Abstract
The relationship between extracellular poly(3-hydroxybutyrate) (PHB) depolymerase synthesis and the unusual properties of a succinate uptake system was investigated in Pseudomonas lemoignei. Growth on and uptake of succinate were highly pH dependent, with optima at pH 5.6. Above pH 7, growth on and uptake of succinate were strongly reduced with concomitant derepression of PHB depolymerase synthesis. The specific succinate uptake rates were saturable by high concentrations of succinate, and maximal transport rates of 110 nmol/mg of cell protein per min were determined between pH 5.6 and 6.8. The apparent KS0.5 values increased with increasing pH from 0.2 mM succinate at pH 5.6 to more than 10 mM succinate at pH 7.6. The uptake of [14C]succinate was strongly inhibited by several monocarboxylates. Dicarboxylates also inhibited the uptake of succinate but only at pH values near the dissociation constant of the second carboxylate function (pKa2). We conclude that the succinate carrier is specific for the monocarboxylate forms of various carboxylic acids and is not able to utilize the dicarboxylic forms. The inability to take up succinate2− accounts for the carbon starvation of P. lemoignei observed during growth on succinate at pH values above 7. As a consequence the bacteria produce high levels of extracellular PHB depolymerase activity in an effort to escape carbon starvation by utilization of PHB hydrolysis products.
Pseudomonas lemoignei was isolated in 1965 as one of the first poly(3-hydroxybutyrate) (PHB)-degrading bacteria and was named in honor of Maurice Lemoigne, who had discovered PHB as a constituent of Bacillus megaterium in 1925 (5, 13). P. lemoignei belongs to the beta subclass of proteobacteria and is related to the Burkholderia-Ralstonia rRNA sublineage. Recently, P. lemoignei (strain A62) has been reisolated by application of a specific enrichment procedure with poly(3-hydroxyvalerate) as a sole source of carbon and energy (16). The metabolic capabilities of P. lemoignei are restricted to the utilization of a few organic acids (acetate, butyrate, valerate, pyruvate, succinate, and 3-hydroxybutyrate) and polyesters such as PHB and related polyhydroxyalkanoates (PHA). Sugars, alcohols, and amino acids are not utilized (5, 18).
The extracellular degradation of PHA depends on the secretion of specific PHA depolymerases which hydrolyze the polymer to water-soluble monomers and/or oligomers. Many PHA depolymerase proteins, as well as their structural genes, have been studied (recently reviewed in references 7 and 10). The synthesis of extracellular PHB depolymerases is highly regulated in most PHA-degrading bacteria, with the expression being generally repressed in the presence of utilizable soluble carbon sources such as glucose or organic acids (9, 12, 17, 22). In contrast, PHB depolymerase production by P. lemoignei is maximal during growth on succinate in batch culture, and the isolation of PHB depolymerase from P. lemoignei usually is done from succinate-grown cells instead of PHB-grown cells (5, 18, 19). It was found that synthesis of PHB depolymerase on succinate was pH dependent and occurred only above pH 7. Below pH 7 formation of PHB depolymerase was impaired (21).
Succinate is the only dicarboxylic acid that can be used by P. lemoignei as a sole source of carbon and energy. This is astonishing since typical dicarboxylate carriers are rather unspecific and utilize several dicarboxylates, such as succinate, malate, and fumarate. The inability of P. lemoignei to metabolize malate and fumarate and the reduced growth rates on succinate at pHs above 7 indicate the presence of an unusual carrier system and prompted us to investigate the relationship between succinate transport and PHB depolymerase synthesis.
MATERIALS AND METHODS
Strain, medium, and culture conditions.
P. lemoignei LMG2207 was grown in mineral salts medium (19) supplemented with 20 mM succinate or other carbon sources as indicated below at 30°C. Alternatively, a 50 mM 3-(morpholino)propanesulfonic acid (MOPS)- or 2-(N-morpholino)ethanesulfonic acid (MES)-based medium with reduced (0.29 g of K2HPO4 per liter) phosphate content was used for cultivation at high (>7.5) or low (<6.5) pH, respectively. Late-exponential-phase cells were harvested by centrifugation (4°C) and resuspended in ≈0.02 volumes of 50 mM MES-NaOH (pH 6.5) containing 1.25 g of MgSO4 · 7H2O (MES buffer) per liter. Cells were stored on ice until use. Growth at a low concentration of succinate (3 mM) was performed by cultivating P. lemoignei in dialysis bags containing 10 ml of the growth medium. The dialysis bags with the inoculated bacteria were shaken in 1-liter flasks filled with 500 ml of growth medium containing 3 mM succinate in order to keep the succinate at 3 mM. The dialysis bags were transferred to fresh medium (500 ml) after 5 h of growth.
Uptake of radioactive compounds.
Cells were diluted in 10 ml of 50 mM MES buffer to 0.2 to 1 mg of cell protein/ml. For the transport assay, the cells were shaken at 30°C for 2 min before 10 μl of [2,3-14C]succinate was added (47.8 mCi/mmol; 0.1 mCi/ml). Samples (0.5 ml) were taken at 20-s intervals over a period of 2 min. Cells were filtered through a nitrocellulose filter (0.45-μm pore size; 25-mm diameter; Sartorius, Göttingen, Germany), washed once with 4 ml of ice-cold MES buffer, and counted in 5 ml of Quick-szint 212 (Zinsser Analytik, Frankfurt, Germany) by using a scintillation counter. For the assay of metabolism, 3 mM unlabeled succinate was added to the cells, and the bacteria were shaken for 30 min at 30°C. The reaction was started by the addition of 10 μl of [2,3-14C]succinate (47.8 mCi/mmol; 0.1 mCi/ml), and 0.5-ml samples were taken over a period of 30 min and treated as described above. Experiments at pH values above 7 were carried out in MOPS buffer.
pH shift during transport assay.
MES-stored cells were diluted in 10 ml of distilled water to 0.2 to 1 mg of protein/ml. The resulting pH was 7.2. In one experiment, cells were used directly (pH 7.2 control). In a second experiment, 0.5 ml of 1 M MES buffer (pH 4) was added to the water-diluted cells before the reaction was started. The resulting pH was 4.9 (pH 4.9, control). The shift experiment was started with water-diluted cells (pH 7.2) by the addition of the label at zero time. After 60 s, 0.5 ml of 1 M MES buffer (pH 4) was added, resulting in a sudden shift of pH to 4.9. The uptake of label was monitored in 20-s intervals for 2 min.
Osmotic shock.
Cells were diluted to 0.2 to 1 mg of protein/ml in 10 ml of MES buffer containing 1.3 mM EDTA and 17% (wt/vol) saccharose and were shaken at room temperature for 15 min. Cells were centrifuged, resuspended in the same volume of 0.5 mM MgCl2, and shaken at room temperature for 15 min (“shock”). These shocked cells were collected by centrifugation and resuspended in the same volume of ice-cold MES buffer. The initial uptake of succinate was measured immediately as described above.
Determination of succinate concentration.
The concentration of succinate was determined enzymatically in a coupled optical assay. The reaction mixture (25°C) contained 50 μl of coenzyme A (CoA; 10 mg/ml), 25 μl of GTP (6 mg/ml), 25 μl of phosphoenolpyruvate (4 mg/ml), 50 μl of NADH2 (10 mg/ml), 10 μl of lactate dehydrogenase-pyruvate kinase (4 mg/ml), 100 mM glycylglycine-KOH buffer (pH 8.4) plus 5 mM MgCl2, and 20 μl of the sample. The reaction was started by the addition of 10 μl of succinyl-CoA synthetase (5 mg/ml) (all biochemicals were from Boehringer Mannheim, Mannheim, Germany).
Assay for PHB depolymerase activity.
PHB depolymerase activity of filter-concentrated (10-kDa exclusion size) cell-free culture fluid was assayed by the initial decrease of optical density at 650 nm of PHB suspensions as described earlier (8, 9). Clearing-zone formation upon incubation at 37°C indicated PHB depolymerase activity.
RESULTS
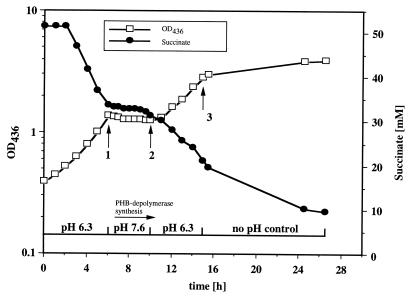
P. lemoignei is able to grow between pH 5.6 and 8.5, and high growth rates were determined during growth with 3-hydroxybutyrate (doubling time [td], 2.5 ± 0.3 h), butyrate (td, 3.5 ± 0.4 h), or acetate (td, 3.2 ± 0.4 h) as a carbon source at pH 8. Extracellular PHB depolymerase activity was not detectable during growth on 3-hydroxybutyrate, butyrate, or acetate. With succinate as a carbon source, high growth rates (td, 1.9 to 2.1 [±0.3] h) were obtained at pH 5.6, 5.8, 6.0, 6.2, 6.4, and 6.6. Extracellular PHB depolymerase activity was hardly detectable during exponential growth. However, the doubling times on succinate increased with increasing pH from 2 ± 0.4 h (pH 5.6 to 6.6) to 3.2 ± 0.4 h (pH 6.8), to 4.8 ± 0.5 h (pH 7.0), and to 8.7 ± 1 h (pH 7.2), and high levels of extracellular PHB depolymerase activity were found in the culture fluid after growth at pH ≥ 6.8. At pHs above 7.5 or below 5.5, P. lemoignei did not grow on succinate. The relationship between pH, growth, and succinate consumption during growth on succinate is shown in Fig. 1. The cells grew very fast at pH 6.3 and consumed succinate. Extracellular PHB depolymerase activity was not detectable. After 6 h of growth the pH was shifted from 6.3 to 7.6 and kept constant for 4 h. Growth and succinate consumption stopped immediately, and PHB depolymerase activity appeared ca. 1 h after the pH shift from pH 6.3 to 7.6. After reacidification to pH 6.3, growth and succinate consumption resumed (Fig. 1).
FIG. 1.
pH dependence of growth and of succinate consumption. Cells were grown in mineral salts medium supplemented with 50 mM sodium succinate at 30°C. The pH was measured continuously and kept constant at 6.3 by the addition of HCl. After 6 h (arrow 1) the pH was shifted to 7.6 by the addition of NaOH and was kept constant for 4 h before being shifted back to 6.3 (arrow 2). After 15 h (arrow 3) the pH control was switched off.
When the bacteria were grown at a very low but almost constant succinate concentration of 3 mM at different constant pH values (see Materials and Methods), high growth rates were obtained only at pHs below 6.5. Growth decreased already at pH ≥ 6.5, and PHB depolymerase appeared in the culture fluid (data not shown). Apparently, the pH above which PHB depolymerase is produced depended on the succinate concentration. These results are in agreement with similar observations of Stinson and Merrick (21) and suggest that the uptake of succinate is impaired at high pH, resulting in decreasing growth rates. In order to test this hypothesis, the uptake of [14C]succinate was studied.
Uptake of [14C]succinate.
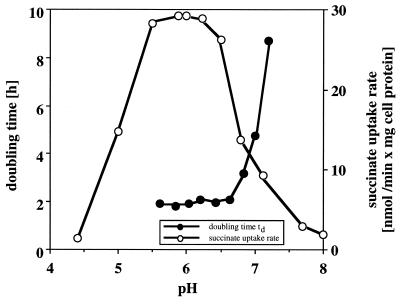
The uptake of the [14C]succinate by succinate-grown cells was linear for at least 30 min under all conditions tested and indicated the active metabolic state of the cells (data not shown). The uptake of succinate was measured at different pH values between pH 4.4 and 8. The slopes of the resulting graphs, i.e., the rates of succinate uptake, were calculated and are shown in Fig. 2. The highest uptake rates were measured between pH 5.5 and 6.5. The uptake rates decreased above pH 6.5 and below pH 5.5, and at pH 8 the succinate uptake rate was only 5% of the rate at pH 6. These results are in agreement with the pH dependence of the doubling times (Fig. 2). The growth rates on succinate were maximal and almost constant (td ≈ 2 ± 0.4 h) between pH 5.6 and 6.6 but decreased at pH above 6.6. These results suggest that the uptake of succinate is not growth limiting at pH 5.6 to 6.6 and that the increase of doubling times with increasing pH is due to carbon limitation at pHs above 6.6 because of insufficient uptake of succinate. The results are in agreement with the lack of PHB depolymerase activity between pH 5.6 and 6.6 (no carbon limitation) and the high levels of PHB depolymerase activity after growth at pH 6.8 or above (carbon limitation). Since P. lemoignei is able to grow at pH 8 on carbon sources other than succinate (e.g., acetate, butyrate, and 3-hydroxybutyrate), a pH effect on general metabolism could not be responsible for the reduced growth rates on succinate under alkaline conditions.
FIG. 2.
pH dependence of growth and succinate uptake. The uptake of [14C]succinate by succinate-grown cells of P. lemoignei was monitored for 30 min at various pH values under steady-state conditions (simultaneous uptake and consumption of succinate). The rates of succinate uptake were calculated from the slopes of the graphs (not shown) and were plotted against pH together with the doubling times of the bacteria on succinate.
To analyze the substrate specificity of the succinate uptake system, competition experiments with [14C]succinate in the presence of a sixfold excess of an unlabeled carbon source were performed (Table 1). The addition of unlabeled succinate, which served as the control, reduced the succinate uptake rate to 20% (corresponding to an inhibition of 80%). A very high degree of inhibition was obtained with maleate (97%). Other dicarboxylates inhibited the succinate uptake rate partially (fumarate [25%] and malate [20%]). Acetate and all other monocarboxylic acids tested inhibited the uptake rates by more than 90% (Table 1). We assume that the transport system is even more specific for monocarboxylates than for most dicarboxylates.
TABLE 1.
Influence of unlabeled carbon sources on [14C]succinate transporta
| Competitive substrate | Growth of P. lemoignei on substrate | pKa1 | pKa2 | pKa3 | Inhibition of succinate uptake (%) |
|---|---|---|---|---|---|
| Dicarboxylates | |||||
| None | 0 | ||||
| Succinate | + | 4.17 | 5.64 | 80 | |
| d,l-Malate | ± | 3.42 | 5.10 | 20 | |
| Fumarate | − | 3.00 | 4.50 | 25 | |
| Maleate | − | 1.90 | 6.50 | 97 | |
| Monocarboxylates | |||||
| Acetate | + | 4.76 | 90 | ||
| Propionate | ± | 4.88 | 95 | ||
| Lactic acid | − | 3.87 | 90 | ||
| Pyruvate | + | 2.49 | 90 | ||
| Butyric acid | + | 4.82 | 95 | ||
| 4-Hydroxybutyric acid | + | 90 | |||
| 3-Hydroxybutyrate | + | 4.29 | 90 | ||
| Valerate | + | 4.81 | 90 | ||
| Octanoate | − | 4.85 | 95 | ||
| Tricarboxylate (citrate) | ± | 3.13 | 4.80 | 6.39 | 25 |
| Amino acids | |||||
| l-Alanine | − | 2.34 | 9.69 | 40 | |
| l-Aspartate | − | 1.88 | 3.86 | 9.82 | <2 |
| l-Glutamate | − | 2.19 | 4.25 | 9.67 | 5 |
| l-Lysine | − | 2.18 | 8.95 | 10.5 | 10 |
| Sugars and related compounds | |||||
| d-Glucose | − | 5 | |||
| d-Gluconate | − | 3.76 | <2 | ||
| Esters | |||||
| Acetic acid ethyl ester | ND | 40 | |||
| Formic acid methyl ester | ND | 10 | |||
| Succinic acid diethyl ester | ND | 80 | |||
| Lactic acid methyl ester | ND | 60 | |||
| d-3-Hydroxybutyric acid | ND | 85 | |||
| Methyl ester |
The uptake rates of [14C]succinate (3 mM) were determined in the absence or presence of 17 mM competitive unlabeled compound at pH 6.5. ND, not determined; +, good growth; ±, poor growth; −, no growth.
The degree of inhibition by competing with other substrates was low (<10%) for glucose, gluconate, and charged amino acids such as glutamate, aspartate, and lysine. These substrates cannot be utilized by P. lemoignei (Table 1). We conclude that the succinate transport system has no specificity for these compounds. The degree of inhibition by esters was variable (10 to 85%).
Characterization of the succinate transporter.
The measurements of [14C]succinate uptake described above were performed under metabolic steady-state conditions; i.e., the label was added after preincubation (30 min) with unlabeled succinate. Under these conditions the uptake rate of succinate is expected to be equal to the metabolic consumption rate for succinate. All subsequent experiments were performed under non-steady-state conditions in order to measure the maximal capacity of the succinate transporter, and the initial rates of succinate uptake were monitored for 2 min (see Materials and Methods).
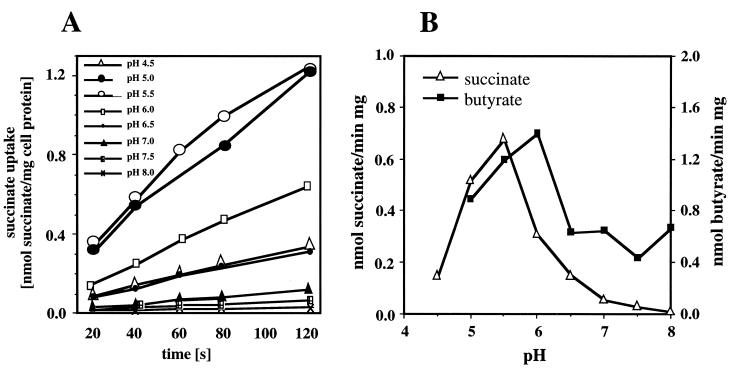
The initial uptake of [14C]succinate was linear for 2 min (Fig. 3A) and was strongly pH dependent, with an optimum at pH 5.5 (Fig. 3A and B). The rates were only 7 and 1% at pH 7.5 and 8, respectively, compared to the maximum rate. However, in contrast to the experiments performed under metabolic steady-state conditions (Fig. 2), a peak of the maximal succinate uptake rates was obtained at pH 5.5 (Fig. 3) instead of a plateau between pH 5.5 and 6.5. This indicated that the uptake of succinate is coupled to the metabolic consumption of succinate (metabolic steady state) and that the uptake is high enough to permit maximal growth (constant uptake and constant doubling time between pH 5.6 and ≈6.5 [Fig. 2]). Apparently, the bacteria were not carbon limited, and therefore expression of PHB depolymerase is not necessary. Above pH 5.5 the capacity of the succinate carrier decreased (Fig. 3), and at a pH above ≈6.5 the uptake of succinate became growth limiting, resulting in increasing doubling times and initiation of PHB depolymerase synthesis.
FIG. 3.
pH dependence of succinate and butyrate transport. (A) The initial uptake of [14C]succinate by succinate-grown cells of P. lemoignei was measured at various pH values. (B) The rates of succinate transport were calculated from the slopes. Additionally, the specific transport rates of [14C]butyrate (2 μM labeled and 100 μM unlabeled butyrate) by succinate-grown cells are shown.
Similar results were obtained for the uptake of [14C]butyrate: a maximal transport rate was measured at pH 6, and lower rates were found between pH 6.5 and pH 8 (Fig. 3B). However, a continuous decrease of the butyrate transport rate with increasing pH was not found. The butyrate transport rates were similar between pH 6.5 and 8 and are in agreement with the ability of P. lemoignei to utilize butyrate at pH 8.
Inhibitors.
The inhibition of the initial succinate uptake rates by various inhibitors was analyzed (Table 2). Inhibitors of the respiratory chain, NaN3 and KCN (each at 1 mM), inhibited the uptake rate to 50 and 70%, respectively. A complete inhibition was obtained when the bacteria were preincubated with KCN or NaN3 for 15 min. Inhibitors of the proton motive force, such as the ionophores tetrachlorosalicylanilide (TCS; 0.1 mM) and nigericin (0.1 mM), inhibited the uptake of succinate completely and confirmed the energy dependence of the succinate carrier. A partial inhibition (40%) was obtained with the potassium ionophore valinomycin (0.1 mM). Vanadate, a potent inhibitor of P-type ATPases and ATP-binding-protein-dependent carriers (ABC transporter), also strongly inhibited the uptake of succinate. Evidence for the presence of essential thiol groups was obtained by measuring the sensitivity of the cells to 1 mM N-ethylmaleimide (90% inhibition at 0.1 mM).
TABLE 2.
Inhibition of [14C]succinate uptakea
| Inhibitor | Inhibitor concn (mM) | Inhibition (%) |
|---|---|---|
| None | 0 | |
| KCN | 0.1 | 40 |
| 1 | 70 | |
| 1b | >95 | |
| NaN3 | 0.1 | 30 |
| 1 | 50 | |
| 1b | >95 | |
| TCS | 0.01 | 95 |
| 0.1 | >95 | |
| 1 | >95 | |
| Valinomycin | 0.1 | 5 |
| 0.05b | 15 | |
| 0.1b | 40 | |
| Nigericin | 0.001 | 5 |
| 0.005 | 30 | |
| 0.01 | 60 | |
| 0.05 | 95 | |
| 0.1 | >95 | |
| Vanadate | 0.1 | <5 |
| 0.1b | 40 | |
| 0.5b | 60 | |
| 1b | 80 | |
| 5b | >95 | |
| N-Ethylmaleimide | 0.1 | 90 |
| 1 | >95 |
The degree of inhibition of the succinate uptake rate after 2 min of preincubation in the presence of the inhibitor was determined.
Value was obtained after 15 min of preincubation in the presence of the inhibitor.
Kinetic aspects.
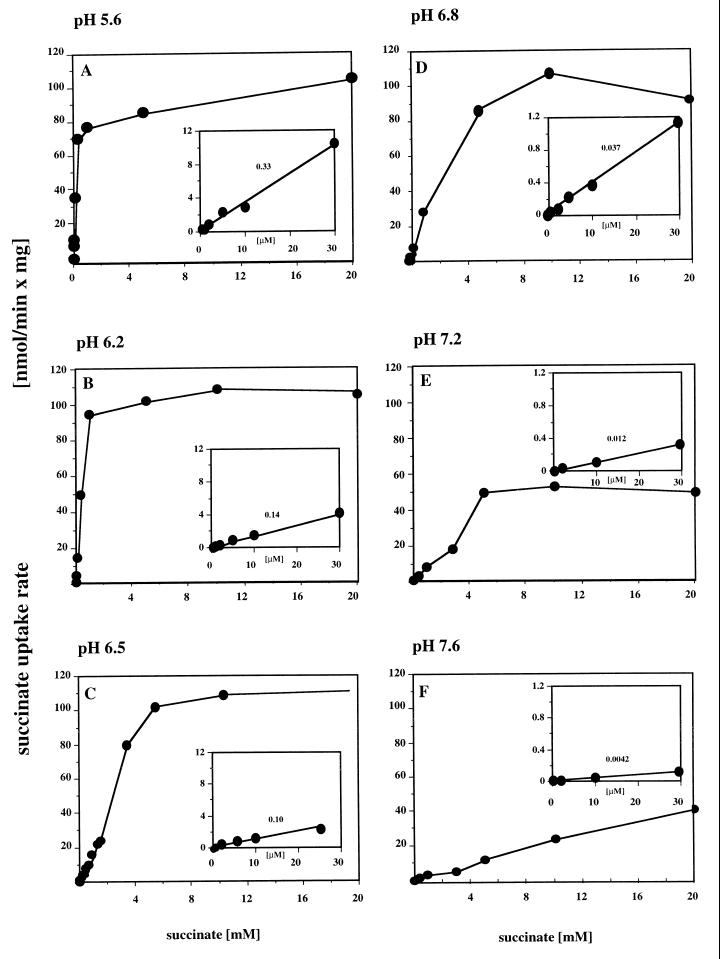
The initial transport rates were measured at various succinate concentrations between 0.5 μM and 20 mM at six different pH values (pH 5.6, 6.2, 6.5, 6.8, 7.2, and 7.6). As shown in Fig. 4A to D, saturation kinetics with plateau values between 100 and 120 nmol of succinate/mg of cell protein × min were obtained for pH values between 5.6 and 6.8. At pH 7.2 the saturation value was lower (≈50 nmol/min × mg), and at pH 7.6 a saturation of the succinate uptake rate was not obtained even at 20 mM succinate (Fig. 4E and F). A linear dependence of the uptake rate on succinate concentration was obtained for all pH values at succinate concentrations below 1 mM (see insets in Fig. 4A to F). The slopes were strongly pH dependent and decreased with increasing pH by about 2 orders of magnitude from 0.33 nmol/(min × mg of protein)/μM concentration of succinate to 0.0042 nmol/(min × mg of protein)/μM concentration of succinate (see values above the graphs in the insets [Fig. 4]). Therefore, the succinate concentration necessary for a maximal transport rate increased with increasing pH. When the data were plotted as Lineweaver-Burk plots (1/V versus 1/S), the resulting graphs hit the x axis around zero or even at positive 1/S values. Apparently, the succinate uptake system of P. lemoignei cannot be described by Michaelis-Menten kinetics. The apparent kS0.5 values for succinate were estimated from Fig. 4A to E and amounted to 0.2 mM (pH 5.6), 0.4 mM (pH 6.2), 2 mM (pH 6.5), 3 mM (pH 6.8), and 5 mM (pH 7.2). At pH 7.6 the apparent kS0.5 value could not be determined because saturation of succinate uptake was not obtained even at 20 mM succinate (Fig. 4F). However, the apparent kS0.5 value at pH 7.6 should be far above 10 mM succinate.
FIG. 4.
Dependence of succinate transport on succinate concentration at different pH values. The initial uptake of [14C]succinate by succinate-grown cells of P. lemoignei was measured at different pH values and at different succinate concentrations. The rates of succinate transport were calculated from the slopes (not shown) and were plotted against the succinate concentration. The insets provide the values for the micromolar concentration range and give the slopes of the graphs as follows: [nanomoles/(minute × milligram of protein)/micromolar concentration of succinate]. Please note the different scales in the insets in panels A to C and D to E, respectively. The experiments were performed at pH 5.6 (A), pH 6.2 (B), pH 6.5 (C), pH 6.8 (D), pH 7.2 (E), and pH 7.6 (F).
Mechanism of succinate transport.
A diffusion mechanism of succinate transport could be excluded because KCN- and NaN3-inhibited cells were completely impaired in succinate uptake. We conclude that diffusion does not contribute significantly to the uptake of succinate. The energy dependence of the succinate uptake system was strongly evidenced by its sensitivity to ionophores (TCS, nigericin, and valinomycin), to inhibitors of the respiratory chain, and to vanadate (see above). The effect of a sudden acidification (shifting the pH from 7.2 to 4.9) on transport activity was analyzed. No increase in the uptake of succinate compared to the controls was measured after the pH shift. Apparently, succinate is not transported by a proton symport mechanism. The dependence of succinate uptake on a shock-sensitive binding protein was tested by osmotic shock (see Materials and Methods). The succinate uptake rates of shocked cells were reduced to about 10 to 30% of those of untreated control cells, depending on the particular batch of cells (eight independent assays; data not shown). The sensitivity of the succinate transport system to osmotic shock, vanadate, and N-ethylmaleimide indicated the presence of a binding-protein-dependent transport system.
DISCUSSION
PHB depolymerase expression is repressed in most PHB-degrading bacteria if water-soluble monomeric carbon sources such as glucose or organic acids are available (9, 10, 12, 17, 22). P. lemoignei seems to be an exception because it produces high amounts of PHB depolymerase during growth in batch culture on succinate at pHs above 7 (21). We found that P. lemoignei grew on succinate at pH values between 5.6 and 7.2 and on 3-hydroxybutyrate in a range from pH 6 to 8.5. PHB depolymerase expression was repressed during exponential growth on 3-hydroxybutyrate independent of pH, but with succinate as a carbon source repression of PHB depolymerase synthesis occurred only at a pH of <6.6. As soon as the pH became 6.8 or higher, the uptake of [14C]succinate decreased and the doubling times increased (Fig. 1 and 2). As a consequence of insufficient carbon supply, the synthesis of PHB depolymerase is derepressed to escape starvation by utilization of PHB hydrolysis products.
But why is P. lemoignei unable to take up succinate efficiently at pHs above 7? The dicarboxylate carriers of other bacteria work efficiently at pH 7.5. The kinetic experiments in this study demonstrated that the succinate transport rates decrease drastically from pH 5.6 to 7.6 (Fig. 4). If we consider the pKa values of both carboxyl groups of succinate (pKa1, 4.2; pKa2, 5.6), the first carboxyl group is deprotonated almost completely at pH 5.6 (96%) and the second is deprotonated to about 50%. The chemical balance between [H-succinate1−] and [succinate2−] can be described by Henderson-Hasselbalch equations 1 and 2, which show that [H-succinate1−] decreases above pH 5.6 (pKa2) with increasing pH. The experimental data are in agreement with the assumption that the succinate carrier transports H-succinate1− and is unable to utilize succinate2−. In the pH shift experiment (Fig. 1), the total succinate concentration amounted to 33 mM when the pH was shifted from 6.3 to 7.6. According to equation 2, the actual concentration of H-succinate1− decreased from 6 mM at pH 6.3 to 0.36 mM at pH 7.6 and the uptake of H-succinate1− decreased drastically. Growth of the bacteria stopped, and PHB depolymerase activity appeared in the culture fluid. If the concentration of H-succinate1− is the parameter that controls the uptake and thus regulates the growth rate and PHB depolymerase synthesis, then the pH values above which growth is reduced and PHB depolymerase is derepressed should be dependent on the absolute concentration of succinate. This was exactly the case: when a constant low succinate concentration (3 mM) was used, reduced growth and carbon starvation-induced synthesis of PHB depolymerase were shifted to lower pH, apparently because [H-succinate1−] is the same at pH 6.5 and 7.6 (0.36 mM) when the total succinate concentrations are 3 and 33 mM, respectively.
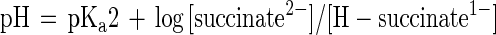 |
 |
1 |
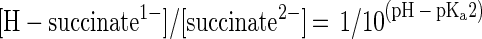 |
2 |
We obtained further evidence for the hypothesis that the succinate carrier is unable to utilize succinate2− but is specific for the monocarboxylate forms of dicarboxylic acids from the competition experiments with dicarboxylates and monocarboxylates: if the dicarboxylate forms of dicarboxylic acids were not a substrate of the succinate carrier, then the inhibition effect of maleate should be much higher than that of its trans isomer, fumarate. Experimental results (Table 1) clearly confirmed this assumption. Maleate and fumarate differ in their pKa2 values by 2 pH units (6.5 versus 4.5). At pH 6.5 (pH of the assay), only 1% of the molecules are present as fumarate1− and the degree of succinate uptake inhibition is low (25%). In contrast, ca. 50% of the maleate molecules are present as maleate1− at pH 6.5, and the degree of inhibition is high (97%). A similar calculation can be made for malate.
Monocarboxylates have pKa values below 5 and thus are ionized almost completely at pH 6.5. All monocarboxylates tested strongly interfered with the uptake of succinate (inhibition of ≥90%). We assume that the carrier has a broad substrate specificity for short-chain fatty acids and dicarboxylates with relative high pKa2 values.
Negatively charged amino acids such as aspartate and glutamate had no effect on succinate uptake, apparently because both carboxyl groups are deprotonated almost completely at pH 6.5. Accordingly, alanine, which has its one carboxyl function ionized at pH 6.5, interfered partially (40%) with the carrier, although alanine does not support growth of P. lemoignei.
Only a little information is available on the uptake of fatty acids by bacteria. In Escherichia coli the uptake of short-chain fatty acids and medium-chain fatty acids requires the ato genes (4). The uptake of long-chain fatty acids is mediated by the FadL/FadD system (1): FadL represents an outer membrane-associated transport protein, and FadD constitutes (inner) membrane-bound acyl-CoA synthetase. Recently, some properties of the energy-dependent uptake of octanoic acid by Pseudomonas putida have been described (3). A biochemical and molecular-biological analysis of the uptake mechanism of short-chain fatty acids is not well established. In E. coli several dicarboxylate carriers have been studied. Under aerobic conditions the uptake of C4 dicarboxylates is mediated by the dct system, which was proposed to be driven by the electrochemical H+ gradient (6) but which might constitute a binding-protein-dependent carrier (14). Three FNR-dependent secondary carriers for C4 dicarboxylates (dcuA, dcuB, and dcuC) have been analyzed under anaerobic conditions (see reference 23 and references therein).
Clear evidence for the energy dependence of the succinate carrier of P. lemoignei was provided by the sensitivity of succinate uptake to inhibitors of the respiratory chain (cyanide and azide) and of the proton motive force (TCS and nigericin). A diffusion mechanism could be excluded. However, the carrier differs from the C4-dicarboxylate or fatty acid carriers of other bacteria in its biochemical properties, such as its unusually high pH dependence and broad substrate specificity for mono- and dicarboxylates. The uptake of succinate could not be stimulated by artificial ΔpH as has been described for carriers of other bacteria (11, 20). Therefore, a proton symport mechanism is unlikely, and we assume that the P. lemoignei succinate carrier does not belong to the superfamily of transmembrane facilitators (15). Some evidence for the presence of a binding-protein-dependent transport system was obtained by its sensitivity to vanadate and to osmotic shock. Vanadate and shock sensitivity are characteristics of the growing group of ATP-dependent carriers (ABC transporters) (2). Furthermore, the uptake of succinate was inhibited by N-ethylmaleimide, which indicates the presence of essential thiol groups located outside of the cytoplasmic membrane.
ACKNOWLEDGMENTS
We thank B. Bowien and V. Müller for helpful advice and discussion.
This work was supported by the Deutsche Forschungsgemeinschaft and the Graduiertenkolleg “Chemische Aktivitäten von Mikroorganismen.”
REFERENCES
- 1.Black P N, Di Russo C C. Molecular and biochemical analysis of fatty acid transport, metabolism, and gene regulation in Escherichia coli. Biochim Biophys Acta. 1994;1210:123–145. doi: 10.1016/0005-2760(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 2.Boos W, Lucht J M. Periplasmic binding protein-dependent ABC transporters. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 1175–1209. [Google Scholar]
- 3.Canicero D, Fernández-Valverde M, Cañedo L M, Schleissner C, Luengo J M. Octanoic acid uptake in Pseudomonas putida U. FEMS Microbiol Lett. 1997;149:51–58. [Google Scholar]
- 4.Clark D, Cronan J E. Two-carbon compounds and fatty acids as carbon sources. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 343–357. [Google Scholar]
- 5.Delafield F P, Doudoroff M, Palleroni N J, Lusty C J, Contopoulos R. Decomposition of poly-β-hydroxybutyrate by pseudomonads. J Bacteriol. 1965;90:1455–1466. doi: 10.1128/jb.90.5.1455-1466.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutowski S J, Rosenberg H. Succinate uptake and related proton movements in Escherichia coli K12. Biochem J. 1975;152:647–654. doi: 10.1042/bj1520647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jendrossek D. Microbial degradation of polyesters: a review on extracellular poly(hydroxyalkanoic acid) depolymerases. Polym Degrad Stab. 1998;59:317–325. [Google Scholar]
- 8.Jendrossek D, Frisse A, Behrends A, Andermann M, Kratzin H D, Stanislawski T, Schlegel H G. Biochemical and molecular characterization of the Pseudomonas lemoignei polyhydroxyalkanoate depolymerase system. J Bacteriol. 1995;177:596–607. doi: 10.1128/jb.177.3.596-607.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jendrossek D, Knoke I, Habibian R B, Steinbüchel A, Schlegel H G. Degradation of poly(3-hydroxybutyrate), PHB, by bacteria and purification of a novel PHB-depolymerase from Comamonas sp. J Environ Polym Degrad. 1993;1:53–63. [Google Scholar]
- 10.Jendrossek D, Schirmer A, Schlegel H G. Biodegradation of polyhydroxyalkanoic acids. Appl Microbiol Biotechnol. 1996;49:451–463. doi: 10.1007/s002530050844. [DOI] [PubMed] [Google Scholar]
- 11.Kashket E R, Wilson T H. Proton-coupled accumulation of galactoside in Streptococcus lactis 7962. Proc Natl Acad Sci USA. 1973;70:2866–2869. doi: 10.1073/pnas.70.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasuya K, Doi Y. Enzymatic degradation of poly[(R)-3-hydroxybutyrate] by Comamonas testosteroni ATSU of soil bacterium. Polym Degrad Stab. 1994;45:379–386. [Google Scholar]
- 13.Lemoigne M. Produits de déshydration et de polymerisation de l’acide β-oxybutyrique. Bull Soc Chim Biol. 1926;8:770–782. [Google Scholar]
- 14.Lo T C Y. The molecular mechanism of dicarboxylic acid transport in Escherichia coli K 12. J Supramol Struct. 1977;7:463–480. doi: 10.1002/jss.400070316. [DOI] [PubMed] [Google Scholar]
- 15.Marger M D, Saier M H., Jr A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem Sci. 1993;18:13–20. doi: 10.1016/0968-0004(93)90081-w. [DOI] [PubMed] [Google Scholar]
- 16.Mergaert J, Schirmer A, Hauben L, Mau M, Hoste B, Kerstens K, Jendrossek D, Swings J. Isolation and identification of poly(3-hydroxyvalerate)-degrading strains of Pseudomonas lemoignei. Int J Syst Bacteriol. 1996;46:769–773. doi: 10.1099/00207713-46-3-769. [DOI] [PubMed] [Google Scholar]
- 17.Mukai K, Yamada K, Doi Y. Efficient hydrolysis of polyhydroxyalkanoates by Pseudomonas stutzeri YM1414 isolated from lake water. Polym Degrad Stab. 1994;43:319–327. [Google Scholar]
- 18.Müller B, Jendrossek D. Purification and properties of poly(3-hydroxyvaleric acid) depolymerase from Pseudomonas lemoignei. Appl Microbiol Biotechnol. 1993;38:487–492. [Google Scholar]
- 19.Nakayama K, Saito T, Fukui T, Shirakura Y, Tomita K. Purification and properties of extracellular poly(3-hydroxybutyrate) depolymerases from Pseudomonas lemoignei. Biochim Biophys Acta. 1985;827:63–72. doi: 10.1016/0167-4838(85)90101-3. [DOI] [PubMed] [Google Scholar]
- 20.Olson E B, Russel J B, Kling T H. Electrogenic l-malate transport by Lactobacillus plantarum: a basis for energy derivation from malolactatic fermentation. J Bacteriol. 1991;173:6199–6206. doi: 10.1128/jb.173.19.6199-6206.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stinson M W, Merrick J M. Extracellular enzyme secretion by Pseudomonas lemoignei. J Bacteriol. 1974;119:152–161. doi: 10.1128/jb.119.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada K, Mukai K, Doi Y. Enzymatic degradation of poly(hydroxyalkanoates) by Pseudomonas pickettii. Int J Biol Macromol. 1993;15:215–220. doi: 10.1016/0141-8130(93)90040-s. [DOI] [PubMed] [Google Scholar]
- 23.Zientz E, Six S, Unden G. Identification of a third secondary carrier (DcuC) for anaerobic C4-dicarboxylate transport in Escherichia coli: roles of the three Dcu carriers in uptake and exchange. J Bacteriol. 1996;178:7241–7247. doi: 10.1128/jb.178.24.7241-7247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]