for propionate under reactor conditions, the sludge was sampled at day 152 and put in two small 80-ml EGSB reactors with 30 g of VSS liter of sludge−1 operated in batch mode at 10°C at an upflow velocity of 6 m h−1. At time zero, the substrate concentration in the reactor was set at 0.3 g of propionate COD (CODprop) liter−1 or 0.2 g of propionate liter−1. The EGSB batch experiment lasted for a 14-h period. Samples (0.2 ml) of supernatants were taken every 20 min until the substrate was completely depleted. The Km value was calculated by fitting the substrate depletion data to the integrated Michaelis-Menten equation, by nonlinear least-squares analysis as described previously (17).
Isotope experiments were performed at 10°C with granular sludge from the second stage. One month before the experiment, sludge was fed once with sodium propionate (final concentration, 0.06 g of CODprop liter−1 or 0.04 g of propionate liter−1). One day before the experiment, 12.5-ml portions of sludge were placed into 25-ml serum bottles, flushed with nitrogen, and preincubated for 24 h with 0.12 g of COD of sodium propionate liter−1 at 10°C. On the day of the experiment, 1 h before the isotope experiment, solutions of labeled [14C]acetate and [14C]bicarbonate were added, and sodium propionate was added at a final concentration of about 0.12 g of COD liter−1. For isotope experiments, VFAs were analyzed by ion-exchange chromatography (1). Radioactive methane and carbon dioxide were measured by a modified method of Zehnder et al. (28).
Analyses.
The pH and redox potential were determined in situ at the effluent line with a Microprocessor WTW 196 pH/mV-meter (Weilheim, Germany). Measurement of pH was conducted with a Schott Nederland N61 double electrode (Tiel, The Netherlands). Redox potential was measured with combined platinum indicator and silver chloride reference electrodes (Schott Nederland PT 6180). Samples of influent and the effluent of both modules were taken three times per week in duplicate, except for the last 10 days, when the samples were taken daily. Analyses of VFAs and the biogas compositions (CH4 and H2) in the reactor and batch experiments were performed as described previously (16, 17).
The CODs for VFAs were determined by standard methods as previously reported (17). The COD conversion factors were 1.07, 1.52, and 1.82 g/g for acetate, propionate, and butyrate, respectively. The COD factor for methane was 2.597 g of O2 liter−1 (20°C), at which temperature, methane was recorded. The VSS content of the sludge was determined by subtracting the ash content from the dry weight after the sludge had been incubated for 24 h at 103°C. The ash content was determined after dry sludge had been heated at 550°C for 120 min.
Microbiological experiments with diluted biomass from the second stage.
Granular sludge from the second stage was sampled at day 182 and stored for 6 months in a refrigerator at 4°C before the microbiological experiments were started. For long-term batch experiments, granular sludge of the EGSB reactor was crushed in a glass mortar under a nitrogen flow. The modified Pfennig medium with 0.12 or 1.2 g of CODprop liter−1 as the substrate was used for dilution of the cell suspension (10). The experiments were performed in 32-ml serum bottles with 20 ml of medium flushed with a nitrogen-carbon dioxide (70%/30%) gas mixture. The initial biomass concentrations were 5, 0.5, and 0.05% (vol/vol). The experiments were performed at 5, 10, 15, 20, 25, and 30°C in duplicate. The temperature dependence of the maximum methane formation and propionate oxidation rate were fitted by using an Arrhenius-derived model and Ratkowsky’s square root empirical model, respectively (17). Bromoethanesulfonic acid was added to a final concentration of 35 mM to inhibit methanogenesis. Gases and VFAs were analyzed by gas chromatography (10). Microscopic observations were performed with a phase-contrast microscope, MBI-3 (Russia).
Calculation.
The maximum rates were calculated from the steepest linear decline in the substrate concentration or the linear increase of methane production, which represented at the minimum 50% of the initial substrate concentrations.
The COD removal efficiency is calculated according to the equation
 |
where l is liter.
RESULTS
Performance of the system.
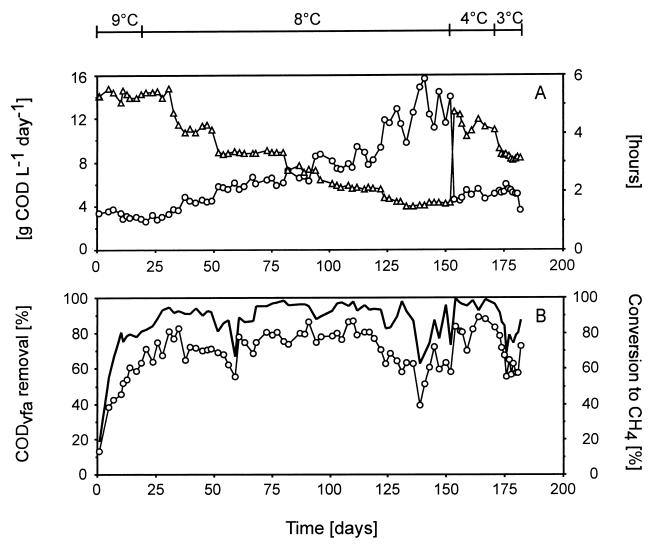
The performance of the two-stage EGSB reactor system is shown in Fig. 2. The OLR of the system was gradually increased from 3.5 to 15.5 g of COD liter−1 day−1 by decreasing the HRT from 5.3 to 1.5 h (Fig. 2A) at an average temperature of 8°C. The loading rate was only increased when the COD removal efficiency reached 90%. The system maintained remarkable stability and high efficiency over the period between days 33 and 133, where the HRT was decreased from 5 to 2 h, and consequently the OLR was raised accordingly up to 12.5 g of COD liter−1 day−1. At an HRT of 1.5 to 1.6 h during the period between days 133 and 152, the system received peak loads of 12 to 15.5 g of COD liter−1 day−1. This resulted in stronger variations in the COD removal efficiency (63 to 92%). The influent contained 12 mg of O2 liter−1, giving rise to maximum oxygen loads of 0.2 g of O2 liter−1 day−1, which is relatively low compared to the OLR. The redox potential of the reactor effluent always remained at −350 to −380 mV, indicating that satisfactory anaerobic conditions prevailed in both modules of the system (data not shown). During the period between days 154 and 171, the system was operated at a temperature of 4°C and at an HRT of only 4 to 5 h. This corresponded to an OLR as high as 4 to 5 g of COD liter−1 day−1. Even under these conditions, the removal efficiency exceeded 90%. When the temperature of the system was further lowered to 3°C between days 173 and 181 and at an HRT of 3 h, corresponding to an OLR of about 5.5 g of COD liter−1 day−1, the treatment efficiency still could be maintained at about 80%.
FIG. 2.
Operation parameters and efficiency of the two-module EGSB reactor system fed with a VFA mixture. (A) ○, OLR; ▵, HRT. (B) ——, CODvfa removal; ○, conversion to CH4.
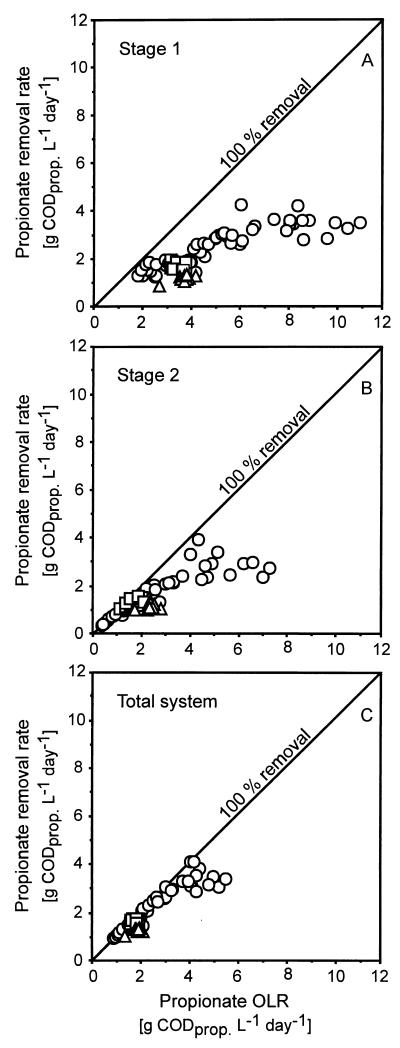
The acetate removal efficiency was 90 to 100% throughout the whole experiment, and the system could accommodate acetate loading rates of 10 g of acetate COD (CODacet) liter−1 day−1 (Table 1). The butyrate removal efficiency gradually increased to 100% and remained stable over the experimental period (Table 1). The system clearly shows some problems with propionate. This can be deduced from the results depicted in Fig. 3, which shows the degradation of propionate in each stage of the system as a function of the propionate OLR. The propionate degradation was far from complete in the first stage (Fig. 3A), but the total system was capable of accommodating propionate loading rates up to 4 g of CODprop liter−1 day−1 with 90 to 95% degradation at an average temperature of 8°C. At 4°C, removal efficiencies over 80% were achieved at propionate loading rates of 2 g of CODprop liter−1 day−1.
TABLE 1.
Average removal efficiency of acetate and butyrate as a percentage of the influent COD of a particular VFA
| Temp (°C) | Avg removal efficiency (%)
|
|||||
|---|---|---|---|---|---|---|
| Acetate
|
Butyrate
|
|||||
| 1st stage | 2nd stage | Total system | 1st stage | 2nd stage | Total system | |
| 8 | 84 | 94 | 97 | 59 | 86 | 90 |
| 4 | 80 | 99 | 100 | 94 | 100 | 100 |
| 3 | 57 | 86 | 86 | 83 | 97 | 99 |
FIG. 3.
Propionate removal rate versus propionate OLR in each module (A and B) and the total system (C). ○, Propionate removal rate at 8°C; □, propionate removal rate at 4°C; ▵, propionate removal rate at 3°C.
Metabolic characteristics of the granule sludge.
Table 2 presents the maximum specific degrading activities (Amax) at 10°C of the inoculum and of sludge samples from each of the modules for propionate, butyrate, and a VFA mixture composed of acetate, propionate, and butyrate in a ratio of 1:1.5:1.8 based on COD. The specific substrate-degrading activities of the sludge increased in time, indicating a good enrichment of methanogens and acetogens under the low-temperature conditions despite the very short liquid retention times applied. The specific activity of the sludge for the VFA mixture and butyrate had doubled after 152 days of operation. Most of the increase in butyrate-degrading activity occurred between days 48 and 152. During this period, a high butyrate removal efficiency had already been achieved in the first module. The specific propionate-degrading activity of the sludge did not increase substantially.
TABLE 2.
Maximum specific substrate-degrading activities at 10°C of inoculum and the EGSB sludge
| Time | Maximum specific substrate-degrading activity (g of COD g of VSS−1 day−1)a
|
||
|---|---|---|---|
| Propionate | Butyrate | VFA mixture | |
| Day 0 | 0.097 (0.007) | 0.053 (0.001) | 0.106 (0.000) |
| 1st stage | |||
| Day 48 | 0.082 (0.000) | 0.056 (0.006) | 0.140 (0.005) |
| Day 152 | 0.093 (0.017) | 0.139 (0.001) | 0.214 (0.002) |
| 2nd stage | |||
| Day 48 | 0.111 (0.008) | 0.068 (0.002) | 0.143 (0.002) |
| Day 152 | 0.110 (0.000) | 0.112 (0.009) | 0.205 (0.002) |
Standard deviations are presented in parentheses.
Psychrophilic propionate degradation.
From the results presented above, it is clear that propionate oxidation is most sensitive in a psychrophilic anaerobic treatment. Therefore, propionate degradation was studied in more detail.
To determine the methane formation rates from acetate and from bicarbonate, during propionate degradation, experiments with the addition of traces of [14C]acetate and [14C]bicarbonate and nonlabeled propionate were performed. A linear methane formation rate in the presence of the isotope traces was observed during the first 10 h of the experiment. The rate of methanogenesis from [14C]acetate was three to five times lower than that from [14C]bicarbonate: 0.015 to 0.020 and 0.058 to 0.072 g of methane COD (CODmeth) g of VSS−1 day−1 of sludge, respectively.
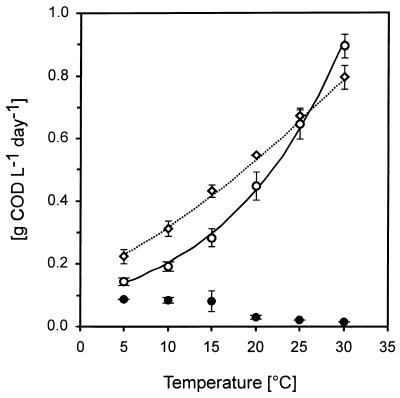
Methane formation from propionate was investigated in batch experiments at 5 to 30°C with 5% (vol/vol) of the reactor biomass (Fig. 4). The maximum methane production rate from 1.25 g of CODprop liter−1 was reached within 1 day at 30°C, and after 2 days of incubation, no propionate could be detected anymore in the medium. The rate of propionate degradation and methane formation was lower at lower temperatures, but still, a relatively high rate (0.15 g of CODmeth liter−1 day−1) of methanogenesis was measured at 5°C. The maximum concentration of acetate detected during the propionate degradation at 25°C was 38 mg of CODacet liter−1, and at 5°C, a value of 178 mg of CODacet liter−1 was found. These values for acetate reflect the difference in the rate of acetate formation and degradation and are in accordance with the results of the labelling experiment described above. The results in Fig. 4 illustrate that the temperature optima of propionate degradation and methane formation are 30°C or higher, despite the fact that the biomass had been grown at 3 to 8°C for 180 days.
FIG. 4.
Temperature characteristics of mesophilic biomass (20 times diluted) exposed for a prolonged period of time to psychrophilic conditions. ○, methane production rate (grams of COD per liter per day); ◊, propionate degradation rate (grams of COD per liter per day); ●, acetate accumulation rate (grams of COD per liter per day). The lines were computed by using the Arrhenius model for the methane production rate (solid line) and the square root model for the propionate degradation rate (dashed line).
The apparent half-saturation constant (Km) for propionate of the sludge present in the second module was estimated in reactor batch mode experiments with sludge taken after 152 days of operation. The Km was 3.75 ± 0.56 mg of CODprop liter−1.
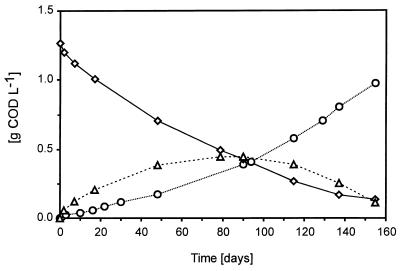
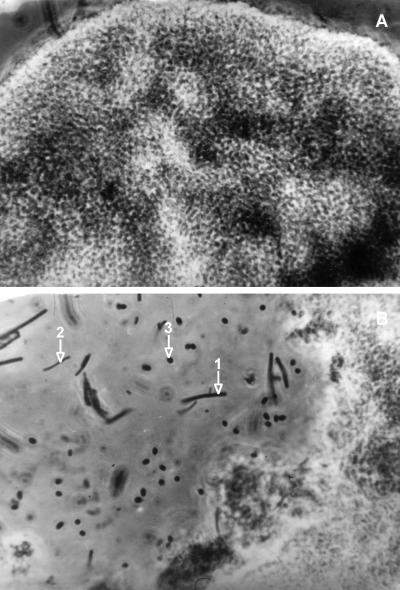
Dilution of the sludge had a strong effect on propionate degradation. In experiments at 5°C with 5% (vol/vol) biomass, it took about 12 days to degrade 1.25 g of CODprop liter−1. This corresponded to a propionate degradation rate of 0.22 g of COD liter−1 day−1. When a lower inoculum concentration (0.5% [vol/vol]) was used, it took more than 160 days to degrade 1.25 g of CODprop liter−1 (see Fig. 6). From this figure, a propionate degradation rate of 0.009 g of COD liter−1 day−1 can be determined. When 0.05% (vol/vol) biomass was used, only half of the initial propionate concentration (1.25 g of CODprop liter−1) was degraded after 300 days of incubation (data not shown). In that case, the estimated rate was 0.004 g of COD liter−1 day−1. Propionate-degrading consortia could be enriched at 10°C. These enrichments were examined microscopically. The microorganisms did not grow suspended, but mainly grew as aggregates (Fig. 5). Some typical morphologies can be recognized. Methanosaeta-like and Methanospirillum-like cells were visible. A highly enriched culture of the Methanospirillum-like cells was obtained at 10°C with H2 or CO2 as a substrate. This methanogen was also able to grow on formate. In addition, oval cells were visible in the propionate-degrading enrichments. These bacteria most likely are the propionate-oxidizing bacteria.
FIG. 6.
Conversion of propionate at 5°C with 200-times-diluted biomass. ◊, propionate; ○, methane; ▵, acetate.
FIG. 5.
Anaerobic cell aggregate and microbial cells. (A) Anaerobic cell aggregate at the end of cultivation at 10°C with a 0.5% inoculum size. Magnification, 100×3.2×2. (B) Methanosaeta-like cells (arrow 1), Methanospirillum-like cells (arrow 2), and presumably propionate-oxidizing bacteria (arrow 3) at the end of cultivation at 10°C with a 0.5% inoculum size. Magnification, 100×3.2×2.
DISCUSSION
Our results indicate that a high-rate anaerobic treatment in a two-stage EGSB system is feasible under very-low-temperature conditions (i.e., down to 3°C). For a VFA mixture as the substrate, COD removal efficiencies of 90% and higher can be achieved at 8 and 4°C at OLRs of 12 and 5 g of COD liter−1 day−1, respectively. The two-stage EGSB concept was capable of accommodating OLRs 5 to 10 times higher at a 90% VFA COD (CODvfa) removal efficiency than those reported so far for psychrophilic anaerobic wastewater treatment (2, 8). Propionate oxidation, which was found to be the most problematic step in anaerobic digestion at low temperature (17), was satisfactory. The specific propionate-degrading activity was high enough and the Km for propionate was low enough to make an anaerobic treatment of dilute cold wastewaters feasible. The low apparent Km values probably can be attributed to excellent mixing conditions prevailing in EGSB reactor systems (9, 17).
Compared to a single-stage reactor system (17), the degradation of propionate improved significantly in a two-stage EGSB system (Fig. 3). The good degradation of fatty acids like acetate and butyrate in the first module clearly improved the overall propionate degradation. Similar observations were previously made for suboptimal thermophilic processes (55 to 65°C) (23, 27). This distinct enhancement of the biodegradation of propionate in a properly designed and operated staged reactor system can be attributed to (i) the development of a balanced microecosystem in the sludge in the separate reactor modules and (ii) the improvement of environmental conditions, such as the lower extent of product inhibition in the conversion of propionate. Particularly in the second stage, where acetate can be maintained at a relatively low level, the conditions for propionate degradation are much more optimal (3). As a consequence of staging, a sludge with a high level of specific propionate-degrading and methanogenic activity will develop in the second stage. This will lead to a substantial increase in the organic loading potential of the system.
The observed increase in specific activities of the granular sludge in time (Table 2) indicates a good enrichment of methanogens and butyrate oxidizers at the low temperatures applied. For the sludge of both stages, the specific activities at 10°C, i.e., for butyrate and for the VFA mixture (Table 2) were higher than the specific activities of sludge in a single-stage system after 235 days of continuous operation on a VFA substrate at 10°C (17). On the other hand, a net growth of propionate oxidizers did not occur in either module (Table 2 and Fig. 6), while propionate-oxidizing consortia could be enriched in batch cultures. Because acetate was accumulated during propionate degradation (Fig. 4 and 6), and propionate oxidizers are sensitive to changes in environmental conditions (3), their growth might have been inhibited by acetate (24), due to the low activity of acetoclastic methanogens at low temperatures. Contrary to propionate oxidizers, the butyrate-oxidizing organisms grew very well, even in the first module. This low butyrate activity of the seed sludge may be attributed to the fact that the seed sludge was cultivated on malting wastewater, which hardly contained butyrate (16) (<0.030 g of butyrate COD [CODbut] liter−1).
The rates of propionate oxidation and methane formation of the sludge were highest at 30°C (Fig. 4). Apparently even after 1.5 years of operation below 10°C, no specialized psychrophilic consortia developed. Moreover, the results also indicate that mesophilic sludge is well able to perform at low temperature, provided that the environmental conditions in the reactor are optimized. In addition to the entrapment of newly grown organisms in the immobilized biomass, the good and stable enrichment of methanogens and butyrate oxidizers can be attributed to the prevailing very low decay rates (Kd) under psychrophilic conditions (25). These features facilitate practical implementation of high-rate anaerobic reactors for application at low temperature, because there is no need to develop specialized psychrophilic populations.
At temperatures below 15°C (Fig. 4 and 6), the observed accumulation of acetate could be due to a low activity of acetoclastic methanogens and/or to an increased activity of homoacetogenic bacteria. The latter seems less likely, because the hydrogen concentration in the reactor modules was much lower (less than 5 nM) than can be reached by homoacetogenic bacteria (about 300 nM) (6). In addition, formate- and hydrogen-utilizing Methanospirillum-like cells were enriched together with propionate oxidizers, indicating that reducing equivalents (hydrogen or formate) during propionate oxidation were mainly utilized by methanogens and not by homoacetogens. The fact that during propionate oxidation, rates of production of 14C-labeled methane from labeled bicarbonate were three- to fivefold higher than those from labeled acetate also indicates that acetate accumulation is not the result of increased homoacetogenic activity.
The rate of acetoclastic methanogenesis in the reactor sludge was much lower than the rate of autotrophic methanogenesis, as measured with labeled substrates. The acetoclastic methanogenesis is more strongly affected by decreasing temperature (10, 14). This explains the observed accumulation of acetate during propionate degradation at low temperatures (Fig. 6). However, for fast propionate degradation, a high rate of acetoclastic methanogenesis is required. Thus, the prevalence of a high density of acetoclastic methanogens in the sludge of the second stage is essential for efficient propionate degradation in that stage (Table 1 and Fig. 3B).
From our experiments, we may conclude that in the two-module reactor system, methanogenic communities were obtained that degraded VFAs, including propionate, completely to CH4 and CO2 at low temperature. A psychrophilic population of microorganisms was not obtained. However, the EGSB reactor configuration enabled a high rate of conversion at a low temperature.
ACKNOWLEDGMENTS
This work was supported by Bavaria B. V., The Netherlands, The Netherlands Science Foundation (NWO) and the Ministry of Science, Russia.
We are grateful to Klaas-Jan Kramer for excellent assistance in performing reactor experiments.
REFERENCES
- 1.Ammann A A, Ruttimenn T B. Simultaneous determination of small organic and inorganic anions in environmental water samples by ion-exchange chromatography. J Chromatogr. 1995;706:259–269. [Google Scholar]
- 2.Banik G C, Dague R R. Proceedings of the 69th Annual Water Environmental Conference, Dallas, Tex. 1996. ASBR treatment of dilute wastewater at psychrophilic temperatures; pp. 235–246. [Google Scholar]
- 3.Boone D R, Bryant M P. Propionate-degrading bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from methanogenic ecosystems. Appl Environ Microbiol. 1980;40:626–632. doi: 10.1128/aem.40.3.626-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conrad R, Wetter B. Influence of temperature on energetics of hydrogen metabolism in homoacetogenic, methanogenic, and other anaerobic bacteria. Arch Microbiol. 1990;155:94–98. [Google Scholar]
- 5.Conrad R, Bak F, Seitz H J, Thebrath B, Mayer H P. Hydrogen turnover by psychrotrophic homoacetogenic and mesophilic methanogenic bacteria in anoxic paddy soil and lake sediment. FEMS Microbiol Ecol. 1989;62:285–294. [Google Scholar]
- 6.Cord-Ruwisch R, Seitz H J, Conrad R. The capacity of hydrogenotrophic anaerobic bacteria to compete for traces of hydrogen depends on redox potential of terminal electron acceptor. Arch Microbiol. 1988;149:350–357. [Google Scholar]
- 7.De Man A W A, Van der Last A R M, Lettinga G. The use of EGSB and UASB anaerobic systems for low strength soluble and complex wastewaters at temperatures ranging from 8 to 30°C. In: Hall E R, Hobson P N, editors. Proceedings of the Fifth International Symposium on Anaerobic Digestion, Bologna, Italy. 1988. pp. 197–209. [Google Scholar]
- 8.Grant S, Lin K C. Effects of temperature and organic loading on the performance of upflow anaerobic sludge blanket reactors. Can J Civ Eng. 1995;22:143–149. [Google Scholar]
- 9.Kato T M, Field J A, Versteeg P, Lettinga G. Feasibility of expanded granular sludge bed reactors for the anaerobic treatment of low strength soluble wastewaters. Biotechnol Bioeng. 1994;44:469–479. doi: 10.1002/bit.260440410. [DOI] [PubMed] [Google Scholar]
- 10.Kotsyurbenko O R, Nozhevnikova A N, Soloviova T I, Zavarzin G A. Methanogenesis at low temperature by microflora of tundra wet land soil. Antonie van Leeuwenhoek. 1996;69:75–86. doi: 10.1007/BF00641614. [DOI] [PubMed] [Google Scholar]
- 11.Kotsyurbenko O R, Simankova M V, Nozhevnikova A N, Zhilina T N, Bolotina N P, Lysenko A M, Osipov G A. New species of psychrophilic acetogens: Acetobacterium bakii sp. nov., A. paludosum sp. nov., A. fimetarium sp. nov. Arch Microbiol. 1995;163:429–434. [Google Scholar]
- 12.Lettinga G. Anaerobic digestion and wastewater treatment systems. Antonie van Leeuwenhoek. 1995;67:3–28. doi: 10.1007/BF00872193. [DOI] [PubMed] [Google Scholar]
- 13.Matsushige K, Inamori Y, Mizuochi M, Hosomi M, Sudo R. The effects of temperature on anaerobic filter treatment for low-strength organic wastewater. Environ Technol. 1990;11:899–910. [Google Scholar]
- 14.Nozhevnikova A N, Holliger C, Ammann A, Zehnder A J B. Psychrophilic methanogenesis in sediments of deep lakes. Water Sci Technol. 1997;36:57–64. [Google Scholar]
- 15.Parshina S N, Nozhevnikova A N, Kalyuzhnyi S V. Decomposition of protein substrates at low temperature by the microflora of pig manure. Microbiology. 1993;62:121–129. [Google Scholar]
- 16.Rebac S, Van Lier J B, Janssen M G J, Dekkers F, Swinkels K T M, Lettinga G. High-rate anaerobic treatment of malting waste water in a pilot-scale EGSB system under psychrophilic conditions. J Chem Technol Biotechnol. 1997;68:135–146. [Google Scholar]
- 17.Rebac S, Ruskova J, Gerbens S, Van Lier J B, Stams A J M, Lettinga G. High-rate anaerobic treatment of wastewater under psychrophilic conditions. J Ferment Bioeng. 1995;80:499–506. [Google Scholar]
- 18.Romesser J A, Wolfe R S, Mayer F, Spiess E, Walther-Mauruschat A. Methanogenium, a new genus of marine methanogenic bacteria, and characterization of Methanogenium cariaci sp. nov. Methanogenium marisnigri sp. nov. Arch Microbiol. 1979;121:147–153. [Google Scholar]
- 19.Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev. 1997;61:262–280. doi: 10.1128/mmbr.61.2.262-280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stams A J M. Metabolic interactions between anaerobic bacteria in methanogenic environments. Antonie van Leeuwenhoek. 1994;66:271–294. doi: 10.1007/BF00871644. [DOI] [PubMed] [Google Scholar]
- 21.Switzenbaum M S, Jewell W J. Proceedings of the 51st Annual WPCF Conference, Anaheim, Calif. 1978. Anaerobic attached film expanded bed reactor treatment of dilute organics; pp. 1–164. [Google Scholar]
- 22.Van der Last A R M, Lettinga G. Anaerobic treatment of domestic sewage under moderate climatic (Dutch) conditions using upflow reactors at increased superficial velocities. Water Sci Technol. 1992;25:167–178. [Google Scholar]
- 23.Van Lier J B, Boersma F, Debets M M W H, Lettinga G. High-rate thermophilic anaerobic wastewater treatment in compartmentalized upflow reactors. Water Sci Technol. 1994;30:251–261. [Google Scholar]
- 24.Van Lier J B, Grolle K C F, Frijters C T M J, Stams A J M, Lettinga G. Effect of acetate, propionate, and butyrate on the thermophilic anaerobic degradation of propionate by methanogenic sludge and defined cultures. Appl Environ Microbiol. 1993;57:1003–1011. doi: 10.1128/aem.59.4.1003-1011.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Lier J B, Rebac S, Lens P, Van Bijnen F, Oude Elferink S J W, Stams A J M, Lettinga G. Anaerobic treatment of partly acidified wastewater in a two-stage expanded granular sludge bed (EGSB) system at 8°C. Water Sci Technol. 1997;36:317–324. [Google Scholar]
- 26.Wheatley A, editor. Anaerobic digestion: a waste treatment technology. London, United Kingdom: Elsevier Applied Science; 1990. [Google Scholar]
- 27.Wiegant W M, Hennink M, Lettinga G. Separation of the propionate degradation to improve the efficiency of thermophilic anaerobic treatment of acidified wastewaters. Water Res. 1986;20:517–524. [Google Scholar]
- 28.Zehnder A J B, Huser B, Brock T D. Measuring radioactive methane with the liquid scintillation counter. Appl Environ Microbiol. 1979;37:897–899. doi: 10.1128/aem.37.5.897-899.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]