Abstract
Background
Messenger RNA coronavirus disease 2019 (COVID-19) vaccines have been associated with allergic reactions. A history of anaphylaxis has been suggested as a risk factor for such reactions. Polyethylene glycol (PEG) has been proposed as a possible culprit allergen.
Objective
To investigate possible PEG or polysorbate allergy among patients reporting prior reactions to COVID-19 vaccines or PEG and to report their subsequent tolerance of COVID-19 vaccines.
Methods
From January 1, 2021, to October 31, 2021, adult patients referred to the McGill University Health Centre allergy clinics who were considered at risk of anaphylaxis were prospectively recruited. The entry criteria were any documented history of reaction to a COVID-19 vaccine or reported allergy to PEG or polysorbate. Evaluated patients underwent skin prick testing (SPT) with PEG and polysorbate. After SPT, placebo-controlled vaccine challenges were carried out.
Results
Of the 44 patients recruited, 40 (90.1%) had reacted to the first vaccine dose, with 18 (45%) of them had anaphylactic reaction. All patients underwent SPT and 5 (11.3%) had a positive test result. A total of 39 patients (88.6%) underwent COVID-19 vaccine challenge at the allergy clinic. Most tolerated the vaccine, with 18 (40.1%) received a single full dose, 20 (45.4%) 2 split doses, and 6 (13.6%) a graded dosing protocol. Of the 40 patients who reacted to the first dose, 2 had immediate nonsevere allergic reactions to the second dose.
Conclusion
In this cohort of patients with a history of anaphylaxis and increased risk of allergic reactions to the COVID-19 vaccines, after allergist evaluation, including negative PEG skin testing result, the vaccine was safely administered without any serious adverse events.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic presents an enormous challenge for public health and clinicians globally. Using different platform technologies, vaccines have been developed and approved by various governmental agencies to help fight the COVID-19 disease. Vaccines, in general, carry the risk of anaphylaxis at a rate of 1.31 cases/1 million doses.1 The newly manufactured messenger RNA (mRNA) vaccines are associated with allergic reactions at the rate of 2.5 to 7.91 cases/1 million doses.2, 3, 4 The causal agents for the allergic reactions to mRNA COVID-19 vaccines are not clearly determined, but the excipient polyethylene glycol (PEG) 2000 has recently gained worldwide attention.5 mRNA vaccines manufactured by Pfizer-BioNTech and Moderna contain PEG 2000. The viral vector vaccines manufactured by AstraZeneca and Johnson & Johnson contain excipients such as polysorbate 80. PEG is a hydrophilic polymer with different molecular weights, widely used as an excipient in medications and skin care products.6 The rate of true allergic reactions to PEG is not known, but it is likely to be under-reported.6 Currently, there is no consensus about the utility of the skin prick testing (SPT) to PEG in determining patients at risk or who may have reacted to the mRNA COVID-19 vaccines.
At the beginning of the vaccination campaign, there have been early reports of anaphylaxis after vaccination in some patients with previous history of anaphylaxis, and at that time, it was not yet understood whether or not patients with a history of anaphylaxis are at higher risk of severe hypersensitivity reaction to COVID-19 vaccination.7 , 8 This led to the initial recommendation of the Medicines and Healthcare Products Regulatory Agency (MHRA) in the United Kingdom to exclude from vaccination any person with a history of food, drug, or vaccine anaphylaxis. Although this recommendation has been amended, there are recent publications describing COVID-19 vaccine reactions in patients with history of self-reported allergic reactions. Patients who have reported anaphylaxis to the vaccine are more likely to be women with prior history of allergic reactions.7, 8, 9 The overall recommendation for the reported allergy symptoms after receiving the COVID-19 vaccines did not impede the completion of the 2-dose vaccine in patients with prior history of allergies, supporting the overall safety of mRNA COVID-19 vaccine.10, 11, 12, 13
The primary outcome of our study was to investigate possible PEG or polysorbate allergy among patients reporting prior reactions to COVID-19 vaccines or PEG. The secondary outcomes were (1) to evaluate whether patients with a prior history of adverse reactions to the excipients found in the COVID-19 vaccines (ie, PEG or PEG chemically related compounds) can safely tolerate one of the COVID-19 vaccines; (2) to evaluate whether patients with adverse reaction to their first COVID-19 vaccine may safely tolerate the second dose; and (3) to evaluate the number of confirmed allergies to excipients including PEG, polysorbate 80, and Cremophor EL (PEG 35) (Milipore Sigma cat # 238470, Darmstadt, Germany).
Methods
Study Design and Patient Recruitment
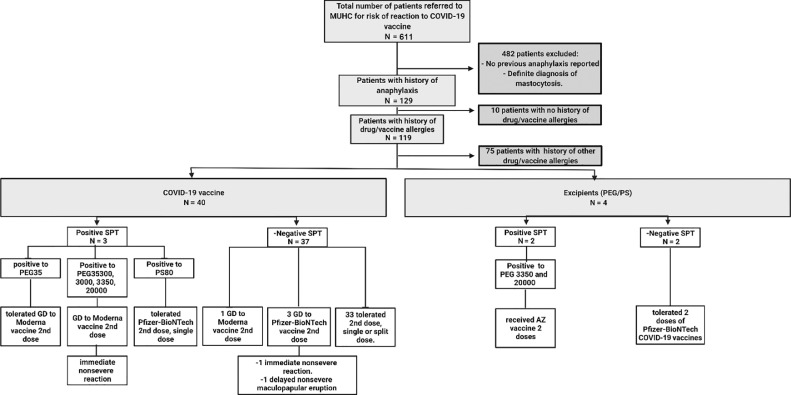
This is a prospective study conducted from January 1, 2021, to October 31, 2021. All adult patients who were referred to the McGill University Health Centre (MUHC) allergy clinic with a history of anaphylaxis to medications, vaccines, food, or Hymenoptera venom, suspected PEG or polysorbate allergy, or a prior hypersensitivity reaction to COVID-19 vaccination were approached for potential participation in the study. However, we only report the patients with allergic reaction to the COVID-19 vaccine or with history of reaction to PEG-related compounds. The inclusion criteria were any documented history of anaphylaxis (involvement of 2 organs or more with abnormal vital signs), or suspected past reactions or documented reactions to PEG, or the actual COVID-19 vaccine (Fig 1) . Written and oral informed consent was obtained from all the patients. The study was approved by MUHC institutional review board committee (ARCOV/2021-7510). The study procedures included a (1) SPT to PEG chemically related compounds, (2) a placebo-controlled challenge to the COVID-19 vaccine, and (3) a survey 1 week after the vaccination for the assessment of delayed symptoms.
Figure 1.
Flowchart of patients recruited and exclusion criteria. Most of the patients reported an allergic reaction to more than 1 agent. AZ, AstraZeneca; COVID-19, coronavirus disease 2019; GD, graded dosing; MUHC, McGill University Health Centre; PEG, polyethylene glycol; PS, polysorbate; SPT, skin prick test.
Skin Prick Testing
PEG chemically related compounds were prepared in sterile water at the laboratory of the Research Institute of the McGill University Health Centre (MUHC-IR), Montréal, Quebec, Canada (eTable 1). Evaluated patients underwent PEG SPT with lower molecular weight (MW) PEGs: PEG 35 (Cremophor EL), PEG 300, PEG 3000, polysorbate 80, and high MW PEG 20,000.6 PEG 3350 (50% wt/vol) (Lax-A-Day; Pendopharm, Montreal, Quebec, Canada) was also used for SPT. The dilutions were performed as per previously published protocols.6 SPT was performed on the forearm with a positive control of histamine 10 mg/mL and a negative control of normal saline, with results recorded 15 minutes after the test. A positive reaction was defined as a recorded wheal size measuring 3 mm in diameter or more compared with the negative control (normal saline). Excipient skin testing alone was used in our cohort considering the global shortage of vaccine supply during the pandemic.
eTable 1.
Preparation Protocols for Different Molecular Weight PEGs and Polysorbate Used for SPT6
| Compound | Manufacturer and cat# | Dilution | Example for 10 mL | Details |
|---|---|---|---|---|
| PEG 300 | Sigma cat# 81162 |
No dilution | PEG is used undiluted | |
| PEG 3000 | Sigma cat# 8190151 |
50% (w/v) | 5 g compound | 5 g in 5 mL sterile water: Incubator 37°C for 2 h to dissolve. Centrifuge 500 g, 5 min. Adjust final volume to 10 mL with sterile water. |
| PEG 3350 | Lax-a-Day | 50% (w/v) | 5 g compound | 17 g diluted in 34 mL water. |
| PEG 20,000 | Sigma cat# 813300 |
.1%-20% (w/v) | 4 g compound | 4 g in 14 mL sterile water: Incubator 37°C for 2 h to dissolve. Centrifuge 500 g, 5 min. Adjust final volume to 20 mL with sterile water. 10% PEG 20,000: mix 8 mL of 20% PEG 20,000 with 8 mL sterile water 1% PEG 20,000: mix 2 mL of 10% PEG 20,000 with 18 mL sterile water 0.1% PEG 20,000: mix 2 mL of 1% PEG 20,000 with 18 mL sterile water 0.01% PEG 20,000: mix 2 mL of 0.1% PEG 20,000 with 18 mL sterile water |
| Polysorbate 80 (tween80) | Sigma cat# P1754 | 20% (v/v) | 5 mL compound | 5 mL + 20 mL sterile water. Vortex |
| Cremophor EL (PEG35) | Sigma cat#238470-1set | 50% (v/v) | 1 bottle 25 mL (25 g compound) | 25 mL + 25 mL ethanol 50% Final concentration: 527 mg/mL |
Abbreviations: PEG, polyethylene glycol; SPT, skin prick test.
Challenge
After SPT, the patients underwent a single-blinded placebo-controlled challenge to either Lax-A-Day (oral laxative containing PEG 3350), before the availability of the vaccine in our vaccine provocation unit, or 1 of the mRNA COVID-19 vaccines (1- or 2-split doses or graded dosing). The placebo consisted of normal saline 0.5 mL. It was administered intramuscularly to the patient in the deltoid muscle, before the actual vaccine dose, followed by a 30-minute observation period before administering the vaccine. Patients were observed for 30 minutes between doses and for 1 hour after the previous dose. If the result of the challenge was negative, the patients were instructed to receive the vaccines or their subsequent doses in a vaccination center. In a subcohort of the Allergic Reaction to COVID-19 Vaccine study, patients who underwent graded dosing administration are reported in another published article.14
None of the patients received AstraZeneca in the allergy clinic due to unavailability.
One-Week Survey
For the patients who received the COVID-19 vaccine, the presence or absence of a nonimmediate reaction to the vaccine was assessed after 1 week by telephone or an electronic survey.
Data Analysis
All statistical analyses were done using R version 4.1.0 (R Core Team [2013]; R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria), and figures were generated using BioRender (BioRender.com) program. The patient's demographics and clinical characteristics were described by percentage for categorical data and by median (interquartile range [IQR]) for continuous data. All variables, except age, were dichotomized.
Results
Among the 129 patients who met the enrolment criteria, 44 reported hypersensitivity reaction to COVID-19 vaccine or PEG-related compounds, 39 (88.7%) were of female sex (median age, 55.5 years), and most were White (N = 35, 79.5%) (Table 1 ). The history of anaphylaxis was reported in the context of reactions to the COVID-19 vaccines (N = 40 from 44, 91%), PEG, or polysorbate (N = 4 from44, 9%). Anaphylaxis was defined using the Brighton (N = 28, 63.6%) and the National Institute of Allergy and Infectious Diseases (NIAID) and Food Allergy and Anaphylaxis Network criteria (N = 18, 41%); 64.2% of the patients diagnosed with anaphylaxis according to the Brighton criteria also met the anaphylaxis criteria according to the NIAID15 , 16 (Table 2 ). There were 6 patients who underwent graded dosing to the second dose in the allergy unit. They reported immediate pruritus (N = 6), hives (N = 6), angioedema (N = 6), and difficulty in breathing (N = 2) after the first dose of the COVID-19 vaccine.
Table 1.
Basic Clinical Characteristics for the Recruited Patients (N = 44)
| Variable | N (%) |
|---|---|
| Age, y (median, IQR) | 55.50 (39.75-65.00) |
| Sex (female) | 39 (88.7%) |
| Ethnicity | |
| White | 35 (79.5%) |
| East Asian | 4 (9.1%) |
| African | 2 (4.5%) |
| Middle East/Aboriginal/Latino | 3 (6.8%) |
| Past medical history of any allergic reaction | 44 (100%) |
| Drug allergy label | 44 (100%) |
| Food allergy label | 15 (34.1%) |
| Allergic rhinitis | 15 (34.1%) |
| Asthma | 9 (20.5%) |
| Atopic dermatitis | 2 (4.5%) |
| Idiopathic urticaria or angioedema | 8 (18.2%) |
| History of anaphylaxis | 12 (27.3%) |
| Charlson Comorbidity Index score | |
| Mild1,2 | 22 (50%) |
| Moderate3,4 | 12 (27.2%) |
| Severe (≥5) | 10 (22.7%) |
| Current medications | |
| Beta-blockers | 3 (6.8%) |
| ACE inhibitors | 4 (9.1%) |
| Angiotensin-receptor blockers | 4 (9.1%) |
| Immunosuppressive medications | 5 (11.4%) |
| Prednisone | 2 (4.5%) |
| Anticoagulants | 2 (4.5%) |
Abbreviations: ACE, angiotensin-converting enzyme; IQR, interquartile range.
Table 2.
Reported Allergic Reactions (N = 44)
| Reported allergic reactions | |
| Antibiotic or antifungal | 8 (18.2%) |
| Anti-inflammatory | 3 (6.8%) |
| Neurologic agents | 1 (2.3%) |
| Chemotherapy agent | 1 (2.3%) |
| Vaccine | 5 (11.4%) |
| Drug that contains similar ingredients as the COVID-19 vaccine | 4 (9.1%) |
| SARS-CoV-2 vaccine | 40 (90.9%) |
| Latency of allergic reaction | |
| Less than 6 mo | 37 (84.1%) |
| More than 6 mo | 7 (15.9%) |
| Reported symptoms | |
| Dermatologic | 28 (63.6%) |
| Respiratory | 24 (54.5%) |
| Cardiovascular | 11 (25%) |
| Gastrointestinal | 4 (9.1%) |
| Time between exposure to allergen and reported reactions | |
| Less than 1 h | 31 (70.4%) |
| More than 1 h | 13 (29.5%) |
| Hospitalization for the allergic episode ICU |
3 (6.8%) 1 (33.3%) |
| Treatment administered | |
| Epinephrine | 9 (20.5%) |
| Antihistamines | 36 (81.8%) |
| Inhalers | 1 (2.3%) |
| Cortisone | 7 (15.9%) |
| Intravenous fluids | 2 (4.5%) |
| No treatment | 6 (13.6%) |
| Do not know | 1 (2.3%) |
Abbreviations: COVID-19, coronavirus disease 2019; FAAN, Food Allergy and Anaphylaxis Network; ICU, intensive care unit; NIAID, National Institute of Allergy and Infectious Diseases; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Latency: time between the reaction and the allergy evaluation.
Skin Prick Testing
All 44 patients underwent SPT to PEG chemically related compounds, and 2 (5.9%) had immediate positive SPT result (Table 3 ). There were 3 (6.8%) patients who had delayed positive SPT result to PEG chemically related compounds (3-6 hours after SPT in the clinic); In our cohort, no patient had positive reactivity to SPT for both polysorbate 80 and PEGs.
Table 3.
Characteristics of the Patients With Positive SPT Result
| Age and sex | History of HSR | Clinical manifestations of PEG allergy | Treatment for the HSR | Positive PEG skin testing | COVID-19 vaccine administered | Setting of COVID-19 vaccine administration | Clinical manifestations after vaccination |
|---|---|---|---|---|---|---|---|
| 57/M | PEG 3350 (Lax-a-day) | Throat tightness, chest tightness, and facial swelling. | Cetirizine 20 mg | SPT positive to PEG 3350: 10 mm, PEG 20,000 10%: 9 mm, PEG 20,000 1%: 9 mm, PEG 20,000 0.1%: 7 mm, PEG 20,000 0.01%: 9 mm. Flare was not recorded Tested 1 wk after the second vaccination. |
AstraZeneca | He received both doses in the vaccination center | No reaction |
| 69/F |
PEG 3350 (PEGylate) | Angioedema of the lips and tongue with generalized hives. | Spontaneous resolution | SPT positive to PEG 3350: W: 7 mm, F: 7 mm, PEG 20,000 10%: W: 10 mm, F: 30 mm, 1%: W: 8 mm, F: 20 mm, 0.1%: W: 8 mm, F: 10 mm, 0.01%: W: 6 mm, F: 8 mm DPT + PEG 3350 (Lax-a-Day): diffuse urticaria and lip angioedema 10 min after ingesting 7 g. Treated with cetirizine. Tested 1 wk before the first vaccination. |
AstraZeneca | Received both doses in the vaccination center | No reaction |
| 39/F | COVID-19 vaccine Pfizer-BioNTech | Palpitation, generalized hives, erythema, throat globus sensation, and vomiting. | Epi-pen, Benadryl, and Hydrocortisone injection. | SPT delayed positive to polysorbate 80, after 4 h. Tested 2 mo after the second vaccination. |
Pfizer-BioNTech | Received both doses in the vaccination center | First dose: uneventful. Second dose: as described. |
| 49/F | -COVID-19 vaccine (Moderna) first dose -Cefazolin -Amoxicillin |
Generalized erythema and urticaria. | Cetirizine 10 mg | SPT delayed positive to Cremophor EL (PEG 35) wheal 9 mm after 3 h. DPT with PEG 3350 (Lax-A-Day) was negative. Tested 3 mo before the second vaccination. |
Moderna | Graded dosing | Pruritus and subjective throat globus sensation, managed with cetirizine. |
| 66/F | -Moderna COVID-19 vaccine -Anileridine -Prevnar vaccine -Shingrix vaccine |
Generalized hives with angioedema and pruritus. | Benadryl and rupatadine. | SPT delayed positive to Cremophor EL (PEG 35): 5 mm, PEG 300: 6 mm, PEG 3000: 5 mm, PEG 3350: 6 mm, PEG 20,000 (10%, 1%, 0.1%, and 0.01%) 5 mm. Tested 1 mo before the second vaccination. |
Moderna | Graded dosing | Reported small itchy spot, after the completion of the protocol. No objective finding. |
Abbreviations: COVID-19, coronavirus disease 2019; DPT, drug provocation test; F, female; HSR, hypersensitivity reaction; M, male; PEG, polyethylene glycol; SPT, skin prick test.
Polyethylene Glycol 3350 Oral Challenge
After the SPT to PEG chemically related compounds, 18 (41%) patients underwent oral challenge to PEG 3350, before we had access to the COVID-19 vaccine for administration in the allergy clinic. Among the 18 patients challenged to oral PEG 3350 in the form of laxative, 1 patient presented with hives and angioedema; this patient had immediate positive skin testing result to PEG 3350 and PEG 20,000 (eFig 1). She received AstraZeneca vaccine safely. Patients with a negative result to PEG 3350 challenge were safely vaccinated in the vaccination center using Pfizer-BioNTech (N = 1), Moderna (N = 1), or AstraZeneca vaccines (N = 2) (Table 4 ).
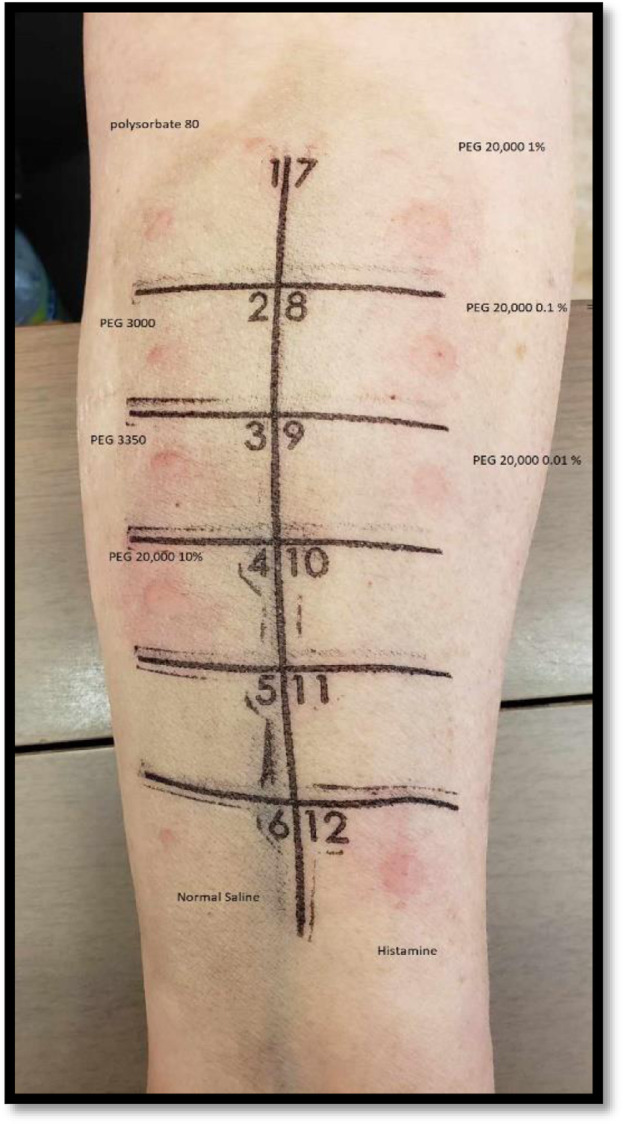
eFigure 1.
The results illustrated here were slightly positive for normal saline (dermographism), but the patient had larger reaction with PEG 20,000 (10%, 1%, 0.1%, 0.01%), PEG 3350, PEG 3000. The difference from the normal saline was more than 3 mm. PEG, polyethylene glycol.
Table 4.
Interventions variable
| Interventions Variable | N (%) |
|---|---|
| Vaccine challenge at the allergy vaccination unit | 40 (100%) |
| Placebo dose administered | 37 (92.5%) |
| Reaction to placebo | 2 (5%) |
| Pfizer-BioNTech COVID-19 vaccine | 34 (85%) |
| Moderna's COVID-19 vaccine | 6 (15%) |
| Protocol for dose administration for challenge | |
| Single dose 100%a | 16 (40%) |
| Split dose 30% and 70%b | 18 (45%) |
| Vaccine administered using a graded dosing protocolc | 6 (15%) |
| Vaccine challenge outcome | |
| Positive test | 2 (5%) |
| Negative test | 38 (95%) |
Abbreviation: COVID-19, coronavirus disease 2019.
Single dose: the whole dose of the vaccine administered at once; example: Pfizer-BioNTech COVID-19 vaccine 0.3 mL.
Split dose: 2-step administration of the vaccine, with 30 min observation between the doses; example: Pfizer-BioNTech COVID-19 vaccine 0.1 mL and then 0.2 mL.
Graded dosing protocol: administering the vaccine in more than 3 steps with 15 to 20 min observation between the doses.
Among the 4 patients with history of reaction to PEG chemically related compounds, 1 was defined as level 1 (N = 1, 25%), 2 as level 2 (N = 2, 50%), and 1 as level 3 Brighton classification (N = 1, 25%). In addition, 2 patients (N = 2, 50%) had immediate positive skin testing result to PEG 3350 and PEG 20,000. All the patients tolerated the COVID-19 vaccine as single full dose (N = 2, 50%) or 2 split doses (N = 2, 50%) (Table 5 ).
Table 5.
Patients With History of Reaction to COVID-19 Vaccine and PEG Chemically Related Products
| Variables | Reaction to COVID-19 vaccineN = 40 (%) | Reaction to PEG chemically related compoundsN = 4 (%) |
|---|---|---|
| Brighton criteria classificationa | ||
| 1 | 4 (10%) | 1 (25%) |
| 2 | 11 (27.5%) | 2 (50%) |
| 3 | 9 (22.5%) | 1 (25%) |
| 4 | 3 (7.5%) | 0 |
| 5 | 13 (32.5%) | 0 |
| NIAID and FAAN criteriab | 15 (37.5%) | 3 (75%) |
| Immediate reactionc | 27 (67.5%) | 4 (100%) |
| Delayed reactiond | 13 (32.5%) | 0 |
| Positive SPT Negative SPT |
3 (7.5%) 37 (92.5%) |
2 (50%) 2 (50%) |
| Method of second dose of vaccine being administered | ||
|
16 (40%) | 2 (50%) |
|
18 (45%) | 2 (50%) |
|
6 (15%) | 0 |
| Reacted to second dose of the COVID-19 vaccine14 | ||
|
0 | 0 |
|
0 | 0 |
|
2 (5%) | 0 |
Abbreviations: COVID-19, coronavirus disease 2019; FAAN, Food Allergy and Anaphylaxis Network; NIAID, National Institute of Allergy and Infectious Diseases; PEG, polyethylene glycol; SPT, skin prick test.
The Brighton Collaboration case definition uses combinations of symptoms to define levels of diagnostic certainty. Brighton level 1 represents the highest level of diagnostic certainty that a reported case represents anaphylaxis; levels 2 and 3 are successively lower levels of diagnostic certainty; level 4 is a case reported as anaphylaxis but that does not meet the Brighton Collaboration case definition; and level 5 is a case that was neither reported as anaphylaxis nor meets the case definition. This study considered Brighton level 1 or 2 anaphylaxis cases.
NIAID/FAAN clinical criteria for the diagnosis of anaphylaxis must meet 1 of the following criteria: (1) acute onset with involvement of skin or mucosal tissue and either (a) respiratory compromise or (b) reduced blood pressure or associated symptoms of end-organ dysfunction; (2) 2 or more of the following occur after exposure to a likely allergen for that patient: (a) involvement of skin or mucosal tissue, (b) respiratory compromise, (c) reduced blood pressure or associated symptoms, or (d) persistent gastrointestinal symptoms; and (3) reduced blood pressure after exposure to a known allergen for that patient.
Immediate reaction: within the first hour after receiving the vaccine, the patients reported urticaria angioedema and/or throat tightness and/or bronchospasm and/or drowsiness.
Delayed reaction: after 1 h from receiving the vaccine the patient reported urticaria angioedema and/or maculopapular rash.
Coronavirus Disease 2019 Vaccine Administration
A total of 40 patients were administered the COVID-19 vaccine in the allergy clinic under observation, with most receiving placebo before the first dose (37 from 40, 92.5%). Of the 37 patients who received the placebo, 2 had reaction (throat tightness, itchiness, or difficulty in breathing). Among the 40 patients with history of immediate reaction to the COVID-19 vaccine, 11 had symptoms consistent with level 2 Brighton classification (N = 11, 27.5%). Furthermore, 16 (40%) tolerated the second dose of the vaccine as single dose, 18 (45%) tolerated the vaccine in 2 split doses, and 6 (15%) tolerated graded doses. Among the 40 patients who reported allergy to the COVID-19 vaccine and had a negative SPT result to PEG, most tolerated the second dose and 2 had nonsevere reactions (Fig 1). Among the 3 patients with positive SPT result, 1 patient presented with nonsevere reaction after the second dose (Table 5).
There were 2 (4.5%) patients who experienced mild symptoms during COVID-19 vaccine graded dosing. In addition, 1 patient reacted to the first dose of Moderna COVID-19 vaccine with immediate hives and subjective throat tightness. Skin testing result was positive for Cremophor EL (PEG 35), and they underwent graded dosing to the second dose of Moderna mRNA-1273 vaccine without any premedication. She did not report any reaction after placebo administration. During the first split dose of 0.05 mL, she reported subjective pruritus. The pruritus worsened after the second split dose of 0.05 mL, so she was given cetirizine 20 mg. Her symptoms improved after 15 minutes, then she received the third and fourth split doses that were well tolerated. The other patient reacted to the first dose of Pfizer-BioNTech COVID-19 vaccine with immediate hives, bronchospasm, and drowsiness. Skin testing result to PEG was negative, and she had graded dosing to Pfizer-BioNTech vaccine. There was no reaction after the administration of the 2 placebo doses; however, 10 minutes after her third split dose (0.15 mL) of the vaccine, she reported pruritus and redness over both her hands and arms and a pressure in the right ear. She was treated with cetirizine 20 mg orally, and her symptoms improved within 30 minutes. Furthermore, 2 hours after the previous dose, she reported pressure in her ears and lightheadedness and her systolic blood pressure level dropped from 122 mm Hg to 101 mm Hg. She was managed with 250 mL of intravenous normal saline with rapid improvement of her symptoms. She did not require epinephrine.
One-Week Post-Coronavirus Disease 2019 Vaccine Survey
The most common reported adverse effects were arm tenderness (N = 28 from 44, 63.6%), headache and fatigue (N = 10 from 44, 22.7%), and swelling on the lips/face (N = 1 from 44, 2.3%) (Table 6 ). There was 1 patient who had initially reported anaphylaxis to her first dose of Pfizer-BioNTech vaccine (Brighton classification grade 2) and had negative skin testing result. She received her second dose by a graded dosing protocol and then developed an isolated delayed whole-body generalized maculopapular rash which started 1 week after the vaccine injection and was managed with oral desloratadine and mometasone topical cream. This eruption lasted for 3 weeks and was accompanied by a mild skin desquamation but no criteria for severe cutaneous reaction. We believe that this was a delayed nonsevere generalized maculopapular eruption secondary to the vaccine.
Table 6.
One-Week Survey After Vaccine Challenge
| Variable (N = 44) | N (%) |
|---|---|
| Did you present with soreness, swelling, or redness at the site of COVID-19 vaccine injection? | |
| Yes | 28 (63.6%) |
| No | 16 (36.3%) |
| Did you present any swelling of your lips/face/elsewhere? | |
| Yes | 1 (2.3%) |
| No | 43 (97.7%) |
| Did you present any other reactions or adverse effects after the evaluation at the allergy clinic? | |
| Yes | 10 (22.7%) |
| No | 34 (77.2%) |
| Have you had any medical problems or needed to go back to hospital since your clinic visit? | |
| Yes | 0 (0%) |
| No | 44 (100%) |
| Have you used any other health services since your clinic visit (eg, general practitioner)? | |
| Yes | 0 (0%) |
| No | 44 (100%) |
Abbreviation: COVID-19, coronavirus disease 2019.
Discussion
The mechanism associated with COVID-19 vaccine adverse reactions is not well understood.17 Our study reveals the safety of COVID-19 vaccine challenges in patients with suspected COVID-19 vaccine allergy. We suspect that a non–immunoglobulin E-mediated or nonimmune mechanism was possibly responsible for the first reaction in most patients who tolerated the second dose without issues.8 There was anxiety caused by the idea of vaccination, as 2 of our patients had reaction to normal saline. This is in accordance with other studies, where most reactions to the COVID-19 vaccines were considered to be related to either anxiety, vasovagal reaction, or possible vocal cord dysfunction.18 We assessed the rate of anaphylaxis using both NIAID (N = 18) and Brighton criteria (N = 28). These results are in keeping with other studies which suggest that Brighton criteria may overdiagnose anaphylaxis.19 Given the reassuring outcome results in our cohort of patients, we believe that true anaphylactic reaction to the COVID-19 vaccine is rare, similar to other published studies.20
Studies suggest that the prevalence of PEG allergy is low.6 In our cohort, we had 2 patients with an immediate positive PEG-SPT or oral challenge to PEG. Therefore, in patients with suspected multiple allergic reactions to unrelated products, a careful review of the ingredients is paramount. In this cohort involving patients who were considered to be at high risk of developing an allergic reaction to the COVID-19 vaccine, SPT with the excipients such as PEG and its chemically related compounds is not a useful diagnostic tool. However, for patients with a concerning history of a reported reaction to PEG, the skin testing was useful in confirming the evidence of sensitization to PEG.21 , 22 A total of 17 patients with suspicion of PEG hypersensitivity underwent PEG skin testing, which was negative, and subsequently had negative challenges to PEG 3350. Thus, we report a 100% negative predictive value of PEG skin testing in this small cohort. It has been reported in a recent study that patients with confirmed sensitization to PEG tolerated mRNA COVID-19 vaccines and that this tolerance could be because of the small amount of PEG 2000 in the mRNA vaccine.23 We only had 1 patient who had a positive SPT result to PEG and underwent a challenge to PEG 3350 (Lax-A-Day), which was positive. As expected from other published data, our cohort revealed that patients who have an immediate positive SPT result to any low MW PEG will also be positive to PEG with higher MW.6 This is also described in an article published by Bruusgaard-Mouritsen et al,6 where the skin testing with different PEG molecular weights had good reproducibility among their cohort. We did not have any evidence of cross-sensitization among PEGs or polysorbate 80 in contrary to the findings of Bruusgaard-Mouritsen et al6; however, a larger study including patients with definite sensitization to PEG is needed to evaluate the usefulness of the PEG SPT to predict COVID-19 vaccine hypersensitivity.
In patients with positive delayed results, it is difficult to evaluate the significance of the SPT to PEG chemically related compounds. Allergic contact dermatitis to PEG has been reported, and it is typically diagnosed through patch testing. Delayed SPT may yield similar results because the primed T cells may need longer time to be reactivated.24 In our cohort, there were 2 patients who had delayed positive SPT result to lower MW PEG but negative to high MW PEG.
The placebo-controlled challenge protocol was an important tool allowing the exclusion of false-positive reactions with nonallergic subjective symptoms, mostly related to anxiety. The outcomes reported on the 1-week follow-up survey bring reassurance and confirm the absence of delayed adverse reactions to the COVID-19 vaccines. Other nonsevere delayed reactions, such as cutaneous events after COVID-19 vaccination, are generally minor and self-limited and should not discourage subsequent vaccination.25
Regarding limitations, this study is limited by the small single-center cohort with limited history information regarding the initial reported adverse reactions to the COVID-19 vaccine. Furthermore, most of the recruited patients had reported mild-to-moderate reactions and no severe anaphylaxis cases were rechallenged with the COVID-19 vaccine.
This study was conducted during the time of uncertainty about the usefulness of PEG skin testing in the management of suspected allergic reaction to the COVID-19 vaccine. International guidelines have provided evolving recommendations since the beginning of the vaccination campaign regarding the vaccination of high-risk patients, and we expect that recommendations may be updated with further information.
In conclusion, our study adds to the current existing evidence that PEG testing is not required when assessing for allergy to the COVID-19 vaccine. In our cohort of patients with a history of PEG allergy or prior reported allergic reaction to a COVID-19 vaccine, following allergist evaluation, the vaccine administration was safe and not associated with severe adverse events. Severe adverse reactions to the COVID-19 vaccines are rare events, and most patients that present a reaction to the first dose are not precluded from revaccination with a second dose, either by challenge or graded dosing. Detailed history can help guide patient risk stratification based on the reported reaction, the clinician assessment, and the availability of resources. Our work has allowed us to participate in the COVID-19 massive vaccination campaign in the fight against the COVID-19 pandemic. Large cohort studies challenging all patients with suspected COVID-19 vaccine hypersensitivities are needed to establish the true risk of COVID-19 vaccine allergy.
Supplementary Data
Footnotes
Dr Copaescu and Dr Isabwe are senior co-authors.
Disclosures: The authors have no conflicts of interest to report.
Funding: Dr Copaescu received support from the Montreal General Hospital Foundation and Research Institute of the McGill University Health Centre.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.anai.2022.05.014.
References
- 1.McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137(3):868–878. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020-January 18, 2021. JAMA. 2021;325(11):1101–1102. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326(14):1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenhawt M, Abrams EM, Shaker M, Chu DK, Khan D, Akin C, et al. The risk of allergic reaction to SARS-CoV-2 vaccines and recommended evaluation and management: a systematic review, meta-analysis, GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract. 2021;9(10):3546–3567. doi: 10.1016/j.jaip.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sellaturay P, Nasser S, Islam S, Gurugama P, Ewan PW. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin Exp Allergy. 2021;51(6):861–863. doi: 10.1111/cea.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruusgaard-Mouritsen MA, Jensen BM, Poulsen LK, Johansen JD, Garvey LH. Optimizing investigation of suspected allergy to polyethylene glycols. J Allergy Clin Immunol. 2022;149(1):168–175. doi: 10.1016/j.jaci.2021.05.020. e4. [DOI] [PubMed] [Google Scholar]
- 7.Shavit R, Maoz-Segal R, Iancovici-Kidon M, Offengenden I, Yahia SH, Maayan DM, et al. Prevalence of allergic reactions after Pfizer-BioNTech COVID-19 vaccination among adults with high allergy risk. JAMA Netw Open. 2021;4(8) doi: 10.1001/jamanetworkopen.2021.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumenthal KG, Robinson LB, Camargo CA, Jr, Shenoy ES, Banerji A, Landman AB, et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325(15):1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macy E, Pandya S, Sheikh J, Burnette A, Shi JM, Chung J, et al. Population-based incidence, severity, and risk factors associated with treated acute-onset COVID-19 mRNA vaccination-associated hypersensitivity reactions. J Allergy Clin Immunol Pract. 2022;10(3):827–836. doi: 10.1016/j.jaip.2021.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Robinson LB, Patel R, Landman AB, Fu X, Shenoy ES, et al. Association of self-reported high-risk allergy history with allergy symptoms after COVID-19 vaccination. JAMA Netw Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.31034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glover RE, Urquhart R, Lukawska J, Blumenthal KG. Vaccinating against covid-19 in people who report allergies. BMJ. 2021;372:n120. doi: 10.1136/bmj.n120. [DOI] [PubMed] [Google Scholar]
- 12.Krantz MS, Stone CA, Jr, Rolando LA, Nobis AE, Koo G, Corey KB, et al. An academic hospital experience screening mRNA COVID-19 vaccine risk using patient allergy history. J Allergy Clin Immunol Pract. 2021;9(10):3807–3810. doi: 10.1016/j.jaip.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbaud A, Garvey LH, Arcolaci A, Brockow K, Mori F, Mayorga C, et al. Allergies and COVID-19 vaccines: an ENDA/EAACI position paper [e-pub ahead of print]. Allergy. doi: 10.1111/all.15241, accessed February 2, 2022. [DOI]
- 14.AlMuhizi F, Ton-Leclerc S, Fein M, Tsoukas C, Garvey LH, Lee D, et al. Successful desensitization to mRNA COVID-19 vaccine in a case series of patients with a history of anaphylaxis to the first vaccine dose. Front Allergy. 2022;3 doi: 10.3389/falgy.2022.825164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampson HA, Muñoz-Furlong A, Campbell RL, Franklin Adkinson N, Jr, Allan Bock S, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 16.Rüggeberg JU, Gold MS, Bayas JM, Blum MD, Bonhoeffer J, Friedlander S, et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5675–5684. doi: 10.1016/j.vaccine.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 17.Krantz MS, Kwah JH, Stone CA, Jr, Phillips EJ, Ortega G, Banerji A, et al. Safety evaluation of the second dose of messenger RNA COVID-19 vaccines in patients with immediate reactions to the first dose. JAMA Intern Med. 2021;181(11):1530–1533. doi: 10.1001/jamainternmed.2021.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krantz MS, Bruusgaard-Mouritsen MA, Koo G, Phillips EJ, Jr Stone CA, Garvey LH. Anaphylaxis to the first dose of mRNA SARS-CoV-2 vaccines: don't give up on the second dose! Allergy. 2021;76(9):2916–2920. doi: 10.1111/all.14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hourihane JO, Byrne AM, Blümchen K, Turner PJ, Greenhawt M. Ascertainment bias in anaphylaxis safety data of COVID-19 vaccines. J Allergy Clin Immunol Pract. 2021;9(7):2562–2566. doi: 10.1016/j.jaip.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfson AR, Robinson LB, Li L, McMahon AE, Cogan AS, Fu X, et al. First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021;9(9):3308–3320. doi: 10.1016/j.jaip.2021.06.010. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wenande E, Garvey LH. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46(7):907–922. doi: 10.1111/cea.12760. [DOI] [PubMed] [Google Scholar]
- 22.Stone CA, Jr, Liu Y, Relling MV, Krantz MS, Pratt AL, Abreo A, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7(5):1533–1540. doi: 10.1016/j.jaip.2018.12.003. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picard M, Drolet JP, Masse MS, Filion CA, ALmuhizi F, Fein M, et al. Safety of COVID-19 vaccination in patients with polyethylene glycol allergy: a case series. J Allergy Clin Immunol Pract. 2022;10(2):620–625. doi: 10.1016/j.jaip.2021.11.021. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conejero C, Loidi L, Hervella M. Contact dermatitis caused by polyethylene glycol-7 monooleate. Contact Dermatitis. 2015;72(3):185–186. doi: 10.1111/cod.12340. [DOI] [PubMed] [Google Scholar]
- 25.McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]