Abstract
Because of their long half-lives and highly nucleophilic tails, histones are particularly susceptible to accumulating nonenzymatic covalent modifications, such as glycation. The resulting modifications can have profound effects on cellular physiology due to the regulatory role histones play in all DNA-templated processes; however, the complexity of Maillard chemistry on proteins makes tracking and enriching for glycated proteins a challenging task. Here, we characterize glyoxal (GO) modifications on histones using quantitative proteomics and an aniline-derived GO-reactive probe. In addition, we leverage this chemistry to demonstrate that the glycation regulatory proteins DJ-1 and GLO1 reduce levels of histone GO adducts. Finally, we employ a two-round pull-down method to enrich histone H3 GO glycation and map these adducts to specific chromatin regions.
Graphical Abstract
Eukaryotic chromatin is comprised of nucleosomal core particles (NCPs) that contain 147 base pairs of DNA spooled around an octamer of histones. The physiochemical interactions between the histones and the DNA dictate chromatin architecture and accessibility of DNA to effector proteins.1 This is often regulated through the dynamic installation of post-translational modifications (PTMs) on the N-terminal tails of histones.1 Histone tails are unstructured, accessible, and contain an overrepresentation of lysine and arginine, the major targets for PTMs.1 Most of these PTMs are tightly regulated by enzymes that write and erase them, determining the combination of PTMs at a certain genomic loci and thus its epigenetic state.1,2 Dysregulated histone PTMs are implicated in aberrant cellular function and disease states including cancer3
In addition to enzymatically regulated PTMs, histone tails are susceptible to nonenzymatic covalent modifications (NECMs).2,4 These modifications are derived from both endogenously and exogenously generated electrophilic metabolites.4 One of the most well-characterized NECMs on histones is glycation, a condensation of aldose monosaccharides or byproducts of glycolysis via the Maillard reaction.5 Initial glycation adducts can further rearrange to form advanced glycation end products (AGEs). In hyperglycemic states, as observed in diabetes or cancers, AGE precursors such as glyoxal (GO) and methylglyoxal (MGO) are elevated and have been demonstrated to glycate proteins leading to significantly altered protein function.6–8.
Along with their abundance, histones have long half-lives,9 which can contribute to the accumulation of adducts such as glycation.2 Recently, several sites on histones have been found to be glycated including ones known to carry key regulatory enzymatic PTMs.10,11 We have previously demonstrated that MGO, a reactive byproduct of glycolysis, modifies histones, especially H3.12 In addition, we showed that glycation of H3 by MGO is elevated in breast cancer and affects chromatin architecture and transcription. These effects are due to both competition with enzymatic PTMs as well as alteration of the biophysical properties of chromatin.12 Protein glycation serves as a challenge in identification and characterization, as it yields a mixture of species due to the complex cascade of reactions that follows initial glycation. While antibodies exist to detect several types of AGEs, they are either not targeted to specific adducts or insufficient for tracking and enriching earlier species. Recently, antibodies targeting an established stable intermediate of the MGO-glycation cascade have been generated.13 An alternative approach involves the design of small chemical probes that can be employed to capture these modifications in cells. Our lab and others have developed several chemical probes to track and enrich for MGO-14–16 as well as ribose-derived17 glycation products, providing a framework to study these challenging histone PTMs.
Similarly, an aniline-derived chemical probe was developed to capture ferroptotic carbonylation products.18 The probe leverages aniline’s chemoselectivity with aldeyhydes and contains an alkynyl moiety for enrichment.18 The formation of AGEs by GO-derived modifications is initiated by an unstable glyoxal-imine intermediate that contains a free aldehyde group.19 This imine eventually forms the AGE carboxymethyllysine (CML) via a Cannizzaro reaction.20 Thus, Chen et al. used this aniline probe to capture early GO-modified adducts on proteins from cell lysate.21 Here we characterize GO glycation using quantitative mass spectrometry (MS) and apply aniline chemistry to label and enrich for GO-derived histones.
RESULTS
To better understand GO glycation on histones we first set out to determine the abundance, chemical composition, and site specificity of these PTMs. Using the MS method Quantitative Analysis of Arginine and Lysine Modifications (QuARKMod), we quantified GO-derived modifications on free histones and chromatin fractions isolated from HEK293T cells treated with increasing concentrations of GO.22 As shown in Figure 1a–c and Figure S1 & S2, GO-derived arginine modifications (CMA, GH-1) exist at levels far lower than GO-derived lysine modifications (CML). In contrast, the profile of MGO-derived PTMs demonstrates a preference for arginine modifications over lysine.11
Figure 1.

Mass spectrometry-based characterization of GO-glycation. (a–c) QuARKMod quantification of total dicarbonyl PTMs in chromatin (error bars represent SEM). (d) Representative MS/MS spectra of H3K79 CML adduct. (e) Sites of glyoxal modification on core histones as determined by MS.
We next sought to evaluate the site specificity of GO modification on histones. Briefly, chromatin was isolated from HEK293T cells treated with GO and separated via sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Histones were then excised and subjected to proteomic preparation (Figure 1d, Figure S3a–j).11,23 A compilation of all sites identified on each of the four core histones is summarized in Figure 1e. It is noteworthy that most of the sites identified are known to carry enzymatic modifications used to regulate chromatin structure.24 For example, H3K79 methylation is associated with actively transcribed chromatin regions and promoting DNA damage repair.25 Blocking of these sites by glycation could therefore disrupt gene accessibility and chromatin repair. Across the core histones, modification of lysine residues was more prominent, reflecting the detected levels of GO adducts we described above.
Because of the general harmful nature of protein glycation events, cells have developed various mechanisms to prevent and remove them. One such example is the glyoxalase cycle. The glyoxalase cycle enzymes glyoxalase 1 (GLO1) and 2 (GLO2) metabolize MGO, preventing its accumulation and modification of proteins.26 Rabbani and Thornalley have alluded that GO can be metabolized in a similar way.26 Metabolism of these dicarbonyls is executed in three sequential steps. First, glutathione reacts with the dicarbonyl, yielding a labile hemithioacetal (HTA). Thereafter, GLO1 catalyzes the isomerization of the HTA into a stable acylglutathione (i.e., glycoylglutathione, GGSH). Finally, GLO2 hydrolyzes LGSH and GGSH to yield glycolate (Scheme S1).26 To characterize the specificity of GLO1 toward MGO and GO, we performed a GLO1 activity assay using liquid chromatography with tandem mass spectrometry (LC-MS/MS). Our data (Figure S4) reveal that GLO1 prefers MGO over GO; that is, GLO1 is slower at catalyzing the conversion of GO-HTA to GGSH when compared to the conversion of MGO-HTA to LGSH. These data indicate that free GO may persist longer than MGO in the cell and underscore the need to further characterize GO adducts and study their effects.
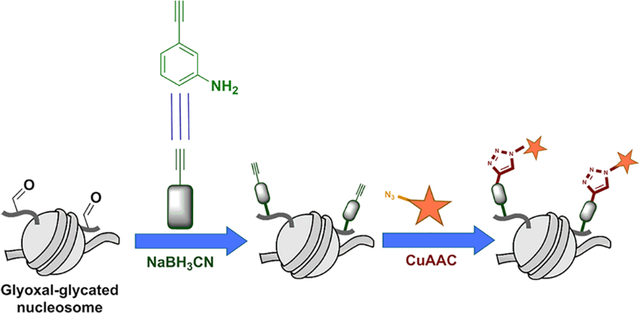
Since a lysine modification, CML, is the most prominent GO adduct we detected, we turned toward tracking this modification. As seen in Scheme 1, an early intermediate of the GO glycation of lysine residues involves a free aldehyde. This carbonyl-containing intermediate is thus ideally suited for capture by the aniline-derived probe m-aminophenylacetylene (m-APA) allowing us to further investigate GO-derived modifications.21 Previous work has established m-APA as more efficient at capturing free aldehydes in cells than other aldehyde-directed probes.18,21 These works have also determined the optimal conditions of use for saturation of the labeling reaction;18,21 we therefore employed these same conditions with modifications for our biochemical analyses and target protein enrichment.
Scheme 1. Protein Glycation by GO (blue), Deglycation by DJ-1, and GO Capture by m-APA (red).

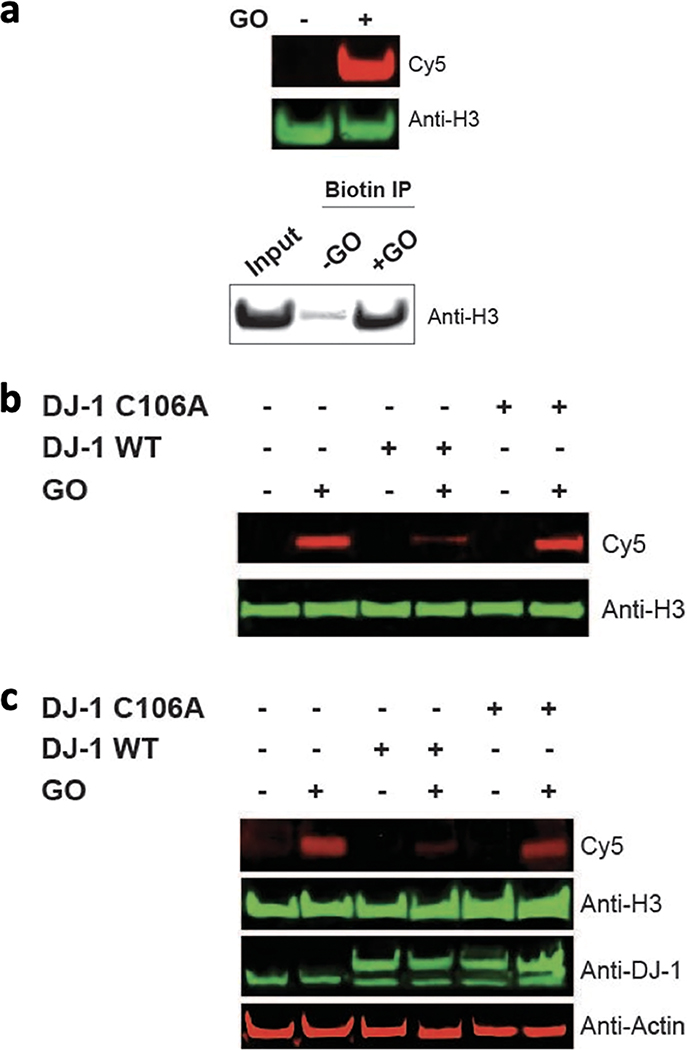
Our previous work demonstrated that H3 is a prime target of glycation by MGO; thus, we chose to focus on H3 as a model substrate.12 We first set out to confirm that H3 glycation by GO could be captured by the m-APA probe. Free H3 was treated with GO followed by an incubation with mAPA. H3 was then subjected to Cu-catalyzed click chemistry, separated by SDS-PAGE, and analyzed by Cy5 fluorescence and western blot analyses as well as biotin–streptavidin enrichment (Figure 2a). As expected, in the presence of GO, histone H3 was labeled with Cy5 and biotin. These data highlight not only that H3 is glycated by GO but also that the resulting adducts can be tracked and enriched through this approach.
Figure 2.
Visualization of GO modification on H3. (a) Free H3 was treated with 2 mM of GO and 5 mM m-APA then clicked with Cy5-azide or biotin azide. The biotinylated H3 was then subjected to a biotin IP. Analysis was done by SDS-PAGE followed by a western blot (Cy5–red and Anti-H3–green). (b) NCPs were coincubated with 2 mM GO in the absence or presence of WT or catalytically inactive (C106A)DJ-1. The NCPs were then treated with 5 mM m-APA and clicked with Cy5-azide. Analysis was done by SDS-PAGE followed by a western blot (Cy5–red and Anti-H3–green). (c) HEK293T cells were transfected with myc-tagged WT or catalytically inactive DJ-1 and then treated with 0.8 mM GO overnight at 37 °C. Lysates were treated with 5 mM m-APA, and histones were extracted followed by click of Cy5-azide. Analysis was performed by SDS-PAGE followed by a western blot. IP, immunoprecipitation.
Recently, we reported that the enzyme DJ-1 removes early histone MGO glycation adducts.11,12 DJ-1 is a multifaceted protein with established roles as a deglycase, and it has been implicated in many diseases such as Parkinson’s and cancer.12,27,28 DJ-1 has also been shown to be promiscuous based on its ability to serve as a deglycase on nucleic acids and peptide substrates alike as well as its possession of a broad esterase functionality.29 On the basis of MGO and GO’s structural similarities and metabolism by GLO1, we hypothesized that DJ-1 would erase GO glycation adducts. To test this, we assembled recombinant NCPs12 containing octamers of the four core histones wrapped in a double-stranded DNA segment and treated them with GO in the presence of DJ-1 or its catalytically inactive mutant (DJ-1 C106A). NCPs were then subjected to the same m-APA capture and subsequent click reactions with Cy5-azide, separated on a native gel, and analyzed by Cy5 fluorescence and western blot analysis (Figure 2b). Our data indicate that the Cy5 glycation signal is dramatically reduced in the presence of DJ-1 but remains unchanged in the presence of DJ-1 C106A. To further validate our findings, we examined the capacity of DJ-1 to rescue GO-glycation in live cells. HEK293T cells were transfected with DJ-1 or DJ-1 C106A and then incubated with GO overnight. Cells were similarly processed as described above; the histone fraction was separated on an SDS-PAGE and analyzed by western blot (Figure 2c). Cy5 glycation signal was, as expected, substantially reduced in cells overexpressing DJ-1 but remained persistent in cells expressing DJ-1 C106A. Together with our previous reports on MGO, these data indicate that DJ-1 plays a significant role in preventing and reversing glycation damage on chromatin.12,30
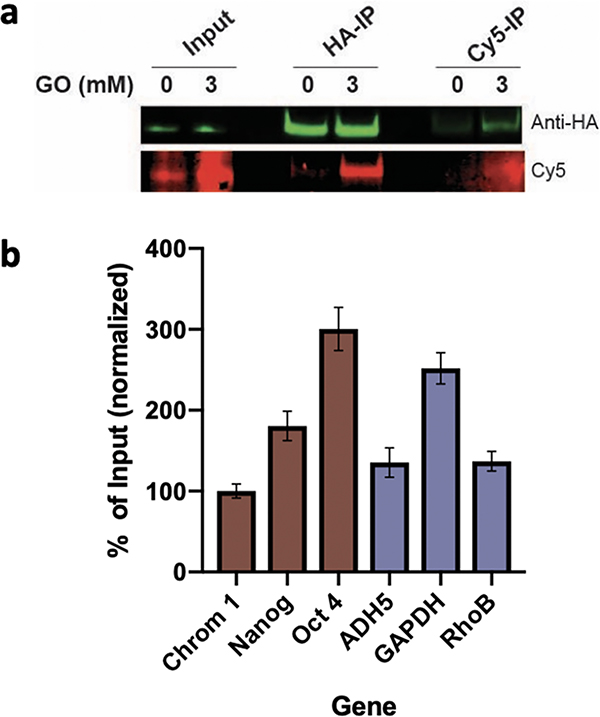
To further explore the effects of GO glycation on chromatin, we employed the m-APA probe to enrich for GO-modified H3 and its associated genes. HEK293T cells were transfected with a hemagglutinin (HA)-tagged H3 construct (Figure S5). Histones expressed through transient transfection are integrated into chromatin similarly to endogenous histones, with no material physiological consequences.17,31 Nuclei of the transfected cells were then isolated and treated with GO for 1 hour before the addition of the m-APA probe. Following fixation and clicking of Cy5, nuclei were sonicated to shear the DNA, and H3 was enriched via HA immunoprecipitation allowing for isolation of H3 (and its associated genes) from all the other potentially glycated proteins. This was followed by a second enrichment step through a Cy5 immunoprecipitation to isolate glycated from nonglycated H3 (Figure 3a). DNA was de-cross-linked, eluted from the beads, and analyzed using quantitative polymerase chain reaction (qPCR) to determine the enrichment of specific representative chromatin regions including centromeric, (constitutive heterochromatin), developmentally repressed (facultative chromatin), and actively transcribed (euchromatin).32 We selected genes representing these regions to determine if histones populating more accessible euchromatic regions accumulate greater levels of GO glycation than those in the less-accessible heterochromatin regions. Our results, presented in Figure 3b, suggest a heterogeneous distribution and diversified abundance of the GO adducts along the entire chromatin. The fact that these regions show a similarly heterogeneous distribution suggests that the accessibility of the histones is not a significant factor in determining the distribution of this mark.
Figure 3.
Click-ChIP and qPCR of glycated H3. (a) Western blot analysis of untreated and treated (3 mM GO) HEK293T nuclei after Cy5-azide click (lanes 1&2), HA-IP (lanes 3&4), and Cy5-IP (lanes 5&6). (b) qPCR of the enrichment over input of treated nuclei following two-round IP (lane 4) for representative genes (brown = heterochromatin, purple = euchromatin), normalized to chromosome 1 centromere (N = 3, ±SEM). IP, immunoprecipitation.
DISCUSSION
NECMs represent an emerging point of interest in the investigation of protein regulation and function.12,31 Despite a lack of direct programing by the cell, NECMs are prominent modifications, and their significance can be seen both in the disruption of normal cellular processes and the regulatory mechanisms that govern them.2 Since NECMs are directly affected by the local concentration of certain metabolites, they serve as an important new link between cellular microenvironment, metabolism, and fate. However, since the installation of NECMs is not enzymatically directed, it is challenging to control, and thus interrogate, their presence and effects. Glycation is particularly challenging due to the chemical complexity and diversity of the accumulated modifications. Therefore, specialized tools and methods are necessary to examine glycation modifications and its consequences.
In this work, we report the characterization of GO glycation on histones. Using quantitative LC-MS/MS, we identified lysine residues as the primary target of GO glycation. Proteomics data identified several key sites of GO modifications on the core histones; all but one of these sites are modified by enzymatically driven PTMs (i.e., methylation and acetylation) involved in the regulation of chromatin states.24 Furthermore, the identification of GO as a lesspreferred substrate of GLO1 potentially leads to a persistence of GO in the cell compared to MGO. By employing the m-APA probe, we were able to investigate histone GO glycation along with their regulation by the deglycase DJ-1. This probe enables the tagging, enrichment, and subsequent analysis of H3 glycation and the localization at certain genomic regions. Further work to characterize a global genomic profile could reveal if certain chromatin regions are differentially modified.
Levels of GO fluctuate depending on physiological states of cells and their environments. Best estimates have stated concentrations in the micromolar range, although these numbers can increase in pathological states.33 Furthermore, these concentrations are at best rough estimates, having been obtained after cell lysis, which can alter the metabolism of GO. Recently, new probes have been developed to more precisely measure in cell concentrations, such as the use of a fluorescent probe to measure MGO, but this has not yet been extended to GO.15
Here we applied higher concentrations of GO than have previously been measured. This relies on our preceding studies on the related molecule MGO demonstrating that the shortterm treatment of high concentrations induces similar effects of long-term treatment with low concentrations.12 This is the basis for mimicking metabolic disorders through a treatment with high levels of corresponding metabolites.31,34 Thus, the use of a higher concentration of GO in this case was used to simulate the accumulation of GO.
GO glycation leads to the formation of AGEs, which are present in numerous disease states, most significantly, diabetes and cancer6–8. Indeed, plasma levels of GO and MGO have been shown to be elevated in diabetes and renal failure.35 This makes GO adducts attractive candidates as biomarkers and potential players in mechanisms for disease promotion, supporting the need for methods to track and characterize them. The ability to generate and utilize chemical probes, as the one described here, has allowed for a much more detailed investigation into these modifications.14,17 In the future, m-APA can be utilized to further characterize the mechanism of GO-glycation and the disruption it induces to histone function and regulation under physiological and pathophysiological states.
Supplementary Material
ACKNOWLEDGMENTS
We extend our gratitude to the members of the David and Galligan laboratories for their invaluable support. We especially thank N. Prescott for qPCR technical assistance R. Pihl for idea refinement. Work in the David lab is supported by the Josie Robertson Foundation, the Pershing Square Sohn Cancer Research Alliance, the NIH (CCSG core Grant No. P30 CA008748, MSK SPORE P50 CA192937, and R35 GM138386), the Parker Institute for Cancer Immunotherapy, the STARR Cancer Alliance award, and the Anna Fuller Trust. In addition, the David lab is supported by W. H. Goodwin, A. Goodwin, and the Commonwealth Foundation for Cancer Research and the Center for Experimental Therapeutics at MSKCC. J.G. is supported by R35 GM137910, and Q.Z. is supported by the OSUCCC start-up funds. D.M.R. is supported by an MSTP grant from the National Institute of General Sciences of the NIH (T32 GM007739) to the Weill Cornell/Rockefeller/Sloan Kettering Tri-Institutional MD-PhD Program. E.J. is supported by NIH training Grant No. T32 ES007091.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschembio.1c00864.
Materials used, quantification of GLO1 activity, QuARKMod PTM analysis, preparation of nucleosomes, glycation and click labeling assays, enrichment of modified histones, Click-ChIP, and qPCR (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acschembio.1c00864
Contributor Information
Devin M. Ray, Tri-Institutional MD-PhD Program, New York, New York 10065, United States; Tri-Institutional PhD Program in Chemical Biology, New York, New York 10065, United States; Chemical Biology Program, Memorial Sloan Kettering Cancer Center, New York, New York 10065, United States.
Erin Q. Jennings, Department of Pharmacology and Toxicology, College of Pharmacy, University of Arizona, Tucson, Arizona 85721, United States.
Igor Maksimovic, Tri-Institutional PhD Program in Chemical Biology, New York, New York 10065, United States; Chemical Biology Program, Memorial Sloan Kettering Cancer Center, New York, New York 10065, United States.
Xander Chai, Department of Pharmacology and Toxicology, College of Pharmacy, University of Arizona, Tucson, Arizona 85721, United States.
James J. Galligan, Department of Pharmacology and Toxicology, College of Pharmacy, University of Arizona, Tucson, Arizona 85721, United States.
Yael David, Tri-Institutional PhD Program in Chemical Biology, New York, New York 10065, United States; Chemical Biology Program, Memorial Sloan Kettering Cancer Center, New York, New York 10065, United States; Department of Pharmacology and Department of Physiology, Biophysics and Systems Biology, Weill Cornell Medical College, New York, New York 10065, United States.
Qingfei Zheng, Department of Radiation Oncology, College of Medicine and Center for Cancer Metabolism, James Comprehensive Cancer Center, The Ohio State University, Columbus, Ohio 43210, United States.
REFERENCES
- (1).Jenuwein T; Allis CD Translating the Histone Code. Science 2001, 293, 1074–1080, DOI: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- (2).Zheng Q; Prescott NA; Maksimovic I; David Y (De)Toxifying the Epigenetic Code. Chem. Res. Toxicol 2019, 32 (5), 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Chi P; Allis CD; Wang GG Covalent Histone Modifications-Miswritten, Misinterpreted and Mis-Erased in Human Cancers. Nat. Rev. Cancer 2010, 10, 457–469, DOI: 10.1038/nrc2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Zheng Q; Maksimovic I; Upad A; David Y Non-Enzymatic Covalent Modifications: A New Link between Metabolism and Epigenetics. Protein Cell 2020, 11 (6), 401–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Hellwig M; Henle T Baking, Ageing, Diabetes: A Short History of the Maillard Reaction. Angew. Chemie Int. Ed 2014, 53 (39), 10316–10329. [DOI] [PubMed] [Google Scholar]
- (6).Gugliucci A; Bendayan M Histones from Diabetic Rats Contain Increased Levels of Advanced Glycation End Products. Biochem. Biophys. Res. Commun 1995, 212 (1), 56–62. [DOI] [PubMed] [Google Scholar]
- (7).Ang SH; Thevarajah M; Alias Y; Khor SM Current Aspects in Hemoglobin A1c Detection: A Review. Clin. Chim. Acta 2015, 439, 202–211, DOI: 10.1016/j.cca.2014.10.019. [DOI] [PubMed] [Google Scholar]
- (8).Volpe CMO; Villar-Delfino PH; Dos Anjos PMF; Nogueira-Machado JA Cellular Death, Reactive Oxygen Species (ROS) and Diabetic Complications Review-Article. Cell Death Diss 2018, 9 (2). DOI: 10.1038/s41419-017-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Commerford SL; Carsten AL; Cronkite EP Histone Turnover within Nonproliferating CellsProc. Natl. Acad. Sci. USA, 1982, 79 DOI: 10.1073/pnas.79.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Guedes S; Vitorino R; Domingues MRM; Amado F; Domingues P Glycation and Oxidation of Histones H2B and H1: In Vitro Study and Characterization by Mass Spectrometry. Anal. Bioanal. Chem 2011, 399 (10), 3529–3539. [DOI] [PubMed] [Google Scholar]
- (11).Galligan JJ; Wepy JA; Streeter MD; Kingsley PJ; Mitchener MM; Wauchope OR; Beavers WN; Rose KL; Wang T; Spiegel DA; et al. J. Methylglyoxal-Derived Posttranslational Arginine Modifications Are Abundant Histone Marks. Proc. Natl. Acad. Sci. U. S. A 2018, 115 (37), 9228–9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Zheng Q; Omans ND; Leicher R; Osunsade A; Agustinus AS; Finkin-Groner E; D’Ambrosio H; Liu B; Chandarlapaty S; Liu S; David Y Reversible Histone Glycation Is Associated with Disease-Related Changes in Chromatin Architecture. Nat. Commun 2019, 10 (1). DOI: 10.1038/s41467-019-09192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Wang T; Streeter MD; Spiegel DA Generation and Characterization of Antibodies against Arginine-Derived Advanced Glycation Endproducts. Bioorg. Med. Chem. Lett 2015, 25 (21), 4881–4886. [DOI] [PubMed] [Google Scholar]
- (14).Zheng Q; Maksimovic I; Upad A; Guber D; David Y Synthesis of an Alkynyl Methylglyoxal Probe to Investigate Non-enzymatic Histone Glycation. J. Org. Chem 2020, 85 (3), 1691–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Wang T; Douglass EF; Fitzgerald KJ; Spiegel DAA “Turn-On” Fluorescent Sensor for Methylglyoxal. J. Am. Chem. Soc 2013, 135 (33), 12429–12433. [DOI] [PubMed] [Google Scholar]
- (16).Sibbersen C; Schou Oxvig AM; Bisgaard Olesen S; Nielsen CB; Galligan JJ; Jørgensen KA; Palmfeldt J; Johannsen M Profiling of Methylglyoxal Blood Metabolism and Advanced Glycation End-Product Proteome Using a Chemical Probe. ACS Chem. Biol 2018, 13 (12), 3294–3305. [DOI] [PubMed] [Google Scholar]
- (17).Maksimovic I; Zheng Q; Trujillo MN; Galligan JJ; David Y An Azidoribose Probe to Track Ketoamine Adducts in Histone Ribose Glycation. J. Am. Chem. Soc 2020, 142 (22), 9999–10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chen Y; Liu Y; Lan T; Qin W; Zhu Y; Qin K; Gao J; Wang H; Hou X; Chen N; Friedmann Angeli JP; Conrad M; Wang C Quantitative Profiling of Protein Carbonylations in Ferroptosis by an Aniline-Derived Probe. J. Am. Chem. Soc 2018, 140 (13), 4712–4720. [DOI] [PubMed] [Google Scholar]
- (19).Glomb MA; Pfahler C Amides Are Novel Protein Modifications Formed by Physiological Sugars. J. Biol. Chem 2001, 276 (45), 41638–41647. [DOI] [PubMed] [Google Scholar]
- (20).Glomb MA; Monnier VM Mechanism of Protein Modification by Glyoxal and Glycolaldehyde, Reactive Intermediates of the Maillard Reaction. J. Biol. Chem 1995, 270 (17), 10017–10026. [DOI] [PubMed] [Google Scholar]
- (21).Chen Y; Qin W; Li Z; Guo Z; Liu Y; Lan T; Wang C Site-Specific Chemoproteomic Profiling of Targets of Glyoxal. Future Med. Chem 2019, 11 (23), 2979–2987. [DOI] [PubMed] [Google Scholar]
- (22).Galligan JJ; Kingsley PJ; Wauchope OR; Mitchener MM; Camarillo JM; Wepy JA; Harris PS; Fritz KS; Marnett LJ Quantitative Analysis and Discovery of Lysine and Arginine Modifications. Anal. Chem 2017, 89 (2), 1299–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Garcia BA; Mollah S; Ueberheide BM; Busby SA; Muratore TL; Shabanowitz J; Hunt DF Chemical Derivatization of Histones for Facilitated Analysis by Mass Spectrometry. Nat. Protoc 2007, 2 (4), 933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Huang H; Sabari BR; Garcia BA; David Allis C; Zhao Y SnapShot: Histone Modifications. Cell; Cell Press, 2014; pp 458–458.e1. DOI: 10.1016/j.cell.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Ljungman M; Parks L; Hulbatte R; Bedi K The Role of H3K79 Methylation in Transcription and the DNA Damage Response. Mutation Research - Reviews in Mutation Research; Elsevier B.V, 2019; pp 48–54. DOI: 10.1016/j.mrrev.2017.11.001. [DOI] [PubMed] [Google Scholar]
- (26).Gaffney DO; Jennings EQ; Anderson CC; Marentette JO; Shi T; Schou Oxvig AM; Streeter MD; Johannsen M; Spiegel DA; Chapman E; et al. Non-Enzymatic Lysine Lactoylation of Glycolytic Enzymes. Cell Chem. Biol 2020, 27 (2), 206–213e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Richarme G; Mihoub M; Dairou J; Bui LC; Leger T; Lamouri A Parkinsonism-Associated Protein DJ-1/Park7 Is a Major Protein Deglycase That Repairs Methylglyoxal- and Glyoxal-Glycated Cysteine, Arginine, and Lysine Residues. J. Biol. Chem 2015, 290 (3), 1885–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Scumaci D; Olivo E; Fiumara CV; La Chimia M; De Angelis MT; Mauro S; Costa G; Ambrosio FA; Alcaro S; Agosti V; et al. DJ-1 Proteoforms in Breast Cancer Cells: The Escape of Metabolic Epigenetic Misregulation. Cells 2020, 9 (9), 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Maksimovic I; Finkin-Groner E; Fukase Y; Zheng Q; Sun S; Michino M; Huggins DJ; Myers RW; David Y Deglycase-Activity Oriented Screening to Identify DJ-1 Inhibitors. RSC Med. Chem 2021, 12 (7), 1232–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Richarme G; Liu C; Mihoub M; Abdallah J; Leger T; Joly N; Liebart JC; Jurkunas UV; Nadal M; Bouloc P; Dairou J; et al. Guanine Glycation Repair by DJ-1/ Park7 and Its Bacterial Homologs. Science (80-.) 2017, 357 (6347), 208–211. [DOI] [PubMed] [Google Scholar]
- (31).Bollong MJ; Lee G; Coukos JS; Yun H; Zambaldo C; Chang JW; Chin EN; Ahmad I; Chatterjee AK; Lairson LL; et al. A Metabolite-Derived Protein Modification Integrates Glycolysis with KEAP1–NRF2 Signalling. Nature 2018, 562 (7728), 600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).David Y; Vila-Perelló M; Verma S; Muir TW Chemical Tagging and Customizing of Cellular Chromatin States Using Ultrafast Trans-Splicing Inteins. Nat. Chem 2015, 7 (5), 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Thornalley PJ Protein and Nucleotide Damage by Glyoxal and Methylglyoxal in Physiological Systems - Role in Ageing and Disease. Drug Metabolism and Drug Interactions; Freund Publishing House Ltd, 2008; pp 125–150. DOI: 10.1515/DMDI.2008.23.1-2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Sanghvi VR; Leibold J; Mina M; Mohan P; Berishaj M; Li Z; Miele MM; Lailler N; Zhao C; de Stanchina E; et al. The Oncogenic Action of NRF2 Depends on De-Glycation by Fructosamine-3-Kinase. Cell 2019, 178 (4), 807–819e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Odani H; Shinzato T; Usami J; Matsumoto Y; Brinkmann Frye E; Baynes JW; Maeda K Imidazolium Crosslinks Derived from Reaction of Lysine with Glyoxal and Methylglyoxal Are Increased in Serum Proteins of Uremic Patients: Evidence for Increased Oxidative Stress in Uremia. FEBS Lett 1998, 427 (3), 381–385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.